Profiling of 2-Acetyl-1-Pyrroline and Other Volatile Compounds in Raw and Cooked Rice of Traditional and Improved Varieties of India
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rice Samples
2.2. Chemicals
2.3. Optimization of HS-SPME Conditions
2.4. HS-SPME Based Extraction of Volatiles from Raw and Cooked Rice
2.5. Determination of 2-AP Using HS-SPME-GC-MS/MS
2.6. Profiling, Identification, and Relative Quantification of Volatile Compounds Using HS-SPME-GC-MS
2.7. Data Processing and Statistical Analysis
3. Results and Discussion
3.1. Optimization of HS-SPME Parameters
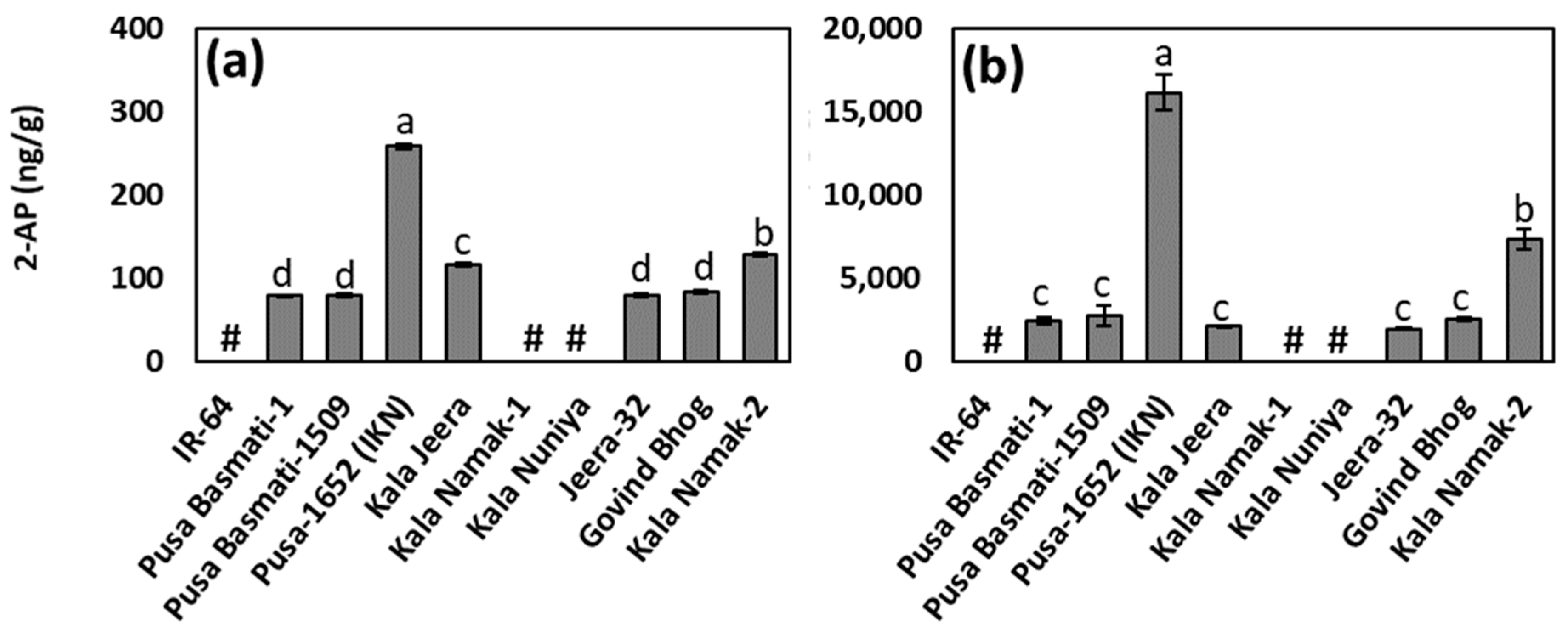
3.2. 2-AP Contents in Raw and Cooked Rice
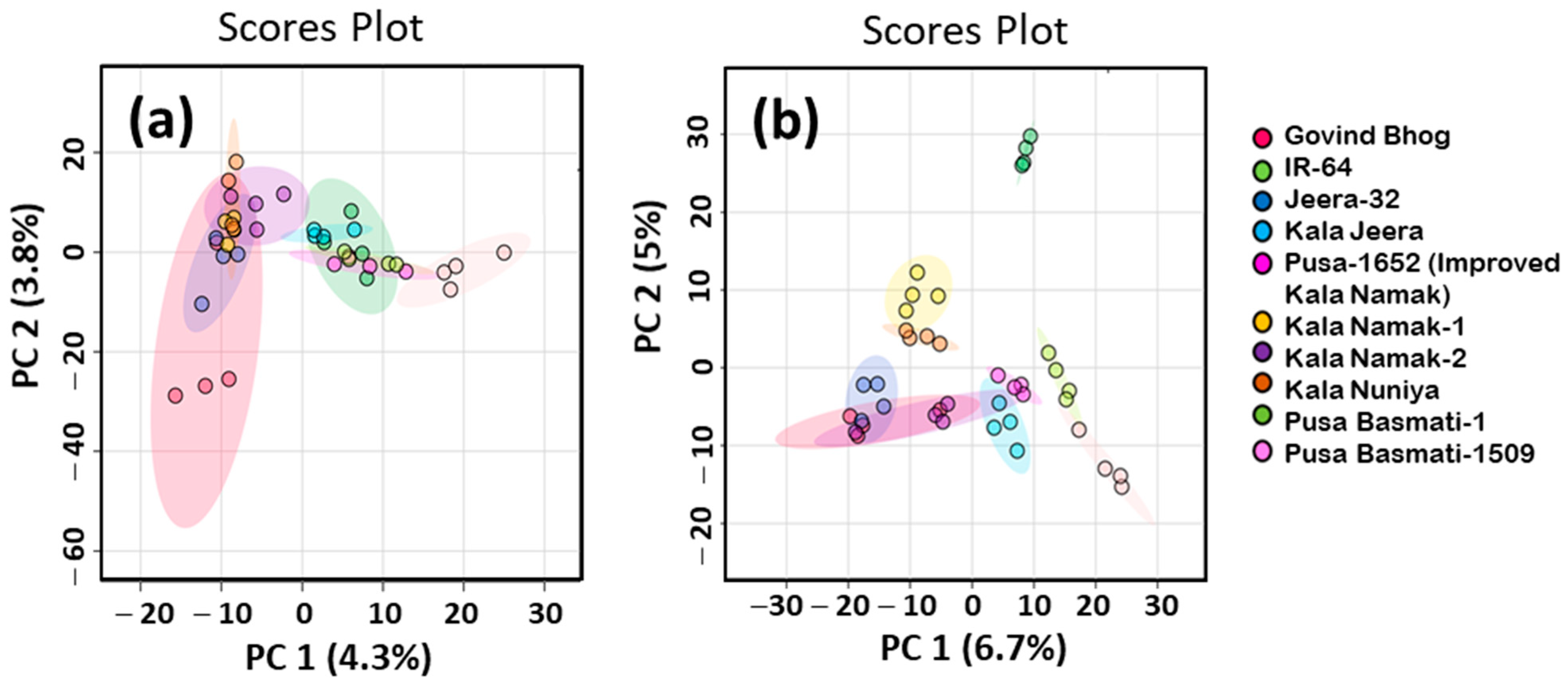
3.3. Untargeted Analysis of Volatile Profiles of Raw and Cooked Rice Samples
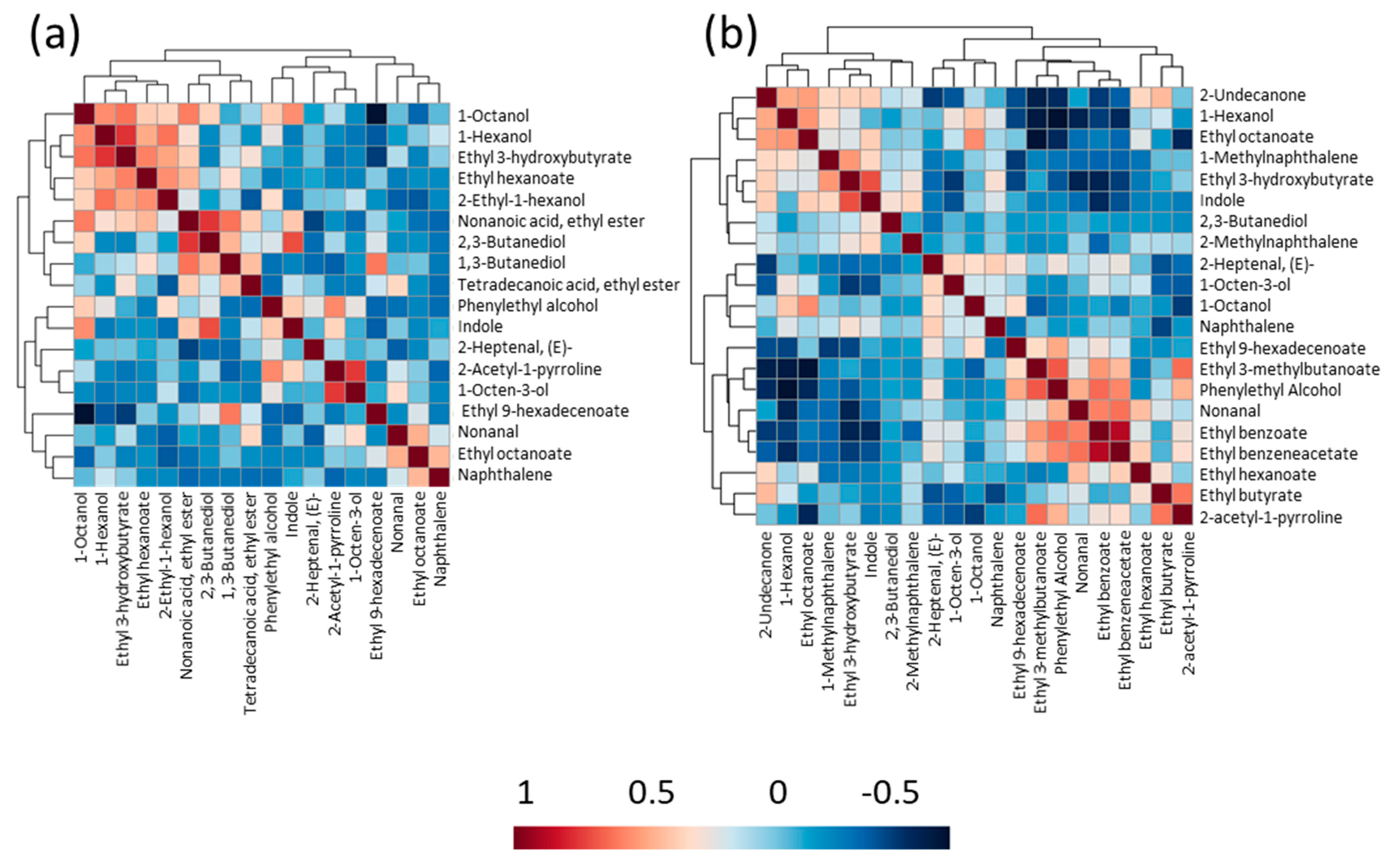
3.4. Volatile Profiles of Raw and Cooked Rice Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jinakot, I.; Jirapakkul, W. Volatile aroma compounds injasmine rice as affected by degrees of milling. J. Nutr. Sci. Vitaminol. 2019, 65, S231–S234. [Google Scholar] [CrossRef] [Green Version]
- Mahattanatawee, K.; Rouseff, R.L. Comparison of aroma active and sulfur volatiles in three fragrant rice cultivars using GC–Olfactometry and GC–PFPD. Food Chem. 2014, 154, 1–6. [Google Scholar] [CrossRef]
- Yang, D.S.; Lee, K.-S.; Jeong, O.-Y.; Kim, K.-J.; Kays, S.J. Characterization of volatile aroma compounds in cooked black rice. J. Agric. Food Chem. 2008, 56, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhang, H.; Zhang, T.; Tamogami, S.; Chen, J.Y. Analysis of flavor volatiles of glutinous rice during cooking by combined gas chromatography–mass spectrometry with modified headspace solid-phase microextraction method. J. Food Compos. Anal. 2009, 22, 347–353. [Google Scholar] [CrossRef]
- Champagne, E.T. Rice aroma and flavor: A literature review. Cereal Chem. 2008, 85, 445–454. [Google Scholar] [CrossRef]
- Hu, X.; Lu, L.; Guo, Z.; Zhu, Z. Volatile compounds, affecting factors and evaluation methods for rice aroma: A review. Trends Food Sci. Technol. 2020, 97, 136–146. [Google Scholar] [CrossRef]
- Ma, R.; Tian, Y.; Chen, L.; Jin, Z. Impact of cooling rates on the flavor of cooked rice during storage. Food Biosci. 2020, 35, 100563. [Google Scholar] [CrossRef]
- Bryant, R.; McClung, A. Volatile profiles of aromatic and non-aromatic rice cultivars using SPME/GC–MS. Food Chem. 2011, 124, 501–513. [Google Scholar] [CrossRef]
- Hopfer, H.; Jodari, F.; Negre-Zakharov, F.; Wylie, P.L.; Ebeler, S.E. HS-SPME-GC-MS/MS method for the rapid and sensitive quantitation of 2-acetyl-1-pyrroline in single rice kernels. J. Agric. Food Chem. 2016, 64, 4114–4120. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Handoko, D.D.; Pather, L.; Methven, L.; Elmore, J.S. Evaluation of 2-acetyl-1-pyrroline in foods, with an emphasis on rice flavour. Food Chem. 2017, 232, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jayaprakasha, G.; Rush, C.M.; Crosby, K.M.; Patil, B.S. Production system influences volatile biomarkers in tomato. Metabolomics 2018, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Lucero, M.; Estell, R.; Tellez, M.; Fredrickson, E. A retention index calculator simplifies identification of plant volatile organic compounds. Phytochem. Anal. 2009, 20, 378–384. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Rojo-Poveda, O.; Ferrocino, I.; Giordano, M.; Zeppa, G. Analytical dataset on volatile compounds of cocoa bean shells from different cultivars and geographical origins. Data Brief. 2019, 25, 104268. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.K.; Mo, C.; Lee, D.-K.; Long, N.P.; Lim, J.; Kwon, S.W. Non-destructive profiling of volatile organic compounds using HS-SPME/GC–MS and its application for the geographical discrimination of white rice. J. Food Drug Anal. 2018, 26, 260–267. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinform. 2016, 55, 14.10.11–14.10.91. [Google Scholar] [CrossRef] [PubMed]
- Ghiasvand, A.R.; Setkova, L.; Pawliszyn, J. Determination of flavour profile in Iranian fragrant rice samples using cold-fibre SPME–GC–TOF–MS. Flavour Fragr. J. 2007, 22, 377–391. [Google Scholar] [CrossRef]
- Goufo, P.; Duan, M.; Wongpornchai, S.; Tang, X. Some factors affecting the concentration of the aroma compound 2-acetyl-1-pyrroline in two fragrant rice cultivars grown in South China. Front. Agric. China 2010, 4, 1–9. [Google Scholar] [CrossRef]
- Choi, S.; Lee, J. Volatile and sensory profiles of different black rice (Oryza sativa L.) cultivars varying in milling degree. Food Res. Int. 2021, 141, 110150. [Google Scholar] [CrossRef] [PubMed]
- Yoshihashi, T. Quantitative analysis on 2-acetyl-1-pyrroline of an aromatic rice by stable isotope dilution method and model studies on its formation during cooking. J. Food Sci. 2002, 67, 619–622. [Google Scholar] [CrossRef]
- Grimm, C.C.; Bergman, C.; Delgado, J.T.; Bryant, R. Screening for 2-acetyl-1-pyrroline in the headspace of rice using SPME/GC-MS. J. Agric. Food Chem. 2001, 49, 245–249. [Google Scholar] [CrossRef]
- Wongpornchai, S.; Dumri, K.; Jongkaewwattana, S.; Siri, B. Effects of drying methods and storage time on the aroma and milling quality of rice (Oryza sativa L.) cv. Khao Dawk Mali 105. Food Chem. 2004, 87, 407–414. [Google Scholar] [CrossRef]
- Lubes, G.; Goodarzi, M. Analysis of volatile compounds by advanced analytical techniques and multivariate chemometrics. Chem. Rev. 2017, 117, 6399–6422. [Google Scholar] [CrossRef]
- Sansenya, S.; Hua, Y.; Chumanee, S. The correlation between 2-acetyl-1-pyrroline content, biological compounds and molecular characterization to the aroma intensities of Thai local rice. J. Oleo Sci. 2018, 67, 893–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.; Nuijten, E.; Shewfelt, R.L.; Kays, S.J. Aroma chemistry of African Oryza glaberrima and Oryza sativa rice and their interspecific hybrids. J. Sci. Food Agric. 2014, 94, 727–735. [Google Scholar] [CrossRef]
- Dias, L.; Duarte, G.; Mariutti, L.; Bragagnolo, N. Aroma profile of rice varieties by a novel SPME method able to maximize 2-acetyl-1-pyrroline and minimize hexanal extraction. Food Res. Int. 2019, 123, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Griglione, A.; Liberto, E.; Cordero, C.; Bressanello, D.; Cagliero, C.; Rubiolo, P.; Bicchi, C.; Sgorbini, B. High-quality Italian rice cultivars: Chemical indices of ageing and aroma quality. Food Chem. 2015, 172, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhong, Y.; Yu, W.; Yue, J.; Liu, Z.; Zheng, Y.; Zhao, Y. Effect of hydrostatic high pressure pretreatment on flavor volatile profile of cooked rice. J. Cereal Sci. 2013, 58, 479–487. [Google Scholar] [CrossRef]
- Hinge, V.; Patil, H.; Nadaf, A. Comparative characterization of aroma volatiles and related gene expression analysis at vegetative and mature stages in basmati and non-basmati rice (Oryza sativa L.) cultivars. Appl. Biochem. Biotechnol. 2016, 178, 619–639. [Google Scholar] [CrossRef]
| Compound | RIcal | a RIrep | Identi-fication Details | b Odor Description | Concentration in ng/g | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IR-64 | Pusa Basmati-1 | Pusa Basmati-1509 | Pusa-1652 (Improved Kala Namak) | Kala Jeera | Kala Namak-1 | Kala Nuniya | Jeera-32 | Govind Bhog | Kala Namak-2 | |||||
| Ethyl hexanoate | 1242 | 1240 | MS, RI | Fruity, apple peel | n.d. | n.d. | 1.2 ± 0.1a | n.d. | n.d. | n.d. | n.d. | 1.1 ± 0.0a | n.d. | n.d. |
| 2-Heptenal, (E)- | 1338 | 1338 | MS, RI | Fruity, green, fatty | n.d. | 2.4 ± 0.5a | n.d. | 1.7 ± 0.1a | 1.8 ± 0.1a | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2-Acetyl-1-pyrroline | 1354 | 1343 | MS, ST | Popcorn, toasted grain, nuty | n.d. | n.d. | n.d. | 1.8 ± 0.2a | 0.5 ± 0.0c | n.d. | n.d. | n.d. | n.d. | 0.9 ± 0.1b |
| 1-Hexanol | 1361 | 1363 | MS, RI, ST | Green, herbaceous, woody, sweet | 1.4 ± 0.1d | 2.6 ± 0.1abcd | 1.7 ± 0.6cd | 2.9 ± 0.0abc | 2.3 ± 0.4abcd | 1.7 ± 0.0cd | 3.2 ± 0.3ab | 2.5 ± 0.0abcd | 3.5 ± 0.1a | 2.0 ± 0.2bcd |
| Nonanal | 1401 | 1396 | MS, RI | Green, fatty, citrus | 1.3 ± 0.1c | 2.5 ± 0.2ab | 2.4 ± 0.4abc | 2.6 ± 0.1ab | n.d. | 1.2 ± 0.0c | n.d. | n.d. | 1.5 ± 0.2bc | 2.8 ± 0.6a |
| Ethyl octanoate | 1444 | 1444 | MS, RI | Fruity, fatty, brandy | n.d. | 2.2 ± 0.2a | 1.9 ± 0.3a | 1.6 ± 0.1ab | n.d. | n.d. | n.d. | n.d. | 1.2 ± 0.1b | 1.3 ± 0.1b |
| 1-Octen-3-ol | 1455 | 1460 | MS, RI | Green, mushroom, earthy, oily | n.d. | n.d. | 1.6 ± 0.2b | 2.8 ± 0.2a | 1.8 ± 0.3b | 1.2 ± 0.0b | n.d. | n.d. | n.d. | 3.1 ± 0.3a |
| 2-Ethyl-1-hexanol | 1494 | 1490 | MS, RI | Citrus, fresh floral, oily, sweet | n.d. | 25.9 ± 3.3a | 26.2 ± 0.6a | 28.0 ± 0.4a | 24.1 ± 3.5a | 32.3 ± 0.4a | 29.3 ± 1.8a | 28.2 ± 4.0a | n.d. | 30.5 ± 0.9a |
| Ethyl 3-hydroxybutyrate | 1529 | 1524 | MS, RI | Green, fruity, waxy, apple skin | 2.7 ± 0.2a | 2.7 ± 0.2a | 2.3 ± 0.1a | 2.0 ± 0.2a | 2.7 ± 0.1a | 2.6 ± 0.2a | 2.2 ± 0.1a | 2.6 ± 0.1a | 2.4 ± 0.1a | 2.0 ± 0.0a |
| Nonanoic acid, ethyl ester | 1542 | 1541 | MS, RI | Waxy, fruity, nutty, wine-note | n.d. | n.d. | 2.2 ± 0.1b | 2.8 ± 0.2a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2,3-Butanediol | 1548 | 1545 | MS, RI | Creamy, fruity, buttery | n.d. | n.d. | 3.9 ± 0.7b | 7.6 ± 0.0a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1-Octanol | 1564 | 1565 | MS, RI | Waxy, green citrus | n.d. | n.d. | n.d. | 1.5 ± 0.2a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1,3-Butanediol | 1582 | 1578 | MS, RI | - | n.d. | n.d. | 7.6 ± 2.2a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Naphthalene | 1764 | 1755 | MS, ST | Pungent, tarry | n.d. | 2.4 ± 0.5 | n.d. | 2.3 ± 0.3a | 0.9 ± 0.5ab | 1.3 ± 0.0bc | n.d. | n.d. | 2.0 ± 0.2ab | n.d. |
| Phenylethyl alcohol | 1929 | 1924 | MS, RI | Floral, sweet, rosey, honey | n.d. | 1.3 ± 0.1c | n.d. | 6.7 ± 0.1a | 1.8 ± 0.1b | n.d. | 1.3 ± 0.0c | n.d. | n.d. | 1.7 ± 0.1b |
| Tetradecanoic acid, ethyl ester | 2060 | 2067 | MS | Waxy, sweet | 3.2 ± 0.2b | 2.6 ± 0.3bc | 8.1 ± 0.3a | 1.7 ± 0.1d | 1.9 ± 0.0cd | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ethyl 9-hexadecenoate | 2267 | 2267 | MS, RI | - | n.d. | 25.9 ± 2.5b | 65.8 ± 0.7a | 7.8 ± 0.4cd | 11.1 ± 0.5c | 2.4 ± 0.3fg | 2.0 ± 0.1fg | 2.9 ± 0.4efg | 4.4 ± 0.2def | 6.7 ± 0.2de |
| Indole | 2475 | 2478 | MS, RI, ST | Animal, floral, mothball | 3.6 ± 0.2b | n.d. | n.d. | 60.9 ± 0.4a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Compound | RIcal | a RIrep | Identi-Fication Details | b Odor Description | Concentration in ng/g | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IR-64 | Pusa Basmati-1 | Pusa Basmati-1509 | Pusa-1652 (Improved Kala Namak) | Kala Jeera | Kala Namak-1 | Kala Nuniya | Jeera-32 | Govind Bhog | Kala Namak-2 | |||||
| Ethyl butyrate | 1046 | 1041 | MS, RI | Fruity, green, apple, fatty | n.d. | 5.1 ± 1.1abc | 8.1 ± 1.4a | 7.0 ± 0.9ab | 3.7 ± 0.5b | n.d. | 3.4 ± 0.5cd | n.d. | n.d. | 4.1 ± 0.9bc |
| Ethyl 3-methylbutanoate | 1077 | 1078 | MS, RI | Fruity, sweet apple, pineapple | n.d. | n.d. | n.d. | 8.1 ± 0.4a | n.d. | n.d. | n.d | 5.7 ± 0.8ab | 6.5 ± 1.7ab | 3.9 ± 0.6b |
| Ethyl hexanoate | 1242 | 1240 | MS, RI | Fruity, apple peel | 11.1 ± 0.2def | 33.8 ± 1.4ab | 44.5 ± 1.9a | 19.8 ± 1.9cd | 22.7 ± 3.4bc | 8.2 ± 0.4ef | 12.9 ± 3.0 cde | 15.0 ± 0.8cde | 33.5 ± 4.6ab | n.d. |
| 2-Heptenal, (E)- | 1338 | 1338 | MS, RI | Fruity, green, fatty | n.d. | 5.6 ± 0.5ab | 4.1 ± 0.4bc | n.d. | n.d. | 5.2 ± 1.2abc | 4.0 ± 0.8bc | 6.6 ± 2.8ab | 10.0 ± 1.5a | 5.2 ± 0.1abc |
| 2-Acetyl-1-pyrroline | 1354 | 1343 | MS, ST | Popcorn, toasted grain, nuty | n.d. | 15.6 ± 1.2d | 16.2 ± 0.8d | 70.4 ± 2.0a | 21.1 ± 1.3c | n.d. | n.d. | 14.7 ± 1.3d | 20.3 ± 1.6c | 25.0 ± 0.8b |
| 1-Hexanol | 1361 | 1363 | MS, RI, ST | Green, herbaceous, woody, sweet | 7.7 ± 0.5cd | 37.7 ± 1.7ab | 9.2 ± 0.2cd | 4.8 ± 0.4cd | 35.9 ± 1.8ab | 28.4 ± 1.8abc | 45.1 ± 15.9a | n.d. | n.d. | 18.2 ± 2.7bcd |
| Nonanal | 1401 | 1396 | MS, RI | Green, fatty, citrus | n.d. | n.d. | 34.8 ± 0.7a | 10.6 ± 0.4b | n.d. | n.d. | n.d. | 14.2 ± 1.7b | 10.2 ± 2.5b | n.d. |
| Ethyl octanoate | 1444 | 1444 | MS, RI | Fruity, fatty, brandy | 8.9 ± 1.4ab | 11.1 ± 0.8ab | 10.9 ± 1.1ab | n.d. | 12.4 ± 0.3a | 6.4 ± 0.6b | 10.9 ± 0.7ab | 7.2 ± 2.1ab | 8.5 ± 1.7ab | 9.5 ± 0.4ab |
| 1-Octen-3-ol | 1455 | 1460 | MS, RI | Green, mushroom, earthy, oily | n.d. | 5.7 ± 0.3ab | 5.4 ± 0.8ab | n.d. | 5.8 ± 0.8ab | 7.6 ± 2.7a | 5.7 ± 0.8ab | 6.3 ± 0.6ab | 9.7 ± 0.8a | 2.6 ± 0.4bc |
| Ethyl 3-hydroxybutyrate | 1529 | 1524 | MS, RI | Green, fruity, waxy, apple skin | 9.1 ± 0.6a | 6.2 ± 0.8ab | n.d. | 6.8 ± 0.5ab | 5.7 ± 1.2ab | 4.1 ± 1.4b | 4.6 ± 0.1b | 3.8 ± 0.6b | 3.5 ± 0.3bc | 5.6 ± 0.7ab |
| 2,3-Butanediol | 1548 | 1545 | MS, RI | Creamy, fruity, buttery | 5.0 ± 0.0a | 5.7 ± 0.4a | 6.6 ± 1.1a | 4.9 ± 0.6a | 3.1 ± 0.6ab | 4.7 ± 1.7a | n.d. | 6.0 ± 1.7a | 4.5 ± 0.6a | 5.0 ± 0.4a |
| 1-Octanol | 1564 | 1565 | MS, RI | Waxy, green citrus | 5.0 ± 0.7bc | 9.6 ± 1.7ab | 11.1 ± 0.5ab | n.d. | 9.2 ± 0.7ab | 7.8 ± 1.7ab | 5.5 ± 0.5abc | 8.3 ± 0.5ab | 9.0 ± 1.2ab | 12.7 ± 4.0a |
| 2-Undecanone | 1606 | 1596 | MS | Waxy, fruity creamy, fatty, floral | 7.1 ± 1.3abc | 10.3 ± 0.4a | 10.3 ± 1.4a | 5.2 ± 0.4bc | 7.9 ± 1.1ab | 2.9 ± 1.7cd | 5.0 ± 0.8ab | n.d. | n.d. | 4.7 ± 0.6bc |
| Ethyl benzoate | 1682 | 1677 | MS, RI | Sweet, fruity, wintergreen, medicinal, | n.d. | 5.6 ± 1.2de | 16.1 ± 3.1ab | 14.7 ± 1.0bc | 11.1 ± 0.9bcd | 4.6 ± 0.5de | 8.6 ± 0.9cd | 22.2 ± 2.5a | 17.5 ± 0.9ab | 7.9 ± 0.1cd |
| Naphthalene | 1764 | 1755 | MS, ST | Pungent, tarry | 6.6 ± 1.1b | 8.8 ± 0.7ab | 8.3 ± 0.6ab | 8.2 ± 0.6ab | 7.1 ± 0.7ab | 8.3 ± 0.5ab | 7.8 ± 0.8ab | 10.2 ± 0.8a | 8.6 ± 0.3ab | 7.4 ± 1.0ab |
| Ethyl benzeneacetate | 1798 | 1782 | MS | - | n.d. | n.d. | 6.2 ± 1.0abc | 5.7 ± 0.6abc | 4.0 ± 0.3bc | n.d. | 3.2 ± 0.2c | 6.7 ± 1.4ab | 7.6 ± 0.5a | n.d. |
| 2-Methylnaphthalene | 1878 | 1877 | MS, RI | Sweet, floral, woody | 3.8 ± 0.4bc | n.d. | 5.9 ± 0.5a | 4.2 ± 0.1b | 2.5 ± 0.3cd | 3.2 ± 0.8bcd | n.d. | n.d. | 4.6 ± 0.2ab | 2.2 ± 0.1d |
| 1- Methylnaphthalene | 1916 | MS | Naphthyl, medicinal | 5.8 ± 1.2a | 4.9 ± 0.7a | 3.8 ± 0.1a | 5.0 ± 1.0a | 5.4 ± 0.4a | 4.1 ± 0.6a | 3.2 ± 0.4ab | 4.8 ± 0.9a | n.d. | 4.9 ± 0.7a | |
| Phenylethyl Alcohol | 1929 | 1924 | MS, RI | Floral, sweet, rosey, honey | n.d. | n.d. | 4.5 ± 0.2a | 5.1 ± 0.3a | n.d. | n.d. | n.d. | 5.0 ± 0.7a | 4.8 ± 0.3a | 5.6 ± 0.8a |
| Ethyl 9-hexadecenoate | 2267 | 2267 | MS, RI | - | n.d. | n.d. | 5.3 ± 1.0a | n.d. | n.d. | n.d. | n.d | 5.4 ± 0.3a | 8.6 ± 2.7a | 6.9 ± 1.3a |
| Indole | 2475 | 2478 | MS, RI, ST | Animal, floral, mothball | 28.6 ± 7.3b | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 13.0 ± 1.7a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasote, D.; Singh, V.K.; Bollinedi, H.; Singh, A.K.; Sreenivasulu, N.; Regina, A. Profiling of 2-Acetyl-1-Pyrroline and Other Volatile Compounds in Raw and Cooked Rice of Traditional and Improved Varieties of India. Foods 2021, 10, 1917. https://doi.org/10.3390/foods10081917
Kasote D, Singh VK, Bollinedi H, Singh AK, Sreenivasulu N, Regina A. Profiling of 2-Acetyl-1-Pyrroline and Other Volatile Compounds in Raw and Cooked Rice of Traditional and Improved Varieties of India. Foods. 2021; 10(8):1917. https://doi.org/10.3390/foods10081917
Chicago/Turabian StyleKasote, Deepak, Vivek Kumar Singh, Haritha Bollinedi, Ashok Kumar Singh, Nese Sreenivasulu, and Ahmed Regina. 2021. "Profiling of 2-Acetyl-1-Pyrroline and Other Volatile Compounds in Raw and Cooked Rice of Traditional and Improved Varieties of India" Foods 10, no. 8: 1917. https://doi.org/10.3390/foods10081917
APA StyleKasote, D., Singh, V. K., Bollinedi, H., Singh, A. K., Sreenivasulu, N., & Regina, A. (2021). Profiling of 2-Acetyl-1-Pyrroline and Other Volatile Compounds in Raw and Cooked Rice of Traditional and Improved Varieties of India. Foods, 10(8), 1917. https://doi.org/10.3390/foods10081917