Comparison of the Technological Application Potential of Functional Ingredients for the Meat Industry Based upon a Novel Fast Screening Tool
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Manufacturing of Lean Meat and Fatty Liver-Based Model Systems
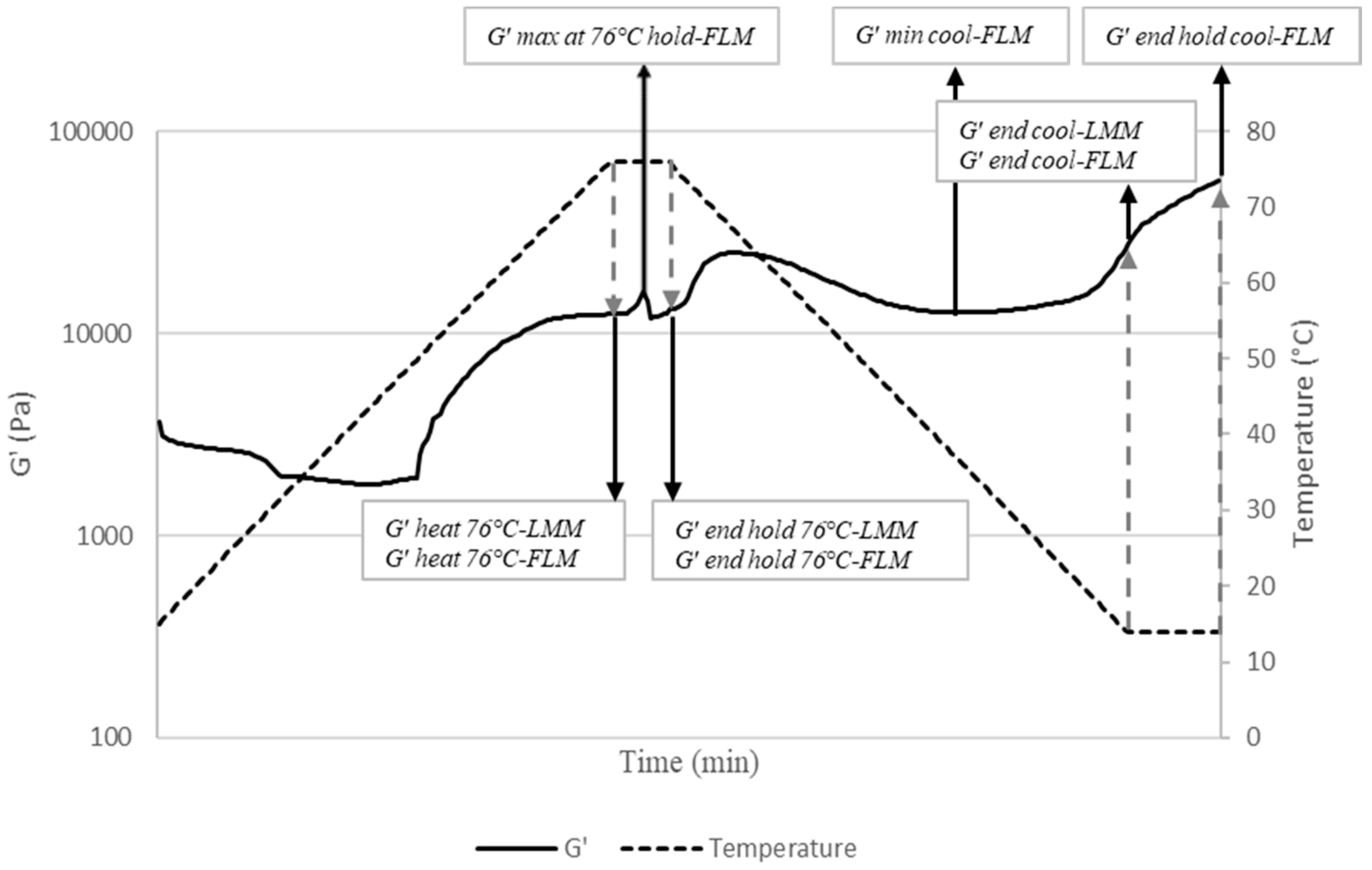
2.3. Dynamic Viscoelastic Properties
2.4. Emulsion Stability
2.5. Drip Loss
2.6. Cooking Loss
2.7. Syneresis
2.8. pH
2.9. Texture
2.10. Cluster Analysis
3. Results
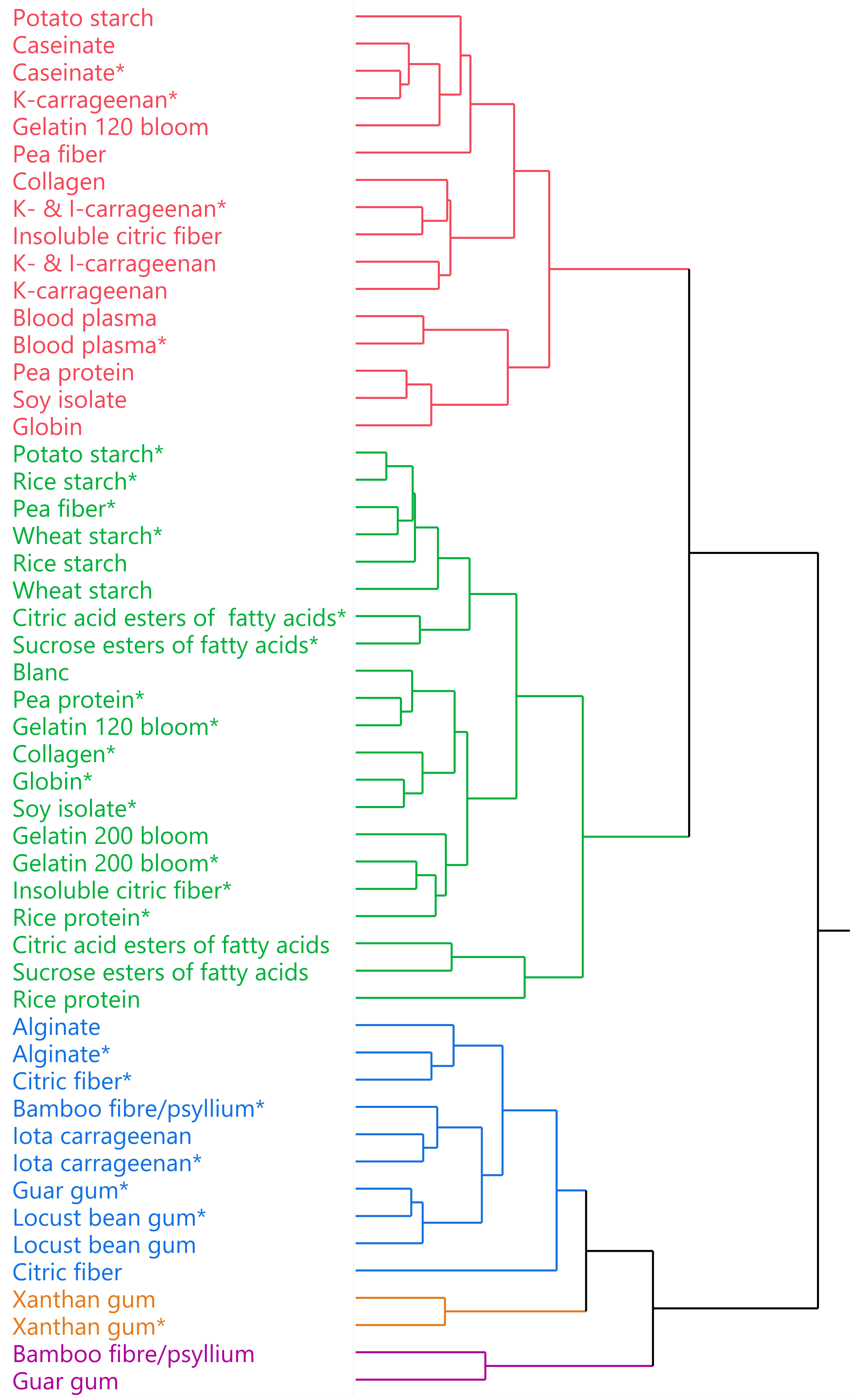
3.1. Overview Hierarchical Clustering
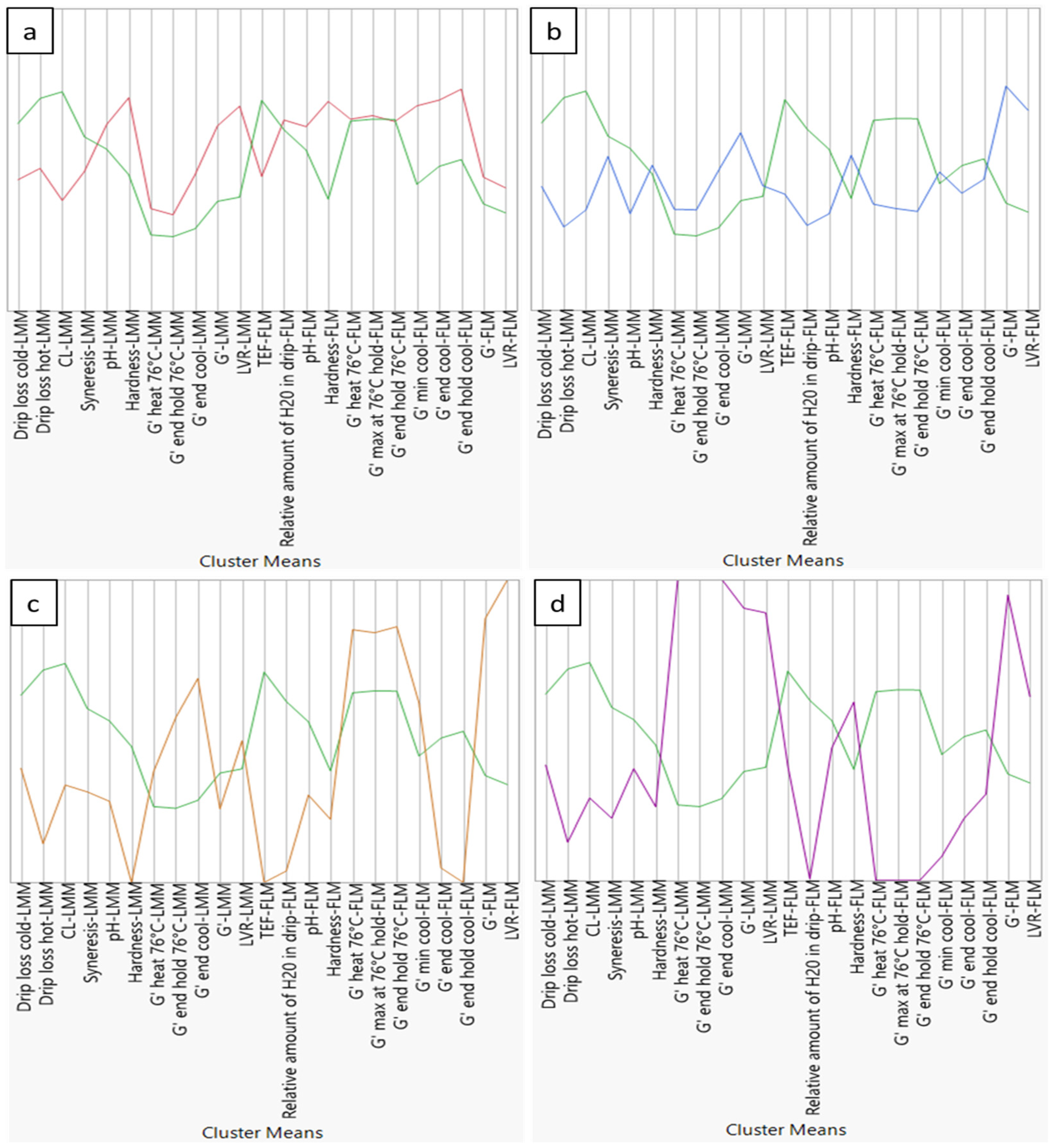
3.2. Green Reference Cluster
3.3. Red Cluster
3.4. Blue Cluster
3.5. Orange Cluster
3.6. Purple Cluster
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Balestra, F.; Petracci, M. Technofunctional Ingredients for Meat Products: Current Challenges. In Sustainable Meat Production and Processing; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 45–68. [Google Scholar] [CrossRef]
- Toldrà, M.; Lynch, S.A.; Couture, R.; Álvarez, C. Blood Proteins as Functional Ingredients. In Sustainable Meat Production and Processing; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 85–101. [Google Scholar] [CrossRef]
- Petracci, M.; Bianchi, M.; Mudalal, S.; Cavani, C. Functional ingredients for poultry meat products. Trends Food Sci. Technol. 2013, 33, 27–39. [Google Scholar] [CrossRef]
- Tarté, R. Ingredients in Meat Products: Properties, Functionality and Applications, 1st ed.; Tarté, R., Ed.; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Thangavelu, K.P.; Kerry, J.P.; Tiwari, B.K.; McDonnell, C.K. Novel processing technologies and ingredient strategies for the reduction of phosphate additives in processed meat. Trends Food Sci. Technol. 2019, 94, 43–53. [Google Scholar] [CrossRef]
- Weiss, J.; Gibis, M.; Schuh, V.; Salminen, H. Advances in ingredient and processing systems for meat and meat products. Meat Sci. 2010, 86, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Skrede, G. Comparison of various types of starch when used in meat sausages. Meat Sci. 1989, 25, 21–36. [Google Scholar] [CrossRef]
- Pietrasik, Z.; Janz, J.A.M. Utilization of pea flour, starch-rich and fiber-rich fractions in low fat bologna. Food Res. Int. 2010, 43, 602–608. [Google Scholar] [CrossRef]
- Zhang, L.; Barbut, S. Effects of regular and modified starches on cooked pale, soft, and exudative; normal; and dry, firm, and dark breast meat batters. Poult. Sci. 2005, 84, 789–796. [Google Scholar] [CrossRef]
- Youssef, M.K.; Barbut, S. Effects of two types of soy protein isolates, native and preheated whey protein isolates on emulsified meat batters prepared at different protein levels. Meat Sci. 2011, 87, 54–60. [Google Scholar] [CrossRef]
- Egbert, W.R.; Payne, C.T. Plant Proteins. In Ingredients in Meat Products: Properties, Functionality and Applications; Tarté, R., Ed.; Springer: New York, NY, USA, 2009; pp. 111–129. [Google Scholar] [CrossRef]
- Sánchez, V.E.; Bartholomai, G.B.; Pilosof, A.M.R. Rheological Properties of Food Gums as related to their water binding capacity and to soy protein interaction. LWT Food Sci. Technol. 1995, 28, 380–385. [Google Scholar] [CrossRef]
- Gordon, A.; Barbut, S.; Schmidt, G. Mechanisms of meat batter stabilization: A review. Crit. Rev. Food Sci. Nutr. 1992, 32, 299–332. [Google Scholar] [CrossRef]
- Glorieux, S.; Goemaere, O.; Steen, L.; Fraeye, I. Phosphate Reduction in Emulsified Meat Products: Impact of Phosphate Type and Dosage on Quality Characteristics. Food Technol. Biotechnol. 2017, 55, 390–397. [Google Scholar] [CrossRef]
- Perez-Santaescolastica, C.; Goemaere, O.; Hanskens, J.; Lorenzo, J.M.; Fraeye, I. Effect of stabiliser classes (animal proteins, vegetable proteins, starches, hydrocolloids and dietary fibre) on the physicochemical properties of a model lean meat product. Int. J. Food Sci. Tech. 2020, 55, 970–977. [Google Scholar] [CrossRef]
- Johnson, R.A.; Wichern, D.W. Applied Multivariate Statistical Analysis, 6th ed.; Prentice Hall: Hoboken, NJ, USA, 2007. [Google Scholar]
- Sultana, S.; Ali, M.E.; Ahamad, M.N.U. Gelatine, collagen, and single cell proteins as a natural and newly emerging food ingredients. In Preparation and Processing of Religious and Cultural Foods; Ali, M.E., Nizar, N.N.A., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 215–239. [Google Scholar] [CrossRef]
- Phillips, G.; Williams, P. Handbook of Hydrocolloids, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2009; pp. 1–924. [Google Scholar]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Schilling, M.W. Emulsifier Applications in Meat Products. In Food Emulsifiers and Their Applications; Hasenhuettl, G.L., Hartel, R.W., Eds.; Springer: New York, NY, USA, 2019; pp. 347–377. [Google Scholar] [CrossRef]
- Gu, R.; Xiao, X.; Sun, J.; Shi, L.; Yang, H. Effects of rice residue on physicochemical properties of silver carp surimi gels. Int. J. Food Prop. 2018, 21, 1743–1754. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Zhang, L.; Li, R.; Zheng, B.; Rea, M.C.; Miao, S. Effect of plant protein mixtures on the microstructure and rheological properties of myofibrillar protein gel derived from red sea bream (Pagrosomus major). Food Hydrocoll. 2019, 96, 537–545. [Google Scholar] [CrossRef]
- Prabhu, G.A. Utilizing functional meat-based proteins in processed meat applications. In Proceedings of the 55th Annual Reciprocal Meat Conference, Savoy, IL, USA, 29 July 2002; pp. 29–34. [Google Scholar]
- O’Regan, J.; Ennis, M.P.; Mulvihill, D.M. Milk proteins. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 298–358. [Google Scholar] [CrossRef]
- Lynch, S.A.; Mullen, A.M.; O’Neill, E.E.; García, C.Á. Harnessing the Potential of Blood Proteins as Functional Ingredients: A Review of the State of the Art in Blood Processing. Compr. Rev. Food Sci. Food Saf. 2017, 16, 330–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youssef, M.K.; Barbut, S. Effects of caseinate, whey and milk proteins on emulsified beef meat batters prepared with different protein levels. J. Muscle Foods 2010, 21, 785–800. [Google Scholar] [CrossRef]
- Tarté, R. Meat-Derived Protein Ingredients. In Ingredients in Meat Products: Properties, Functionality and Applications; Tarté, R., Ed.; Springer: New York, NY, USA, 2009; pp. 145–171. [Google Scholar] [CrossRef]
- Sarteshnizi, R.; Hosseini, H.; Mousavi Khaneghah, A.; Narges, K. A review on application of hydrocolloids in meat and poultry products. Int. Food Res. J. 2015, 22, 872–887. [Google Scholar]
- Ayadi, M.A.; Kechaou, A.; Makni, I.; Attia, H. Influence of carrageenan addition on turkey meat sausages properties. J. Food Eng. 2009, 93, 278–283. [Google Scholar] [CrossRef]
- Lamkey, J.W. Nonstarch Hydrocolloids. In Ingredients in Meat Products: Properties, Functionality and Applications; Tarté, R., Ed.; Springer: New York, NY, USA, 2009; pp. 57–82. [Google Scholar] [CrossRef]
- Imeson, A.P. Carrageenan and furcellaran. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 164–185. [Google Scholar] [CrossRef]
- DeFreitas, Z.; Sebranek, J.G.; Olson, D.G.; Carr, J.M. Carrageenan Effects on Salt-Soluble Meat Proteins in Model Systems. J. Food Sci. 1997, 62, 539–543. [Google Scholar] [CrossRef]
- Verbeken, D.; Neirinck, N.; Van Der Meeren, P.; Dewettinck, K. Influence of κ-carrageenan on the thermal gelation of salt-soluble meat proteins. Meat Sci. 2005, 70, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Foegeding, E.A.; Ramsey, S.R. Rheological and Water-Holding Properties of Gelled Meat Batters Containing Iota Carrageenan, Kappa Carrageenan or Xanthan gum. J. Food Sci. 1987, 52, 549–553. [Google Scholar] [CrossRef]
- Mehta, N.; Ahlawat, S.S.; Sharma, D.P.; Dabur, R.S. Novel trends in development of dietary fiber rich meat products-a critical review. J. Food Sci. Technol. 2015, 52, 633–647. [Google Scholar] [CrossRef] [Green Version]
- Yegin, S.; Kopec, A.; Kitts, D.D.; Zawistowski, J. Dietary fiber: A functional food ingredient with physiological benefits. In Dietary Sugar, Salt and Fat in Human Health; Preuss, H.G., Bagchi, D., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 531–555. [Google Scholar] [CrossRef]
- Whiting, R.C. Addition of Phosphates, Proteins, and Gums to Reduced-Salt Frankfurter Batters. J. Food Sci. 1984, 49, 1355–1357. [Google Scholar] [CrossRef]
- Ramírez, J.A.; Barrera, M.; Morales, O.G.; Vázquez, M. Effect of xanthan and locust bean gums on the gelling properties of myofibrillar protein. Food Hydrocoll. 2002, 16, 11–16. [Google Scholar] [CrossRef]
- Montero, P.; Hurtado, J.L.; Pérez-Mateos, M. Microstructural behaviour and gelling characteristics of myosystem protein gels interacting with hydrocolloids. Food Hydrocoll. 2000, 14, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.L.; Blanchard, S.P. Viscoelastic Properties of Myofibrillar Protein-Polysaccharide Composite Gels. J. Food Sci. 1993, 58, 164–167. [Google Scholar] [CrossRef]
- Demirci, Z.O.; Yılmaz, I.; Demirci, A. Effects of xanthan, guar, carrageenan and locust bean gum addition on physical, chemical and sensory properties of meatballs. J. Food Sci. Technol. 2014, 51, 936–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Product Class/Tested Dosage (LD–HD) | Product Name |
|---|---|
| Animal protein 1–2% | Collagen |
| Gelatin 120 bloom | |
| Gelatin 200 bloom | |
| Blood plasma | |
| Globin | |
| Caseinate | |
| Vegetable protein 1–2% | Pea protein |
| Rice protein | |
| Soy isolate | |
| Starch 1.5–3% | Potato starch |
| Rice starch | |
| Wheat starch | |
| (in)Soluble fiber 1–3% | Insoluble citric fiber |
| Citric fiber | |
| Bamboo fiber/psyllium | |
| Pea fiber | |
| Gum 0.5–1% | Guar gum |
| Xanthan gum | |
| Locust bean gum | |
| Alginate | |
| κ-carrageenan | |
| κ-ι-carrageenan | |
| ι-carrageenan | |
| Emulsifier sensu stricto 0.3–1% | Citric acid esters of fatty acids |
| Sucrose esters of fatty acids |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goemaere, O.; De Ketelaere, B.; Hanskens, J.; Masijn, Q.; Pérez Santaescolastica, C.; Fraeye, I. Comparison of the Technological Application Potential of Functional Ingredients for the Meat Industry Based upon a Novel Fast Screening Tool. Foods 2021, 10, 2078. https://doi.org/10.3390/foods10092078
Goemaere O, De Ketelaere B, Hanskens J, Masijn Q, Pérez Santaescolastica C, Fraeye I. Comparison of the Technological Application Potential of Functional Ingredients for the Meat Industry Based upon a Novel Fast Screening Tool. Foods. 2021; 10(9):2078. https://doi.org/10.3390/foods10092078
Chicago/Turabian StyleGoemaere, Olivier, Bart De Ketelaere, Jana Hanskens, Quinten Masijn, Cristina Pérez Santaescolastica, and Ilse Fraeye. 2021. "Comparison of the Technological Application Potential of Functional Ingredients for the Meat Industry Based upon a Novel Fast Screening Tool" Foods 10, no. 9: 2078. https://doi.org/10.3390/foods10092078
APA StyleGoemaere, O., De Ketelaere, B., Hanskens, J., Masijn, Q., Pérez Santaescolastica, C., & Fraeye, I. (2021). Comparison of the Technological Application Potential of Functional Ingredients for the Meat Industry Based upon a Novel Fast Screening Tool. Foods, 10(9), 2078. https://doi.org/10.3390/foods10092078






