Chocolate as Carrier to Deliver Bioactive Ingredients: Current Advances and Future Perspectives
Abstract
1. Introduction
2. Chocolate and Its Natural Flavonoid Composition
3. Technological Properties to Consider When Developing Functional Chocolate Formulation
4. Chocolate as Carrier for the Delivery of Bioactive Ingredients
4.1. Phenolic Compounds
4.2. Omega-3 Polyunsaturated Fatty Acids (ω-3 PUFAs)
4.3. Probiotics
4.4. Vitamins and Minerals
5. Sugar-Free Chocolates as Carrier for the Delivery of Bioactive Ingredients
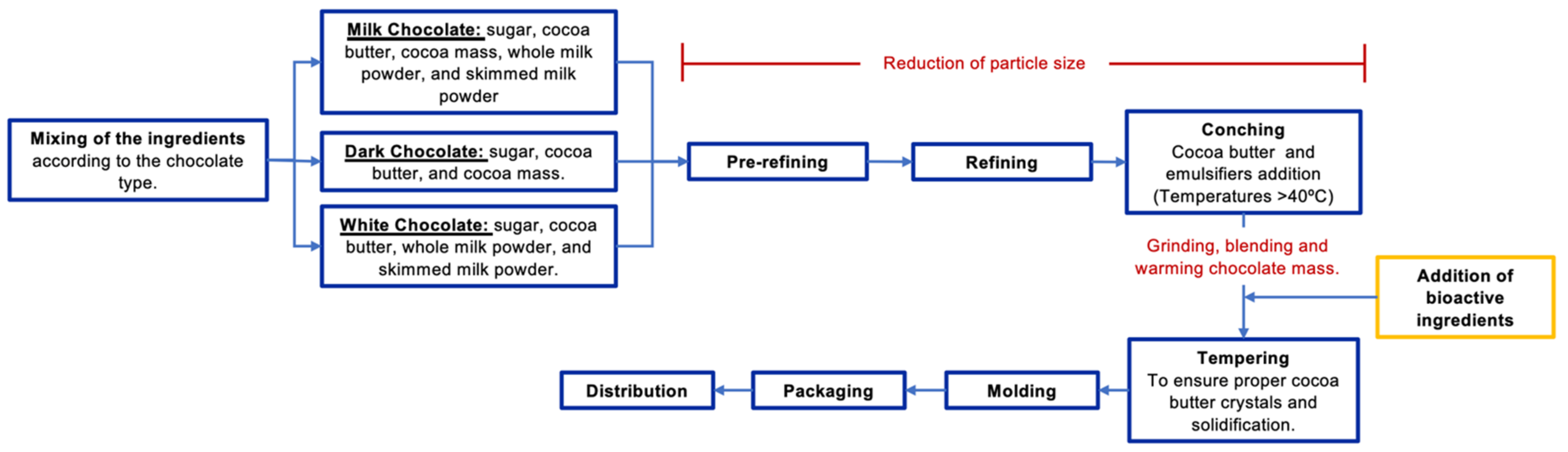
6. The Chocolate Making Process: Where to Add the Bioactive Ingredients?
7. General Protocol to Develop Functional Chocolates to Be Commercialized in the Food Supplement of Nutraceutical Markets
8. Conclusions and Further Research Needs
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santini, A.; Novellino, E. Nutraceuticals—Shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharmacol. 2018, 11, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. A practical guide for designing effective nutraceutical combinations in the form of foods, beverages, and dietary supplements against chronic degenerative diseases. Trends Food Sci. Technol. 2019, 88, 179–193. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Santana-Gálvez, J.; Cisneros-Zevallos, L. Designing next-generation functional food and beverages: Combining nonthermal processing technologies and postharvest abiotic stresses. Food Eng. Rev. 2020. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Coelho, M.S.; Salas-Mellado, M.D.L.M. Bioactive Compounds as Ingredients of Functional Foods: Polyphenols, carotenoids, peptides from animal and plant sources new. In Bioactive Compounds: Health Benefits and Potential Applications; Segura-Campos, M.R., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 129–142. [Google Scholar] [CrossRef]
- Cencic, A.; Chingwaru, W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef]
- Ackar, D.; Lendić, K.V.; Valek, M.; Šubarić, D.; Miličević, B.; Babić, J.; Nedić, I. Cocoa polyphenols: Can we consider cocoa and chocolate as potential functional food? J. Chem. 2013, 2013, 289392. [Google Scholar] [CrossRef]
- Annunziata, A.; Vecchio, R.; Kraus, A. Factors affecting parents’ choices of functional foods targeted for children. Int. J. Consum. Stud. 2016, 40, 527–535. [Google Scholar] [CrossRef]
- Konar, N.; Palabiyik, I.; Toker, O.S.; Polat, D.G.; Kelleci, E.; Pirouzian, H.R.; Akcicek, A.; Sagdic, O. Conventional and sugar-free probiotic white chocolate: Effect of inulin DP on various quality properties and viability of probiotics. J. Funct. Foods 2018, 43, 206–213. [Google Scholar] [CrossRef]
- Konar, N.; Toker, O.S.; Pirouzian, H.R.; Oba, S.; Polat, D.G.; Palabiyik, I.; Poyrazoglu, E.S.; Sağdiç, O. Enrichment of milk chocolate by using EPA and DHA originated from various origins: Effects on product quality. Sugar Tech. 2018, 20, 745–755. [Google Scholar] [CrossRef]
- Foong, Y.J.; Lee, S.T.; Ramli, N.; Tan, Y.N.; Ayob, M.K. Incorporation of potential probiotic Lactobacillus plantarum Isolated from fermented cocoa beans into dark chocolate: Bacterial viability and physicochemical properties analysis. J. Food Qual. 2013, 36, 164–171. [Google Scholar] [CrossRef]
- Yoon, M.-H.; Kim, K.-H.; Hwang, H.-R.; Jo, J.-E.; Kim, M.-S.; Yook, H.-S. Quality characteristics and antioxidant activity of chocolate containing flowering cherry (Prunus serrulata L. var. spontanea Max. wils.) Fruit Powder. J. Korean Soc. Food Sci. Nutr. 2009, 38, 1600–1605. [Google Scholar] [CrossRef]
- Jung, K.-M.; Park, S.-G.; Lee, Y.; Kim, S.R. Development and characterization of peach powder-added chocolate and chocolate-covered freeze-dried peach snack. J. East Asian Soc. Diet. Life 2017, 27, 521–528. [Google Scholar] [CrossRef]
- Hwang, M.-H.; Jeon, H.-L.; Kim, H.-D.; Lee, S.-W.; Kim, M.-R. Quality characteristics and antioxidant activities of chocolate added with mulberry pomace. Korean J. Food Cook. Sci. 2012, 28, 479–487. [Google Scholar] [CrossRef][Green Version]
- Katz, D.L.; Doughty, K.; Ali, A. Cocoa and chocolate in human health and disease. Antioxid. Redox Signal. 2011, 15, 2779–2811. [Google Scholar] [CrossRef]
- Meng, C.C.; Jalil, A.M.M.; Ismail, A. Phenolic and theobromine contents of commercial dark, milk and white chocolates on the malaysian market. Molecules 2009, 14, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Paranjape, A.; Sonawane, S.; Patil, S. Development of sugar free and fortified chocolates with D-optimal design approach. J. Food Eng. Technol. 2021, 10, 28–33. [Google Scholar] [CrossRef]
- Gómez-Fernández, A.R.; Santacruz, A.; Jacobo-Velázquez, D.A. The complex relationship between metabolic syndrome and sweeteners. J. Food Sci. 2021, 86, 1511–1531. [Google Scholar] [CrossRef]
- Sarfarazi, M.; Mohebbi, M. An investigation into the crystalline structure, and the rheological, thermal, textural and sensory properties of sugar-free milk chocolate: Effect of inulin and maltodextrin. J. Food Meas. Charact. 2020, 14, 1568–1581. [Google Scholar] [CrossRef]
- Gonçalves, E.V.; Lannes, S. Chocolate rheology. Food Sci. Technol. 2010, 30, 845–851. [Google Scholar] [CrossRef]
- Jalil, A.M.M.; Ismail, A. Polyphenols in cocoa and cocoa products: Is there a link between antioxidant properties and health? Molecules 2008, 13, 2190–2219. [Google Scholar] [CrossRef]
- Sokolov, A.N.; Pavlova, M.A.; Klosterhalfen, S.; Enck, P. Chocolate and the brain: Neurobiological impact of cocoa flavanols on cognition and behavior. Neurosci. Biobehav. Rev. 2013, 37, 2445–2453. [Google Scholar] [CrossRef]
- El-Kalyoubi, M.; Khallaf, M.; Abdelrashid, A.; Mostafa, E.M. Quality characteristics of chocolate—Containing some fat replacer. Ann. Agric. Sci. 2011, 56, 89–96. [Google Scholar] [CrossRef]
- Glicerina, V.; Balestra, F.; Rosa, M.D.; Romani, S. Effect of manufacturing process on the microstructural and rheological properties of milk chocolate. J. Food Eng. 2015, 145, 45–50. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M. Factors influencing rheological and textural qualities in chocolate—A review. Trends Food Sci. Technol. 2007, 18, 290–298. [Google Scholar] [CrossRef]
- Rezaei, F.; VanderGheynst, J.S. Critical moisture content for microbial growth in dried food-processing residues. J. Sci. Food Agric. 2010, 90, 2000–2005. [Google Scholar] [CrossRef] [PubMed]
- De Melo, L.; Bolini, H.; Efraim, P. Sensory profile, acceptability, and their relationship for diabetic/reduced calorie chocolates. Food Qual. Prefer. 2009, 20, 138–143. [Google Scholar] [CrossRef]
- Silva, M.; Tulini, F.; Marinho, J.F.; Mazzocato, M.C.; De Martinis, E.C.; Luccas, V.; Favaro-Trindade, C.S. Semisweet chocolate as a vehicle for the probiotics Lactobacillus acidophilus LA3 and Bifidobacterium animalis subsp. lactis BLC1: Evaluation of chocolate stability and probiotic survival under in vitro simulated gastrointestinal conditions. LWT 2017, 75, 640–647. [Google Scholar] [CrossRef]
- Méndez, A.S.; Pérez, L.A.C. Adición de hierro hemo, proveniente de hemoglobina bovina a un chocolate de consumo directo. Bistua Rev. Fac. Cienc. Básicas 2011, 9, 21–31. [Google Scholar]
- Didar, Z. Enrichment of dark chocolate with vitamin D3 (free or liposome) and assessment quality parameters. J. Food Sci. Technol. 2020, 58, 3065–3072. [Google Scholar] [CrossRef] [PubMed]
- Faccinetto-Beltrán, P.; Gómez-Fernández, A.; Orozco-Sánchez, N.; Pérez-Carrillo, E.; Marín-Obispo, L.; Hernández-Brenes, C.; Santacruz, A.; Jacobo-Velázquez, D. Physicochemical properties and sensory acceptability of a next-generation functional chocolate added with omega-3 polyunsaturated fatty acids and probiotics. Foods 2021, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Fernández, A.; Faccinetto-Beltrán, P.; Orozco-Sánchez, N.; Pérez-Carrillo, E.; Santacruz, A.; Jacobo-Velázquez, D. Physicochemical properties and sensory acceptability of sugar free dark chocolate formulations added with probiotics. Rev. Mex. Ing. Quim. 2021, 20, 697–709. [Google Scholar] [CrossRef]
- Gómez-Fernández, A.R.; Faccinetto-Beltrán, P.; Orozco-Sánchez, N.E.; Pérez-Carrillo, E.; Marín-Obispo, L.M.; Hernández-Brenes, C.; Santacruz, A.; Jacobo-Velázquez, D.A. Sugar-free milk chocolate as a carrier of omega-3 polyunsaturated fatty acids and probiotics: A potential functional food for the diabetic population. Foods 2021, 10, 1866. [Google Scholar] [CrossRef]
- Gültekin-Özgüven, M.; Karadag, A.; Duman, Ş.; Ozkal, B.; Özçelik, B. Fortification of dark chocolate with spray dried black mulberry (Morus nigra) waste extract encapsulated in chitosan-coated liposomes and bioaccessability studies. Food Chem. 2016, 201, 205–212. [Google Scholar] [CrossRef]
- Yoo, K.-M.; Song, M.-R.; Ji, E.-J. Preparation and sensory characteristics of chocolate with added coffee waste. Korean J. Food Nutr. 2011, 24, 111–116. [Google Scholar] [CrossRef][Green Version]
- Toker, O.S.; Konar, N.; Pirouzian, H.R.; Oba, S.; Polat, D.G.; Palabiyik, I.; Poyrazoglu, E.S.; Sagdic, O. Developing functional white chocolate by incorporating different forms of EPA and DHA—Effects on product quality. LWT 2018, 87, 177–185. [Google Scholar] [CrossRef]
- Toker, O.S.; Konar, N.; Palabiyik, I.; Pirouzian, H.R.; Oba, S.; Polat, D.G.; Poyrazoglu, E.S.; Sagdic, O. Formulation of dark chocolate as a carrier to deliver eicosapentaenoic and docosahexaenoic acids: Effects on product quality. Food Chem. 2018, 254, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Zarić, D.B.; Bulatović, M.L.; Rakin, M.B.; Krunić, T.; Lončarević, I.S.; Pajin, B.S. Functional, rheological and sensory properties of probiotic milk chocolate produced in a ball mill. RSC Adv. 2016, 6, 13934–13941. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Nebesny, E.; Motyl, I.; Libudzisz, Z. Effect of milk chocolate supplementation with lyophilised Lactobacillus cells on its attributes. Czech J. Food Sci. 2010, 28, 392–406. [Google Scholar] [CrossRef]
- Mandal, S.; Hati, S.; Puniya, A.K.; Singh, R.; Singh, K. Development of synbiotic milk chocolate using encapsulated Lactobacillus casei NCDC 298. J. Food Process. Preserv. 2012, 37, 1031–1037. [Google Scholar] [CrossRef]
- Marcial-Coba, M.S.; Saaby, L.; Knøchel, S.; Nielsen, D.S. Dark chocolate as a stable carrier of microencapsulated Akkermansia muciniphila and Lactobacillus casei. FEMS Microbiol. Lett. 2018, 366, fny290. [Google Scholar] [CrossRef]
- Kemsawasd, V.; Chaikham, P.; Rattanasena, P. Survival of immobilized probiotics in chocolate during storage and with an in vitro gastrointestinal model. Food Biosci. 2016, 16, 37–43. [Google Scholar] [CrossRef]
- Denkova-Kostova, R.S.; Goranov, B.G.; Teneva, D.G.; Tomova, T.G.; Denkova, Z.R.; Shopska, V.; Mihaylova-Ivanova, Y. Bio-preservation of chocolate mousse with free and immobilized cells of Lactobacillus plantarum D2 and lemon (Citrus lemon L.) or grapefruit (Citrus paradisi L.) zest essential oils. Acta Sci. Pol. Technol. Aliment. 2015, 20, 5–16. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Jacobo-Velázquez, D.A. Classification of phenolic compounds. In Phenolic Compounds in Food, 1st ed.; Nollet, L.M.L., Gutierrez-Uribe, J.A., Eds.; CRC Press: New York, NY, USA, 2017; pp. 3–21. [Google Scholar]
- Caleja, C.; Ribeiro, A.; Barreiro, M.F.; Ferreira, I.C. Phenolic compounds as nutraceuticals or functional food ingredients. Curr. Pharm. Des. 2017, 23, 2787–2806. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Villela-Castrejón, J.; Serna-Saldívar, S.O.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Synergistic combinations of curcumin, sulforaphane, and dihydrocaffeic acid against human colon cancer cells. Int. J. Mol. Sci. 2020, 21, 3108. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Robinson, J.G.; Ijioma, N.; Harris, W. Omega-3 fatty acids and cognitive function in women. Women Health 2010, 6, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Orellana, P.; Valenzuela, R.; Valenzuela, A.; Morales, G.I. Efectos neuro protectores del ácido araquidónico y del ácido docosahexaenoico en las etapas extremas de la vida: Una visión integradora. Rev. Chil. Nutr. 2018, 45, 80–88. [Google Scholar] [CrossRef]
- Gao, H.; Geng, T.; Huang, T.; Zhao, Q. Fish oil supplementation and insulin sensitivity: A systematic review and meta-analysis. Lipids Health Dis. 2017, 16, 1–9. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Metian, M. Fish Matters: Importance of aquatic foods in human nutrition and global food supply. Rev. Fish. Sci. 2013, 21, 22–38. [Google Scholar] [CrossRef]
- Punia, S.; Sandhu, K.S.; Siroha, A.K.; Dhull, S.B. Omega 3-metabolism, absorption, bioavailability and health benefits—A review. PharmaNutrition 2019, 10, 100162. [Google Scholar] [CrossRef]
- Valdés-Ramos, R.; Guadarrama-López, A.L.; Martínez-Carrillo, B.E.; Harbige, L.S. n-3 Polyunsaturated fatty acids in type 2 diabetes mellitus. In Bioactive Food as Dietary Interventions for Diabetes, 2nd ed.; Watson, R.R., Preedy, V.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 193–209. [Google Scholar] [CrossRef]
- Neethirajan, S.; Jayas, D.S. Nanotechnology for the food and bioprocessing industries. Food Bioprocess Technol. 2010, 4, 39–47. [Google Scholar] [CrossRef]
- Cheng, L.-H.; Liu, Y.-W.; Wu, C.-C.; Wang, S.; Tsai, Y.-C. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J. Food Drug Anal. 2019, 27, 632–648. [Google Scholar] [CrossRef]
- Sarkar, S.R.; Banerjee, S. Gut microbiota in neurodegenerative disorders. J. Neuroimmunol. 2019, 328, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Agahi, A.; Hamidi, G.A.; Daneshvar, R.; Hamdieh, M.; Soheili, M.; Alinaghipour, A.; Taba, S.M.E.; Salami, M. Does severity of alzheimer’s disease contribute to its responsiveness to modifying gut microbiota? A double blind clinical trial. Front. Neurol. 2018, 9, 662. [Google Scholar] [CrossRef]
- Wong, C.B.; Kobayashi, Y.; Xiao, J.-Z. Probiotics for Preventing Cognitive Impairment in Alzheimer’s Disease. In Gut Microbiota Brain Axis; Evrensel, A., Ünsalver, B.Ö., Eds.; Intech Open: London, UK, 2018; pp. 85–104. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.; Van Den Berg, F.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Abu Al-Soud, W.; Sørensen, S.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Kim, S.S. Probiotics and prebiotics: Present status and future perspectives on metabolic disorders. Nutrients 2016, 8, 173. [Google Scholar] [CrossRef]
- Balakumar, M.; Prabhu, D.; Sathishkumar, C.; Prabu, P.; Rokana, N.; Kumar, R.; Raghavan, S.; Soundarajan, A.; Grover, S.; Batish, V.K.; et al. Improvement in glucose tolerance and insulin sensitivity by probiotic strains of Indian gut origin in high-fat diet-fed C57BL/6J mice. Eur. J. Nutr. 2016, 57, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.-C.; Strassburger, K.; Nowotny, B.; Kolb, H.; Nowotny, P.; Burkart, V.; Zivehe, F.; Hwang, J.-H.; Stehle, P.; Pacini, G.; et al. Intake of Lactobacillus reuteri improves incretin and insulin secretion in glucose-tolerant humans: A proof of concept. Diabetes Care 2015, 38, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Ahrén, B.; Schmitz, O. GLP-1 Receptor agonists and DPP-4 inhibitors in the treatment of type 2 diabetes. Horm. Metab. Res. 2004, 36, 867–876. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, X.; Li, J.; Li, Z.; Hu, Q.; Li, L.; Hao, X.; Song, M.; Li, C. Using probiotics for type 2 diabetes mellitus intervention: Advances, questions, and potential. Crit. Rev. Food Sci. Nutr. 2019, 60, 670–683. [Google Scholar] [CrossRef]
- Tardy, A.-L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and minerals for energy, fatigue and cognition: A narrative review of the biochemical and clinical evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Gastélum-Estrada, A.; Serna-Saldívar, S.O.; Jacobo-Velázquez, D.A. Fighting the COVID-19 pandemic through biofortification: Innovative approaches to improve the immunomodulating capacity of foods. ACS Food Sci. Technol. 2021, 1, 480–486. [Google Scholar] [CrossRef]
- Kruszewski, B.; Obiedziński, M.W. Multivariate analysis of essential elements in raw cocoa and processed chocolate mass materials from three different manufacturers. LWT 2018, 98, 113–123. [Google Scholar] [CrossRef]
- Aidoo, R.P.; Afoakwa, E.O.; Dewettinck, K. Optimization of inulin and polydextrose mixtures as sucrose replacers during sugar-free chocolate manufacture—Rheological, microstructure and physical quality characteristics. J. Food Eng. 2014, 126, 35–42. [Google Scholar] [CrossRef]
- Skytte, U.P.; Kaylegian, K.E. Ingredients from milk. In Beckett’s Industrial Chocolate Manufacture and Use; Beckett, S.T., Fowler, M.S., Ziegler, G.R., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 102–134. [Google Scholar]
- Hartel, R.W.; Von Elbe, J.H.; Hofberger, R. Fats, Oils and Emulsifiers. In Confectionery Science and Technology; Hartel, R.W., von Elbe, J.H., Hofberger, R., Eds.; Springer: Cham, Switzerland, 2017; pp. 85–124. [Google Scholar] [CrossRef]
- Yeganehzad, S.; Pahlevanloo, A.; Kiumarsi, M.; Zayerzadeh, A.; Sadjadi, S.A.; Shahidi, M.; Nadali, N. Effects of sugar-free, αs1-casein–enriched chocolate on stress: Based on salivary cortisol measurement and questionnaire data collection. J. Nutr. Sci. Diet. 2018, 4, 112–119. [Google Scholar]
- Konar, N.; Palabiyik, I.; Toker, O.S.; Polat, D.G.; Sener, S.; Akcicek, A.; Sagdic, O. Effect of inulin DP on various properties of sugar-free dark chocolates containing Lactobacillus paracasei and Lactobacillus acidophilus. Int. J. Food Eng. 2017, 13, 20170045. [Google Scholar] [CrossRef]
- Konar, N. Influence of conching temperature and some bulk sweeteners on physical and rheological properties of prebiotic milk chocolate containing inulin. Eur. Food Res. Technol. 2012, 236, 135–143. [Google Scholar] [CrossRef]
- Aidoo, R.P.; Afoakwa, E.O.; Dewettinck, K. Rheological properties, melting behaviours and physical quality characteristics of sugar-free chocolates processed using inulin/polydextrose bulking mixtures sweetened with stevia and thaumatin extracts. LWT 2015, 62, 592–597. [Google Scholar] [CrossRef]
- De Melo, L.L.M.M.; Bolini, H.; Efraim, P. Equisweet Milk chocolates with intense sweeteners using time-intensity method. J. Food Qual. 2007, 30, 1056–1067. [Google Scholar] [CrossRef]
- Palazzo, A.; Carvalho, M.; Efraim, P.; Bolini, H. The determination of isosweetness concentrations of sucralose, rebaudioside and neotame as sucrose substitutes in new diet chocolate formulations using the time-intensity analysis. J. Sens. Stud. 2011, 26, 291–297. [Google Scholar] [CrossRef]
- Shah, A.B.; Jones, G.P.; Vasiljevic, T. Sucrose-free chocolate sweetened with Stevia rebaudiana extract and containing different bulking agents—Effects on physicochemical and sensory properties. Int. J. Food Sci. Technol. 2010, 45, 1426–1435. [Google Scholar] [CrossRef]
- Kiumarsi, M.; Rafe, A.; Yeganehzad, S. Effect of different bulk sweeteners on the dynamic oscillatory and shear rheology of chocolate. Appl. Rheol. 2017, 27, 64123. [Google Scholar] [CrossRef]
- Rad, A.H.; Pirouzian, H.R.; Toker, O.S.; Konar, N. Application of simplex lattice mixture design for optimization of sucrose-free milk chocolate produced in a ball mill. LWT 2019, 115, 108435. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Komes, D.; Dujmović, M.; Karlović, S.; Biškić, M.; Brnčić, M.; Ježek, D. Physical, bioactive and sensory quality parameters of reduced sugar chocolates formulated with natural sweeteners as sucrose alternatives. Food Chem. 2015, 167, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Toker, O.S.; Palabiyik, I.; Konar, N. Chocolate quality and conching. Trends Food Sci. Technol. 2019, 91, 446–453. [Google Scholar] [CrossRef]
- Kinta, Y.; Hartel, R.W. Bloom formation on poorly-tempered chocolate and effects of seed addition. J. Am. Oil Chem. Soc. 2009, 87, 19–27. [Google Scholar] [CrossRef]
- Hough, G.; Langohr, K.; Gomez, G.; Curia, A. Survival analysis applied to sensory shelf life of foods. J. Food Sci. 2003, 68, 359–362. [Google Scholar] [CrossRef]
- Paz-Yépez, C.; Peinado, I.; Heredia, A.; Andrés, A. Lipids digestibility and polyphenols release under in vitro digestion of dark, milk and white chocolate. J. Funct. Foods 2018, 52, 196–203. [Google Scholar] [CrossRef]
- Medicated Confectionary Market—Growth, Trends, Covid-19 Impact, and Forecasts (2021–2026). Available online: https://www.mordorintelligence.com/industry-reports/medicated-confectionery-market (accessed on 21 August 2021).
- Shahidan, N.; Salleh, N.; Zakaria, Z.; Anwar, N.R. Glycemic index of chocolate fortified with pumpkin (Cucurbita moshata) and taro (Colocasia esculenta) powder and its effect on mood and cognitive functions of female students. J. Fundam. Appl. Sci. 2018, 9, 876. [Google Scholar] [CrossRef]
- Allen, R.R.; Carson, L.; Kwik-Uribe, C.; Evans, E.M.; Erdman, J.W. Daily consumption of a dark chocolate containing flavanols and added sterol esters affects cardiovascular risk factors in a normotensive population with elevated cholesterol. J. Nutr. 2008, 138, 725–731. [Google Scholar] [CrossRef]
- Milagres, M.P.; Silva, D.M.; Pereira, I.d.O.; Senhorinho, L.M.; Sant’Ana, A.E.G.; Matos, T.B. Health benefits of chocolate consumption with high concentration of cocoa incorporated from triterpenic acids, isolated from Mansoa Hirsuta DC. Food Sci. Technol. 2020, 40, 305–311. [Google Scholar] [CrossRef]

| Bioactive Ingredient | Chocolate Type | Concentration of Bioactive Ingredient Added to the Formulation | Main Findings | Reference |
|---|---|---|---|---|
| Phenolic compounds | White and dark | Phenolic extract from cherry fruit was added as powder at 0, 3, 6 or 9% (w/w). | Total phenol content and DPPH radical scavenging activity increased as the concentration of the phenolic extract increased. Color, hardness, smell, taste, texture, and overall acceptability was significatively higher when the phenolic extract from peach was added at 3% in the chocolate formulation. | [11] |
| Phenolic extract from mulberry pomace powder was added as powder at 0, 0.5, 1.5 or 2.5% (w/w). | Mulberry addition at 1.5% showed the higher score in sensory acceptability. The higher the % of mulberry powder, the higher was the chocolate’s hardness, phenolic and flavonoid content. Phenolic extract addition resulted on a formulation with higher antioxidant activity. | [13] | ||
| Dark | Phenolic extract from peach (Fantasia cultivar) was added as powder at 0, 5, 10 or 15% (w/w). | Phenolic extract addition (15%) showed a significant increase in flavonoid content and reducing power. Sensory test did not show favorable results (score < 6), because of the bitterness of the peach cultivar. | [12] | |
| Black mulberry extract (BME) spray-dried powder (0.05% w/v) encapsulated in chitosan-coated liposomes, was added to natural (pH 4.5) and alkalized cocoa liquors (pH 6 and 7.5) during the last hour of conching at temperatures of 40, 60, and 80 °C, respectively. | Chitosan-coated liposomal powders provided better protection of anthocyanins than spray-dried extract and enhanced in vitro bioaccessability. | [33] | ||
| Phenolic extract from coffee waste was added as powder at 0, 1, 2, 3, and 4% (w/w). | Total flavonoid content and radical scavenging activity increased as the coffee powder ratio increased in chocolate formulation. Optimal sensory acceptability was observed in chocolate added with coffee extract at 2%. | [34] | ||
| ω-3 PUFAs | White | Five chocolate formulations added with ω-3 PUFAs (EPA/DHA) from different sources and presentations were evaluated: control, MAP (5.97 g/100 g chocolate), MAO (2.88 g/100 g), TGO (4.04 g/100 g), and TGM (6.78 g/100 g). | Different ω-3 PUFAs sources affected aftertaste, overall acceptability, and color saturation. Sensory evaluation was within acceptable limits. It was possible to produce functional chocolate added with the different ω-3 PUFAs sources. EPA/DHA addition did not affect melting profiles of chocolate formulations. | [35] |
| ω-3 PUFAs | Milk | Five chocolate formulations added with ω-3 PUFA (EPA/DHA) from different sources and presentations were evaluated: control, MAP (5.97 g/100 g chocolate), MAO (2.88 g/100 g), TGO (4.04 g/100 g), and TGM (6.78 g/100 g). | Milk chocolate proved to be an effective delivery system of EPA/DHA. Oil from microalgae rich in ω-3 PUFA had a mild impact in rheological parameters. Sligh variation on melting profiles were observed on chocolates added with different sources of ω-3 PUFA. The chocolate formulation with ω-3 PUFAs added as microencapsulated form (TGM) showed the highest acceptability, due to its masking property. | [9] |
| Dark | Five chocolate formulations added with ω-3 PUFA (EPA/DHA) from different sources and presentations were evaluated: control, MAP (5.97 g/100 g chocolate), MAO (2.88 g/100 g), TGO (4.04 g/100 g), and TGM (6.78 g/100 g). | Chocolate’s fortification with EPA/DHA was feasible without generating a negative effect on quality. Considering taste and bitterness parameters, microencapsulated source was the closest to the control. Microencapsulated forms did not significantly affect quality and may be a potential source to produce a functional dark chocolate with adequate acceptability. | [36] | |
| ω-3 PUFAs and probiotics | Milk | Each chocolate portion (12 g) contained ω-3 PUFA from fish oil [76.0 ± 5.2 mg or 195.8 ± 6.5 mg] and probiotics mix (L. plantarum 299v and L. rhamnosus GG, >1 × 106 CFU). | Probiotics and fish oil addition at 76.0 ± 5.2 mg per chocolate portion showed optimal results in the sensory, rheological, texture, and color analysis. Milk chocolate proved to be a suitable vehicle to deliver probiotics, maintaining 1 × 106 CFU per 12 g and an adequate amount of ω-3 PUFA. If higher concentrations of ω-3 PUFA are needed, alternative sources must be studied. | [30] |
| Probiotics | Milk | Freeze-dried strains of Lactobacillus acidophilus NCFM, Lactobacillus rhamnosus HN001, and Bifidobacterium lactis HN019 were added to milk chocolate formulation. | Final concentration 1 × 108 CFU per g of chocolate was achieved. Probiotics’ addition increased rheological parameters affecting chocolate flow properties. and its addition at 40 °C improved the sensory properties of chocolate and the survival of L.acidophilus and L.rhamnosus. Survival of the strains was >90%, six months after storage at 20 °C. | [37] |
| Lyophilized probiotics (Lactobacillus casei and Lactobacillus paracasei) were added at 3.33 g/100 g (w/w) and 40 °C providing at least 1 × 106–1 × 107 CFU/g. | No significant differences in sensory properties were detected between the treatment and probiotic-free chocolates. Bacterial viability maintained between the functional level of 1 × 106–1 × 108 CFU/g to 12 months of storage at 18 °C. Dark chocolate was an adequate and stable carrier for both Lactobacillus strains. | [38] | ||
| Probiotics | Milk | Dry mix of skim milk powder, cocoa powder, sodium benzoate, inulin (5% w/w), Lactobacillus casei NCDC 298 free or microencapsulated in sodium alginate (4%), and maize starch (2%) were added to milk chocolate ingredients previously mixed. | The final product (free and microencapsulated probiotics) showed probiotics at 1 × 108 CFU/g during 60 days of storage at 7 °C. Solids, fat, and protein in milk chocolate generated a protective matrix for probiotics. In vivo study using adult male albino mice with encapsulated probiotic chocolate supplementation increased the fecal levels of lactobacilli and decremented total coliforms. Probiotics addition either free or microencapsulated did not affect the sensory quality of chocolate. | [39] |
| Dark | Probiotic Lactobacillus plantarum isolated from fermented cocoa beans was added at an initial concentration of 1 × 108 CFU/g | Dark chocolate showed to be a suitable carrier for probiotics with an 81.25% survival rate at 84 days of storage at 4 °C; no changes in physicochemical properties were detected due to probiotics addition. | [10] | |
| The viability of probiotics added as free freeze-dried powder to dark chocolate formulations was evaluated during the shelf-life of the product (60 days at 4 or 15 °C) and under in vitro gastric passage conditions. The probiotic strains used were Akkermansia muciniphila and Lactobacillus casei. | Dark chocolate conferred efficient protection to A. muciniphila (a strict anaerobic strain) and L. casei showing a final concentration ≥ 1 × 107 CFU/g of chocolate after 60 days of storage at 4 °C and 15 °C. A high survival rate was observed after in vitro gastric transit at pH 3. Probiotic-added dark chocolate had no significant difference between two commercial chocolates in sensory test. | [40] | ||
| Milk, white, and dark | Three different types of chocolate (white, milk, and dark) were added with immobilized Lactobacillus casei 01 and Lactobacillus acidophilus LA5. In the tempering step, probiotics powder diluted in skim milk solution (10% w/w) were added to chocolate. The viability of probiotics was tested under gastrointestinal conditions and during storage at 4 °C or 25 °C for 60 days. | The three types of chocolates were appropriate to protect the viability of probiotics under gastrointestinal conditions (1 × 102 CFU/mL of probiotics at the final stage of the in vitro study) and during storage of the product (>1 × 106 CFU/g of chocolate). Dark chocolate showed higher levels of probiotics than milk or white chocolate, due to its high concentration of cocoa and antioxidant compounds. Also, the addition of probiotics did not affect consumers’ acceptability. | [41] | |
| Semi-sweet | Formulation of semi-sweet chocolate was added with freeze-dried probiotics (Lactobacillus acidophilus LA3 and Bifidobacterium animalis subsp. lactis BLC1). Probiotics were added during tempering process at a ratio of 1 × 1010 CFU/100 g. Chocolate stability and probiotic viability was evaluated under gastrointestinal conditions and during storage (120 days at 25 °C). | Incorporation of freeze-dried probiotics was successful in the chocolate matrix resulting in 1 × 108 CFU/g. The formulation showed bacterial viability with a slight reduction of 1.4 and 0.7 logarithmic cycles during storage for 120 days at 25 °C and under in vitro simulated gastrointestinal conditions. No significant differences were shown in the sensory evaluation between chocolate formulations added with probiotics and the control. | [27] | |
| Minerals | Milk | Chocolate formulation was added with desiccated bovine hemoglobin (DBH) at four different concentrations (0, 4.7, 5.7, and 6.7% w/w), to obtain 10, 12 and 14% of the required daily intake of iron for children (18 mg) per portion (25 g). | Iron-rich chocolates 4.7% and 5.7% had no significant effect in the overall acceptability as compared with the control. Results showed that it is possible to add 5.7% of DBH with a mixing time of 20 min, obtaining 2.2 mg of iron per portion (12% of the recommended daily intake). The product showed to be microbiologically suitable for consumption, and an alternative functional chocolate to contribute to the increase of iron consumption in children. | [28] |
| Vitamins | Dark | Formulation of dark chocolates was enriched with vitamin D3 in two presentations: free and liposome. Final amount of vitamin D3 was 5 µg per 10 g of chocolate. | No significant changes in color, sensory, rheological, and melting properties were detected due to the addition of free vitamin D3 and liposome vitamin D3. Vitamin retention during 15, 30, 45, and 60 days of storage at 25 °C was higher when added as liposomes than the free compounds. Addition of vitamin D3 in liposomes is a successful strategy for the fortification of dark chocolate. | [29] |
| Chocolate Type | Sweetners Added | Concentration of Bioactive Compound Added to the Formulation | Main Findings | Reference |
|---|---|---|---|---|
| White | Inulin (DP < 10 and DP > 23; 9% w/w) | Probiotics Lactobacillus paracasei Lpc-37 ATCC SD5275 (4 × 108 CFU/g) and L. acidophilus La-14 ATCC SD5212 (2 × 108 CFU/g) were added to a sugar-free white chocolate formulation. | Probiotics viability resulted on 4 × 106 CFU/g after storage (90 days at 20 °C). Inulin and probiotics addition affected the quality and rheological parameters, while maintained the acceptability by consumers. | [8] |
| Milk | Isomalt (32 or 35%, w/w) and stevia (0.03%, w/w) | Microencapsulated probiotics ((Lactobacillus plantarum 299v (L.p299v) and Lactobacillus acidophilus La 3 20 (DSMZ 17742)) were added to chocolate after tempering at 29 °C at a ratio of 1 × 1013 CFU/g. Chocolates were formulated adding 790 mg of FO per serving size (12 g), expecting to obtain 200 mg of ω-3 PUFAs. | Probiotic viability showed >2 × 107 CFU per serving size in the final product. Sugar replacement by isomalt and stevia, along with probiotics addition resulted on a chocolate formulation with similar hardness values as compared with the control. The product contained >130 mg of ω-3 PUFAs per portion. Fish oil addition decreased the acceptability of chocolate. However, when fish oil was added in sugar-free formulations, the product showed higher acceptability as compared with the control (with sugar) added with ω-3 PUFAs. | [32] |
| Dark | Optimum concnetration of erythritol, stevia, and isomalt was determines by response surface methodology (RSM) | Beetroot, Jamun seed, and pink pitahaya powder (4 and 7% w/w) and coffee (0.79% w/w) to mask aftertaste. | The formulation optimized by RSM consisted of 21.9 g cocoa butter, 5.1 g erythritol, 0.10 g stevia, and of 14.9 g isomalt per 100 g of chocolate. The overall acceptability of sugar-free chocolates was 8.9. Fortification of sugar-free formulation with beetroot powder resulted in an increase of protein and fiber content as compared with the other extracts added. | [16] |
| Mixtures of Polydextrose (26.36%) + Inulin (12% w/w); or Isomalt (38.32% w/w) + Stevia (0.03% w/w), | Microencapsulated probiotics L. acidophilus La3 (DSMZ 17742) and L. plantarum 299v (L.p299v) were added to a dark chocolate formulation after the tempering step (29 °C) at a ratio of 1 × 1013 CFU/g. | Results demonstrated that the chocolate formulation maintained adequate viability of probiotics, resulting on a final product with 1 × 106 CFU per portion of 12 g. The sugar-free dark cholate formulation with the highest acceptability and with nearest physicochemical properties to the control, contained Isomalt+ Stevia + Probiotics. | [31] | |
| Dark | Isomalt, inulin and maltitol (38% w/w) | Addition of αs1-casein peptide (150 mg) before the conching process. | A clinical trial was performed to determine if daily consumption of to evaluate the effectiveness of daily consumption of 12 g of sugar-free added with 150 mg of αs1-casein peptide in alleviating stress in healthy, normal-weight participants (N = 75). Salivary cortisol was measured as and indicator of stress, along with a self-reportingquestionnaire. Consumption of chocolate added with of αs1-casein peptide reduced stress in the Iranian population. The peptide showed stability during the chocolate elaboration process. | [71] |
| Sucrose (32.5% w/w), inulin (9% w/w DP > 23 or 9% w/w DP < 10) and maltitol (32.5% w/w). | (N = 14) Half of the samples contained sucrose, inulin, and probiotics. The other half contained maltitol, inulin, and probiotics (Lactobacillus paracasei and Lactobacillus acidophilus). Physicochemical, probiotic viability, and shelf-life analyses were performed. | Inulin type significantly influenced the viability of probiotics during shelf-life. After 90 days of storage, it was obtained 1 × 106 CFU probiotics per 25 g of chocolate. Inulin DP < 10 was more suitable for probiotic addition and chocolate production. The combination of probiotics and inulin (DP < 10) did not affect the physicochemical properties. | [72] |
| Sweeteners Added | Study Details | Experimental Findings | Reference |
|---|---|---|---|
| Inulin, isomalt, and maltitol | Three milk chocolate samples with inulin (9.0% w/w) (Control (34% w/w sucrose), 34% w/w maltitol, and 34% w/w isomalt) were tested at different conching temperatures (50, 55 and 60 °C). Physical (color, hardness, and water activity) and rheological properties were examined. | Bulk sweeteners with inulin at different conching temperatures induced changes in the physical and rheological properties of chocolate. It was concluded that maltitol is a suitable substitute in milk chocolates containing inulin since showed closer physicochemical properties to the control as compared with the formulation containing isomalt. | [73] |
| Inulin and polydextrose | Three dark chocolate samples were tested with different high intense sweeteners (BS used: 12% w/w inulin and 36% polydextrose), the treatments were control (48% w/w sucrose without BS), stevia (0.24% w/w), and thaumatin (0.06% w/w) extracts. Rheological properties, melting behaviors, and physical properties were tested. | BS increased hardness in chocolates and affected the color parameters. Stevia and thaumatin decreased hardness in chocolates. Sugar-free chocolates showed similar flow and melting properties as the control. | [74] |
| Polydextrose, lactitol, sucralose, and stevia | Five milk chocolate formulations were produced with different sucrose concentrations (40–52%) to determine sweetness by acceptance test. Then sucrose was replaced by BS (60% w/w polydextrose and 40% w/w lactitol) and sweetened with (0.07% w/w) or stevioside (0.03% w/w). Physicochemical analyses (moisture, particle size, and viscosity) were performed. | The use of BS in milk chocolates resulted to be acceptable and have similar sensory characteristics to sucrose. Sucralose is the most appropriate sweetener for replacing sucrose in milk chocolate than stevia. | [75] |
| Neotame, stevia, and sucralose | Four different milk chocolates were tested with a time-intensity method. The samples were control (43% w/w sucrose) and three different sugar-free chocolates with BS (17% w/w polydextrose, 26% w/w erythritol) sweetened with 0.06% w/w sucralose, 0.22% w/w stevia and 0.004% w/w neotame. | The best replacement for sucrose was sucralose meaning to be suitable for diet purposes presenting a similar a time-intensity to the control. | [76] |
| Stevia, polydextrose, maltodextrin, and inulin | Five milk chocolates containing 0.5% w/w stevia, 26.8% w/w polydextrose, 5.0% w/w maltodextrin and with different types of inulin (15% w/w, HPX, GR, and HP) were prepared. Physical, rheological, and sensory tests were evaluated. | Sugar-free chocolate with HP inulin obtained similar physicochemical characteristics as compared with the control. According to the sensory analysis, inulin with the highest degree of polymerization (HP) obtained an overall acceptance compared to the control. | [77] |
| Maltitol, isomalt, and inulin | Four different chocolates formulations were elaborated: control (38% w/w sucrose), maltitol (38% w/w), isomalt (38% w/w), and inulin (38%). Rheological behaviors, as well as chocolate structure, were investigated. | Depending on the molecular structure of BS, the apparent viscosity and yield stress were affected. Inulin and maltitol showed a liquid-like behavior while isomalt and sucrose a solid-like behavior. Temperature influenced the structure network between BS and chocolate matrix. | [78] |
| Inulin and polydextrose | 12 chocolate formulations were evaluated, with different percentages of inulin and polydextrose (0, 25, 50, 75, and 100%). Rheological, microstructure, and physicochemical analysis were performed. | Inulin and polydextrose significantly modify the physicochemical and rheological properties and it depended on their concentration. The optimal concentration was 75% w/w polydextrose and 24% w/w inulin to improve rheological properties. | [68] |
| Maltitol, xylitol, and isomalt | 17 milk chocolate formulations were evaluated with different concentrations of maltitol (0, 16, 33, 50, and 66% w/w), xylitol (0, 16, 33, 50, 66, and 100% w/w), and isomalt (0, 16, 33, 50, 66, and 100% w/w) using a ball mill process. Rheological, physicochemical, and sensory analysis were performed. | BS influences physicochemical characteristics. According to their fitted models 11.16% w/w maltitol, 8.9% w/w xylitol and 12.93% w/w isomalt produced the optimum milk chocolate improving chocolate quality parameters. The sensory analysis showed that stevia significantly modifies the taste of milk chocolates by increasing bitterness and generating an undesirable aftertaste; also, polyols lowered the texture acceptability. | [79] |
| Fructose, sugar alcohols (isomalt and lactitol), inulin, and oligofructose, and natural sweeteners | 5 semi-sweet chocolates were evaluated. Chocolate 1 (sucrose), chocolate 2 (fructose, lactitol, stevioside, inulin, oligofructose, and agave syrup), chocolate 3 (fructose, xylitol, oligofructose, liquorice root, yacon, rice syrup, dried carrot, and black locust flowers), chocolate 4 (fructose, isomalt, stevia leaves, oligofructose, lucuma, agave syrup, and peppermint), chocolate 5 (fructose, maltitol, stevia leaves, yacon, and rice syrup). | Chocolate containing inulin, oligofructose, and agave syrup was characterized with the lowest content of sugars but exhibited calorific value the closest to control chocolate, while chocolate containing yacon, carrot, and black locust flowers exhibited the highest sugar content. Polyols increased hardness in the chocolate matrix due to their large particle size. | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faccinetto-Beltrán, P.; Gómez-Fernández, A.R.; Santacruz, A.; Jacobo-Velázquez, D.A. Chocolate as Carrier to Deliver Bioactive Ingredients: Current Advances and Future Perspectives. Foods 2021, 10, 2065. https://doi.org/10.3390/foods10092065
Faccinetto-Beltrán P, Gómez-Fernández AR, Santacruz A, Jacobo-Velázquez DA. Chocolate as Carrier to Deliver Bioactive Ingredients: Current Advances and Future Perspectives. Foods. 2021; 10(9):2065. https://doi.org/10.3390/foods10092065
Chicago/Turabian StyleFaccinetto-Beltrán, Paulinna, Andrea R. Gómez-Fernández, Arlette Santacruz, and Daniel A. Jacobo-Velázquez. 2021. "Chocolate as Carrier to Deliver Bioactive Ingredients: Current Advances and Future Perspectives" Foods 10, no. 9: 2065. https://doi.org/10.3390/foods10092065
APA StyleFaccinetto-Beltrán, P., Gómez-Fernández, A. R., Santacruz, A., & Jacobo-Velázquez, D. A. (2021). Chocolate as Carrier to Deliver Bioactive Ingredients: Current Advances and Future Perspectives. Foods, 10(9), 2065. https://doi.org/10.3390/foods10092065








