Contamination of Fusarium proliferatum and Aspergillus flavus in the Rice Chain Linked to Crop Seasons, Cultivation Regions, and Traditional Agricultural Practices in Mekong Delta, Vietnam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Location, Sampling, and Data Collection
2.2. Determination of Water Activity
2.3. Isolation and Determination of Fungi
2.3.1. Isolation and Identification by Macroscope and Microscope Observation
2.3.2. Identification of Fungi by PCR Assay
2.4. Determination of Mycotoxigenic Fungi by LC–MS/MS
2.4.1. Extraction of Mycotoxins from Fungi
2.4.2. LC–MS/MS
2.5. Pre- and Post-Harvest Practices in the Vietnamese Mekong Delta Rice Chain
2.6. Data Analysis
3. Results and Discussion
3.1. Agricultural Practices in the Vietnamese Mekong Delta
3.2. Water Activity
3.3. Fungi Contamination in the Vietnamese Mekong Delta Rice Chain
3.3.1. Contamination of A. flavus and F. proliferatum by Crop Seasons
3.3.2. Infection of A. flavus and F. proliferatum by Cultivation Regions
3.4. Impact of Pre-Harvest Practices on the Infection of F. proliferatum and A. flavus
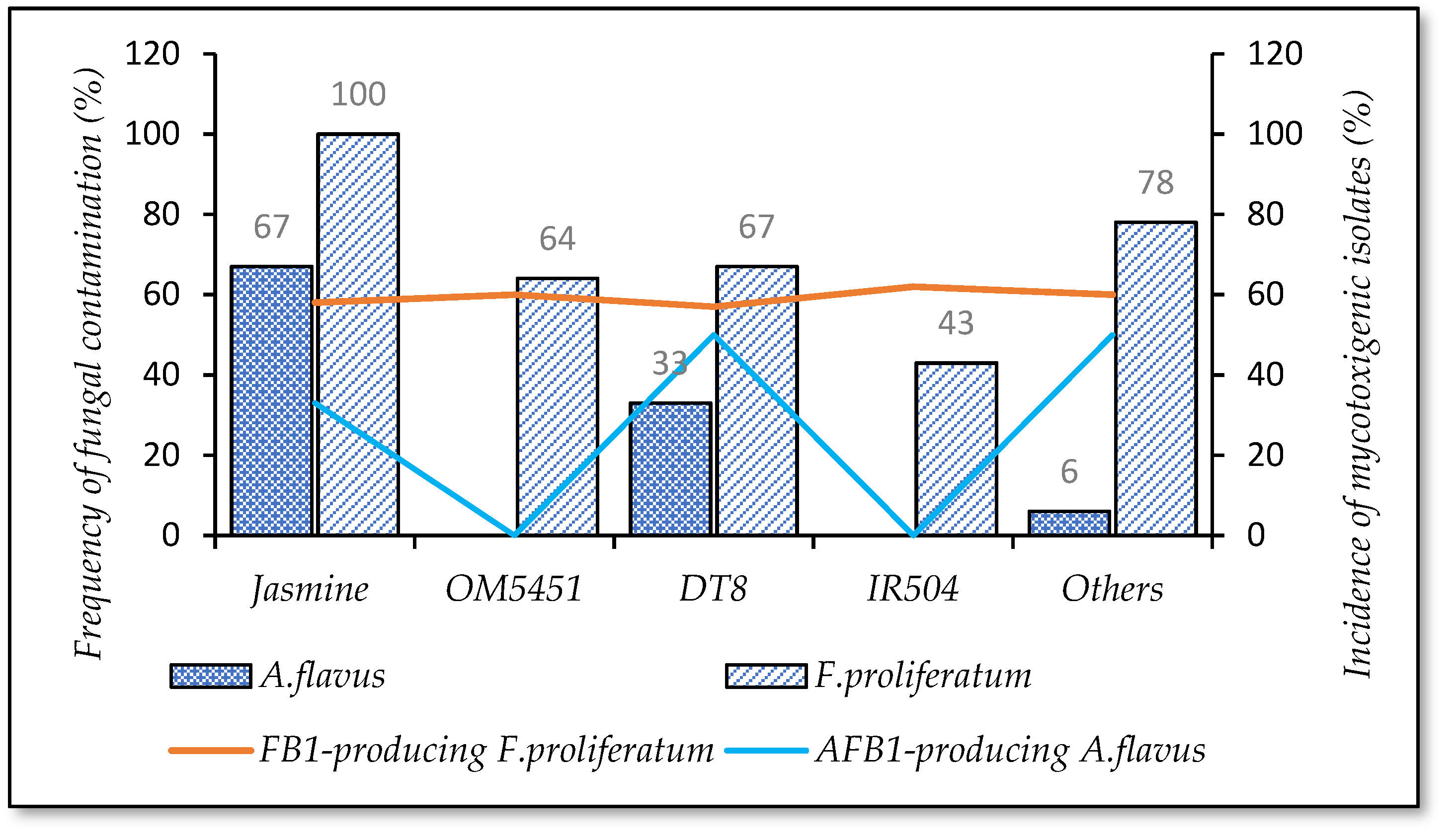
3.4.1. Selection of Paddy Variety
3.4.2. Crop Residue Management
3.4.3. Fertilizer Application
3.5. Impact of Post-Harvest Practices on the Contamination of F. proliferatum and A. flavus
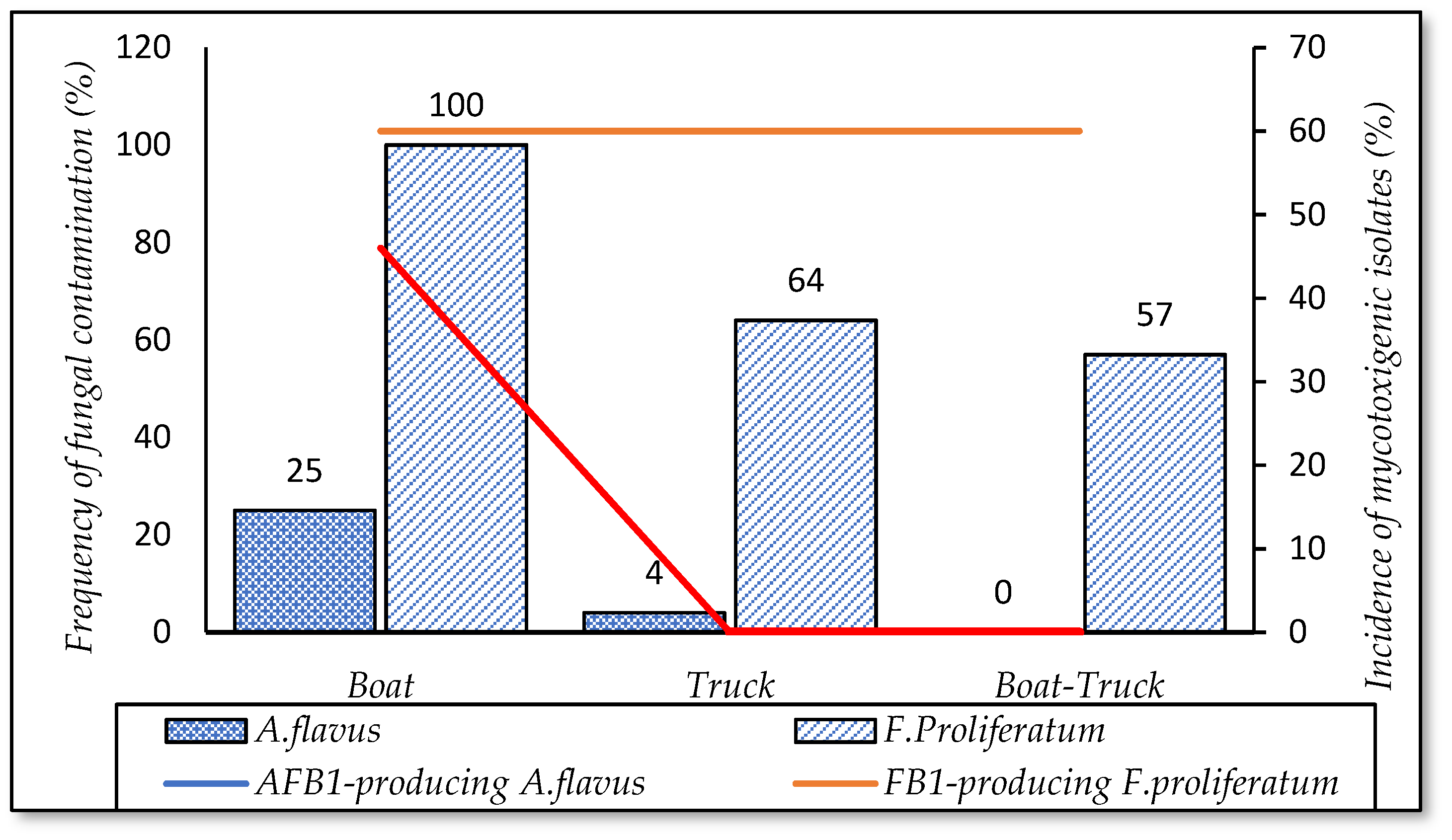
3.5.1. Means of Transportation
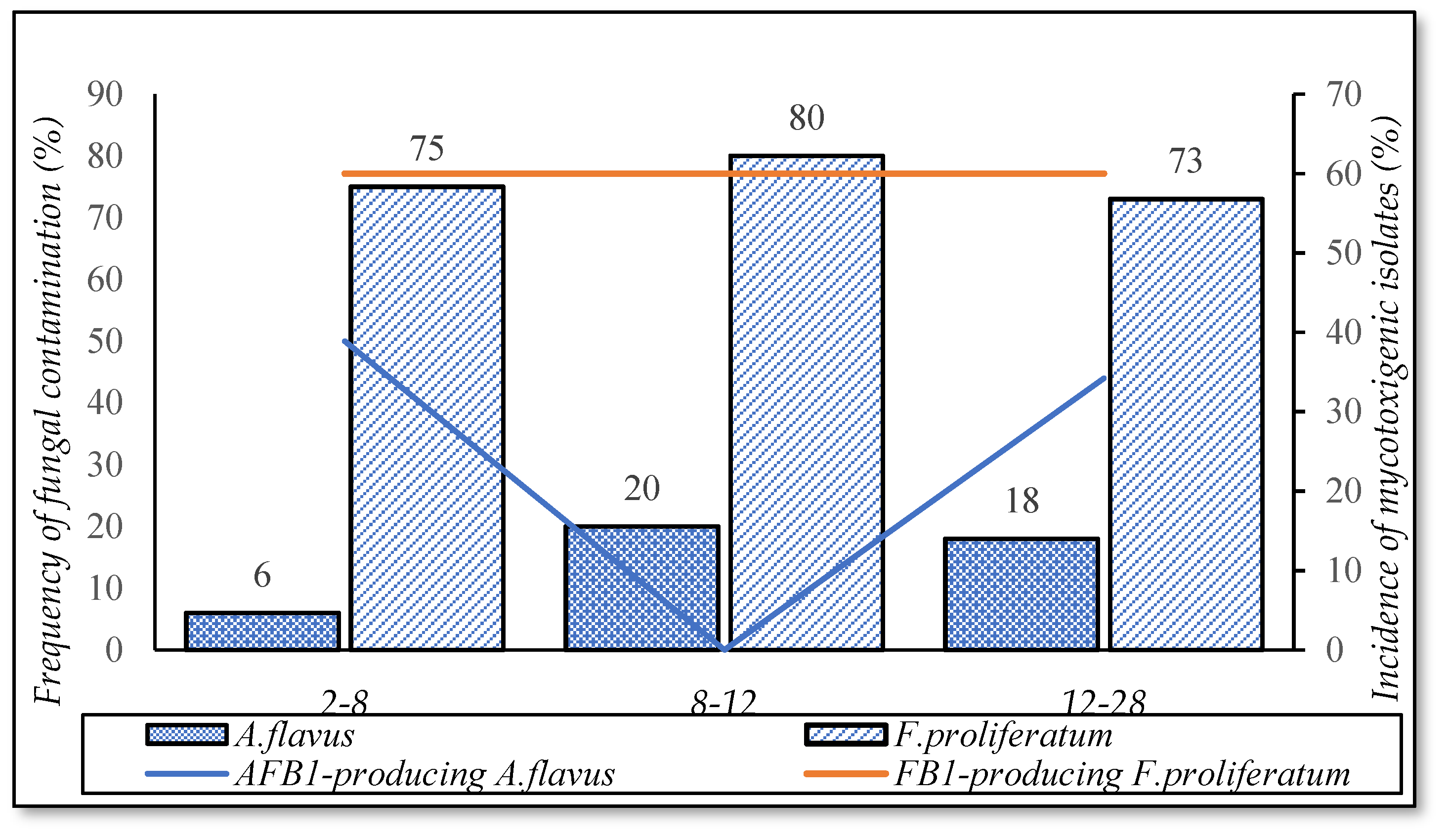
3.5.2. Delayed Drying Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devi, K.S.; Ponnarasi, T. An economic analysis of modern rice production technology and its adoption behaviour in Tamil Nadu. Agric. Econ. Res. Rev. 2009, 22, 341–348. [Google Scholar] [CrossRef]
- Rice Consumption Per Capita in Vietnam. Available online: https://www.helgilibrary.com/indicators/rice-consumption-per-capita/vietnam (accessed on 10 January 2021).
- National Center for Hydrometeorological Forecasting. Available online: https://nchmf.gov.vn/KttvsiteE/en-US/2/index.html (accessed on 16 June 2021).
- Reddy, K.; Reddy, C.; Abbas, H.; Abel, C.; Muralidharan, K. Mycotoxigenic fungi, mycotoxins, and management of rice grains. Toxin Rev. 2008, 27, 287–317. [Google Scholar] [CrossRef]
- Reddy, K.R.; Farhana, N.I.; Salleh, B. Occurrence of Aspergillus spp. and aflatoxin B1 in Malaysian foods used for human consumption. J. Food Sci. 2011, 76, T99–T104. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.; Reddy, C.; Muralidharan, K. Detection of Aspergillus spp. and aflatoxin B1 in rice in India. Food Microbiol. 2009, 26, 27–31. [Google Scholar] [CrossRef]
- Sales, A.C.; Yoshizawa, T. Mold counts and Aspergillus section Flavi populations in rice and its by-products from the Philippines. J. Food Prot. 2005, 68, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Trung, T.S.; Bailly, J.; Querin, A.; Le Bars, P.; Guerre, P. Fungal contamination of rice from south Vietnam, mycotoxinogenesis of selected strains and residues in rice. Rev. De Médecine Vétérinaire 2001, 152, 555–560. [Google Scholar] [CrossRef]
- Kushiro, M. Historical review of researches on yellow rice and mycotoxigenic fungi adherent to rice in Japan. マイコトキシン 2015, 65, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, A.; Gkrillas, A.; Dorne, J.; Dall’Asta, C.; Palumbo, R.; Lima, N.; Battilani, P.; Venâncio, A.; Giorni, P. Pre-and postharvest strategies to minimize mycotoxin contamination in the rice food chain. Compr. Rev. Food Sci. Food Saf. 2019, 18, 441–454. [Google Scholar] [CrossRef]
- General Statistics Office of Vietnam. Available online: https://www.gso.gov.vn/en/px-web/?pxid=E0612&theme=Agriculture%2C%20Forestry%20and%20Fishing (accessed on 16 June 2021).
- Nguyen, H.K.; Chiong, R.; Chica, M.; Middleton, R.; Pham, D.T.K. Contract Farming in the Mekong Delta’s Rice Supply Chain: Insights from an Agent-Based Modeling Study. J. Artif. Soc. Soc. Simul. 2019, 22, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Tran, M.T.; Ameye, M.; Phan, L.T.-K.; Devlieghere, F.; De Saeger, S.; Eeckhout, M.; Audenaert, K. Impact of ethnic pre-harvest practices on the occurrence of Fusarium verticillioides and fumonisin B1 in maize fields from Vietnam. Food Control 2021, 120, 107567. [Google Scholar] [CrossRef]
- Tran, T.M.; Ameye, M.; Phan, L.T.-K.; Devlieghere, F.; De Saeger, S.; Eeckhout, M.; Audenaert, K. Post-harvest contamination of maize by Fusarium verticillioides and fumonisins linked to traditional harvest and post-harvest practices: A case study of small-holder farms in Vietnam. Int. J. Food Microbiol. 2021, 339, 109022. [Google Scholar] [CrossRef]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi; Westerdijk Fungal Biodiversity Institute: Utrecht, The Netherlands, 2019. [Google Scholar]
- Landschoot, S.; Audenaert, K.; Waegeman, W.; De Baets, B.; Haesaert, G. Influence of maize–wheat rotation systems on Fusarium head blight infection and deoxynivalenol content in wheat under low versus high disease pressure. Crop Prot. 2013, 52, 14–21. [Google Scholar] [CrossRef]
- Bennet, J. An Overview of the Genus Aspergillus. In Aspergillus: Molecular BiologyandGenomics; Caister Academic Press: Norfolk, UK, 2010; pp. 1–17. [Google Scholar]
- Huong, B.T.M.; Brimer, L.; Dalsgaard, A. Dietary exposure to aflatoxin B1, ochratoxin A and fuminisins of adults in Lao Cai province, Viet Nam: A total dietary study approach. Food Chem. Toxicol. 2016, 98, 127–133. [Google Scholar] [CrossRef]
- Huong, B.T.M.; Do, T.T.; Madsen, H.; Brimer, L.; Dalsgaard, A. Aflatoxins and fumonisins in rice and maize staple cereals in Northern Vietnam and dietary exposure in different ethnic groups. Food Control 2016, 70, 191–200. [Google Scholar] [CrossRef]
- Do, T.H.; Tran, S.C.; Le, C.D.; Nguyen, H.-B.T.; Le, P.-T.T.; Le, H.-H.T.; Le, T.D.; Thai-Nguyen, H.-T. Dietary exposure and health risk characterization of aflatoxin B1, ochratoxin A, fumonisin B1, and zearalenone in food from different provinces in Northern Vietnam. Food Control 2020, 112, 107108. [Google Scholar] [CrossRef]
- Amatulli, M.T.; Spadaro, D.; Gullino, M.L.; Garibaldi, A. Molecular identification of Fusarium spp. associated with bakanae disease of rice in Italy and assessment of their pathogenicity. Plant Pathol. 2010, 59, 839–844. [Google Scholar] [CrossRef]
- Desjardins, A.; Manandhar, H.; Plattner, R.; Manandhar, G.; Poling, S.; Maragos, C. Fusarium species from Nepalese rice and production of mycotoxins and gibberellic acid by selected species. Appl. Environ. Microbiol. 2000, 66, 1020–1025. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.; Lee, S.-H.; Lee, S.-H.; Shin, J.Y.; Yun, J.-C.; Lee, Y.-W.; Ryu, J.-G. Occurrence of Fusarium mycotoxins in rice and its milling by-products in Korea. J. Food Prot. 2011, 74, 1169–1174. [Google Scholar] [CrossRef]
- Ok, H.E.; Choi, S.-W.; Chang, H.J.; Chung, M.-S.; Chun, H.S. Occurrence of five 8-ketotrichothecene mycotoxins in organically and conventionally produced cereals collected in Korea. Food Control 2011, 22, 1647–1652. [Google Scholar] [CrossRef]
- Sawane, A.; Sawane, M. Mycotoxigenicity of Aspergillus, Penicillium and Fusarium spp. isolated from stored rice. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 116–121. [Google Scholar]
- Reddy, K.; Reddy, C.; Mangala, U.; Muralidharan, K. Site of Infection of Aspergillus sp. in seeds of rice cultivars. J. Mycol. Plant Pathol. 2006, 36, 271–277. [Google Scholar]
- Mousa, W.; Ghazali, F.; Jinap, S.; Ghazali, H.; Radu, S. Modelling the effect of water activity and temperature on growth rate and aflatoxin production by two isolates of Aspergillus flavus on paddy. J. Appl. Microbiol. 2011, 111, 1262–1274. [Google Scholar] [CrossRef]
- Mousa, W.; Ghazali, F.M.; Jinap, S.; Ghazali, H.M.; Radu, S. Modeling growth rate and assessing aflatoxins production by Aspergillus flavus as a function of water activity and temperature on polished and brown rice. J. Food Sci. 2013, 78, M56–M63. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Sago, Y.; Zheng, Y.; Nakagawa, H.; Kushiro, M. Mycotoxins in rice. Int. J. Food Microbiol. 2007, 119, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Samapundo, S.; Devlieghere, F.; De Meulenaer, B.; Geeraerd, A.; Van Impe, J.; Debevere, J. Predictive modelling of the individual and combined effect of water activity and temperature on the radial growth of Fusarium verticilliodes and F. proliferatum on corn. Int. J. Food Microbiol. 2005, 105, 35–52. [Google Scholar] [CrossRef]
- Marín, S.; Sanchis, V.; Teixido, A.; Saenz, R.; Ramos, A.; Vinas, I.; Magan, N. Water and temperature relations and microconidial germination of Fusarium moniliforme and Fusarium proliferatum from maize. Can. J. Microbiol. 1996, 42, 1045–1050. [Google Scholar] [CrossRef]
- Trung, N.H.; Thanh, V.Q. Vulnerability to flood in the Vietnamese Mekong Delta: Mapping and uncertainty assessment. J. Environ. Sci. Eng. B 2013, 2, 229. [Google Scholar]
- Parsons, M.; Munkvold, G. Associations of planting date, drought stress, and insects with Fusarium ear rot and fumonisin B1 contamination in California maize. Food Addit. Contam. 2010, 27, 591–607. [Google Scholar] [CrossRef]
- FAO. 1979. Available online: http://www.fao.org/docrep/014/AM811E/AM811E.pdf (accessed on 10 June 2021).
- Bouras, N.; Holtz, M.D.; Aboukhaddour, R.; Strelkov, S.E. Influence of nitrogen sources on growth and mycotoxin production by isolates of Pyrenophora tritici-repentis from wheat. Crop J. 2016, 4, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Cotten, T.; Munkvold, G. Survival of Fusarium moniliforme, F. proliferatum, and F. subglutinans in maize stalk residue. Phytopathology 1998, 88, 550–555. [Google Scholar] [CrossRef] [Green Version]
- Rossi, V.; Scandolara, A.; Battilani, P. Effect of environmental conditions on spore production by Fusarium verticillioides, the causal agent of maize ear rot. Eur. J. Plant Pathol. 2009, 123, 159–169. [Google Scholar] [CrossRef]
- Nakhro, N.; Dkhar, M. Populations and biomass carbon in paddy field soil. Agron. J. 2010, 9, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Adegoke, G.O.; Letuma, P. Strategies for the prevention and reduction of mycotoxins in developing countries. Mycotoxin Food Saf. Dev. Ctries. 2013, 123–136. [Google Scholar] [CrossRef] [Green Version]
- Sinha, K.K. Detoxification of mycotoxins and food safety. Mycotoxins Agric. Food Saf. 1998, 1, 381–406. [Google Scholar]
- Samapundo, S.; Devlieghere, F.; Geeraerd, A.; De Meulenaer, B.; Van Impe, J.; Debevere, J. Modelling of the individual and combined effects of water activity and temperature on the radial growth of Aspergillus flavus and A. parasiticus on corn. Food Microbiol. 2007, 24, 517–529. [Google Scholar] [CrossRef]
- Channaiah, L.H.; Maier, D.E. Best stored maize management practices for the prevention of mycotoxin contamination. Mycotoxin Reduct. Grain Chain. 2014, 78. [Google Scholar] [CrossRef]
- Pitt, J.; Taniwaki, M.H.; Cole, M. Mycotoxin production in major crops as influenced by growing, harvesting, storage and processing, with emphasis on the achievement of Food Safety Objectives. Food Control 2013, 32, 205–215. [Google Scholar] [CrossRef]
- Makun, H.A.; Gbodi, T.A.; Akanya, O.H.; Salako, E.A.; Ogbadu, G.H. Fungi and some mycotoxins contaminating rice (Oryza sativa) in Niger state, Nigeria. Afr. J. Biotechnol. 2007, 6, 97–104. [Google Scholar] [CrossRef]
- Abbas, H.; Cartwright, R.; Xie, W.; Mirocha, C.; Richard, J.; Dvorak, T.; Sciumbato, G.; Shier, W. Mycotoxin production by Fusarium proliferatum isolates from rice with Fusarium sheath rot disease. Mycopathologia 1999, 147, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.; Cartwright, R.; Shier, W.; Abouzied, M.; Bird, C.; Rice, L.; Ross, P.F.; Sciumbato, G.; Meredith, F. Natural occurrence of fumonisins in rice with Fusarium sheath rot disease. Plant Dis. 1998, 82, 22–25. [Google Scholar] [CrossRef] [Green Version]
- Magan, N.; Aldred, D. Post-harvest control strategies: Minimizing mycotoxins in the food chain. Int. J. Food Microbiol. 2007, 119, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Magan, N.; Aldred, D.; Mylona, K.; Lambert, R.J. Limiting mycotoxins in stored wheat. Food Addit. Contam. 2010, 27, 644–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trucksess, M.; Abbas, H.; Weaver, C.; Shier, W. Distribution of aflatoxins in shelling and milling fractions of naturally contaminated rice. Food Addit. Contam. Part A 2011, 28, 1076–1082. [Google Scholar] [CrossRef]
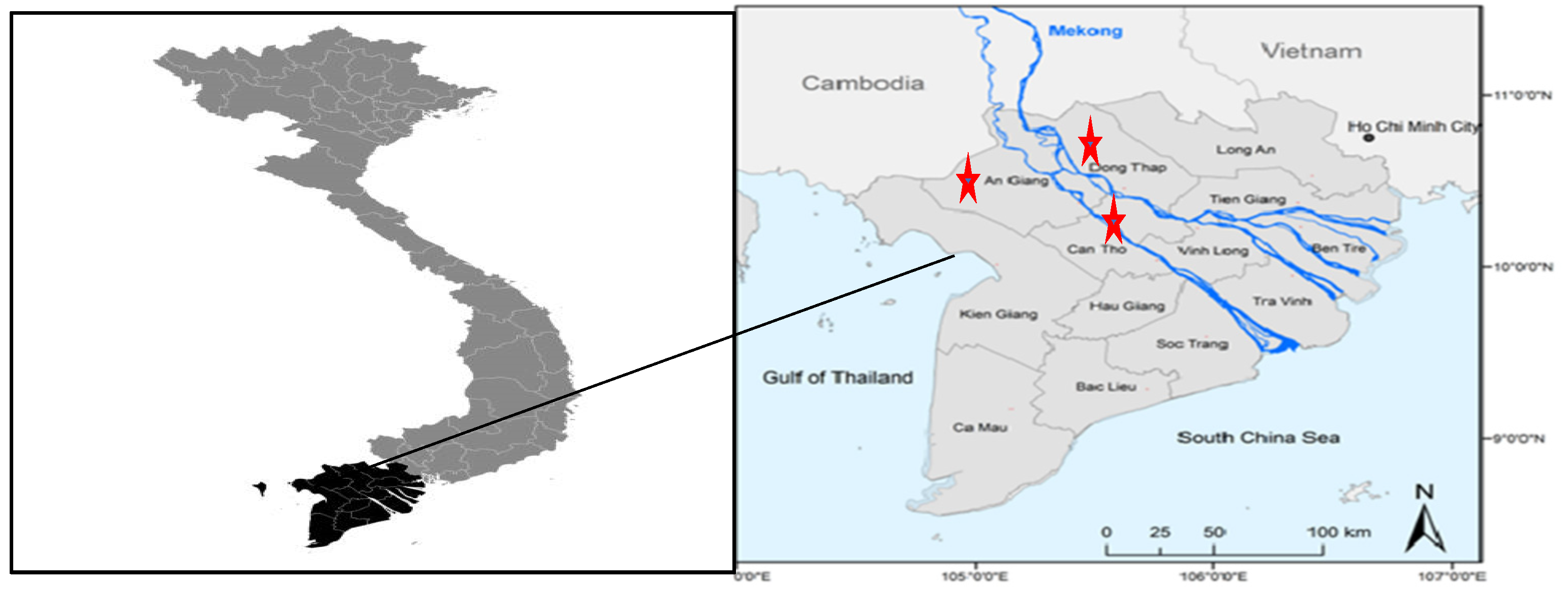
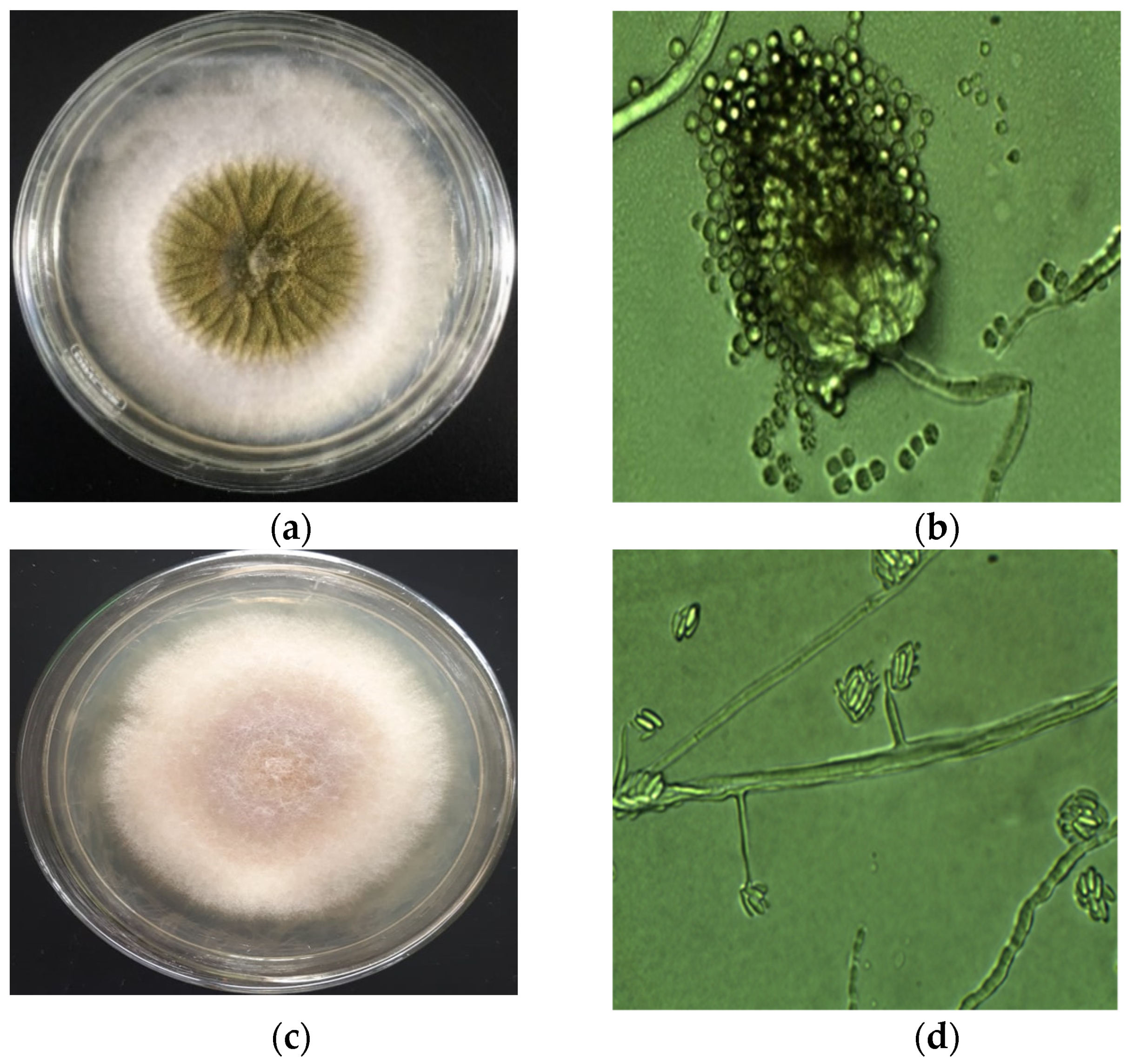
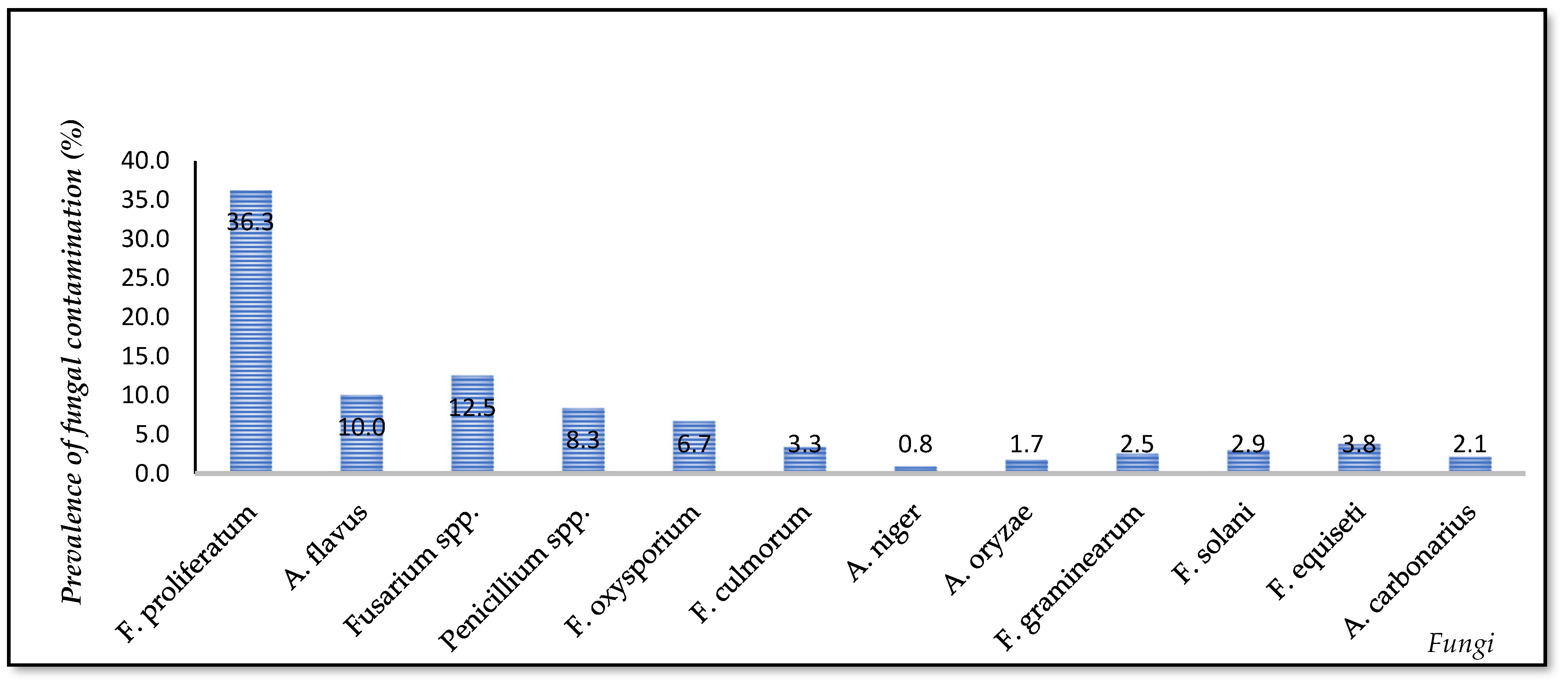

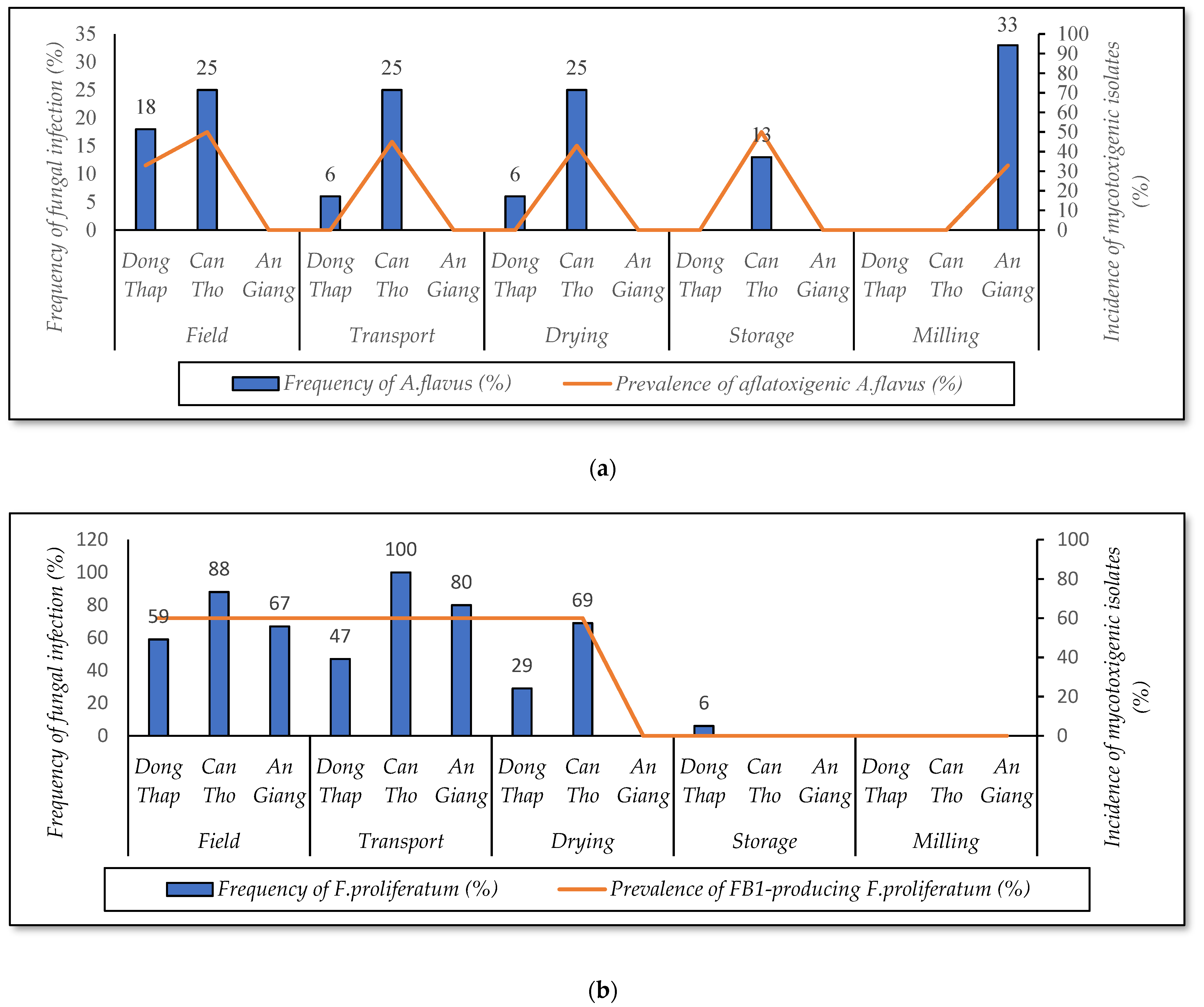


| Stages in the Rice Chain | Dong Thap (n = 85) | Can Tho (n = 80) | An Giang (n = 75) | Total Samples/Stage | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WS17/18 | SA18 | AW18 | WS18/19 | AW19 | WS17/18 | WS18/19 | AW19 | WS17/18 | SA18 | WS18/19 | AW19 | ||
| Field | 7 | 3 | 2 | 3 | 2 | 5 | 4 | 7 | 3 | 4 | 3 | 5 | 48 |
| Transport | 7 | 3 | 2 | 3 | 2 | 5 | 4 | 7 | 3 | 4 | 3 | 5 | 48 |
| Drying | 7 | 3 | 2 | 3 | 2 | 5 | 4 | 7 | 3 | 4 | 3 | 5 | 48 |
| Storage | 7 | 3 | 2 | 3 | 2 | 5 | 4 | 7 | 3 | 4 | 3 | 5 | 48 |
| Milling | 7 | 3 | 2 | 3 | 2 | 5 | 4 | 7 | 3 | 4 | 3 | 5 | 48 |
| Total | 35 | 15 | 10 | 15 | 10 | 25 | 20 | 35 | 15 | 20 | 15 | 25 | 240 |
| Agricultural Practices | Applications | 2017–2019 |
|---|---|---|
| Total (n = 48) % | ||
| Pre-harvest practices | ||
| Paddy varieties | OM 5451 | 23 |
| IR 50404 | 14 | |
| DT 8 | 13 | |
| Jasmine | 13 | |
| Others (OM4900, 7347, ST24, Timthan) | 37 | |
| Fertilizers | Inorganic | 90 |
| Organic–Inorganic (30/70:w/w) | 10 | |
| Crop residue management | Burn | 44 |
| Remove off | 42 | |
| Leave on the field and use bio-decomposer to decompose crop debris | 14 | |
| Post-harvest practices | ||
| Means of transportation | Trucks | 52 |
| Boats | 33 | |
| Boats–Trucks | 15 | |
| Delayed drying duration (hours) | 2–8 | 67 |
| 8–12 | 10 | |
| 12–28 | 23 |
| Describe Statistics | Field (n = 48) | Transport (n = 48) | Drying (n = 48) | Storage (n = 48) | Milling (n = 48) |
|---|---|---|---|---|---|
| Mean ± SD | 0.95 ± 0.03 a | 0.95 ± 0.03 a | 0.72 ± 0.09 b | 0.71 ± 0.07 b | 0.69 ± 0.05 b |
| Median | 0.96 | 0.96 | 0.71 | 0.68 | 0.68 |
| Min | 0.87 | 0.88 | 0.53 | 0.56 | 0.63 |
| Max | 0.98 | 0.98 | 0.92 | 0.91 | 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, L.T.K.; Tran, T.M.; Audenaert, K.; Jacxsens, L.; Eeckhout, M. Contamination of Fusarium proliferatum and Aspergillus flavus in the Rice Chain Linked to Crop Seasons, Cultivation Regions, and Traditional Agricultural Practices in Mekong Delta, Vietnam. Foods 2021, 10, 2064. https://doi.org/10.3390/foods10092064
Phan LTK, Tran TM, Audenaert K, Jacxsens L, Eeckhout M. Contamination of Fusarium proliferatum and Aspergillus flavus in the Rice Chain Linked to Crop Seasons, Cultivation Regions, and Traditional Agricultural Practices in Mekong Delta, Vietnam. Foods. 2021; 10(9):2064. https://doi.org/10.3390/foods10092064
Chicago/Turabian StylePhan, Lien Thi Kim, Trang Minh Tran, Kris Audenaert, Liesbeth Jacxsens, and Mia Eeckhout. 2021. "Contamination of Fusarium proliferatum and Aspergillus flavus in the Rice Chain Linked to Crop Seasons, Cultivation Regions, and Traditional Agricultural Practices in Mekong Delta, Vietnam" Foods 10, no. 9: 2064. https://doi.org/10.3390/foods10092064
APA StylePhan, L. T. K., Tran, T. M., Audenaert, K., Jacxsens, L., & Eeckhout, M. (2021). Contamination of Fusarium proliferatum and Aspergillus flavus in the Rice Chain Linked to Crop Seasons, Cultivation Regions, and Traditional Agricultural Practices in Mekong Delta, Vietnam. Foods, 10(9), 2064. https://doi.org/10.3390/foods10092064








