Revalorization of Cava Lees to Improve the Safety of Fermented Sausages
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Preparation of Cava Lees
2.3. Phenolic Extract Preparation
2.4. Elaboration of Inoculated Fermented Pork Sausages
2.5. Microbiological and Physicochemical Analysis
2.6. Isolation and Monitoring of Starter Culture Strains
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of Cava Lees Applied in Fermented Pork Sausages (Experiment 1)
3.1.1. Characterization of Physicochemical Parameters
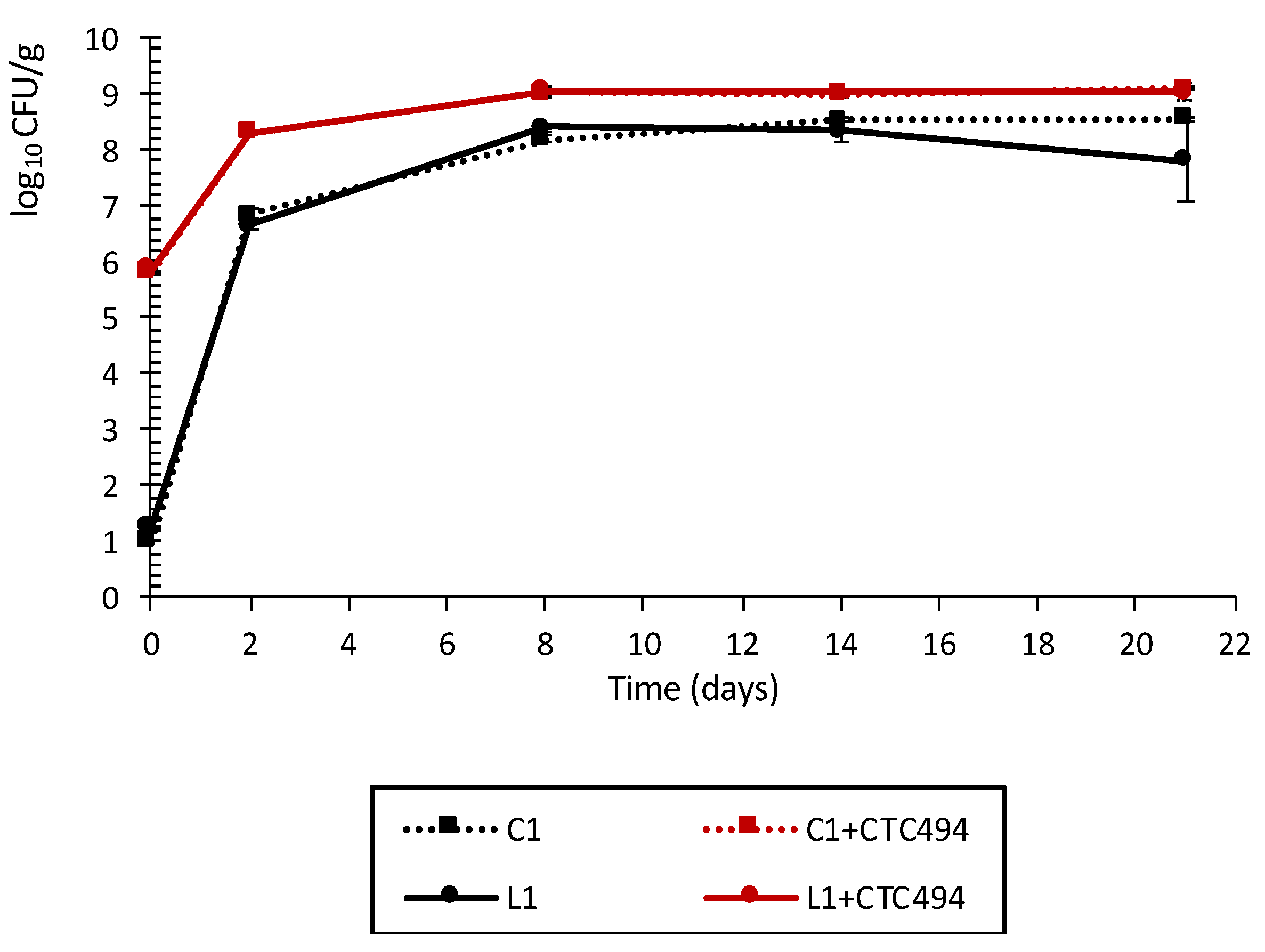
3.1.2. Behavior of LAB during Fermentation and Ripening
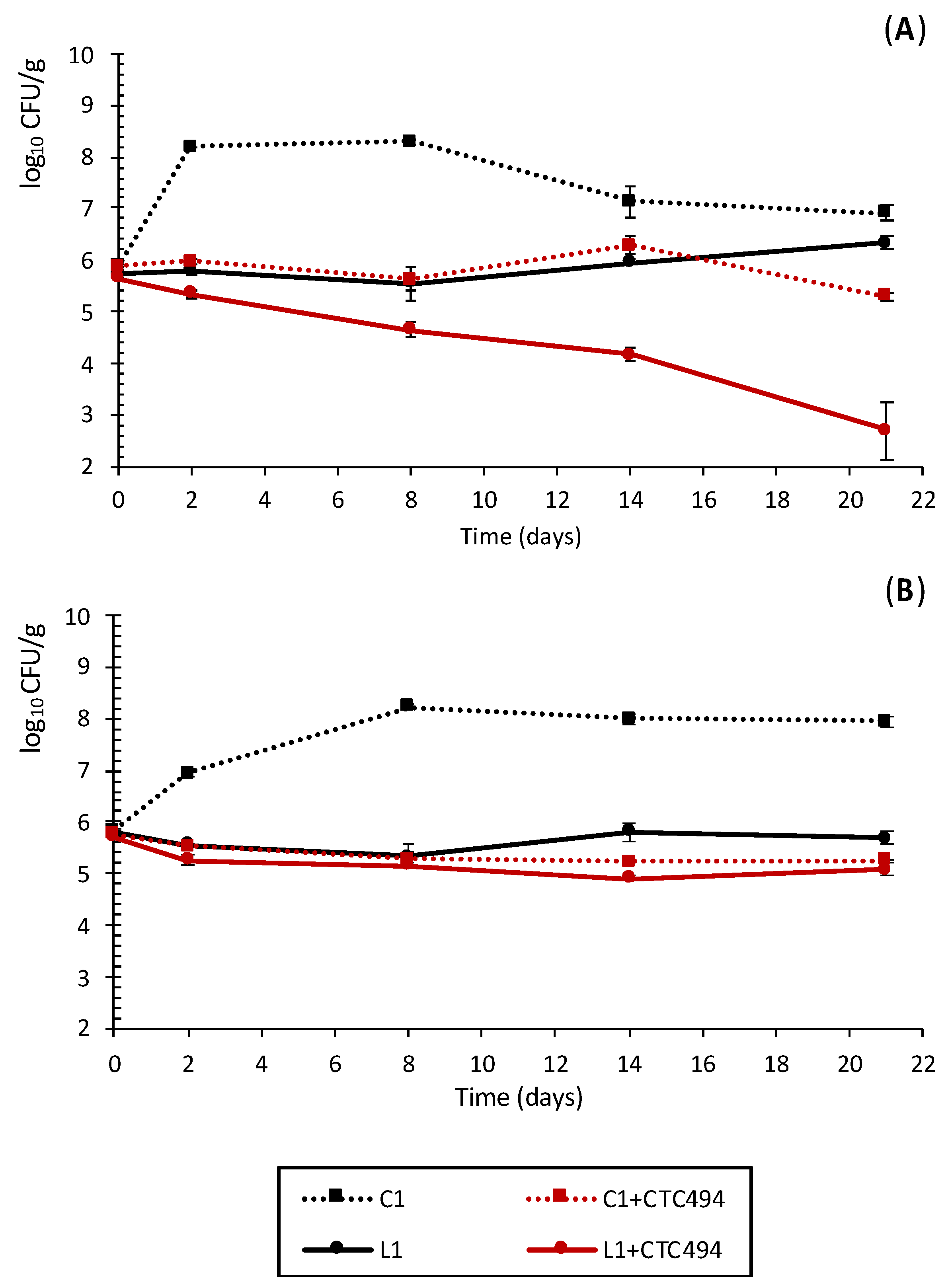
3.1.3. Impact of Cava Lees on Pathogenic Bacteria
3.2. Effect of the LPE in Fermented Pork Sausages (Experiment 2)
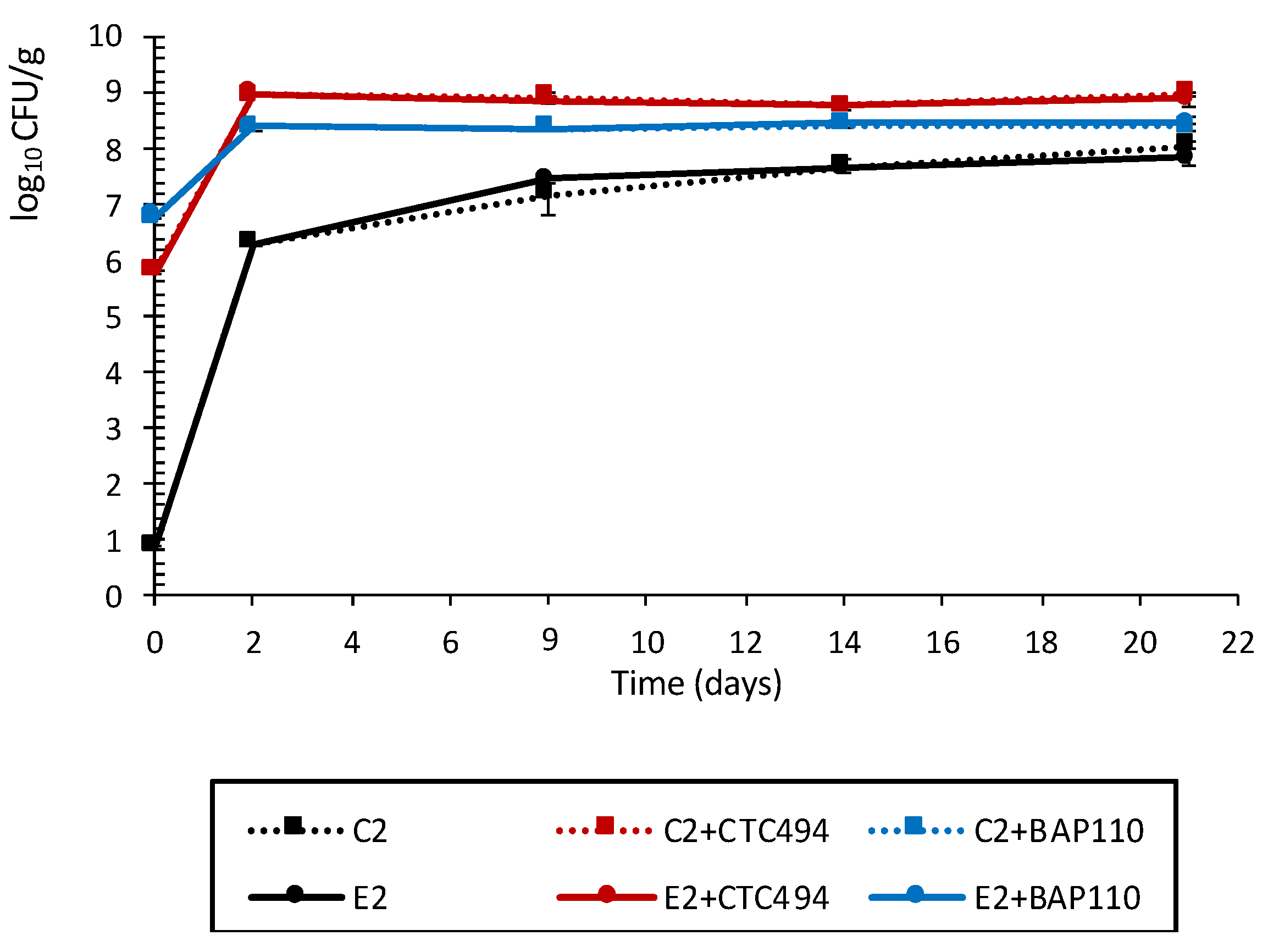
3.2.1. Characterization of Physicochemical Parameters and LAB Counts
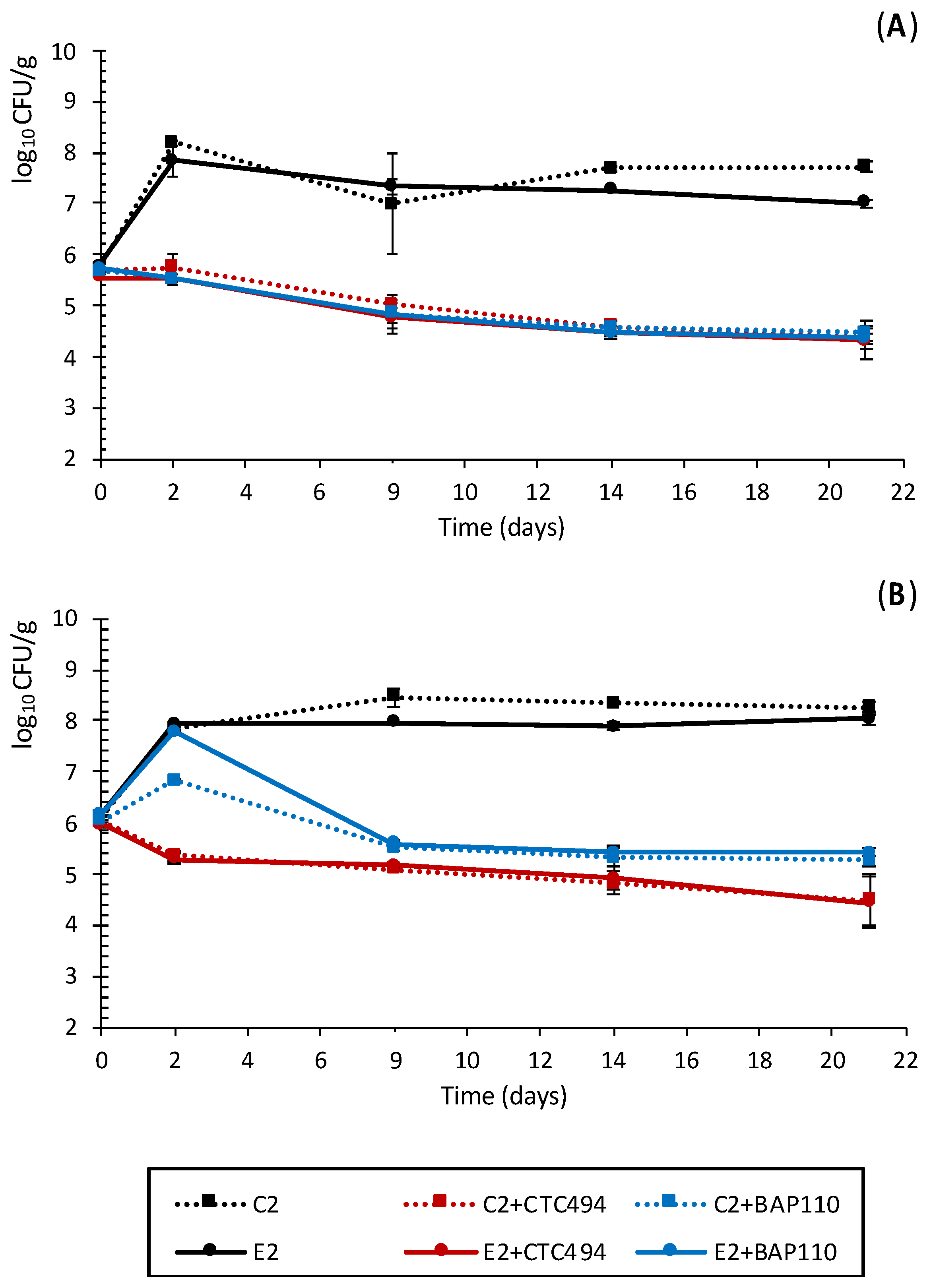
3.2.2. Impact of the LPE on Pathogenic Bacteria
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Iseppi, A.; Lomolino, G.; Marangon, M.; Curioni, A. Current and future strategies for wine yeast lees valorization. Food Res. Int. 2020, 137, 109352. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- García, M.; Dominguez, R.; Galvez, M.; Casas, C.; Selgas, M. Utilization of cereal and fruit fibres in low fat dry fermented sausages. Meat Sci. 2002, 60, 227–236. [Google Scholar] [CrossRef]
- Fernández-Ginés, J.M.; Fernández-López, J.; Sayas-Barberá, E.; Sendra, E.; Pérez-Álvarez, J.A. Lemon albedo as a new source of dietary fiber: Application to bologna sausages. Meat Sci. 2004, 67, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Zapata, E.; Zunino, V.; Pérez-Alvarez, J.A.; Fernández-López, J. Effect of tiger nut fibre addition on the quality and safety of a dry-cured pork sausage (‘Chorizo’) during the dry-curing process. Meat Sci. 2013, 95, 562–568. [Google Scholar] [CrossRef]
- Mendes, A.C.G.; Rettore, D.M.; Ramos, A.d.L.S.; da Cunha, S.d.F.V.; de Oliveira, L.C.; Ramos, E.M. Salames tipo milano elaborados com fibras de subprodutos da produção de vinho tinto. Cienc. Rural 2014, 44, 1291–1296. [Google Scholar] [CrossRef]
- Younis, K.; Ahmad, S. Waste utilization of apple pomace as a source of functional ingredient in buffalo meat sausage. Cogent Food Agric. 2015, 1, 1119397. [Google Scholar] [CrossRef]
- Tremonte, P.; Pannella, G.; Lombardi, S.J.; Iorizzo, M.; Vergalito, F.; Cozzolino, A.; Maiuro, L.; Succi, M.; Sorrentino, E.; Coppola, R. Low-fat and high-quality fermented sausages. Microorganisms 2020, 8, 1025. [Google Scholar] [CrossRef] [PubMed]
- Sayas-Barberá, E.; Viuda-Martos, M.; Fernández-López, F.; Pérez-Alvarez, J.A.; Sendra, E. Combined use of a probiotic culture and citrus fiber in a traditional sausage ‘Longaniza de Pascua’. Food Control 2012, 27, 343–350. [Google Scholar] [CrossRef]
- Yalinkiliç, B.; Kaban, G.; Kaya, M. The effects of different levels of orange fiber and fat on microbiological, physical, chemical and sensorial properties of sucuk. Food Microbiol. 2012, 29, 255–259. [Google Scholar] [CrossRef]
- Mikami, N.; Tsukada, Y.; Pelpolage, S.W.; Han, K.H.; Fukushima, M.; Shimada, K. Effects of sake lees (sake-kasu) supplementation on the quality characteristics of fermented dry sausages. Heliyon 2020, 6, e03379. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, J.; Zhi, N.; Aleson-Carbonell, L.; Pérez-Alvarez, J.A.; Kuri, V. Antioxidant and antibacterial activities of natural extracts: Application in beef meatballs. Meat Sci. 2005, 69, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; González-Rodríguez, R.M.; Sánchez, M.; Amado, I.R.; Franco, D. Effects of natural (grape seed and chestnut extract) and synthetic antioxidants (buthylatedhydroxytoluene, BHT) on the physical, chemical, microbiological and sensory characteristics of dry cured sausage “chorizo”. Food Res. Int. 2013, 54, 611–620. [Google Scholar] [CrossRef]
- Fasolato, L.; Carraro, L.; Facco, P.; Cardazzo, B.; Balzan, S.; Taticchi, A.; Andreani, N.A.; Montemurro, F.; Martino, M.E.; Di Lecce, G.; et al. Agricultural by-products with bioactive effects: A multivariate approach to evaluate microbial and physicochemical changes in a fresh pork sausage enriched with phenolic compounds from olive vegetation water. Int. J. Food Microbiol. 2016, 228, 34–43. [Google Scholar] [CrossRef]
- Marchiani, R.; Bertolino, M.; Belviso, S.; Giordano, M.; Ghirardello, D.; Torri, L.; Piochi, M.; Zeppa, G. Yogurt enrichment with grape pomace: Effect of grape cultivar on physicochemical, microbiological and sensory properties. J. Food Qual. 2016, 39, 77–89. [Google Scholar] [CrossRef]
- Van Ba, H.; Seo, H.W.; Cho, S.H.; Kim, Y.S.; Kim, J.H.; Ham, J.S.; Park, B.Y.; Nam, S.P. Antioxidant and anti-foodborne bacteria activities of shiitake by-product extract in fermented sausages. Food Control 2016, 70, 201–209. [Google Scholar] [CrossRef]
- Van Ba, H.; Seo, H.W.; Cho, S.H.; Kim, Y.S.; Kim, J.H.; Ham, J.S.; Park, B.Y.; Pil-Nam, S. Effects of extraction methods of shiitake by-products on their antioxidant and antimicrobial activities in fermented sausages during storage. Food Control 2017, 79, 109–118. [Google Scholar] [CrossRef]
- Vázquez-Armenta, F.J.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; González-Aguilar, G.A.; Nazzaro, F.; Fratianni, F.; Ayala-Zavala, J.F. Antibacterial and antioxidant properties of grape stem extract applied as disinfectant in fresh leafy vegetables. J. Food Sci. Technol. 2017, 54, 3192–3200. [Google Scholar] [CrossRef]
- Menchetti, L.; Taticchi, A.; Esposto, S.; Servili, M.; Ranucci, D.; Branciari, R.; Miraglia, D. The influence of phenolic extract from olive vegetation water and storage temperature on the survival of Salmonella enteritidis inoculated on mayonnaise. LWT 2020, 129, 109648. [Google Scholar] [CrossRef]
- Serra, A.T.; Matias, A.A.; Nunes, A.V.M.; Leitão, M.C.; Brito, D.; Bronze, R.; Silva, S.; Pires, A.; Crespo, M.T.; San Romão, M.V.; et al. In vitro evaluation of olive- and grape-based natural extracts as potential preservatives for food. Innov. Food Sci. Emerg. Technol. 2008, 9, 311–319. [Google Scholar] [CrossRef]
- Tsukada, M.; Sheng, H.; Kamachi, T.; Niwano, Y. Microbicidal action of photoirradiated aqueous extracts from wine lees. J. Food Sci. Technol. 2016, 53, 3020–3027. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Puig, M.; Moreno, P.; Pina-Pérez, M.C.; Rodrigo, D.; Martínez, A. Combined effect of high hydrostatic pressure (HHP) and antimicrobial from agro-industrial by-products against S. Typhimurium. LWT 2017, 77, 126–133. [Google Scholar] [CrossRef]
- Vazquez-Armenta, F.J.; Bernal-Mercado, A.T.; Lizardi-Mendoza, J.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; Gonzalez-Aguilar, G.A.; Nazzaro, F.; Fratianni, F.; Ayala-Zavala, J.F. Phenolic extracts from grape stems inhibit Listeria monocytogenes motility and adhesion to food contact surfaces. J. Adhes. Sci. Technol. 2018, 32, 889–907. [Google Scholar] [CrossRef]
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.P.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical composition, antioxidant and antimicrobial activity of phenolic compounds extracted from wine industry by-products. Food Control 2018, 92, 516–522. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Santos, R.A.; Queiroz, M.; Leal, C.; Saavedra, M.J.; Domínguez-Perles, R.; Rodrigues, M.; Barros, A.I.R.N.A. Monitoring the antioxidant and antimicrobial power of grape (Vitis vinifera L.) stems phenolics over long-term storage. Ind. Crops Prod. 2018, 126, 83–91. [Google Scholar] [CrossRef]
- Ricci, A.; Bernini, V.; Maoloni, A.; Cirlini, M.; Galaverna, G.; Neviani, E.; Lazzi, C. Vegetable by-product lacto-fermentation as a new source of antimicrobial compounds. Microorganisms 2019, 7, 607. [Google Scholar] [CrossRef]
- Taguri, T.; Tanaka, T.; Kouno, I. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biol. Pharm. Bull. 2004, 27, 1965–1969. [Google Scholar] [CrossRef]
- Papadopoulou, C.; Soulti, K.; Roussis, I.G. Potential antimicrobial activity of red and white wine phenolic extracts against strains of Staphylococcus aureus, Escherichia coli and Candida albicans. Food Technol. Biotechnol. 2005, 43, 41–46. [Google Scholar]
- Cetin-Karaca, H.; Newman, M.C. Antimicrobial efficacy of plant phenolic compounds against Salmonella and Escherichia coli. Food Biosci. 2015, 11, 8–16. [Google Scholar] [CrossRef]
- Mattos, G.N.; Tonon, R.V.; Furtado, A.A.L.; Cabral, L.M.C. Grape by-product extracts against microbial proliferation and lipid oxidation: A review. J. Sci. Food Agric. 2017, 97, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- D.O. Cava Regulatory Board Global Report. 2020. Available online: https://www.cava.wine/documents/258/ENG_KEY_FIGURES_2020.pdf (accessed on 31 May 2021).
- Lavelli, V.; Torri, L.; Zeppa, G.; Fiori, L.; Spigno, G. Recovery of Winemaking By-Products. Ital. J. Food Sci. 2016, 28, 542–564. [Google Scholar]
- Hernández-Macias, S.; Comas-Basté, O.; Jofré, A.; Bover-Cid, S.; Latorre-Moratalla, M.L.; Vidal-Carou, M.C. Growth-promoting effect of cava lees on lactic acid bacteria strains: A potential revalorization strategy of a winery by-product. Foods 2021, 10, 1636. [Google Scholar] [CrossRef]
- Garriga, M.; Aymerich, M.T.; Costa, S.; Monfort, J.M.; Hugas, M. Bactericidal synergism through bacteriocins and high pressure in a meat model system during storage. Food Microbiol. 2002, 19, 509–518. [Google Scholar] [CrossRef]
- Aguilera, M.Á. Characterization of Recovered Lees from Sparkling Wines. Ph.D. Thesis, University of Barcelona, Barcelona, Spain, 2016. [Google Scholar]
- Vallverdú-Queralt, A.; Medina-Remón, A.; Martínez-Huélamo, M.; Jáuregui, O.; Andres-Lacueva, C.; Lamuela-Raventos, R.M. Phenolic profile and hydrophilic antioxidant capacity as chemotaxonomic markers of tomato varieties. J. Agric. Food Chem. 2011, 59, 3994–4001. [Google Scholar] [CrossRef]
- Rubio, R.; Jofré, A.; Aymerich, T.; Guàrdia, M.D.; Garriga, M. Nutritionally enhanced fermented sausages as a vehicle for potential probiotic lactobacilli delivery. Meat Sci. 2014, 96, 937–942. [Google Scholar] [CrossRef]
- Bover-Cid, S.; Izquierdo-Pulido, M.; Vidal-Carou, M.C. Effect of proteolytic starter cultures of Staphylococcus spp. on biogenic amine formation during the ripening of dry fermented sausages. Int. J. Food Microbiol. 1999, 46, 95–104. [Google Scholar] [CrossRef]
- Fernández-López, J.; Viuda-Martos, M.; Sendra, E.; Sayas-Barberá, E.; Navarro, C.; Pérez-Alvarez, J.A. Orange fibre as potential functional ingredient for dry-cured sausages. Eur. Food Res. Technol. 2007, 226, 1–6. [Google Scholar] [CrossRef]
- Yang, B.; Prasad, K.N.; Xie, H.; Lin, S.; Jiang, Y. Structural characteristics of oligosaccharides from soy sauce lees and their potential prebiotic effect on lactic acid bacteria. Food Chem. 2011, 126, 590–594. [Google Scholar] [CrossRef]
- Gómez, B.; Peláez, C.; Martínez-Cuesta, M.C.; Parajó, J.C.; Alonso, J.L.; Requena, T. Emerging prebiotics obtained from lemon and sugar beet byproducts: Evaluation of their in vitro fermentability by probiotic bacteria. LWT 2019, 109, 17–25. [Google Scholar] [CrossRef]
- Fernández-López, J.; Sendra, E.; Sayas-Barberá, E.; Navarro, C.; Pérez-Alvarez, J.A. Physico-chemical and microbiological profiles of ‘salchichón’ (Spanish dry-fermented sausage) enriched with orange fiber. Meat Sci. 2008, 80, 410–417. [Google Scholar] [CrossRef]
- Serra-Castelló, C.; Bover-Cid, S.; Garriga, M.; Hansen, T.B.; Gunvig, A.; Jofré, A. Risk management tool to define a corrective storage to enhance Salmonella inactivation in dry fermented sausages. Int. J. Food Microbiol. 2021, 346, 109160. [Google Scholar] [CrossRef]
- Ramachandraiah, K.; Chin, K.B. Antioxidant, antimicrobial, and curing potentials of micronized celery powders added to pork sausages. Food Sci. Anim. Resour. 2021, 41, 110–121. [Google Scholar] [CrossRef]
- Zokaityte, E.; Cernauskas, D.; Klupsaite, D.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Ruzauskas, M.; Gruzauskas, R.; Juodeikiene, G.; Rocha, J.M.; et al. Bioconversion of milk permeate with selected lactic acid bacteria strains and apple by-products into beverages with antimicrobial properties and enriched with galactooligosaccharides. Microorganisms 2020, 8, 1182. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Zhuang, H.; Li, L.; Chen, X.; Zhang, J. Effects of plant polyphenols and α-tocopherol on lipid oxidation, microbiological characteristics, and biogenic amines formation in dry-cured bacons. J. Food Sci. 2015, 80, C547–C555. [Google Scholar] [CrossRef]
- Leistner, L. Basic aspects of food preservation by hurdle technology. Int. J. Food Microbiol. 2000, 55, 181–186. [Google Scholar] [CrossRef]
- International Commission on Microbiological Specifications for Foods (ICMSF). Microbiological Specifications for Foods (ICMSF). Microbiological specifications of food pathogens. In Microorganisms in Foods 5; Roberts, T.A., Baird-Parker, A.C., Tompkin, R.B., Eds.; Springer: New York, NY, USA, 1996; p. 514. ISBN 9780412473500. [Google Scholar]
- Jofré, A.; Aymerich, T.; Garriga, M. Improvement of the food safety of low acid fermented sausages by enterocins A and B and high pressure. Food Control 2009, 20, 179–184. [Google Scholar] [CrossRef]
- Piekarska-Radzik, L.; Klewicka, E. Mutual influence of polyphenols and Lactobacillus spp. bacteria in food: A review. Eur. Food Res. Technol. 2021, 247, 9–24. [Google Scholar] [CrossRef]
- Romero-Díez, R.; Rodríguez-Rojo, S.; Cocero, M.J.; Duarte, C.M.M.; Matias, A.A.; Bronze, M.R. Phenolic characterization of aging wine lees: Correlation with antioxidant activities. Food Chem. 2018, 259, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Jara-Palacios, M.J. Wine Lees as a Source of Antioxidant Compounds. Antioxidants 2019, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Razmkhab, S.; Lopez-Toledano, A.; Ortega, J.M.; Mayen, M.; Merida, J.; Medina, M. Adsorption of phenolic compounds and browning products in white wines by yeasts and their cell walls. J. Agric. Food Chem. 2002, 50, 7432–7437. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Cell wall anthocyanin adsorption by different Saccharomyces strains during the fermentation of Vitis vinifera L. cv Graciano grapes. Eur. Food Res. Technol. 2005, 220, 341–346. [Google Scholar] [CrossRef]
- Mazauric, J.-P.P.; Salmon, J.-M.M. Interactions between yeast lees and wine polyphenols during simulation of wine aging. II. Analysis of desorbed polyphenol compounds from yeast lees. J. Agric. Food Chem. 2006, 54, 3876–3881. [Google Scholar] [CrossRef]
- Gallardo-Chacón, J.J.; Vichi, S.; Urpí, P.; López-Tamames, E.; Buxaderas, S. Antioxidant activity of lees cell surface during sparkling wine sur lie aging. Int. J. Food Microbiol. 2010, 143, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Barcia, M.T.; Pertuzatti, P.B.; Gómez-Alonso, S.; Godoy, H.T.; Hermosín-Gutiérrez, I.; Teixeira, M.; Becker, P.; Gómez-Alonso, S.; Teixeira, H.; Hermosín-Gutiérrez, I. Phenolic composition of grape and winemaking by-products of Brazilian hybrid cultivars BRS Violeta and BRS Lorena. Food Chem. 2014, 159, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Marcos, B.; Aymerich, T.; Guardia, M.D.; Garriga, M. Assessment of high hydrostatic pressure and starter culture on the quality properties of low-acid fermented sausages. Meat Sci. 2007, 76, 46–53. [Google Scholar] [CrossRef][Green Version]
- Latorre-Moratalla, M.L.; Veciana-Nogués, T.; Bover-Cid, S.; Garriga, M.; Aymerich, T.; Zanardi, E.; Ianieri, A.; Fraqueza, M.J.; Patarata, L.; Drosinos, E.H.; et al. Biogenic amines in traditional fermented sausages produced in selected European countries. Food Chem. 2008, 107, 912–921. [Google Scholar] [CrossRef]
- Curiel, J.A.; Rodríguez, H.; Landete, J.M.; de las Rivas, B.; Muñoz, R. Ability of Lactobacillus brevis strains to degrade food phenolic acids. Food Chem. 2010, 120, 225–229. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Zhuang, H.; Chen, X.; Li, L.; Qiao, W.; Zhang, J. Effects of plant polyphenols and α-tocopherol on lipid oxidation, residual nitrites, biogenic amines, and N-nitrosamines formation during ripening and storage of dry-cured bacon. LWT Food Sci. Technol. 2015, 60, 199–206. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Pan, D.D.; Cao, J.X.; Shao, X.F.; Chen, Y.J.; Sun, Y.Y.; Ou, C.R. Effect of black pepper essential oil on the quality of fresh pork during storage. Meat Sci. 2016, 117, 130–136. [Google Scholar] [CrossRef]
- Sánchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef]
- Aymerich, T.; Rodríguez, M.; Garriga, M.; Bover-Cid, S. Assessment of the bioprotective potential of lactic acid bacteria against Listeria monocytogenes on vacuum-packed cold-smoked salmon stored at 8 °C. Food Microbiol. 2019, 83, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Serra-Castelló, C.; Costa, J.C.C.P.; Jofré, A.; Bolívar, A.; Pérez-Rodríguez, F.; Bover-Cid, S. A mathematical model to predict the antilisteria bioprotective effect of Latilactobacillus sakei CTC494 in vacuum packaged cooked ham. Int. J. Food Microbiol. 2021, in press. [Google Scholar]
- Payne, K.D.; Rico-Munoz, E.; Davidson, P.M. The antimicrobial activity of phenolic compounds against Listeria monocytogenes and their effectiveness in a model milk system. J. Food Prot. 1989, 52, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Klancnik, A.; Guzej, B.; Kolar, M.H.; Abramovic, H.; Mozina, S.S.; Klančnik, A.; Guzej, B.; Kolar, M.H.; Abramovič, H.; Možina, S.S.; et al. In vitro antimicrobial and antioxidant activity of commercial rosemary extract formulations. J. Food Prot. 2009, 72, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Tabasco, R.; Sánchez-Patán, F.; Monagas, M.; Bartolomé, B.; Victoria Moreno-Arribas, M.; Peláez, C.; Requena, T. Effect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: Resistance and metabolism. Food Microbiol. 2011, 28, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
| Experiment | Batch | Ingredient | Starter Culture |
|---|---|---|---|
| 1 | C1 | ||
| L1 | Cava lees | ||
| C1 + CTC494 | L. sakei CTC494 1 | ||
| L1 + CTC494 | Cava lees | L. sakei CTC494 1 | |
| 2 | C2 | ||
| E2 | LPE | ||
| C2 + CTC494 | L. sakei CTC494 | ||
| E2 + CTC494 | LPE | L. sakei CTC494 | |
| C2 + BAP110 | L. sakei BAP110 | ||
| E2 + BAP110 | LPE | L. sakei BAP110 |
| Batch | |||||
|---|---|---|---|---|---|
| Day | C1 | L1 | C1 + CTC494 | L1 + CTC494 | |
| pH | 0 | 5.67 ± 0.01 a | 5.20 ± 0.14 b | 5.66 ± 0.02 a | 5.22 ± 0.09 b |
| 2 | 5.74 ± 0.01 a | 5.43 ± 0.04 b | 5.07 ± 0.01 c | 4.89 ± 0.01 d | |
| 4 | 5.62 ± 0.02 a | 5.39 ± 0.01 b | 4.93 ± 0.01 c | 4.76 ± 0.01 d | |
| 8 | 5.44 ± 0.02 a | 5.35 ± 0.01 b | 5.01 ± 0.02 c | 4.80 ± 0.01 d | |
| 14 | 5.36 ± 0.05 a | 5.18 ± 0.03 ab | 5.40 ± 0.15 a | 4.91 ± 0.00 b | |
| 21 | 6.26 ± 0.38 a | 5.41 ± 0.05 b | 5.28 ± 0.05 b | 5.03 ± 0.05 b | |
| aw | 0 | 0.973 ± 0.001 a | 0.972 ± 0.000 a | 0.973 ± 0.000 a | 0.972 ± 0.001 a |
| 2 | 0.972 ± 0.000 a | 0.971 ± 0.000 a | 0.972 ± 0.000 a | 0.972 ± 0.000 a | |
| 4 | 0.974 ± 0.000 a | 0.972 ± 0.000 a | 0.969 ± 0.000 a | 0.970 ± 0.000 a | |
| 8 | 0.969 ± 0.000 a | 0.969 ± 0.000 a | 0.967 ± 0.000 b | 0.964 ± 0.000 c | |
| 14 | 0.963 ± 0.000 a | 0.964 ± 0.000 b | 0.965 ± 0.000 ab | 0.960 ± 0.000 c | |
| 21 | 0.960 ± 0.000 b | 0.966 ± 0.000 a | 0.962 ± 0.000 ab | 0.961 ± 0.000 b |
| Batch | |||||||
|---|---|---|---|---|---|---|---|
| Day | C2 | E2 | C2 + CTC494 | E2 + CTC494 | C2 + BAP110 | E2 + BAP110 | |
| pH | 0 | 5.88 ± 0.02 a | 5.86 ± 0.01 a | 5.88 ± 0.01 a | 5.86 ± 0.02 a | 5.85 ± 0.01 a | 5.77 ± 0.05 b |
| 2 | 5.84 ± 0.01 a | 5.81 ± 0.02 a | 5.10 ± 0.03 c | 5.10 ± 0.03 c | 5.42 ± 0.10 b | 5.38 ± 0.11 b | |
| 5 | 5.71 ± 0.07 a | 5.73 ± 0.04 a | 4.96 ± 0.03 c | 4.95 ± 0.01 c | 5.24 ± 0.03 b | 5.33 ± 0.04 b | |
| 9 | 5.56 ± 0.07 a | 5.56 ± 0.02 a | 4.98 ± 0.01 b | 4.96 ± 0.01 b | 4.99 ± 0.02 b | 4.98 ± 0.02 b | |
| 14 | 5.38 ± 0.04 b | 5.46 ± 0.03 a | 5.01 ± 0.01 c | 5.00 ± 0.01 cd | 4.96 ± 0.02 cd | 4.95 ± 0.00 d | |
| 21 | 5.28 ± 0.17 a | 5.38 ± 0.09 a | 5.01 ± 0.01 b | 5.01 ± 0.04 b | 5.02 ± 0.02 b | 4.99 ± 0.02 b | |
| aw | 0 | 0.972 ± 0.001 a | 0.971 ± 0.001 a | 0.972 ±0.001 a | 0.971 ± 0.001 a | 0.972 ± 0.001 a | 0.971 ± 0.001 a |
| 2 | 0.970 ± 0.001 a | 0.970 ± 0.001 a | 0.972 ± 0.001 a | 0.971 ± 0.001 a | 0.971 ± 0.001 a | 0.971 ± 0.001 a | |
| 5 | 0.971 ± 0.002 a | 0.969 ± 0.002 a | 0.970 ± 0.001 a | 0.970 ± 0.001 a | 0.969 ± 0.001 a | 0.969 ± 0.001 a | |
| 9 | 0.972 ± 0.001 a | 0.971 ± 0.001 a | 0.971 ± 0.002 a | 0.972 ± 0.001 a | 0.969 ± 0.001 a | 0.970 ± 0.001 a | |
| 14 | 0.966 ± 0.001 bc | 0.970 ± 0.002 ab | 0.968 ± 0.001 abc | 0.971 ± 0.002 a | 0.964 ± 0.002 c | 0.969 ± 0.001 ab | |
| 21 | 0.964 ± 0.001 a | 0.963 ± 0.001 a | 0.963 ± 0.001 a | 0.963 ± 0.002 a | 0.962 ± 0.001 a | 0.963 ± 0.001 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Macias, S.; Ferrer-Bustins, N.; Comas-Basté, O.; Jofré, A.; Latorre-Moratalla, M.; Bover-Cid, S.; Vidal-Carou, M.d.C. Revalorization of Cava Lees to Improve the Safety of Fermented Sausages. Foods 2021, 10, 1916. https://doi.org/10.3390/foods10081916
Hernández-Macias S, Ferrer-Bustins N, Comas-Basté O, Jofré A, Latorre-Moratalla M, Bover-Cid S, Vidal-Carou MdC. Revalorization of Cava Lees to Improve the Safety of Fermented Sausages. Foods. 2021; 10(8):1916. https://doi.org/10.3390/foods10081916
Chicago/Turabian StyleHernández-Macias, Salvador, Núria Ferrer-Bustins, Oriol Comas-Basté, Anna Jofré, Mariluz Latorre-Moratalla, Sara Bover-Cid, and María del Carmen Vidal-Carou. 2021. "Revalorization of Cava Lees to Improve the Safety of Fermented Sausages" Foods 10, no. 8: 1916. https://doi.org/10.3390/foods10081916
APA StyleHernández-Macias, S., Ferrer-Bustins, N., Comas-Basté, O., Jofré, A., Latorre-Moratalla, M., Bover-Cid, S., & Vidal-Carou, M. d. C. (2021). Revalorization of Cava Lees to Improve the Safety of Fermented Sausages. Foods, 10(8), 1916. https://doi.org/10.3390/foods10081916







