Scientometric and Methodological Analysis of the Recent Literature on the Health-Related Effects of Tomato and Tomato Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy, Eligibility Criteria, and Data Extraction
2.2. Statistical Analysis
3. Results
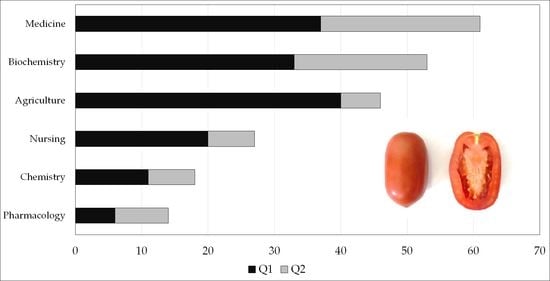
3.1. Geographic Distribution of Authors and Journal Category
3.2. Materials Used as Tester: Fresh, Purified and Formulate Products
3.3. Use of “Special” Tomato Genotypes as a Tester
3.4. Target Material
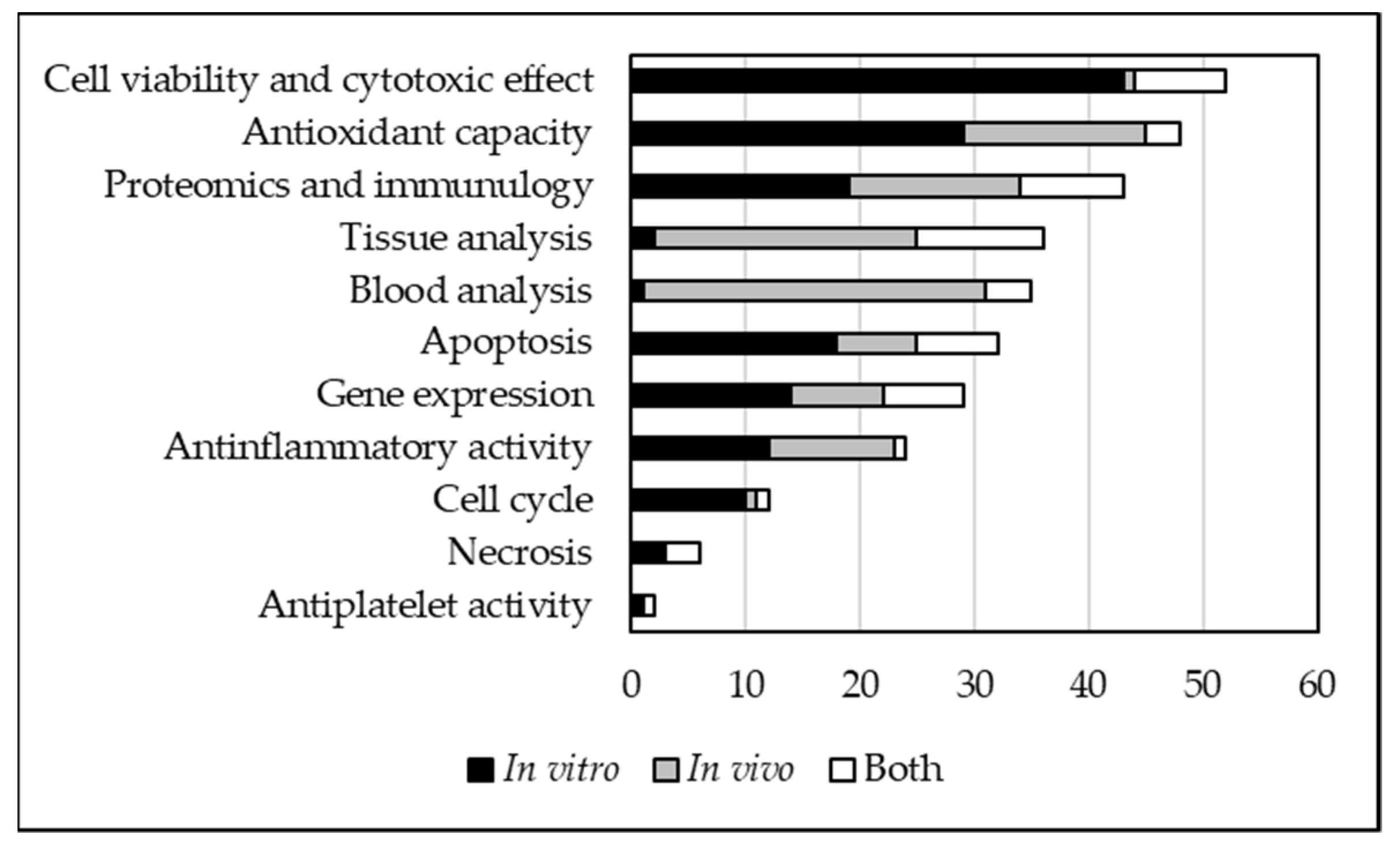
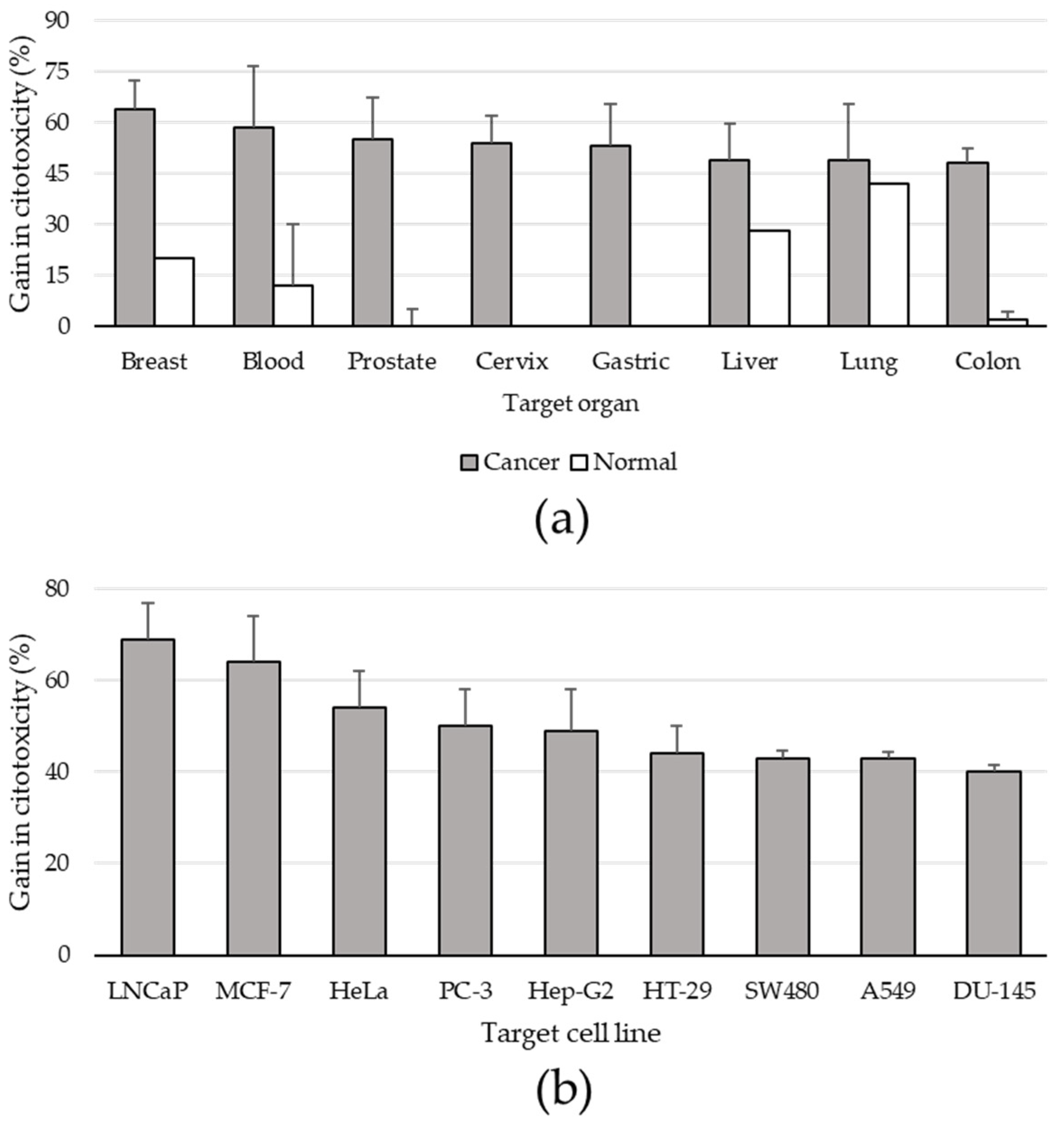
3.5. Type of Analysis and Anti-Proliferative Effects on Human In Vitro Cell Cultures
3.6. Multivariate Analysis
4. Discussion
4.1. Bibliometrics Trends
4.2. Use of Different Testers: Fresh, Formulate, and Purified Products
4.3. The Use of “Special” Tomatoes
4.4. The Target Systems: In Vitro versus In Vivo Experiments and Analyses
4.5. Multivariate Correspondence Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rothan, C.; Diouf, I.; Causse, M. Trait discovery and editing in tomato. Plant J. 2019, 97, 73–90. [Google Scholar] [CrossRef]
- FAOSTAT 2021. Available online: http://www.fao.org/faostat/ (accessed on 31 May 2021).
- Szabo, K.; Cătoi, A.F.; Vodnar, D.C. Bioactive compounds extracted from tomato processing by-products as a source of valuable nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Roointan, A.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial effects and potential risks of tomato consumption for human health: An overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 14, 3202–3242. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, W.; Wang, X.; Zhao, K.; Negi, D.S.; Zhuo, L.; Qi, M.; Wang, X.; Zhang, X. Lycopene and risk of prostate cancer: A systematic review and meta-analysis. Medicine 2015, 94, e1260. [Google Scholar] [CrossRef]
- Thies, F.; Mills, L.M.; Moir, S.; Masson, L.F. Cardiovascular benefits of lycopene: Fantasy or reality? Proc. Nutr. Soc. 2017, 76, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Applegate, C.C.; Rowles, J.L.; Erdman, J.W., Jr. Can Lycopene Impact the Androgen Axis in Prostate Cancer? Systematic Review of Cell Culture and Animal Studies. Nutrients 2019, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Gonzali, S.; Perata, P. Anthocyanins from Purple Tomatoes as Novel Antioxidants to Promote Human Health. Antioxidants 2020, 20, 1017. [Google Scholar] [CrossRef] [PubMed]
- Raiola, A.; Tenore, G.C.; Barone, A.; Frusciante, L.; Rigano, M.M. Vitamin E Content and Composition in Tomato Fruits: Beneficial Roles and Bio-Fortification. Int. J. Mol. Sci. 2015, 16, 29250–29264. [Google Scholar] [CrossRef] [PubMed]
- Green, A.S.; Fascetti, A.J. Meeting the Vitamin A Requirement: The Efficacy and Importance of β-Carotene in Animal Species. Sci. World J. 2016, 2016, 7393620. [Google Scholar] [CrossRef]
- Giorio, G.; Stigliani, A.L.; D’Ambrosio, C. Agronomic performance and transcriptional analysis of carotenoid biosynthesis in fruits of transgenic HighCaro and control tomato lines under field conditions. Transgenic Res. 2007, 16, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Dono, G.; Rambla, J.L.; Frusciante, S.; Granell, A.; Diretto, G.; Mazzucato, A. Color Mutations Alter the Biochemical Composition in the San Marzano Tomato Fruit. Metabolites 2020, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Ronen, G.; Carmel-Goren, L.; Zamir, D.; Hirschberg, J. An alternative pathway to beta -carotene formation in plant chromoplasts discovered by map-based cloning of beta and old-gold color mutations in tomato. Proc. Natl. Acad. Sci. USA 2000, 26, 11102–11107. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.V.; Maluf, W.R.; Márcio de Azevedo, S.; Carvalho Andrade-Júnior, V.; Gomes, L.A.A.; Moretto, P.; Licursi, V. Yield and post-harvest quality of tomato hybrids heterozygous at the loci alcobaça, old gold-crimson or high pigment. Genet. Mol. Res. 2003, 30, 317–327. [Google Scholar]
- Unlu, N.Z.; Bohn, T.; Francis, D.M.; Nagaraja, H.N.; Clinton, S.K.; Schwartz, S.J. Lycopene from heat-induced cis-isomer-rich tomato sauce is more bioavailable than from all-trans-rich tomato sauce in human subjects. Br. J. Nutr. 2007, 98, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Barry, C.S.; Pandey, P. A survey of cultivated heirloom tomato varieties identifies four new mutant alleles at the green-flesh locus. Mol. Breed. 2009, 24, 269–276. [Google Scholar] [CrossRef]
- Pozzo, L.; Russo, R.; Frassinetti, S.; Vizzarri, F.; Árvay, J.; Vornoli, A.; Casamassima, D.; Palazzo, M.; Croce, C.M.D.; Longo, V. Wild Italian Prunus spinosa L. Fruit Exerts In Vitro Antimicrobial Activity and Protects Against In Vitro and In Vivo Oxidative Stress. Foods 2019, 9, 5. [Google Scholar] [CrossRef]
- Mes, P.J.; Boches, P.; Myers, J.R.; Durst, R. Characterization of Tomatoes Expressing Anthocyanin in the Fruit. J. Amer. Soc. Hort. Sci. 2008, 133, 262–269. [Google Scholar] [CrossRef]
- Adato, A.; Mandel, T.; Mintz-Oron, S.; Venger, I.; Levy, D.; Yativ, M.; Domínguez, E.; Wang, Z.; De Vos, R.C.; Jetter, R.; et al. Fruit-surface flavonoid accumulation in tomato is controlled by a SlMYB12-regulated transcriptional network. PLoS Genet. 2009, 5, e1000777. [Google Scholar] [CrossRef]
- Gonzali, S.; Mazzucato, A.; Perata, P. Purple as a tomato: Towards high anthocyanin tomatoes. Trends Plant Sci. 2009, 14, 237–241. [Google Scholar] [CrossRef]
- Colanero, S.; Perata, P.; Gonzali, S. The atroviolacea Gene Encodes an R3-MYB Protein Repressing Anthocyanin Synthesis in Tomato Plants. Front. Plant Sci. 2018, 9, 830. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chen, N.; Huang, Z.; Li, D.; Zhi, J.; Yu, B.; Liu, X.; Cao, B.; Qiu, Z. Anthocyanin Fruit encodes an R2R3-MYB transcription factor, SlAN2-like, activating the transcription of SlMYBATV to fine-tune anthocyanin content in tomato fruit. New Phytol. 2020, 225, 2048–2063. [Google Scholar] [CrossRef] [PubMed]
- Butelli, E.; Titta, L.; Giorgio, M.; Mock, H.P.; Matros, A.; Peterek, S.; Schijlen, E.G.W.M.; Hall, R.D.; Bovy, A.G.; Luo, J.; et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 2008, 26, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.; Buret, M.; Duffé, P.; Garchery, C.; Baldet, P.; Rothan, C.; Causse, M. Candidate genes and quantitative trait loci affecting fruit ascorbic acid content in three tomato populations. Plant Physiol. 2007, 143, 1943–1953. [Google Scholar] [CrossRef]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Kozukue, N.; Han, J.S.; Lee, K.R.; Friedman, M. Dehydrotomatine and alpha-tomatine content in tomato fruits and vegetative plant tissues. J. Agric. Food Chem. 2004, 7, 2079–2083. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Anticarcinogenic, Cardioprotective, and Other Health Benefits of Tomato Compounds Lycopene, α-Tomatine, and Tomatidine in Pure Form and in Fresh and Processed Tomatoes. J. Agric. Food Chem. 2013, 61, 9534–9550. [Google Scholar] [CrossRef]
- Baldina, S.; Picarella, M.E.; Troise, A.D.; Pucci, A.; Ruggieri, V.; Ferracane, R.; Barone, A.; Fogliano, V.; Mazzucato, A. Metabolite profiling of Italian tomato landraces with different fruit types. Front. Plant Sci. 2016, 7, 664. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Bessler, H.; Salman, H.; Bergman, M.; Alcalay, Y.; Djaldetti, M. In Vitro Effect of Lycopene on Cytokine Production by Human Peripheral Blood Mononuclear Cells. Immunol. Investig. 2008, 37, 183–190. [Google Scholar] [CrossRef]
- King-Batoon, A.; Leszczynska, M.J.; Klein, C.B. Modulation of Gene Methylation by Genistein or Lycopene in Breast Cancer Cells. Environ. Mol. Mutagen. 2008, 49, 36–45. [Google Scholar] [CrossRef]
- Tang, F.Y.; Shih, C.J.; Cheng, L.H.; Ho, H.J.; Chen, H.J. Lycopene inhibits growth of human colon cancer cells via suppression of the Akt signaling pathway. Mol. Nutr. Food Res. 2008, 52, 646–654. [Google Scholar] [CrossRef]
- Wood, L.G.; Garg, M.L.; Powell, H.; Gibson, P.G. Lycopene-rich treatments modify noneosinophilic airway inflammation in asthma: Proof of concept. Free Radic. Res. 2008, 42, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Epriliati, I.; D’Arcy, B.; Gidley, M. Nutriomic Analysis of Fresh and Processed Fruit Products. 2. During in Vitro Simultaneous Molecular Passages Using Caco-2 Cell Monolayers. J. Agric. Food Chem. 2009, 57, 3377–3388. [Google Scholar] [CrossRef]
- Di Giacomo, C.; Acquaviva, R.; Sorrenti, V.; Vanella, A.; Grasso, S.; Barcellona, M.L.; Galvano, F.; Vanella, L.; Renis, M. Oxidative and Antioxidant Status in Plasma of Runners: Effect of Oral Supplementation with Natural Antioxidants. J. Med. Food 2009, 12, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.E.; Karrasch, T.; Mühlbauer, M.; Allard, B.; Narula, A.; Herfarth, H.H.; Jobin, C. Tomato Lycopene Extract Prevents Lipopolysaccharide-Induced NF-kB Signaling but Worsens Dextran Sulfate Sodium-Induced Colitis in NF-kBEGFP Mice. PLoS ONE 2009, 4, e4562. [Google Scholar] [CrossRef] [PubMed]
- Markovitch, D.; Tyrrell, R.M.; Tauler, P.; Frystyk, J.; Stokes, K.; Thompson, D. Lycopene supplementation (passata sauce) reduces apoptosis but does not affect oxidant-responsive heme oxygenase-1 in human lymphocytes. Nutrition 2009, 25, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Bellovino, D.; Simone, R.; Boninsegna, A.; Cellini, F.; Monastra, G.; Gaetani, S. Effect of beta-carotene-rich tomato lycopene-beta-ciclase (tlcy-b) on cell growth inhibition in HT-29 colon adenocarcinoma cells. Br. J. Nutr. 2009, 102, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Frank, O.R.; Stocks, N.P. Dark chocolate or tomato extract for prehypertension: A randomised controlled trial. BMC Complement. Altern. Med. 2009, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.Y.; Choa, H.J.; Paib, M.H.; Chena, Y.H. Concomitant supplementation of lycopene and eicosapentaenoic acid inhibits the proliferation of human colon cancer cells. J. Nutr. Biochem. 2009, 20, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Kurcer, M.A.; Kurcer, Z.; Koksal, M.; Baba, F.; Ocak, A.R.; Aksoy, N.; Atessahin, A.; Sahna, E. Effect of lycopene on caspase-3 enzyme activation in liver of methanol-intoxicated rats: Comparison with fomepizole. J. Med. Food. 2010, 13, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Taveira, M.; Pereira, D.M.; Valentao, P.; Andrade, P.B. Tomato (Lycopersicon esculentum) Seeds: New Flavonols and Cytotoxic Effect. J. Agric. Food Chem. 2010, 58, 2854–2861. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Lee, H.S.; Kim, H.J.; Lee, I.S.; Kozukue, N.; Levin, C.E.; Friedman, M. Changes in Free Amino Acid, Phenolic, Chlorophyll, Carotenoid, and Glycoalkaloid Contents in Tomatoes during 11 Stages of Growth and Inhibition of Cervical and Lung Human Cancer Cells by Green Tomato Extracts. J. Agric. Food Chem. 2010, 58, 7547–7556. [Google Scholar] [CrossRef] [PubMed]
- Konijeti, R.; Henning, S.; Moro, A.; Sheikh, A.; Elashoff, D.; Shapiro, A.; Said, J.W.; Heber, D.; Cohen, P.; Aronson, W.J. Chemoprevention of Prostate Cancer with Lycopene in the Tramp Model. Prostate 2010, 70, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Colangelo, M.; Simone, R.; Catalano, A.; Boninsegna, A.; Lanza, P.; Monego, G.; Ranelletti, F.O. Lycopene induces cell growth inhibition by altering mevalonate pathway and Ras signaling in cancer cell lines. Carcinogenesis 2010, 31, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Pannellini, T.; Iezzi, M.; Liberatore, M.; Sabatini, F.; Iacobelli, S.; Rossi, C.; Alberti, S.; Di Ilio, C.; Vitaglione, P.; Fogliano, V.; et al. Dietary Tomato Supplement Prevents Prostate Cancer in TRAMP Mice. Cancer Prev. Res. 2010, 3, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Richelle, M.; Sanchez, B.; Tavazzi, I.; Lambelet, P.; Bortlik, K.; Williamson, G. Lycopene isomerisation takes place within enterocytes during absorption in human subjects. Br. J. Nutr. 2010, 103, 1800–1807. [Google Scholar] [CrossRef][Green Version]
- Shi, J.; Yang, B.; Feng, P.; Lia, D.; Zhua, J. Induction of apoptosis by tomato using space mutation breeding in human colon cancer SW480 and HT-29 cells. J. Sci. Food Agric. 2010, 90, 615–621. [Google Scholar] [CrossRef]
- El-Rouby, D.H. Histological and immunohistochemical evaluation of the chemopreventive role of lycopene in tongue carcinogenesis induced by 4-nitroquinoline-1-oxide. Arch. Oral. Biol. 2011, 56, 664–671. [Google Scholar] [CrossRef]
- Palozza, P.; Simone, R.; Catalano, A.; Parronea, N.; Monego, G.; Ranelletti, F.O. Lycopene regulation of cholesterol synthesis and efflux in human macrophages. J. Nutr. Biochem. 2011, 22, 971–978. [Google Scholar] [CrossRef]
- Lee, S.T.; Wong, P.F.; Cheah, S.C.; Mustafa, M.R. Alpha-Tomatine Induces Apoptosis and Inhibits Nuclear Factor-Kappa B Activation on Human Prostatic Adenocarcinoma PC-3 Cells. PLoS ONE 2011, 6, e18915. [Google Scholar] [CrossRef]
- Palozza, P.; Simone, R.; Catalano, A.; Monego, G.; Barini, A.; Mele, M.C.; Parrone, N.; Trombino, S.; Picci, N.; Ranelletti, F.O. Lycopene prevention of oxysterol-induced proinflammatory cytokine cascade in human macrophages: Inhibition of NF-κB nuclear binding and increase in PPARγ expression. J. Nutr. Biochem. 2011, 22, 259–268. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Sharifi, R.; Viana, M.; Pajkovic, N.; Zhu, D.; Yuan, D.; Yang, Y.; Bowen, P.E.; Stacewicz-Sapuntzakis, M. Antioxidant Effects of Lycopene in African American Men with Prostate Cancer or Benign Prostate Hyperplasia: A Randomized, Controlled. Trial. Cancer Prev. Res. 2011, 4, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Upaganlawar, A.; Patel, V.; Balaraman, R. Tomato lycopene attenuates myocardial infarction induced by isoproterenol: Electrocardiographic, biochemical and anti-apoptotic study. Asian Pac. J. Trop. Biomed. 2012, 2, 345–351. [Google Scholar] [CrossRef]
- Chao, M.W.; Chen, C.H.; Chang, Y.L.; Teng, C.M.; Pan, S.L. α-Tomatine-Mediated Anti-Cancer Activity In Vitro and In Vivo through Cell Cycle- and Caspase-Independent Pathways. PLoS ONE 2012, 7, e44093. [Google Scholar] [CrossRef]
- Harms-Ringdahl, M.; Jenssen, D.; Haghdoost, S. Tomato juice intake suppressed serum concentration of 8-oxodG after extensive physical activity. Nutr. J. 2012, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Mills, L.M.; Wilson, H.; Thies, F. Lycopene inhibits lymphocyte proliferation through mechanisms dependent on early cell activation. Mol. Nutr. Food Res. 2012, 56, 1034–1042. [Google Scholar] [CrossRef]
- Fuentes, E.; Castro, R.; Astudillo, L.; Carrasco, G.; Alarcon, M.; Gutierrez, M.; Palomo, I. Bioassay-Guided Isolation and HPLC Determination of Bioactive Compound That Relate to the Antiplatelet Activity (Adhesion, Secretion, and Aggregation) from Solanum lycopersicum. Evid. Based Complement. Alternat. Med. 2012, 2012, 147031. [Google Scholar] [CrossRef]
- Teodoro, A.J.; Oliveira, L.F.; Martins, B.N.; Maia, G.d.A.; Martucci, R.B.; Borojevic, R. Effect of lycopene on cell viability and cell cycle progression in human cancer cell lines. Cancer Cell Int. 2012, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Forero-Doria, O.; Carrasco, G.; Maricán, A.; Santos, L.S.; Alarcón, M.; Palomo, I. Effect of Tomato Industrial Processing on Phenolic Profile and Antiplatelet Activity. Molecules 2013, 18, 11526–11536. [Google Scholar] [CrossRef]
- Gupta, P.; Pal Bansal, M.; Koul, A. Lycopene modulates initiation of N-nitrosodiethylamine induced hepatocarcinogenesis: Studies on chromosomal abnormalities, membrane fluidity and antioxidant defense system. Chem. Biol. Interact. 2013, 206, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Pal Bansal, M.; Koul, A. Spectroscopic Characterization of Lycopene Extract from Lycopersicum esculentum (Tomato) and Its Evaluation as a Chemopreventive Agent Against Experimental Hepatocarcinogenesis in Mice. Phytother. Res. 2013, 27, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Pal Bansal, M.; Koul, A. Evaluating the effect of lycopene from Lycopersicum esculentum on apoptosis during NDEA induced hepatocarcinogenesis. Biochem. Biophys. Res. Comm. 2013, 434, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Wong, P.F.; He, H.; Hooper, J.D.; Mustafa, M.R. Alpha-Tomatine Attenuation of In Vivo Growth of Subcutaneous and Orthotopic Xenograft Tumors of Human Prostate Carcinoma PC-3 Cells Is Accompanied by Inactivation of Nuclear Factor-Kappa B Signaling. PLoS ONE 2013, 8, e57708. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Wong, P.F.; Hooper, J.D.; Mustafa, M.R. Alpha-tomatine synergises with paclitaxel to enhance apoptosis of androgen-independent human prostate cancer PC-3 cells in vitro and in vivo. Phytomedicine 2013, 20, 1297–1305. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Liu, R.; Loewen, S.; Tsao, R. Carotenoid compositions of coloured tomato cultivars and contribution to antioxidant activities and protection against H2O2-induced cell death in H9c2. Food Chem. 2013, 136, 878–888. [Google Scholar] [CrossRef]
- Mazzucato, A.; Willems, D.; Bernini, R.; Picarella, M.E.; Santangelo, E.; Ruiu, F.; Tilesi, F.; Soressi, G.P. Novel phenotypes related to the breeding of purple-fruited tomatoes and effect of peel extracts on human cancer cell proliferation. Plant Phys. Biochem. 2013, 72, 125–133. [Google Scholar] [CrossRef]
- Müller, L.; Catalano, A.; Simone, R.; Cittadini, A.; Fröhlich, K.; Böhm, V.; Palozza, P. Antioxidant Capacity of Tomato Seed Oil in Solution and Its Redox Properties in Cultured Macrophages. J. Agric. Food Chem. 2013, 61, 346–354. [Google Scholar] [CrossRef]
- Zuniga, K.E.; Clinton, S.K.; Erdman, J.W. The Interactions of Dietary Tomato Powder and Soy Germ on Prostate Carcinogenesis in the TRAMP Model. Cancer Prev. Res. 2013, 6, 548–557. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, D.S.; Kozukue, N.; Kim, H.J.; Nishitani, Y.; Mizuno, M.; Levin, C.E.; Friedman, M. Protein, free amino acid, phenolic, β-carotene, and lycopene content, and antioxidative and cancer cell inhibitory effects of 12 greenhouse-grown commercial cherry tomato varieties. J. Food Comp. Anal. 2014, 34, 115–127. [Google Scholar] [CrossRef]
- Elgass, S.; Cooper, A.; Chopra, M. Lycopene Treatment of Prostate Cancer Cell Lines Inhibits Adhesion and Migration Properties of the Cells. Int. J. Med. Sci. 2014, 11, 948–954. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hazewindus, M.; Haenen, R.M.M.G.; Weseler, A.R.; Bast, A. Protection against Chemotaxis in the Anti-Inflammatory Effect of Bioactives from Tomato Ketchup. PLoS ONE 2014, 9, e114387. [Google Scholar] [CrossRef]
- Navarro-Gonzalez, I.; Perez-Sanchez, H.; Martın-Pozuelo, G.; Garcıa-Alonso, J.; Jesus Periago, M. The Inhibitory Effects of Bioactive Compounds of Tomato Juice Binding to Hepatic HMGCR: In Vivo Study and Molecular Modelling. PLoS ONE 2014, 9, e83968. [Google Scholar] [CrossRef] [PubMed]
- Rigano, M.M.; Raiola, A.; Tenore, G.C.; Monti, D.M.; Del Giudice, R.; Frusciante, L.; Barone, A. Quantitative Trait Loci Pyramiding Can Improve the Nutritional Potential of Tomato (Solanum lycopersicum) Fruits. J. Agric. Food Chem. 2014, 62, 11519–11527. [Google Scholar] [CrossRef]
- Kujawska, M.; Ewertowska, M.; Adamska, T.; Sadowski, C.; Ignatowicz, E.; Jodynis-Liebert, J. Antioxidant effect of lycopene-enriched tomato paste on N-nitrosodiethylamine-induced oxidative stress in rats. J. Physiol. Biochem. 2014, 70, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Deng, Z.; Liu, R.; Loewen, S.; Tsao, R. Bioaccessibility, in vitro antioxidant activities and in vivo anti-inflammatory activities of a purple tomato (Solanum lycopersicum L.). Food Chem. 2014, 159, 353–360. [Google Scholar] [CrossRef]
- Tommonaro, G.; Caporale, A.; De Martino, L.; Popolo, A.; De Prisco, R.; Nicolaus, B.; Abbamondi, G.R.; Saturnino, C. Antioxidant and cytotoxic activities investigation of tomato seed extracts. Nat. Prod. Res. 2014, 28, 764–768. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Wang, Q. Lycopene can reduce prostate--specific antigen velocity in a phase II clinical study in Chinese population. Chin. Med. J. 2014, 127, 2143–2146. [Google Scholar]
- Conlon, L.E.; Wallig, M.A.; Erdman, J.W., Jr. Low-lycopene containing tomato powder diet does not protect against prostate cancer in TRAMP mice. Nutr. Res. 2015, 35, 882–890. [Google Scholar] [CrossRef]
- Grainger, E.M.; Hadley, C.H.; Moran, N.E.; Riedl, K.M.; Gong, M.C.; Pohar, K.; Schwartz, S.J.; Clinton, S.K. A comparison of plasma and prostate lycopene in response to typical servings of tomato soup, sauce or juice in men before prostatectomy. Br. J. Nutr. 2015, 114, 596–607. [Google Scholar] [CrossRef][Green Version]
- Martínez-Huélamo, M.; Tulipani, S.; Estruch, R.; Escribano, E.; Illán, M.; Corella, D.; Lamuela-Raventós, R.M. The tomato sauce making process affects the bioaccessibility and bioavailability of tomato phenolics: A pharmacokinetic study. Food Chem. 2015, 173, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Nam, S.H.; Friedman, M. The Tomato Glycoalkaloid α-Tomatine Induces Caspase-Independent Cell Death in Mouse Colon Cancer CT-26 Cells and Transplanted Tumors in Mice. J. Agric. Food Chem. 2015, 63, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, X.; Li, D.; He, Y.; Li, Y.; Du, Z.; Zhang, K.; Di Paola, R.; Goodin, S.; Zheng, X. Combination of α-Tomatine and Curcumin Inhibits Growth and Induces Apoptosis in Human Prostate Cancer Cells. PLoS ONE 2015, 10, e0144293. [Google Scholar] [CrossRef] [PubMed]
- Kopec, R.E.; Schick, J.; Tober, K.L.; Riedl, K.M.; Francis, D.M.; Young, G.S.; Schwartz, S.J.; Oberyszyn, T.M. Sex differences in skin carotenoid deposition and acute UVB-induced skin damage in SKH-1 hairless mice after consumption of tangerine tomatoes. Mol. Nutr. Food Res. 2015, 59, 2491–2501. [Google Scholar] [CrossRef]
- Navarrete, S.; Alarcón, M.; Palomo, I. Aqueous Extract of Tomato (Solanum lycopersicum L.) and Ferulic Acid Reduce the Expression of TNF-α and IL-1β in LPS-Activated Macrophages. Molecules 2015, 20, 15319–15329. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Ruiz-Cruza, S.; Chaidezb, C.; Ornelas-Pazc, J.J.; López-Matad, M.A.; Márquez-Ríose, E.; Estrada, M.I. Chemical constitution and effect of extracts of tomato plants byproducts on the enteric viral surrogates. Int. J. Environ. Health Res. 2015, 25, 299–311. [Google Scholar] [CrossRef]
- Pourahmadi, Z.; Mahboob, S.; Saedisomeolia, M.; Reykandeh, M.T. The effect of Tomato juice consumption on antioxidant status in overweight and obese females. Women Health 2015, 55, 795–804. [Google Scholar] [CrossRef]
- Raiola, A.; Del Giudice, R.; Monti, D.M.; Tenore, G.C.; Barone, A.; Rigano, M.M. Bioactive Compound Content and Cytotoxic Effect on Human Cancer Cells of Fresh and Processed Yellow Tomatoes. Molecules 2016, 21, 33. [Google Scholar] [CrossRef]
- Stajčić, S.; Ćetković, G.; Čanadanović-Brunet, J.; Djilas, S.; Mandić, A.; Četojević-Simin, D. Tomato waste: Carotenoid content, antioxidant and cell growth activities. Food Chem. 2015, 172, 225–232. [Google Scholar] [CrossRef]
- Di Paola Naranjo, R.D.; Otaiza, S.; Saragusti, A.C.; Baroni, V.; Carranza Adel, V.; Peralta, I.E.; Valle, E.M.; Carrari, F.; Asis, R. Hydrophilic antioxidants from Andean tomato landraces assessed by their bioactivities in vitro and in vivo. Food Chem. 2006, 206, 146–155. [Google Scholar] [CrossRef]
- Treggiari, D.; Zoccatelli, G.; Molesini, B.; Degan, M.; Rotino, G.L.; Sala, T.; Cavallini, C.; MacRae, C.A.; Minuz, P.; Pandolfini, T. A cystine-knot miniprotein from tomato fruit inhibits endothelial cell migration and angiogenesis by affecting vascular endothelial growth factor receptor (VEGFR) activation and nitric oxide production. Mol. Nutr. Food Res. 2015, 59, 2255–2266. [Google Scholar] [CrossRef]
- Del Giudice, R.; Petruk, G.; Raiola, A.; Barone, A.; Monti, D.M.; Rigano, M.M. Carotenoids in fresh and processed tomato (Solanum lycopersicum) fruits protect cells from oxidative stress injury. J. Sci. Food Agric. 2016, 97, 1616–1623. [Google Scholar] [CrossRef]
- Gupta, P.; Bhatia, N.; Bansal, M.P.; Koul, A. Lycopene modulates cellular proliferation, glycolysis and hepatic ultrastructure during hepatocellular carcinoma. World J. Hepatol. 2016, 18, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- Petruk, G.; Raiola, A.; Del Giudice, R.; Barone, A.; Frusciante, L.; Rigano, M.M.; Monti, D.M. An ascorbic acid-enriched tomato genotype to fight UVA-induced oxidative stress in normal human keratinocytes. J. Photochem. Photobiol. B 2016, 163, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, K.; Rudolf, E. Antiproliferative effects of α-tomatine are associated with different cell death modalities in human colon cancer cells. J. Funct. Foods 2016, 27, 491–502. [Google Scholar] [CrossRef]
- Yamashoji, S.; Onoda, E. Detoxification and function of immature tomato. Food Chem. 2016, 209, 171–176. [Google Scholar] [CrossRef]
- Barone, D.; Cito, L.; Tommonaro, G.; Abate, A.A.; Penon, D.; De Prisco, R.; Penon, A.; Forte, I.M.; Benedetti, E.; Cimini, A.; et al. Antitumoral potential, antioxidant activity and carotenoid content of two Southern Italy tomato cultivars extracts: San Marzano and Corbarino. J. Cell Physiol. 2018, 233, 1266–1277. [Google Scholar] [CrossRef]
- Bayomy, N.A.; Elbakary, R.H.; Ibrahim, M.A.A.; Abdelaziz, E.Z. Effect of Lycopene and Rosmarinic Acid on Gentamicin Induced Renal Cortical Oxidative Stress, Apoptosis, and Autophagy in Adult Male Albino Rat. Anat. Rec. 2017, 300, 1137–1149. [Google Scholar] [CrossRef]
- Cha, J.H.; Kim, W.K.; Ha, A.W.; Kim, M.H.; Chang, M.J. Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr. Res. Pract. 2017, 11, 90–96. [Google Scholar] [CrossRef]
- Figueiredo-Gonzalez, M.; Valentao, P.; Pereira, D.M.; Andrade, P.B. Further insights on tomato plant: Cytotoxic and antioxidant activity of leaf extracts in human gastric cells. Food Chem. Toxicol. 2017, 109, 386–392. [Google Scholar] [CrossRef]
- Kapoor, S.; Dharmesh, S.M. Pectic Oligosaccharide from tomato exhibiting anticancer potential on a gastric cancer cell line: Structure-function relationship. Carbohyd. Polym. 2017, 160, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Thomas-Ahner, J.M.; Moran, N.E.; Cooperstone, J.L.; Erdman, J.W., Jr.; Young, G.S.; Clinton, S.K. β-carotene 9′,10′ oxygenase Modulates the Anticancer Activity of Dietary Tomato or Lycopene on Prostate Carcinogenesis in the TRAMP Model. Cancer Prev. Res. 2017, 10, 161–169. [Google Scholar] [CrossRef]
- Ramos-Bueno, R.P.; Romero-González, R.; González-Fernándeza, M.J.; Guil-Guerreroa, J.L. Phytochemical composition and in vitro anti-tumour activities of selected tomato varieties. J. Sci. Food Agric. 2017, 97, 488–496. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.C.; Andrade, P.B.; Valentão, P.; Pereira, D.M. Neurotoxicity of the steroidal alkaloids tomatine and tomatidine is RIP1 kinase- and caspase-independent and involves the eIF2α branch of the endoplasmic reticulum. J. Steroid Biochem. Mol. Biol. 2017, 171, 178–186. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Jiménez-Altayó, F.; Alsina, L.; Onetti, Y.; Rinaldi de Alvarenga, J.F.; Claro, C.; Ogalla, E.; Casals, N.; Lamuela-Raventos, R.M. Mediterranean tomato-based sofrito protects against vascular alterations in obese Zucker rats by preserving NO bioavailability. Mol. Nutr. Food. Res. 2017, 61, 9. [Google Scholar] [CrossRef]
- Yelken, B.O.; Balcı, T.; Süslüer, S.Y.; Kayabaşı, C.; Avcı, C.B.; Kırmızıbayrak, P.B.; Gündüz, C. The effect of tomatine on metastasis related matrix metalloproteinase (MMP) activities in breast cancer cell model. Gene 2017, 627, 408–411. [Google Scholar] [CrossRef]
- Sandoval, V.; Rodríguez-Rodríguez, R.; Martínez-Garza, Ú.; Rosell-Cardona, C.; Lamuela-Raventós, R.M.; Marrero, P.F.; Haro, D.; Relat, J. Mediterranean Tomato-Based Sofrito Sauce Improves Fibroblast Growth Factor 21 (FGF21) Signaling in White Adipose Tissue of Obese ZUCKER Rats. Mol. Nutr. Food Res. 2018, 62, 1700606. [Google Scholar] [CrossRef] [PubMed]
- Arathi, B.P.; Raghavendra-Rao Sowmya, P.; Kuriakose, G.C.; Shilpa, S.; Shwetha, H.J.; Kumar, S.; Raju, M.; Baskaran, V.; Lakshminarayana, R. Fractionation and Characterization of Lycopene-Oxidation Products by LC-MS/MS (ESI)+: Elucidation of the Chemopreventative Potency of Oxidized Lycopene in Breast-Cancer Cell Lines. J. Agric. Food Chem. 2018, 66, 11362–11371. [Google Scholar] [CrossRef]
- Arena, M.P.; Govers, C.; Lotti, C.; Ricciardi, L.; Wichers, H.J.; Mes, J.J. The Effect of Tomatine on Gene Expression and Cell Monolayer Integrity in Caco-2. Molecules 2018, 23, 644. [Google Scholar] [CrossRef] [PubMed]
- Blando, F.; Calabriso, N.; Berland, H.; Maiorano, G.; Gerardi, C.; Carluccio, M.A.; Andersen, Ø.M. Radical Scavenging and Anti-Inflammatory Activities of Representative Anthocyanin Groupings from Pigment-Rich Fruits and Vegetables. Int. J. Mol. Sci. 2018, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Costa-Rodrigues, J.; Fernandes, M.H.; Pinho, O.; Rocha Monteiro, P.R. Modulation of human osteoclastogenesis and osteoblastogenesis by lycopene. J. Nutr. Biochem. 2018, 57, 26–34. [Google Scholar] [CrossRef]
- Fernàndez-Bedmar, Z.; Anter, J.; Alonso Moraga, A. Anti/Genotoxic, Longevity Inductive, Cytotoxic, and Clastogenic-Related Bioactivities of Tomato and Lycopene. Environ. Mol. Mutagen. 2018, 59, 427–437. [Google Scholar] [CrossRef]
- Hurtado-Barroso, S.; Martínez-Huélamo, M.; Rinaldi de Alvarenga, J.F.; Quifer-Rada, P.; Vallverdú-Queralt, A.; Pérez-Fernández, S.; Lamuela-Raventós, R.M. Acute Effect of a Single Dose of Tomato Sofrito on Plasmatic Inflammatory Biomarkers in Healthy Men. Nutrients 2019, 11, 851. [Google Scholar] [CrossRef]
- Nieman, D.C.; Capps, C.L.; Capps, C.R.; Shue, Z.L.; McBride, J.E. Effect of 4-Week Ingestion of Tomato-Based Carotenoids on Exercise-Induced Inflammation, Muscle Damage, and Oxidative Stress in Endurance Runners. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 266–273. [Google Scholar] [CrossRef]
- Campestrini, L.H.; Melo, P.S.; Peres, L.E.P.; Calhelha, R.C.; Ferreira, I.C.F.R.; Alencar, S.M. A new variety of purple tomato as a rich source of bioactive carotenoids and its potential health benefits. Heliyon 2019, 5, e02831. [Google Scholar] [CrossRef]
- Grainger, E.M.; Moran, N.E.; Francis, D.M.; Schwartz, S.J.; Wan, L.; Thomas-Ahner, J.; Kopec, R.E.; Riedl, K.M.; Young, G.S.; Abaza, R.; et al. A Novel Tomato-Soy Juice Induces a Dose-Response Increase in Urinary and Plasma Phytochemical Biomarkers in Men with Prostate Cancer. J. Nutr. 2019, 149, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Groten, K.; Marini, A.; Grether-Beck, S.; Jaenicke, T.; Ibbotson, S.H.; Moseley, H.; Ferguson, J.; Krutmann, J. Tomato Phytonutrients Balance UV Response: Results from a Double-Blind, Randomized, Placebo-Controlled Study. Skin Pharmacol. Physiol. 2019, 32, 101. [Google Scholar] [CrossRef] [PubMed]
- Campra, P.; Aznar-Garcia, M.J.; Ramos-Bueno, R.P.; Gonzalez-Fernandez, M.J.; Khaldi, H.; Garrido-Cardenas, J.A. A whole-food approach to the in vitro assessment of the antitumor activity of gazpacho. Food Res. Int. 2019, 121, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Jesuz, V.A.; Elias Campos, M.B.; Rosse de Souza, V.; Bede, T.P.; Moraes, B.P.T.; Silva, A.R.; de Albuquerque, C.F.G.; Blondet de Azeredo, V.; Teodoro, A.J. Lycopene and Tomato Sauce Improve Hepatic and Cardiac Cell Biomarkers in Rats. J. Med. Food. 2019, 22, 1175–1182. [Google Scholar] [CrossRef]
- Soares, N.D.C.P.; Elias, M.B.; Lima Machado, C.; Trindade, B.B.; Borojevic, R.; Teodoro, A.J. Comparative Analysis of Lycopene Content from Different Tomato-Based Food Products on the Cellular Activity of Prostate Cancer Cell Lines. Foods 2019, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Storniolo, C.E.; Sacanella, I.; Mitjavila, M.T.; Lamuela-Raventos, R.M.; Moreno, J.J. Bioactive Compounds of Cooked Tomato Sauce Modulate Oxidative Stress and Arachidonic Acid Cascade Induced by Oxidized LDL in Macrophage Cultures. Nutrients 2019, 11, 1880. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Diaconeasa, Z.; Cătoi, A.F.; Vodnar, D.C. Screening of Ten Tomato Varieties Processing Waste for Bioactive Components and Their Related Antioxidant and Antimicrobial Activities. Antioxidants 2019, 8, 292. [Google Scholar] [CrossRef]
- Tanambell, H.; Quek, S.Y.; Bishop, K.S. Screening of In Vitro Health Benefits of Tangerine Tomatoes. Antioxidants 2019, 7, 230. [Google Scholar] [CrossRef]
- Antonuccio, P.; Micali, A.; Puzzolo, D.; Romeo, C.; Vermiglio, G.; Squadrito, V.; Freni, J.; Pallio, G.; Trichilo, V.; Righi, M.; et al. Nutraceutical Effects of Lycopene in Experimental Varicocele: An “In Vivo” Model to Study Male Infertility. Nutrients 2020, 12, 1536. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Jiménez, C.; Pinyol, M.; Herreras, Z.; Catalán, M.; Martínez-Huélamo, M.; Lamuela-Raventos, R.M.; Sala-Vila, A.; Cofán, M.; Gilabert, R.; et al. 5-cis-, Trans- and Total Lycopene Plasma Concentrations Inversely Relate to Atherosclerotic Plaque Burden in Newly Diagnosed Type 2 Diabetes Subjects. Nutrients 2020, 12, 1696. [Google Scholar] [CrossRef] [PubMed]
- Marcolongo, P.; Gamberucci, A.; Tamasi, G.; Pardini, A.; Bonechi, C.; Rossi, C.; Giunti, R.; Barone, V.; Borghini, A.; Fiorenzani, P.; et al. Chemical Characterisation and Antihypertensive Effects of Locular Gel and Serum of Lycopersicum esculentum L. var. “Camone” Tomato in Spontaneously Hypertensive Rats. Molecules 2020, 25, 3758. [Google Scholar] [CrossRef]
- Nguenang, G.S.; Ntyam, A.S.M.; Kuete, V. Acute and Subacute Toxicity Profiles of the Methanol Extract of Lycopersicon esculentum L. Leaves (Tomato), a Botanical with Promising In Vitro Anticancer Potential. Evid. Based Complement. Alternat. Med. 2020, 2020, 8935897. [Google Scholar] [CrossRef]
- Ou, S.; Fang, Y.; Tang, H.; Wu, T.; Chen, L.; Jiang, M.; Zhou, L.; Xu, J.; Guo, K. Lycopene protects neuroblastoma cells against oxidative damage via depression of ER stress. J. Food Sci. 2020, 85, 3552–3561. [Google Scholar] [CrossRef]
- Scarano, A.; Olivieri, F.; Gerardi, C.; Liso, M.; Chiesa, M.; Chieppa, M.; Frusciante, L.; Barone, A.; Santino, A.; Rigano, M.M. Selection of tomato landraces with high fruit yield and nutritional quality under elevated temperatures. J. Sci. Food Agric. 2020, 100, 2791–2799. [Google Scholar] [CrossRef]
- Russo, C.; Ferro, Y.; Maurotti, S.; Salvati, M.A.; Mazza, E.; Pujia, R.; Terracciano, R.; Maggisano, G.; Mare, R.; Giannini, S.; et al. Lycopene and bone: An in vitro investigation and a pilot prospective clinical study. J. Transl. Med. 2020, 18, 43. [Google Scholar] [CrossRef]
- Storniolo, C.E.; Sacanella, I.; Lamuela-Raventos, R.M.; Moreno, J.J. Bioactive Compounds of Mediterranean Cooked Tomato Sauce (Sofrito) Modulate Intestinal Epithelial Cancer Cell Growth Through Oxidative Stress/Arachidonic Acid Cascade Regulation. ACS Omega 2020, 5, 17071–17077. [Google Scholar] [CrossRef]
- Tenore, G.C.; Caruso, D.; D’Avino, M.; Buonomo, G.; Caruso, G.; Ciampaglia, R.; Schiano, E.; Maisto, M.; Annunziata, G.; Novellino, E. A Pilot Screening of Agro-Food Waste Products as Sources of Nutraceutical Formulations to Improve Simulated Postprandial Glycaemia and Insulinaemia in Healthy Subjects. Nutrients 2020, 12, 1292. [Google Scholar] [CrossRef] [PubMed]
- Tjahjodjati; Sugandi, S.; Umbas, R.; Satari, M. The Protective Effect of Lycopene on Prostate Growth Inhibitory Efficacy by Decreasing Insulin Growth Factor-1 in Indonesian Human Prostate Cancer Cells. Res. Rep. Urol. 2020, 12, 137–143. [Google Scholar] [CrossRef]
- Zhu, R.; Wei, J.; Liu, H.; Liu, C.; Wang, L.; Chen, B.; Li, L.; Jia, Q.; Tian, Y.; Li, R.; et al. Lycopene attenuates body weight gain through induction of browning via regulation of peroxisome proliferator-activated receptor γ in high-fat diet-induced obese mice. J. Nutr. Biochem. 2020, 78, 108335. [Google Scholar] [CrossRef]
- Shejawal, K.P.; Randive, D.S.; Bhinge, S.D.; Bhutkar, M.A.; Todkar, S.S.; Mulla, A.S.; Jadhav, N.R. Green synthesis of silver, iron and gold nanoparticles of lycopene extracted from tomato: Their characterization and cytotoxicity against COLO320DM, HT29 and Hella cell. J. Mater. Sci. Mater. Med. 2021, 32, 19. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Luo, X.; Chen, T.; Guo, W.; Liang, C.; Tang, S.; Mo, J. Naringenin inhibits migration, invasion, induces apoptosis in human lung cancer cells and arrests tumour progression in vitro. J. Cell Mol. Med. 2021, 25, 2563–2571. [Google Scholar] [CrossRef]
- Santangelo, E.; Carnevale, M.; Migliori, C.A.; Picarella, M.E.; Dono, G.; Mazzucato, A.; Gallucci, F. Evaluation of tomato introgression lines diversified for peel color as a source of functional biocompounds and biomass for energy recovery. Biomass Bioenergy 2020, 141, 105735. [Google Scholar] [CrossRef]
- Wang, H.; Nakamura, M.; Abbott, T.R.; Zhao, D.; Luo, K.; Yu, C.; Nguyen, C.M.; Lo, A.; Daley, T.P.; La Russa, M.; et al. CRISPR-mediated live imaging of genome editing and transcription. Science 2019, 365, 1301–1305. [Google Scholar] [CrossRef]
- Wang, E.; Liu, Y.; Xu, C.; Liu, J. Antiproliferative and proapoptotic activities of anthocyanin and anthocyanidin extracts from blueberry fruits on B16-F10 melanoma cells. Food Nutr. Res. 2017, 61, 1325308. [Google Scholar] [CrossRef]
- Zhou, K.; Raffoul, J.J. Potential anticancer properties of grape antioxidants. J. Oncol. 2012, 2012, 803294. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Cobb, L.J.; Mehta, H.H.; Seeram, N.P.; Heber, D.; Pantuck, A.J.; Cohen, P. Pomegranate extract induces apoptosis in human prostate cancer cells by modulation of the IGF-IGFBP axis. Growth Horm. IGF Res. 2010, 20, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Deng, Z.; Liu, R.; Young, J.C.; Zhu, H.; Loewen, S.; Tsao, R. Characterization of phytochemicals and antioxidant activities of a purple tomato (Solanum lycopersicum L.). J. Agric. Food Chem. 2011, 59, 11803–11811. [Google Scholar] [CrossRef] [PubMed]
- Vanden Berghe, T.; Grootjans, S.; Goossens, V.; Dondelinger, Y.; Krysko, D.V.; Takahashi, N.; Vandenabeele, P. Determination of apoptotic and necrotic cell death in vitro and in vivo. Methods 2013, 61, 117–129. [Google Scholar] [CrossRef] [PubMed]


| Authors’ Country | Country Code 1 | No. of Papers | Mean No. of Authors | Journal Country | ||
|---|---|---|---|---|---|---|
| Total | With Corresponding Author | With International Collaboration (%) | ||||
| Italy | ITA | 22 | 22 | 22.7 | 9.0 | 0 |
| United States of America | USA | 18 | 12 | 38.9 | 7.9 | 37 |
| Spain | ESP | 13 | 11 | 15.4 | 6.2 | 1 |
| India | IND | 9 | 9 | 0.0 | 4.3 | 0 |
| China | CHN | 8 | 6 | 50.0 | 7.5 | 2 |
| Australia | AUS | 6 | 3 | 50.0 | 4.3 | 0 |
| Portugal | PRT | 5 | 4 | 40.0 | 4.6 | 0 |
| Brazil | BRA | 4 | 4 | 25.0 | 6.8 | 0 |
| Republic of Korea | KOR | 4 | 3 | 75.0 | 5.8 | 1 |
| Chile | CHL | 3 | 3 | 0.0 | 5.7 | 0 |
| Great Britain | GBR | 3 | 3 | 33.0 | 4.0 | 23 |
| Malaysia | MYS | 3 | 3 | 66.7 | 4.3 | 0 |
| Taiwan | TWN | 3 | 3 | 0.0 | 4.7 | 0 |
| Other 2 | 27 | 21 | 37.0 | 4.9 | 43 | |
| Total | 107 | 6.3 | 107 | |||
| Type of Tester | Source Organ/Tissue | Extract, Compound, or Class of Compounds 1 | No. of Papers | References | |
|---|---|---|---|---|---|
| Total | With Extract Char. | ||||
| F | Seed | HE | 1 | 1 | [78] |
| Vegetative tissues | Alcohol extract | 1 | 1 | [87] | |
| HE | 1 | 0 | [128] | ||
| HE, LE | 1 | 1 | [101] | ||
| Unripe fruit | HE, LE, alcohol extract | 1 | 1 | [44,97] | |
| Ripe fruit | HE | 6 | 6 | [43,59,61,86,91,95] | |
| LE | 6 | 4 | [67,90,93,98,124,133] | ||
| HE, LE | 7 | 7 | [71,75,77,89,104,127,130] | ||
| Lycopene extract | 9 | 7 | [62,63,64,94,109,116,123,126,136] | ||
| Anthocyanin extract | 2 | 2 | [68,111] | ||
| Digestate | 2 | 2 | [35,39] | ||
| Cistine knot-microprotein | 1 | 1 | [92] | ||
| Pectic oligosaccharides | 1 | 1 | [102] | ||
| Processed (gazpacho, juice, ketchup, paste, passata, soup, sauce, sofrito) | 15 | 14 | [38,49,57,59,74,81,82,88,106,108,114,117,119,121,131] | ||
| FO | Capsule | 6 | 2 | [36,40,54,79,115,118] | |
| Beadlets (lycopene) | 2 | 2 | [80,103] | ||
| Tomato extract | 1 | 1 | [37] | ||
| Powder, paste | 4 | 3 | [47,70,76,85] | ||
| Seed oil | 1 | 1 | [69] | ||
| P | Lycopene | 23 | - | [31,32,33,41,42,46,50,51,53,55,58,60,72,99,100,112,122,125,129,132,134,135,136] | |
| Tomatine | 9 | - | [52,56,65,66,83,84,96,105,107] | ||
| Naringenin | 3 | - | [122,132,137] | ||
| β-Carotene | 2 | - | [122,132] | ||
| Hydroxytyrosol | 2 | - | [122,132] | ||
| Curcumin | 1 | - | [84] | ||
| Tomatidine | 1 | - | [105] | ||
| F and FO | Paste, beadlets | 1 | 1 | [45] | |
| Paste, oleoresins | 1 | 1 | [48] | ||
| Juice, capsule | 1 | 1 | [34] | ||
| F and P | Juice, lycopene | 1 | 0 | [113] | |
| Sauce, lycopene | 1 | 0 | [120] | ||
| HE, ketchup, lycopene, vitamins | 1 | 1 | [73] | ||
| HE, tomatine | 1 | 1 | [97] | ||
| Digestate, tomatine | 1 | 0 | [110] | ||
| Type | Known Enrichment/Variation | Species/Variety/Genotype Name | Known/Putative Mutation | References |
|---|---|---|---|---|
| Wild species | S. habrochaites, S. pimpinellifolium | - | [91] | |
| Breeding lines | S. pennellii introgression lines (IL7-3, 12-4) | [75] | ||
| Ascorbic acid | DHO4 | - | [95] | |
| Landraces | Italian landraces | - | [78,98,130,133] | |
| Romanian landraces | [123] | |||
| Central American landraces | [67,130] | |||
| Argentinian landraces | [91] | |||
| Mutants | Carotenoids—yellow | Totori Gold, Sugar Yellow, Gold Sugar, V062A, Wapsipinicon Peach, San Marzano giallo, GiàGiù, M-4, M-284; Luracatao yellow | r2 | [67,71,78,89,91] |
| Carotenoids—orange | Gold Minichal, V186A, Jaune Flammee Golden Eye | B2 | [67,71,124] | |
| Tangerine line, FG04-169, Olga’s Round Golden Chicken Egg, Golden Green | t | [71,85,124] | ||
| Carotenoids—high lycopene | FG99-218 | ogc hp-2 | [117] | |
| Chlorophylls—brown | Brown fruit: Cherokee Purple, Black Kiss, Black tomato 1,2 Camone, Black plum | gf2 | [67,71,78,127,130] | |
| Zebrino | gf gs2 | [93] | ||
| Carotenoids—chlorophylls, green | Green fruit: Saeng Green Bichuiball, Saeng Green Chorok | r gf2 | [71] | |
| Flavonoids—pink | TI01-0044BABAA, Huachinango | y2 | [67] | |
| Anthocyanins—purple | Purple fruit: Sun Black, V118, TI00-0028ADBB, Purple cherry | Aft atv | [67,68,77,111,116] | |
| M1 and M2 (space mutation breeding) | [49] | |||
| Genetically modified | Carotenoids | tlcy-b overexpressors | [39] |
| Organism | Type of Line | Target Organ | Cell Lines 1 | References |
|---|---|---|---|---|
| Man | Normal | Blood | PBMC (3), macrophages (1), platelets (2), HUVEC (2) | [31,58,59,61,69,73,92] |
| Bone marrow | HMSC (1) | [112] | ||
| Breast | MCF-10A (1) | [32] | ||
| Colon mucosa | NCM-460 (1), CCD-18 (1) | [96,104] | ||
| Kidney | HRCE (1), Hek-293 (3) | [75,89,124] | ||
| Liver | Chang (2), WRL-68 (1) | [44,52,71] | ||
| Lung | Hel-299 (2), MRC-5 (1), WI-38 (1) | [44,71,90,107] | ||
| Prostate | PNT-2 (1), RWPE-1 (3) | [52,66,72,84] | ||
| Skin | HSF (1), HaCaT (1), HMEC-1 (1) | [95,98,111] | ||
| Cancer | Blood | HL-60 (3), K-562 (1), U937 (2), THP-1 (3) | [44,51,53,56,73,74,86,113,122] | |
| Bone | Saos-2 (1) | [131] | ||
| Bone marrow | SH-SY5Y (2) | [105,129] | ||
| Breast | MCF-7 (7), MCF-10A (1), MDA-MB-468 (1) | [32,60,90,93,107,109,116] | ||
| Cervix | HeLa (11) | [44,60,68,71,75,89,90,93,97,116,136] | ||
| Colon | HT29 (10), CaCo-2 (5), SW-480 (3), HCT-116 (2), T-84 (1), LoVo (1), SW-620 (2), SW-48 (1), Colo320D (1) | [33,35,39,41,46,48,49,60,68,96,100,104,110,119,132,136] | ||
| Larynx | HEp-2 (1) | [60] | ||
| Liver | Hep-G2 (5) | [60,71,89,93,116] | ||
| Lung | A549 (4), NCI-H460 (1) | [44,60,71,116,137] | ||
| Prostate | DU-145 (4), LNCaP (4), PC-3 (8), VCaP (1), primary cancer cells (1) | [46,52,60,65,66,72,84,121,124,134] | ||
| Skin | A-375 (1), BEN (1) | [46,78] | ||
| Stomach | AGS (2), YCC-1 (1), -2 (1), -3 (1) | [98,101,102] | ||
| Mouse | Normal | Bone marrow | BMDC (1) | [130] |
| Embryo | BALB/c3T3 (2), NIH-3T3 (2), SV-T2 (2), 3T3-L1 (1)NIH-3T3 (2) | [75,93,97,102,135] | ||
| Heart | H9C2 (1) | [67] | ||
| Intestine | IEC-18 (1) | [37] | ||
| Cancer | Blood | RBL-2H3 (1) | [43] | |
| Brain | C6 (1) | [97] | ||
| Colon | CT-26 (1) | [83] | ||
| Pig | Normal | Liver | PLP2 (1) | [116] |
| Other | [87,91,123] |
| Organism | Type of Sample | Target | References |
|---|---|---|---|
| Man | Healthy | Adults | [38,48,82,114,118,131,133] |
| Sportsmen | [36,57,115] | ||
| Unhealthy | Diabetic patients | [126] | |
| Asthmatic patients | [34] | ||
| Hypertensic patients | [40] | ||
| Obese females | [88] | ||
| Prostate cancer patient | [54,79,81,117] | ||
| Mouse | Healthy | Adults | [37,42,62,63,64,74,99,120,125,127,128] |
| Unhealthy | Hypertensic patients | [127] | |
| Infarcted patients | [55] | ||
| Obese patients | [106,108,135] | ||
| Oxidatively stressed patients | [76,77,85] | ||
| Trombotic patients | [61] | ||
| Blood cancer patients | [56] | ||
| Colon cancer patients | [83] | ||
| Liver cancer patients | [94] | ||
| Prostate cancer patients | [45,47,65,66,70,80,84,103] | ||
| Tongue cancer patients | [50] | ||
| Zebrafish | [92] | ||
| Drosophila | [113] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tilesi, F.; Lombardi, A.; Mazzucato, A. Scientometric and Methodological Analysis of the Recent Literature on the Health-Related Effects of Tomato and Tomato Products. Foods 2021, 10, 1905. https://doi.org/10.3390/foods10081905
Tilesi F, Lombardi A, Mazzucato A. Scientometric and Methodological Analysis of the Recent Literature on the Health-Related Effects of Tomato and Tomato Products. Foods. 2021; 10(8):1905. https://doi.org/10.3390/foods10081905
Chicago/Turabian StyleTilesi, Francesca, Andrea Lombardi, and Andrea Mazzucato. 2021. "Scientometric and Methodological Analysis of the Recent Literature on the Health-Related Effects of Tomato and Tomato Products" Foods 10, no. 8: 1905. https://doi.org/10.3390/foods10081905
APA StyleTilesi, F., Lombardi, A., & Mazzucato, A. (2021). Scientometric and Methodological Analysis of the Recent Literature on the Health-Related Effects of Tomato and Tomato Products. Foods, 10(8), 1905. https://doi.org/10.3390/foods10081905