Almond By-Products: Valorization for Sustainability and Competitiveness of the Industry
Abstract
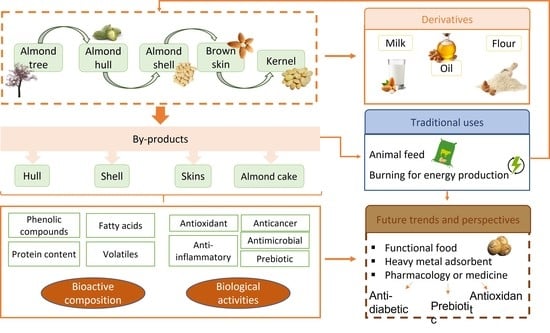
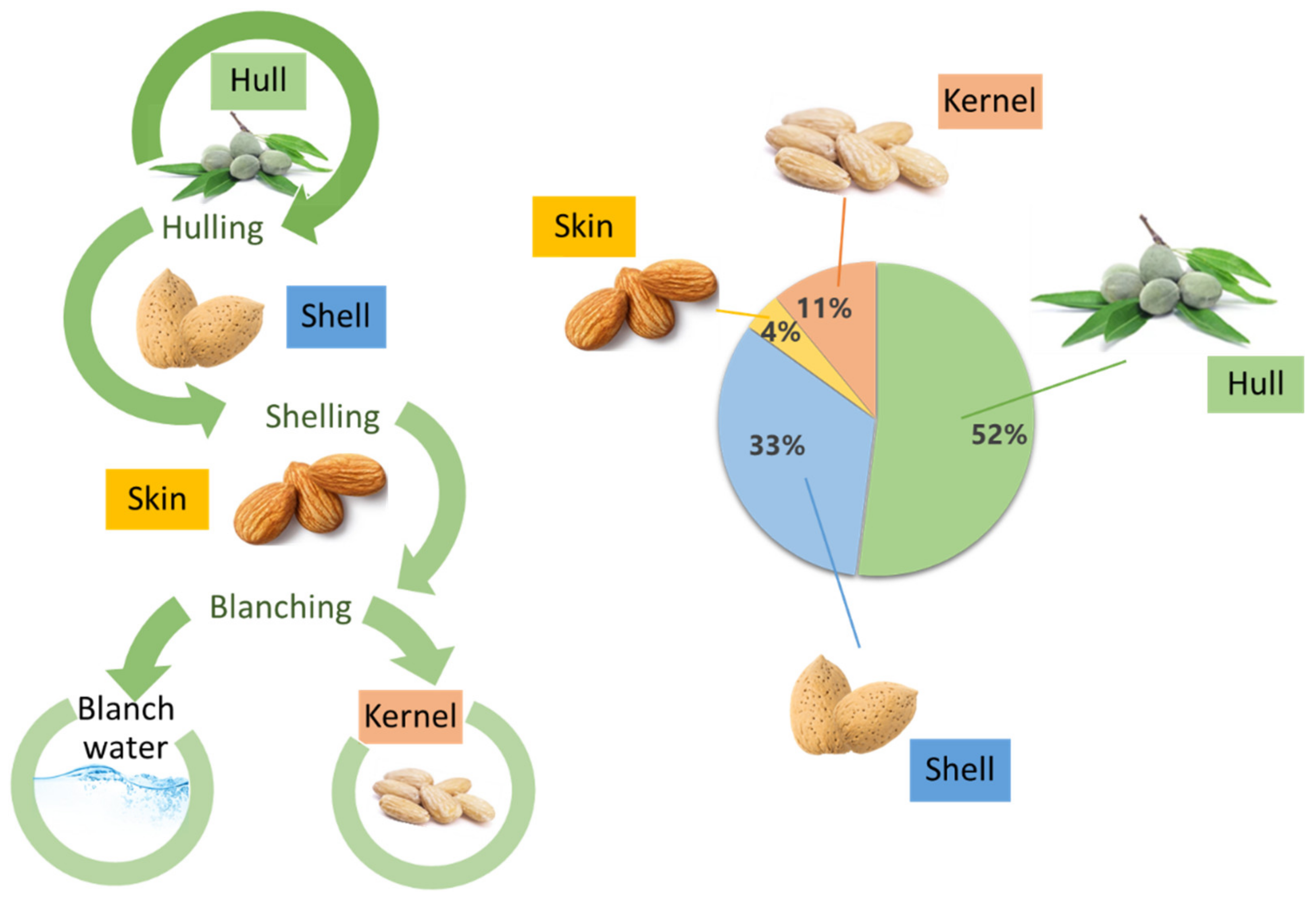
:1. Introduction
2. Bioactive Composition of Almond by-Products
2.1. Phenolic Compounds
2.2. Fatty Acids
2.3. Volatiles
2.4. Protein Content
3. Biological Activities from Almond By-Products
3.1. Antioxidant Activity
3.2. Anticancer Activity
3.3. Anti-Inflammatory Activity
3.4. Antimicrobial Activity
3.5. Prebiotic Activity
3.6. Other Activities
4. Current Trends and Future Perspectives
4.1. Almond and Its By-Products in the Food Industry
4.2. Other Uses for Almond By-Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barreira, J.C.M.; Nunes, M.A.; da Silva, B.V.; Pimentel, F.B.; Costa, A.S.G.; Alvarez-Ortí, M.; Pardo, J.E.; Oliveira, M.B.P.P. Almond cold-pressed oil by-product as ingredient for cookies with potential health benefits: Chemical and sensory evaluation. Food Sci. Hum. Wellness 2019, 8, 292–298. [Google Scholar] [CrossRef]
- Alasalvar, C.; Salvadó, J.S.; Ros, E. Bioactives and health benefits of nuts and dried fruits. Food Chem. 2020, 314, 126192. [Google Scholar] [CrossRef]
- Esfahlan, A.J.; Jamei, R.; Esfahlan, R.J. The importance of almond (Prunus amygdalus L.) and its by-products. Food Chem. 2010, 120, 349–360. [Google Scholar] [CrossRef]
- Bartolomé, B.; Monagas, M.; Garrido, I.; Gómez-Cordovés, C.; Martín-Álvarez, P.J.; Lebrón-Aguilar, R.; Urpí-Sardà, M.; Llorach, R.; Andrés-Lacueva, C. Almond (Prunus dulcis (Mill.) D.A. Webb) polyphenols: From chemical characterization to targeted analysis of phenolic metabolites in humans. Arch. Biochem. Biophys. 2010, 501, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Monagas, M.; Garrido, I.; Lebrón-Aguilar, R.; Bartolome, B.; Gómez-Cordovés, C. Almond (Prunus dulcis (Mill.) D.A. Webb) skins as a potential source of bioactive polyphenols. J. Agric. Food Chem. 2007, 55, 8498–8507. [Google Scholar] [CrossRef]
- Prgomet, I.; Goncalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I.R.N.A. Valorization challenges to almond residues: Phytochemical composition and functional application. Molecules 2017, 22, 1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fornés Comas, J.; Alonso Segura, J.M.; Rafel Socias i Company. Shell hardness in almond: Cracking load and kernel percentage. Sci. Hortic. 2019, 245, 7–11. [Google Scholar] [CrossRef]
- Lammi, C.; Bellumori, M.; Cecchi, L.; Bartolomei, M.; Bollati, C.; Clodoveo, M.L.; Corbo, F.; Arnoldi, A.; Mulinacci, N. Extra virgin olive oil phenol extracts exert hypocholesterolemic effects through the modulation of the LDLR pathway: In vitro and cellular mechanism of action elucidation. Nutrients 2020, 12, 1723. [Google Scholar] [CrossRef]
- Karimi, Z.; Firouzi, M.; Dadmehr, M.; Javad-Mousavi, S.A.; Bagheriani, N.; Sadeghpour, O. Almond as a nutraceutical and therapeutic agent in Persian medicine and modern phytotherapy: A narrative review. Phytother. Res. 2021, 35. [Google Scholar] [CrossRef] [PubMed]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Santos, R.; Saavedra, M.J.; Aires, A.; Pascual-Seva, N.; Barros, A. Irrigation deficit turns almond by-products into a valuable source of antimicrobial (poly)phenols. Ind. Crops Prod. 2019, 132, 186–196. [Google Scholar] [CrossRef]
- Moldero, D.; López-Bernal, Á.; Testi, L.; Lorite, I.J.; Fereres, E.; Orgaz, F. Long-term almond yield response to deficit irrigation. Irrig. Sci. 2021. [Google Scholar] [CrossRef]
- Aktas, T.; Thy, P.; Williams, R.B.; McCaffrey, Z.; Khatami, R.; Jenkins, B.M. Characterization of almond processing residues from the Central Valley of California for thermal conversion. Fuel Process. Technol. 2015, 140, 132–147. [Google Scholar] [CrossRef]
- Urruzola, I.; Robles, E.; Serrano, L.; Labidi, J. Nanopaper from almond (Prunus dulcis) shell. Cellulose 2014, 21, 1619–1629. [Google Scholar] [CrossRef]
- Xie, L.; Bolling, B.W. Characterisation of stilbenes in California almonds (Prunus dulcis) by UHPLC–MS. Food Chem. 2014, 148, 300–306. [Google Scholar] [CrossRef]
- Summo, C.; Palasciano, M.; De Angelis, D.; Paradiso, V.M.; Caponio, F.; Pasqualone, A. Evaluation of the chemical and nutritional characteristics of almonds (Prunus dulcis (Mill). D.A. Webb) as influenced by harvest time and cultivar. J. Sci. Food Agric. 2018, 98, 5647–5655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolling, B.W.; Dolnikowski, G.; Blumberg, J.B.; Chen, C.-Y.O. Polyphenol content and antioxidant activity of California almonds depend on cultivar and harvest year. Food Chem. 2010, 122, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant activity and bioactive compounds of ten Portuguese regional and commercial almond cultivars. Food Chem. Toxicol. 2008, 46, 2230–2235. [Google Scholar] [CrossRef]
- Čolić, S.D.; Fotirić Akšić, M.M.; Lazarević, K.B.; Zec, G.N.; Gašić, U.M.; Dabić Zagorac, D.Č.; Natić, M.M. Fatty acid and phenolic profiles of almond grown in Serbia. Food Chem. 2017, 234, 455–463. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Roto, A.V.; Bolling, B.W. Characterization of ellagitannins, gallotannins, and bound proanthocyanidins from California almond (Prunus dulcis) varieties. J. Agric. Food Chem. 2012, 60, 12151–12156. [Google Scholar] [CrossRef]
- Milbury, P.E.; Chen, C.-Y.; Dolnikowski, G.G.; Blumberg, J.B. Determination of flavonoids and phenolics and their distribution in almonds. J. Agric. Food Chem. 2006, 54, 5027–5033. [Google Scholar] [CrossRef] [PubMed]
- Beltrán Sanahuja, A.; Maestre Pérez, S.E.; Grané Teruel, N.; Valdés García, A.; Prats Moya, M.S. Variability of chemical profile in almonds (Prunus dulcis) of different cultivars and origins. Foods 2021, 10, 153. [Google Scholar] [CrossRef]
- López-Ortiz, C.M.; Prats-Moya, S.; Sanahuja, A.B.; Maestre-Pérez, S.E.; Grané-Teruel, N.; Martín-Carratalá, M.L. Comparative study of tocopherol homologue content in four almond oil cultivars during two consecutive years. J. Food Compos. Anal. 2008, 21, 144–151. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Casal, S.; Ferreira, I.C.F.R.; Peres, A.M.; Pereira, J.A.; Oliveira, M.B.P.P. Supervised chemical pattern recognition in almond (Prunus dulcis) Portuguese PDO cultivars: PCA- and LDA-based triennial study. J. Agric. Food Chem. 2012, 60, 9697–9704. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Almonds and Maintenance of Normal Blood LDL Cholesterol Concentrations (ID 1131) and Maintenance of Normal Erectile Function (ID 2482) Pursuant to Article 13 (1) of Regulation (EC) No 19; Wiley Online Library: Hoboken, NJ, USA, 2011; Volume 9. [Google Scholar]
- Prats-Moya, M.S.; Grané-Teruel, N.; Berenguer-Navarro, V.; Martín-Carratalá, M.L. A chemometric study of genotypic variation in triacylglycerol composition among selected almond cultivars. J. Am. Oil Chem. Soc. 1999, 76, 267–272. [Google Scholar] [CrossRef]
- Cherif, A.; Sebei, K.; Boukhchina, S.; Kallel, H.; Belkacemi, K.; Arul, J. Kernel fatty acid and triacylglycerol composition for three almond cultivars during maturation. J. Am. Oil Chem. Soc. 2004, 81, 901–905. [Google Scholar] [CrossRef]
- Zhu, Y.; Wilkinson, K.L.; Wirthensohn, M. Changes in fatty acid and tocopherol content during almond (Prunus dulcis, cv. Nonpareil) kernel development. Sci. Hortic. 2017, 225, 150–155. [Google Scholar] [CrossRef]
- Nawade, B.; Yahyaa, M.; Reuveny, H.; Shaltiel-Harpaz, L.; Eisenbach, O.; Faigenboim, A.; Bar-Yaakov, I.; Holland, D.; Ibdah, M. Profiling of volatile terpenes from almond (Prunus dulcis) young fruits and characterization of seven terpene synthase genes. Plant Sci. 2019, 287, 110187. [Google Scholar] [CrossRef]
- Xiao, L.; Lee, J.; Zhang, G.; Ebeler, S.E.; Wickramasinghe, N.; Seiber, J.; Mitchell, A.E. HS-SPME GC/MS characterization of volatiles in raw and dry-roasted almonds (Prunus dulcis). Food Chem. 2014, 151, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, I.; Malheiro, R.; Meyer, A.S.; Pereira, J.A.; Gonçalves, B. Application of chemometric tools for the comparison of volatile profile from raw and roasted regional and foreign almond cultivars (Prunus dulcis). J. Food Sci. Technol. 2019, 56, 3764–3776. [Google Scholar] [CrossRef] [Green Version]
- Sathe, S.K.; Wolf, W.J.; Roux, K.H.; Teuber, S.S.; Venkatachalam, M.; Sze-Tao, K.W.C. Biochemical characterization of amandin, the major storage protein in almond (Prunus dulcis L.). J. Agric. Food Chem. 2002, 50, 4333–4341. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, S.; Venkatachalam, M.; Mistry, A.M.; Lapsley, K.; Sathe, S.K. Almond (Prunus dulcis L.) protein quality. Plant Foods Hum. Nutr. 2005, 60, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Roncero, J.M.; Álvarez-Ortí, M.; Pardo-Giménez, A.; Rabadán, A.; Pardo, J.E. Review about non-lipid components and minor fat-soluble bioactive compounds of almond kernel. Foods 2020, 9, 1646. [Google Scholar] [CrossRef] [PubMed]
- De Souza, T.S.P.; Dias, F.F.G.; Oliveira, J.P.S.; de Moura Bell, J.M.L.N.; Koblitz, M.G.B. Biological properties of almond proteins produced by aqueous and enzyme-assisted aqueous extraction processes from almond cake. Sci. Rep. 2020, 10, 10873. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Faulks, R.M.; Bisignano, C.; Waldron, K.W.; Narbad, A.; Wickham, M.S.J. In vitro evaluation of the prebiotic properties of almond skins (Amygdalus communis L.). FEMS Microbiol. Lett. 2010, 304, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, A.A.; Smith, N.E.; Baldwin, R.L. Nutritional value of almond hulls for dairy cows. J. Dairy Sci. 1984, 67, 97–103. [Google Scholar] [CrossRef]
- Valdés, A.; Vidal, L.; Beltrán, A.; Canals, A.; Garrigós, M.C. Microwave-assisted extraction of phenolic compounds from almond skin byproducts (Prunus amygdalus): A multivariate analysis approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef] [Green Version]
- Bottone, A.; Masullo, M.; Montoro, P.; Pizza, C.; Piacente, S. HR-LC-ESI-Orbitrap-MS based metabolite profiling of Prunus dulcis Mill. (Italian cultivars Toritto and Avola) husks and evaluation of antioxidant activity. Phytochem. Anal. 2019, 30, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Musarra-Pizzo, M.; Ginestra, G.; Smeriglio, A.; Pennisi, R.; Sciortino, M.T.; Mandalari, G. The antimicrobial and antiviral activity of polyphenols from almond (Prunus dulcis L.) skin. Nutrients 2019, 11, 2355. [Google Scholar] [CrossRef] [Green Version]
- Truong, V.L.; Bak, M.J.; Jun, M.; Kong, A.N.T.; Ho, C.T.; Jeong, W.S. Antioxidant defense and hepatoprotection by procyanidins from almond (Prunus amygdalus) skins. J. Agric. Food Chem. 2014, 62, 8668–8678. [Google Scholar] [CrossRef]
- Zrig, A.; Mohamed, H.B.; Tounekti, T.; Ahmed, S.O.; Khemira, H. Differential responses of antioxidant enzymes in salt-stressed almond tree grown under sun and shade conditions. J. Plant Sci. Res. 2015, 2, 117–127. [Google Scholar]
- Chen, C.Y.O.; Milbury, P.E.; Blumberg, J.B. Polyphenols in almond skins after blanching modulate plasma biomarkers of oxidative stress in healthy humans. Antioxidants 2019, 8, 95. [Google Scholar] [CrossRef] [Green Version]
- An, J.; Liu, J.; Liang, Y.; Ma, Y.; Chen, C.; Cheng, Y.; Peng, P.; Zhou, N.; Zhang, R.; Addy, M.; et al. Characterization, bioavailability and protective effects of phenolic-rich extracts from almond hulls against pro-oxidant induced toxicity in Caco-2 cells. Food Chem. 2020, 322, 126742. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, N.; Kar, A.; Sharma, R.; Bhasin, S. In-vitro antioxidative potential of different fractions from Prunus dulcis seeds: Vis a vis antiproliferative and antibacterial activities of active compounds. S. Afr. J. Bot. 2017, 108, 184–192. [Google Scholar] [CrossRef]
- Gomaa, E.Z. In vitro antioxidant, antimicrobial, and antitumor activities of bitter almond and sweet apricot (Prunus armeniaca L.) kernels. Food Sci. Biotechnol. 2013, 22, 455–463. [Google Scholar] [CrossRef]
- Khani, A.; Meshkini, A. Anti-proliferative activity and mitochondria-dependent apoptosis induced by almond and walnut by-product in bone tumor cells. Waste Biomass Valoriz. 2021, 12, 1405–1416. [Google Scholar] [CrossRef]
- Mericli, F.; Becer, E.; Kabadayi, H.; Hanoglu, A.; Hanoglu, D.Y.; Yavuz, D.O.; Ozek, T.; Vatansever, S. Fatty acid composition and anticancer activity in colon carcinoma cell lines of Prunus dulcis seed oil. Pharm. Biol. 2017, 55, 1239–1248. [Google Scholar] [CrossRef]
- Dammak, M.I.; Chakroun, I.; Mzoughi, Z.; Amamou, S.; Mansour, H.B.; Le Cerf, D.; Majdoub, H. Characterization of polysaccharides from Prunus amygdalus peels: Antioxidant and antiproliferative activities. Int. J. Biol. Macromol. 2018, 119, 198–206. [Google Scholar] [CrossRef]
- García-Pérez, P.; Barreal, M.E.; Rojo-De Dios, L.; Cameselle-Teijeiro, J.F.; Gallego, P.P. Bioactive natural products from the genus Kalanchoe as cancer chemopreventive agents: A review. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 61, pp. 49–84. [Google Scholar]
- Hikal, D.M.; Awad, N.S.; Abdein, M.A. The anticancer activity of cashew (Anacardium occidentale) and almond (Prunus dulcis) kernels. Adv. Environ. Biol. 2017, 11, 31–41. [Google Scholar]
- Zorrilla, P.; Rodriguez-Nogales, A.; Algieri, F.; Garrido-Mesa, N.; Olivares, M.; Rondón, D.; Zarzuelo, A.; Utrilla, M.P.; Galvez, J.; Rodriguez-Cabezas, M.E. Intestinal anti-inflammatory activity of the polyphenolic-enriched extract Amanda® in the trinitrobenzenesulphonic acid model of rat colitis. J. Funct. Foods 2014, 11, 449–459. [Google Scholar] [CrossRef]
- Müller, A.K.; Schmölz, L.; Wallert, M.; Schubert, M.; Schlörmann, W.; Glei, M.; Lorkowski, S. In vitro digested nut oils attenuate the lipopolysaccharide-induced inflammatory response in macrophages. Nutrients 2019, 11, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisignano, C.; Filocamo, A.; La Camera, E.; Zummo, S.; Fera, M.T.; Mandalari, G. Antibacterial activities of almond skins on cagA-positive and-negative clinical isolates of Helicobacter pylori. BMC Microbiol. 2013, 13. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P.; Bhatt, P.C.; Rahman, M.; Patel, D.K.; Anwar, F.; Al-Abbasi, F.; Verma, A.; Kumar, V. Preclinical renal chemo-protective potential of Prunus amygdalus Batsch seed coat via alteration of multiple molecular pathways. Arch. Physiol. Biochem. 2018, 124, 88–96. [Google Scholar] [CrossRef]
- Mandalari, G.; Bisignano, C.; Genovese, T.; Mazzon, E.; Wickham, M.S.J.; Paterniti, I.; Cuzzocrea, S. Natural almond skin reduced oxidative stress and inflammation in an experimental model of inflammatory bowel disease. Int. Immunopharmacol. 2011, 11, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Taylor, A.M.; Swanson, K.S.; Novotny, J.A.; Baer, D.J. Almond consumption and processing affects the composition of the gastrointestinal microbiota of healthy adult men and women: A randomized controlled trial. Nutrients 2018, 10, 126. [Google Scholar] [CrossRef] [Green Version]
- Rocchetti, G.; Bhumireddy, S.R.; Giuberti, G.; Mandal, R.; Lucini, L.; Wishart, D.S. Edible nuts deliver polyphenols and their transformation products to the large intestine: An in vitro fermentation model combining targeted/untargeted metabolomics. Food Res. Int. 2019, 116, 786–794. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, X.; Huang, G.; Zhang, W.; Rao, P.; Ni, L. Prebiotic effects of almonds and almond skins on intestinal microbiota in healthy adult humans. Anaerobe 2014, 26, 1–6. [Google Scholar] [CrossRef]
- Pasqualone, A.; Laddomada, B.; Spina, A.; Todaro, A.; Guzmàn, C.; Summo, C.; Mita, G.; Giannone, V. Almond by-products: Extraction and characterization of phenolic compounds and evaluation of their potential use in composite dough with wheat flour. LWT Food Sci. Technol. 2018, 89, 299–306. [Google Scholar] [CrossRef]
- Bottone, A.; Montoro, P.; Masullo, M.; Pizza, C.; Piacente, S. Metabolite profiling and antioxidant activity of the polar fraction of Italian almonds (Toritto and Avola): Analysis of seeds, skins, and blanching water. J. Pharm. Biomed. Anal. 2020, 190, 113518. [Google Scholar] [CrossRef] [PubMed]
- Kahlaoui, M.; Vecchia, S.B.D.; Giovine, F.; Kbaier, H.B.H.; Bouzouita, N.; Pereira, L.B.; Zeppa, G. Characterization of polyphenolic compounds extracted from different varieties of almond hulls (Prunus dulcis L.). Antioxidants 2019, 8, 647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeriglio, A.; Mandalari, G.; Bisignano, C.; Filocamo, A.; Barreca, D.; Bellocco, E.; Trombetta, D.; Karimi, Z.; Firouzi, M.; Dadmehr, M.; et al. Polyphenolic content and biological properties of Avola almond (Prunus dulcis Mill. D.A. Webb) skin and its industrial byproducts. Phytother. Res. 2019, 51, 283–293. [Google Scholar] [CrossRef]
- Li, N.; Jia, X.; Chen, C.Y.O.; Blumberg, J.B.; Song, Y.; Zhang, W.; Zhang, X.; Ma, G.; Chen, J. Almond consumption reduces oxidative DNA damage and lipid peroxidation in male smokers. J. Nutr. 2007, 137, 2717–2722. [Google Scholar] [CrossRef] [Green Version]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [Green Version]
- Amico, V.; Barresi, V.; Condorelli, D.; Spatafora, C.; Tringali, C. Antiproliferative terpenoids from almond hulls (Prunus dulcis): Identification and structure-activity relationships. J. Agric. Food Chem. 2006, 54, 810–814. [Google Scholar] [CrossRef]
- Lim, T.K. (Ed.) Prunus dulcis. In Edible Medicinal and Non-Medicinal Plants: Volume 4, Fruits; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 4, p. 491. ISBN 9789400740532. [Google Scholar]
- Udenigwe, C.C.; Je, J.Y.; Cho, Y.S.; Yada, R.Y. Almond protein hydrolysate fraction modulates the expression of proinflammatory cytokines and enzymes in activated macrophages. Food Funct. 2013, 4, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Lauro, M.R.; Marzocco, S.; Rapa, S.F.; Musumeci, T.; Giannone, V.; Picerno, P.; Aquino, R.P.; Puglisi, G. Recycling of almond by-products for intestinal inflammation: Improvement of physical-chemical, technological and biological characteristics of a dried almond skins extract. Pharmaceutics 2020, 12, 884. [Google Scholar] [CrossRef]
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Silva, A.S.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, İ.; et al. Almonds (Prunus dulcis Mill. D. A. webb): A source of nutrients and health-promoting compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theophil Anand, G.; Renuka, D.; Ramesh, R.; Anandaraj, L.; John Sundaram, S.; Ramalingam, G.; Magdalane, C.M.; Bashir, A.K.H.; Maaza, M.; Kaviyarasu, K. Green synthesis of ZnO nanoparticle using Prunus dulcis (Almond Gum) for antimicrobial and supercapacitor applications. Surf. Interfaces 2019, 17, 100376. [Google Scholar] [CrossRef]
- Bouaziz, F.; Koubaa, M.; Helbert, C.B.; Kallel, F.; Driss, D.; Kacem, I.; Ghorbel, R.; Chaabouni, S.E. Purification, structural data and biological properties of polysaccharide from Prunus amygdalus gum. Int. J. Food Sci. Technol. 2015, 50, 578–584. [Google Scholar] [CrossRef]
- Bouaziz, F.; Koubaa, M.; Ellouz Ghorbel, R.; Ellouz Chaabouni, S. Biological properties of water-soluble polysaccharides and hemicelluloses from almond gum. Int. J. Biol. Macromol. 2017, 95, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Bisignano, C.; Mandalari, G.; Smeriglio, A.; Trombetta, D.; Pizzo, M.M.; Pennisi, R.; Sciortino, M.T. Almond skin extracts abrogate HSV-1 replication by blocking virus binding to the cell. Viruses 2017, 9, 178. [Google Scholar] [CrossRef]
- Arena, A.; Bisignano, C.; Stassi, G.; Mandalari, G.; Wickham, M.S.J.; Bisignano, G. Immunomodulatory and antiviral activity of almond skins. Immunol. Lett. 2010, 132, 18–23. [Google Scholar] [CrossRef]
- Mandalari, G.; Bisignano, C.; D’Arrigo, M.; Ginestra, G.; Arena, A.; Tomaino, A.; Wickham, M.S.J. Antimicrobial potential of polyphenols extracted from almond skins. Lett. Appl. Microbiol. 2010, 51, 83–89. [Google Scholar] [CrossRef]
- Singh, R.D.; Nadar, C.G.; Muir, J.; Arora, A. Green and clean process to obtain low degree of polymerisation xylooligosaccharides from almond shell. J. Clean. Prod. 2019, 241, 118237. [Google Scholar] [CrossRef]
- Martins, I.M.; Chen, Q.; Oliver Chen, C.Y. Emerging functional foods derived from almonds. In Wild Plants, Mushrooms and Nuts: Functional Food Properties and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 445–469. ISBN 9781118944653. [Google Scholar]
- Liu, Z.; Wang, W.; Huang, G.; Zhang, W.; Ni, L. In vitro and in vivo evaluation of the prebiotic effect of raw and roasted almonds (Prunus amygdalus). J. Sci. Food Agric. 2016, 96, 1836–1843. [Google Scholar] [CrossRef] [Green Version]
- Pasqualone, A.; Laddomada, B.; Boukid, F.; de Angelis, D.; Summo, C. Use of almond skins to improve nutritional and functional properties of biscuits: An example of upcycling. Foods 2020, 9, 1705. [Google Scholar] [CrossRef]
- Kalita, S.; Khandelwal, S.; Madan, J.; Pandya, H.; Sesikeran, B.; Krishnaswamy, K. Almonds and cardiovascular health: A review. Nutrients 2018, 10, 468. [Google Scholar] [CrossRef] [Green Version]
- Eslampour, E.; Asbaghi, O.; Hadi, A.; Abedi, S.; Ghaedi, E.; Lazaridi, A.V.; Miraghajani, M. The effect of almond intake on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 50, 102399. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Zhang, H.; Qi, J.; Hu, A.; Jiang, Q.; Hou, Y.; Feng, Q.; Ojo, O.; Wang, X. An almond-based low carbohydrate diet improves depression and glycometabolism in patients with type 2 diabetes through modulating gut microbiota and GLP-1: A randomized controlled trial. Nutrients 2020, 12, 3036. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, R.; Sharma, S. Almond. In Antioxidants in Vegetables and Nuts—Properties and Health Benefits; Nayik, G.A., Gull, A., Eds.; Springer: New York, NY, USA, 2020; pp. 423–452. [Google Scholar]
- Li, S.C.; Liu, Y.H.; Liu, J.F.; Chang, W.H.; Chen, C.M.; Chen, C.Y.O. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism 2011, 60, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Arslan, J.; Sultan, F.T.; Siddiqi, H.S.; Qasim, M.; Gilani, A.-u.G. Almond protects the liver in coronary artery disease—A randomized controlled clinical trial. J. Pak. Med. Assoc. 2020, 1–15. [Google Scholar] [CrossRef]
- Gorji, N.; Moeini, R.; Memariani, Z. Almond, hazelnut and walnut, three nuts for neuroprotection in Alzheimer’s disease: A neuropharmacological review of their bioactive constituents. Pharmacol. Res. 2018, 129, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Elamrani, A.; Abid, M.; Drouet, S.; Kiani, R.; Garros, L.; Kabra, A.; Addi, M.; Hano, C. A quick, green and simple ultrasound-assisted extraction for the valorization of antioxidant phenolic acids from Moroccan almond cold-pressed oil residues. Appl. Sci. 2020, 10, 3313. [Google Scholar] [CrossRef]
- Reisman, E. Superfood as spatial fix: The ascent of the almond. Agric. Hum. Values 2020, 37, 337–351. [Google Scholar] [CrossRef]
- Ryan, N.T. World almond market. In Almonds: Botany, Production and Uses; Gradziel, T.M., Ed.; CABI: Wallingford, UK, 2017; pp. 449–459. [Google Scholar]
- Vanga, S.K.; Wang, J.; Orsat, V.; Raghavan, V. Effect of pulsed ultrasound, a green food processing technique, on the secondary structure and in-vitro digestibility of almond milk protein. Food Res. Int. 2020, 137, 109523. [Google Scholar] [CrossRef] [PubMed]
- Čolić, S.D.; Zec, G.; Nati, M.; Fotirić Akšić, M.M. Almond (Prunus dulcis) oil. In Fruit Oils: Chemistry and Functionality; Ramadan, M.F., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 149–180. [Google Scholar]
- Doulati Ardejani, F.; Badii, K.; Limaee, N.Y.; Shafaei, S.Z.; Mirhabibi, A.R. Adsorption of Direct Red 80 dye from aqueous solution onto almond shells: Effect of pH, initial concentration and shell type. J. Hazard. Mater. 2008, 151, 730–737. [Google Scholar] [CrossRef]
- İzgi, M.S.; Saka, C.; Baytar, O.; Saraçoğlu, G.; Şahin, Ö. Preparation and characterization of activated carbon from microwave and conventional heated almond shells using phosphoric acid activation. Anal. Lett. 2019, 52, 772–789. [Google Scholar] [CrossRef]
- Ait Ahsaine, H.; Zbair, M.; Anfar, Z.; Naciri, Y.; El haouti, R.; El Alem, N.; Ezahri, M. Cationic dyes adsorption onto high surface area ‘almond shell’ activated carbon: Kinetics, equilibrium isotherms and surface statistical modeling. Mater. Today Chem. 2018, 8, 121–132. [Google Scholar] [CrossRef]
- Pirayesh, H.; Khazaeian, A. Using almond (Prunus amygdalus L.) shell as a bio-waste resource in wood based composite. Compos. Part B Eng. 2012, 43, 1475–1479. [Google Scholar] [CrossRef]
- Sabbatini, A.; Lanari, S.; Santulli, C.; Pettinari, C. Use of almond shells and rice husk as fillers of poly(methyl methacrylate) (PMMA) composites. Materials 2017, 10, 872. [Google Scholar] [CrossRef] [Green Version]
- Demirbaş, A. Fuel characteristics of olive husk and walnut, hazelnut, sunflower, and almond shells. Energy Sources 2002, 24, 215–221. [Google Scholar] [CrossRef]
- Urrestarazu, M.; Martínez, G.A.; Salas, M.D.C. Almond shell waste: Possible local rockwool substitute in soilless crop culture. Sci. Hortic. 2005, 103, 453–460. [Google Scholar] [CrossRef]
- Krist, S. Almond oil. In Vegetable Fats and Oils; Puri, S., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2021; pp. 77–79. ISBN 9783030303143. [Google Scholar]

| Geographical Origin | Almond Variety | Phenolic Content (mg/g) | References |
|---|---|---|---|
| Australian | Johnston Prolific | 0.8–0.9 | [15] |
| Californian | Butte | 6.1 | [16] |
| Carmel | 6.1 | ||
| Fritz | 3.6 | ||
| Mission | 6.1 | ||
| Monterrey | 4.3 | ||
| Nonpareil | 5.3 | ||
| Texas | 1.5 | [15] | |
| Thompson | 0.8–1.5 | ||
| Italian | Duro | 164 | [17] |
| Filippo Ceo | 1.3–2.7 | [15] | |
| Genco | 2.2–11.0 | ||
| Tuono | 1.3–4.9 | ||
| Spanish | Desmajo Largueta | 1.7–1.8 | [15] |
| Marcona | 0.3–1.0 | ||
| Italian and Spanish | Ferragnès | 2.5–4.2 | [15] |
| Francolì | 2.7–7.3 | ||
| Portuguese | Pegarinhos | 34.2 | [17] |
| Compounds | Blanched Skin (μg/g) | Whole Almonds (μg/g) | Blanch Water (μg/g) |
|---|---|---|---|
| Hydroxybenzoic acids and aldehydes | 26.3–57.3 | 2.4–7.0 | |
| p-Hydroxybenzoic acid | 3.1–7.2 | 0.03–0.06 | |
| Vanillic acid | 11.1–19.2 | 1.1–2.5 | |
| Protocatechuic acid | 6.7–17.2 | 1.3–4.4 | |
| Protocatechuic aldehyde | 5.4–13.7 | - | |
| Hydroxycinnamic acids | 1.8–4.9 | - | |
| trans p-Coumaric acid | 0–1.1 | - | |
| 3-O-Caffeoylquinic acid | 1.8–3.8 | - | |
| Flavan-3-ols | 61.5–134 | 12.7–51.3 | |
| (+)-Catechin | 20.1–38.3 | 9.5–38.6 | |
| (−)-Epicatechin | 7.2–26.5 | 3.2–12.7 | |
| Procyanidin B1-3, B5, B7, C1 | 25.0–46.1 | - | |
| Unknown dimers/trimers A [(epi)catechin → A → (epi)catechin] | 9.2–23.0 | - | |
| Flavonol glycosides | 15.6–130 | 113–231 | |
| Kaempferol-3-O-rutinoside | 5.3–40.7 | 7.1–14.3 | |
| Kaempferol-3-O-glucoside | 0–14.2 | 0.08–0.2 | |
| Kaempferol-3-O-galactoside | - | 0.05–0.2 | |
| Isorhamnetin-3-O-rutinoside | 5.3–58.0 | 99–191 | |
| Isorhamnetin-3-O-glucoside | 5.0–15.3 | - | |
| Isorhamnetin-3-O-galactoside | - | 3.0–7.1 | |
| Quercetin-3-O-glucoside | 0–1.7 | 0.5–1.6 | |
| Quercetin-3-O-galactoside | - | 3.1–12.6 | |
| Quercetin-3-O-rutinoside | - | 0.6–3.7 | |
| Flavanone glycosides | 4.8–28.6 | 0.9–1.9 | |
| Naringenin-7-O-glucoside | 2.3–25.9 | 0.9–1.9 | |
| Eriodictyol-7-O-glucoside | 2.5–2.7 | - | |
| Flavonol aglycones | 9.6–33.0 | 1.1–4.9 | |
| Kaempferol | 2.7–12.1 | 0–0.04 | |
| Quercetin | 1.0–4.9 | 0.2–0.3 | |
| Isorhamnetin | 5.9–16.0 | 0.9–4.6 | |
| Dihydroflavonol aglycones | 0–10.3 | 0.4–3.0 | |
| Dihydroquercetin | 0–10.3 | - | |
| Dihydroxykaempferol | - | 0.4–3.0 | |
| Flavanone aglycones | 6.7–19.9 | 1.2–6.9 | |
| Naringenin | 3.7–12.1 | 0.4–1.2 | |
| Eriodictyol | 3.0–7.8 | 0.8–5.7 | |
| Stilbenes | 0.002 | 0.008 | 0.07–0.11 |
| Polydatin | 1.5–2.2 ng/g | 7.2–8.5 ng/g | 63–84 ng/g |
| Piceatannol + oxyresveratrol | ND | ND | 9.1–25.5 ng/g |
| trans-Resveratrol | ND | <LOD | |
| Pterostilbene | ND | <LOD | |
| Total | 111–418 | 132–306 |
| Mechanisms of Action | References |
|---|---|
| Antioxidant activity | |
| Free-radical scavenging activity: DPPH, ORAC, ABTS | [37,38] |
| Reducing power: FRAP | [37,39] |
| Antioxidant enzymes induction: SOD, CAT, GPx, APX | [40,41,42] |
| Cell antioxidant response modulation: Nrf2, ARE expression | [40] |
| Inhibition of lipid oxidation TBARS | [3] |
| Depletion of oxidative stress markers: ROS, GSH, DNA, and protein degradation | [42,43] |
| Anticancer activity | |
| Effectiveness against MCF-7, MDA-MB-468, HepG2, HCT-116, Saos-2, Colo-320, Colo-741, Caco-2, and B-16 cancer cell lines | [44,45,46,47,48] |
| Oxidative stress alleviation | [49] |
| Cell cycle arrest | [50] |
| Impairment of mitochondrial function and induction of caspase-mediated apoptosis | [46] |
| Inhibition of tumor migration, metastasis, and cell malignancy | [47] |
| Anti-inflammatory activity | |
| Inhibition of immune cell infiltration | [51] |
| Reduction of pro-inflammatory CKs: IL-1β, IL-6, TNF-α, CINC-1, MCP-1 | [51,52] |
| Depletion of inflammatory mediators: PGE2, NFκB, NO, ICAM-1, selectins | [53,54,55] |
| Inhibition of pro-inflammatory enzyme activity: iNOS, COX-2, MPO, PARP | [51,52] |
| Antimicrobial activity | |
| Bacteriostatic effect against both pathogenic Gram-positive and Gram-negative bacteria | [39] |
| Antifungal activity against C. albicans | [39] |
| Antiviral activity HSV-1 and HSV-2: inhibition of viral penetration, suppression of early viral proteins and viral DNA accumulation, enhancement of antiviral immune cell response | [39] |
| Prebiotic activity | |
| Enhancement of bifidobacterial and lactobacilli populations via butyrate production | [56,57] |
| Promotion of β-galactosidase activity and inhibition of β-glucuronidase and azoreductase activities | [9] |
| Suppression of pathogenic bacteria growth | [58] |
| Cholesterol-Lowering and Obesity-Preventing Effects | |
|---|---|
| Reduction of TC, LDL-C, ApoB levels | [9] |
| Improvement of lipoprotein profile and inhibition of LDL-C oxidation | [42] |
| Reduction of body adiposity, body mass index, and body weight | [77] |
| Cardioprotective effects | |
| Reduction of atherogenic index | [80] |
| Reduction of blood pressure | [81] |
| Antidiabetic effects | |
| Reduction of blood glucose level via GLP-1 production | [82] |
| Reduction of carbohydrate absorption | [83] |
| Reduction of insulin resistance in diabetic patients | [84] |
| Hepatoprotective effects | |
| Reduction of serum ALT, AST, and GGT levels | [40,85] |
| Induction of liver antioxidant enzymes: SOD, GPx, CAT | [40] |
| Neuroprotective effects | |
| Alzheimer-preventing mechanisms: anxiolytic, sedative, and memory-enhancing properties | [9,86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barral-Martinez, M.; Fraga-Corral, M.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Almond By-Products: Valorization for Sustainability and Competitiveness of the Industry. Foods 2021, 10, 1793. https://doi.org/10.3390/foods10081793
Barral-Martinez M, Fraga-Corral M, Garcia-Perez P, Simal-Gandara J, Prieto MA. Almond By-Products: Valorization for Sustainability and Competitiveness of the Industry. Foods. 2021; 10(8):1793. https://doi.org/10.3390/foods10081793
Chicago/Turabian StyleBarral-Martinez, Marta, Maria Fraga-Corral, Pascual Garcia-Perez, Jesus Simal-Gandara, and Miguel A. Prieto. 2021. "Almond By-Products: Valorization for Sustainability and Competitiveness of the Industry" Foods 10, no. 8: 1793. https://doi.org/10.3390/foods10081793
APA StyleBarral-Martinez, M., Fraga-Corral, M., Garcia-Perez, P., Simal-Gandara, J., & Prieto, M. A. (2021). Almond By-Products: Valorization for Sustainability and Competitiveness of the Industry. Foods, 10(8), 1793. https://doi.org/10.3390/foods10081793