Impact of Global Climate Change on the European Barley Market Requires Novel Multi-Method Approaches to Preserve Crop Quality and Authenticity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Origin and Storage of the Spring Barley Samples
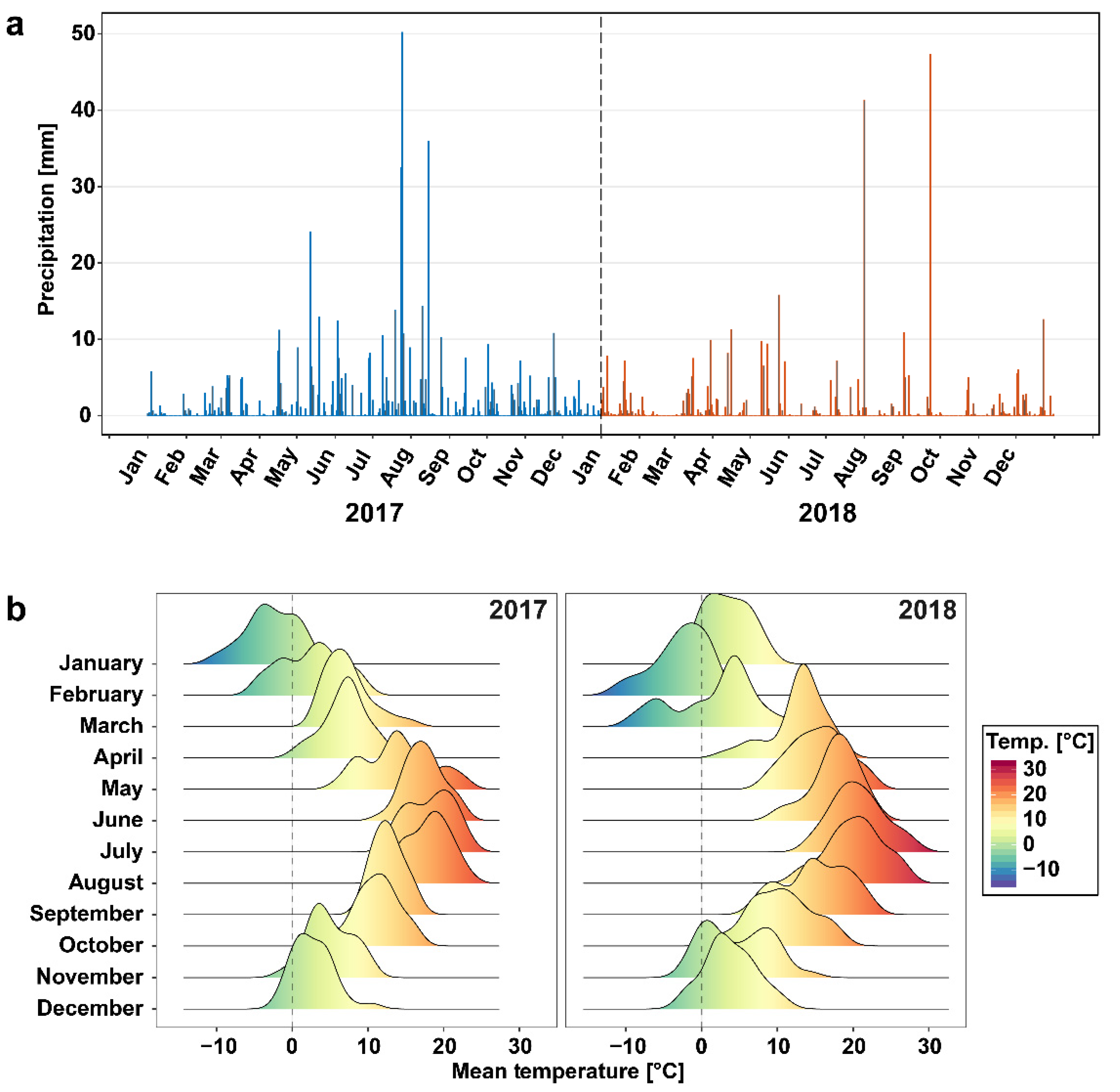
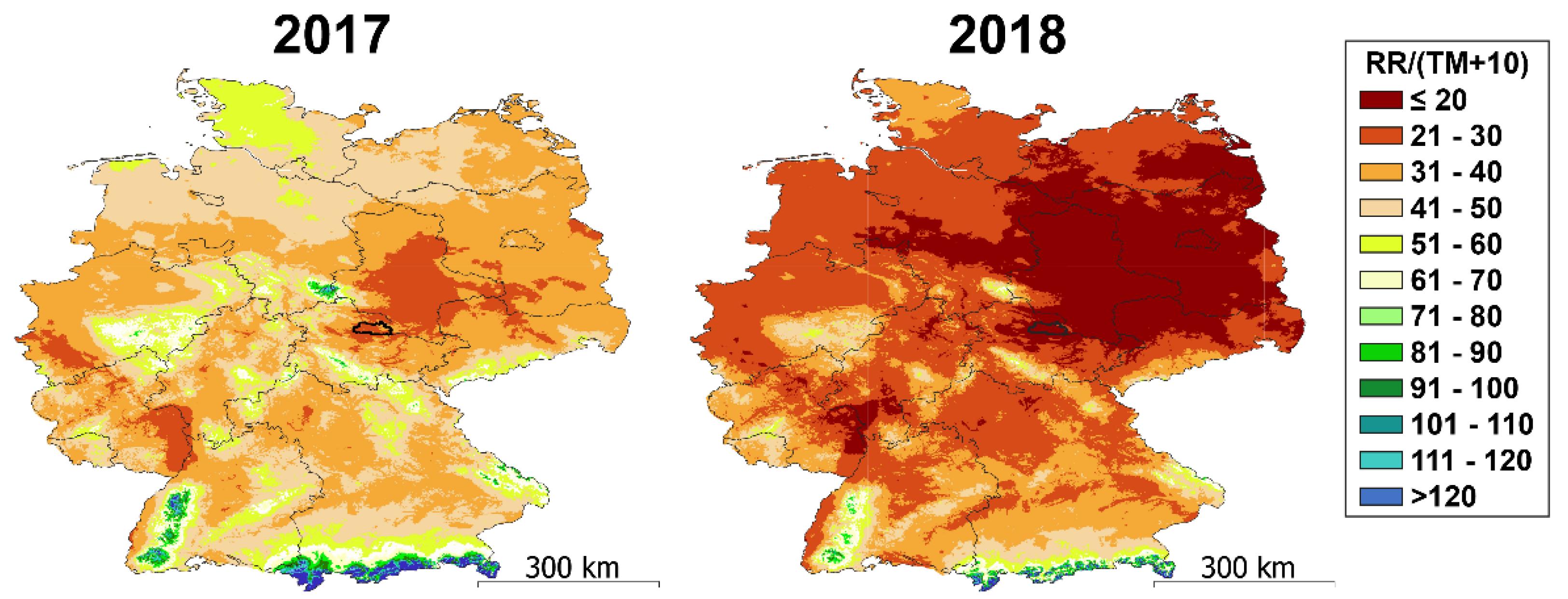
2.2. Weather at the Sampling Site
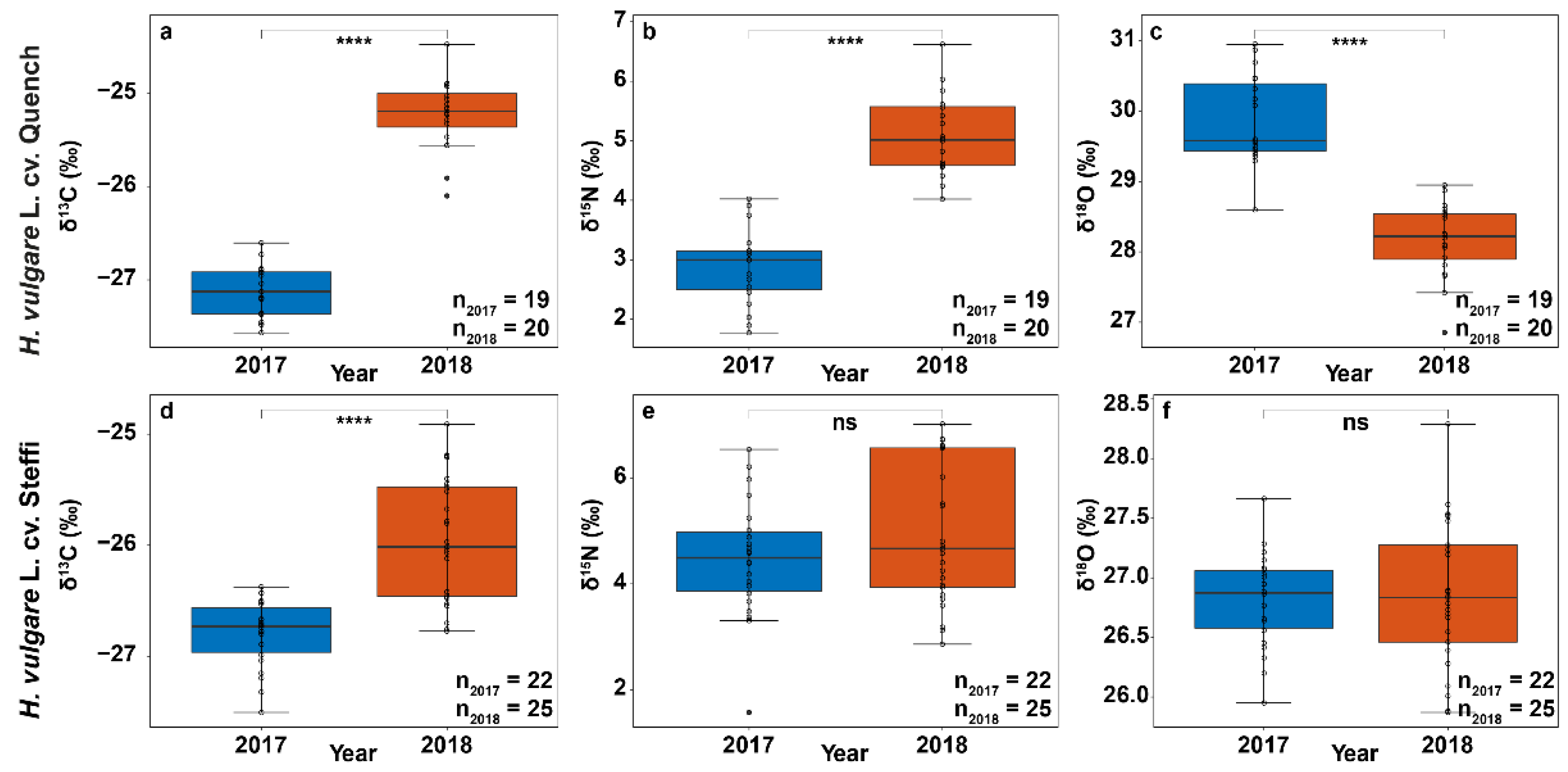
2.3. Isotope-Ratio Mass Spectrometry (IRMS)
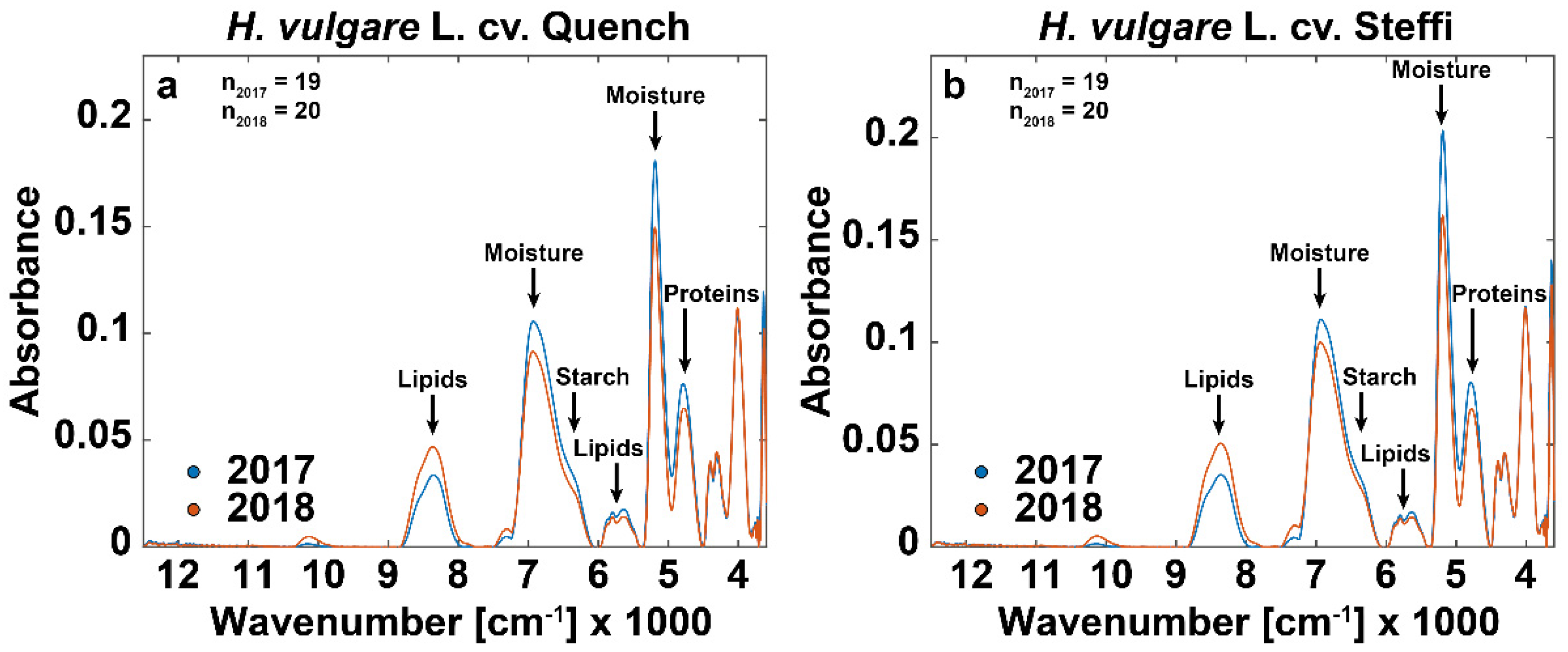
2.4. Fourier-Transform Near-Infrared Spectroscopy (FT-NIR)
2.5. Proton Nuclear Magnetic Resonance (1H-NMR)
2.6. Data Preprocessing and Statistical Analysis
3. Results and Discussion
3.1. Weather at the Sampling Site Differed between 2017 and 2018
3.2. Stable Isotope Abundances Indicate Severe Drought Stress
3.3. FT-NIR Spectra Show Additional Drought-Related Differences
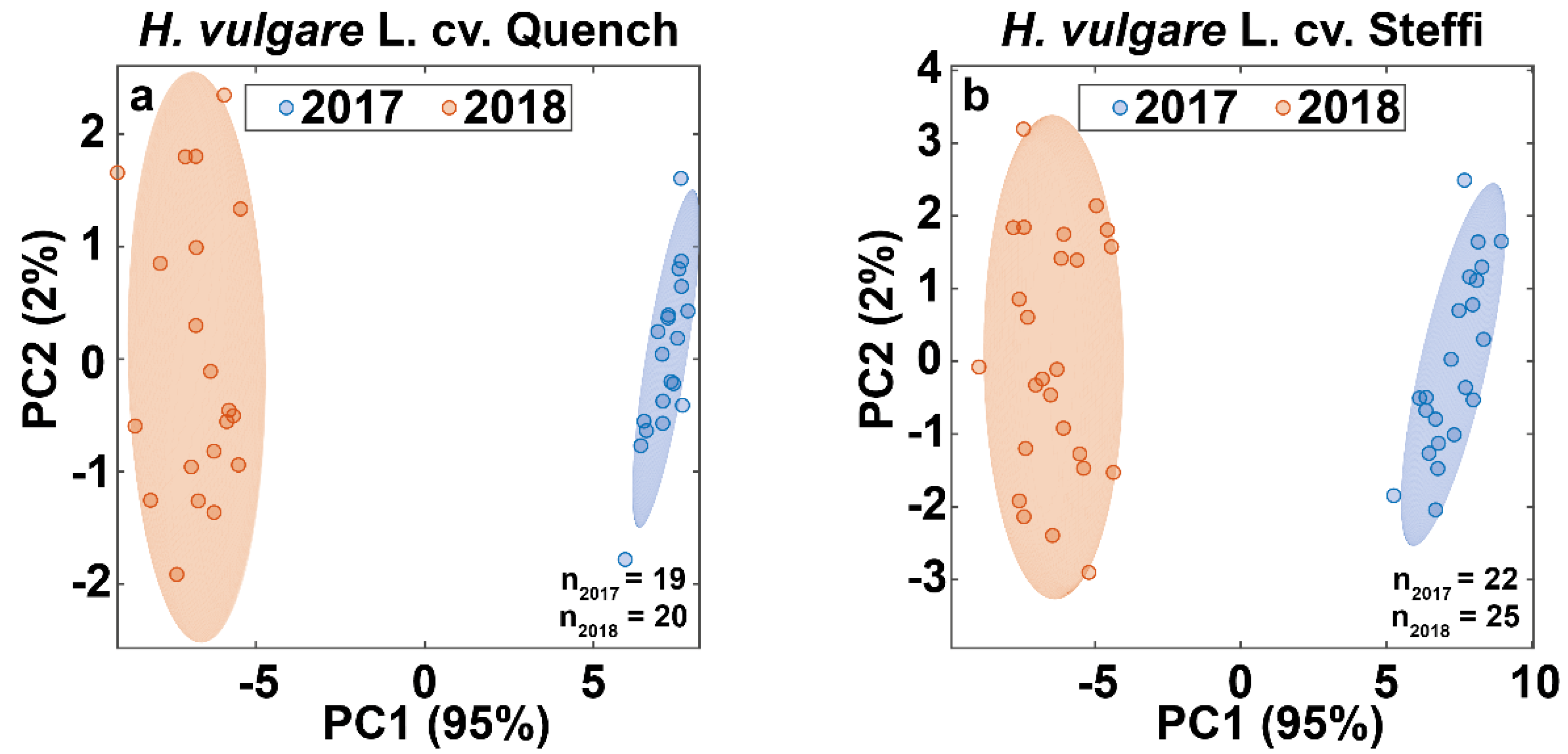
3.4. Data Fusion Enables Differentiation of Barley Harvest Years
3.5. 1H-NMR Confirms Observations by Means of the Marker Betaine
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Q.; Gilgen, A.K.; Signarbieux, C.; Klaus, V.H.; Buchmann, N. Cropping Systems Alter Hydraulic Traits of Barley but not Pea Grown in Mixture. Plant Cell Environ. 2021, 1–13. [Google Scholar] [CrossRef]
- Trnka, M.; Rötter, R.P.; Ruiz-Ramos, M.; Kersebaum, K.C.; Olesen, J.E.; Žalud, Z.; Semenov, M.A. Adverse weather conditions for European wheat production will become more frequent with climate change. Nat. Clim. Chang. 2014, 4, 637–643. [Google Scholar] [CrossRef]
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide research trends on wheat and barley: A bibliometric comparative analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef] [Green Version]
- Paynter, B.H.; Young, K.J. Grain and malting quality in two-row spring barley are influenced by grain filling moisture. Aust. J. Agric. Res. 2004, 55, 539–550. [Google Scholar] [CrossRef]
- Federal Ministry of Food and Agriculture (BMEL)—Erntebericht 2018. Available online: https://www.bmel.de/SharedDocs/Downloads/DE/_Landwirtschaft/Pflanzenbau/Ernte-Bericht/ernte-2018.pdf;jsessionid=E982D6918788080F847DB05B67B27E07.live921?__blob=publicationFile&v=2 (accessed on 23 May 2021).
- Heinrich Durst Malzfabriken GmbH & Co. KG. Requirements and Conditions of Barley Deliveries to Durst Malz—Crop 2020. Available online: http://www.durst-malz.de/uploads/media/Requirements_Crop_20_EN.pdf (accessed on 23 May 2021).
- Xie, W.; Xiong, W.; Pan, J.; Ali, T.; Cui, Q.; Guan, D.; Meng, J.; Mueller, N.D.; Lin, E.; Davis, S.J. Decreases in global beer supply due to extreme drought and heat. Nat. Plants 2018, 4, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, F.; Müller-Westermeier, G.; Penda, E.; Mächel, H.; Zimmermann, K.; Kaiser-Weiss, A.; Deutschländer, T. Monitoring of climate change in Germany–data, products and services of Germany’s National Climate Data Centre. Adv. Sci. Res. 2013, 10, 99–106. [Google Scholar] [CrossRef] [Green Version]
- de Martonne, E. Une nouvelle function climatologique: L’indice d’aridité. Meteorologie 1926, 2, 449–458. [Google Scholar]
- Bidartondo, M.I.; Burghardt, B.; Gebauer, G.; Bruns, T.D.; Read, D.J. Changing partners in the dark: Isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proc. R. Soc. B Biol. Sci. 2004, 271, 1799–1806. [Google Scholar] [CrossRef] [Green Version]
- Gebauer, G.; Preiss, K.; Gebauer, A.C. Partial mycoheterotrophy is more widespread among orchids than previously assumed. New Phytol. 2016, 211, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Gebauer, G.; Schulze, E.D. Carbon and nitrogen isotope ratios in different compartments of a healthy and a declining Picea abies forest in the Fichtelgebirge, NE Bavaria. Oecologia 1991, 87, 198–207. [Google Scholar] [CrossRef]
- Godelmann, R.; Fang, F.; Humpfer, E.; Schütz, B.; Bansbach, M.; Schäfer, H.; Spraul, M. Targeted and nontargeted wine analysis by 1H NMR spectroscopy combined with multivariate statistical analysis. Differentiation of important parameters: Grape variety, geographical origin, year of vintage. J. Agric. Food Chem. 2013, 61, 5610–5619. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, R. Phenotypic, physiological and malt quality analyses of US barley varieties subjected to short periods of heat and drought stress. J. Cereal Sci. 2017, 76, 199–205. [Google Scholar] [CrossRef]
- Mahalingam, R.; Bregitzer, P. Impact on physiology and malting quality of barley exposed to heat, drought and their combination during different growth stages under controlled environment. Physiol. Plant. 2019, 165, 277–289. [Google Scholar] [CrossRef]
- Brooks, J.R.; Buchmann, N.; Phillips, S.; Ehleringer, B.; Evans, R.D.; Lott, M.; Martinelli, L.A.; Pockman, W.T.; Sandquist, D.; Sparks, J.P.; et al. Heavy and light beer: A carbon isotope approach to detect C4 carbon in beers of different origins, styles, and prices. J. Agric. Food Chem. 2002, 50, 6413–6418. [Google Scholar] [CrossRef]
- Ferrio, J.P.; Mateo, M.A.; Bort, J.; Abdalla, O.; Voltas, J.; Araus, J.L. Relationships of grain δ13C and δ18O with wheat phenology and yield under water-limited conditions. Ann. Appl. Biol. 2007, 150, 207–215. [Google Scholar] [CrossRef]
- Hubick, K.; Farquhar, G. Carbon isotope discrimination and the ratio of carbon gained to water lost in barley cultivars. Plant Cell Environ. 1989, 12, 795–804. [Google Scholar] [CrossRef]
- Farquhar, G.D.; O’Leary, M.H.; Berry, J.A. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust. J. Plant Physiol. 1982, 9, 121–137. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Richards, R.A. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust. J. Plant Physiol. 1984, 11, 539–552. [Google Scholar] [CrossRef]
- Sánchez-Díaz, M.; García, J.L.; Antolín, M.C.; Araus, J.L. Effects of soil drought and atmospheric humidity on yield, gas exchange, and stable carbon isotope composition of barley. Photosynthetica 2002, 40, 415–421. [Google Scholar] [CrossRef]
- Liu, H.; Guo, B.; Wei, Y.; Wei, S.; Ma, Y.; Zhang, W. Effects of region, genotype, harvest year and their interactions on δ13C, δ15N and δD in wheat kernels. Food Chem. 2015, 171, 56–61. [Google Scholar] [CrossRef]
- Ivandic, V.; Hackett, C.A.; Zhang, Z.J.; Staub, J.E.; Nevo, E.; Thomas WT, B.; Forster, B.P. Phenotypic responses of wild barley to experimentally imposed water stress. J. Exp. Bot. 2000, 51, 2021–2029. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Cai, K.; Zhang, G.; Zeng, F. Metabolite profiling of barley grains subjected to water stress: To explain the genotypic difference in drought-induced impacts on malting quality. Front. Plant Sci. 2017, 8, 1547. [Google Scholar] [CrossRef]
- Lopes, M.S.; Araus, J.L. Nitrogen source and water regime effects on durum wheat photosynthesis and stable carbon and nitrogen isotope composition. Physiol. Plant. 2006, 126, 435–445. [Google Scholar] [CrossRef]
- Barbour, M.M.; Fischer, R.A.; Sayre, K.D.; Farquhar, G.D. Oxygen isotope ratio of leaf and grain material correlates with stomatal conductance and grain yield in irrigated wheat. Aust. J. Plant Physiol. 2000, 27, 625–637. [Google Scholar] [CrossRef]
- Flood, R.G.; Martin, P.J.; Gardner, W.K. Dry matter accumulation and partitioning and its relationship to grain yield in wheat. Aust. J. Exp. Agric. 1995, 35, 495–502. [Google Scholar] [CrossRef]
- Slafer, G.A.; Satorre, E.H.; Andrade, F.H. Increases in grain yield in bread wheat from breeding and associated physiological changes. In Genetic Improvement of Field Crops, 1st ed.; Slafer, G.A., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1994; pp. 1–54. [Google Scholar]
- De Girolamo, A.; Cortese, M.; Cervellieri, S.; Lippolis, V.; Pascale, M.; Logrieco, A.F.; Suman, M. Tracing the geographical origin of durum wheat by FT-NIR spectroscopy. Foods 2019, 8, 450. [Google Scholar] [CrossRef] [Green Version]
- Shenk, J.S.; Workman, J.J.; Westerhaus, M.O. Application of NIR spectroscopy to agricultural products. In Handbook of Near-Infrared Analysis, 3rd ed.; Burns, D.A., Ciurczak, E.W., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2001; pp. 419–474. [Google Scholar]
- Wenzel, A.; Frank, T.; Reichenberger, G.; Herz, M.; Engel, K.H. Impact of induced drought stress on the metabolite profiles of barley grain. Metabolomics 2015, 11, 454–467. [Google Scholar] [CrossRef]
- Cozzolino, D.; Roumeliotis, S.; Eglinton, J. Exploring the use of near infrared (NIR) reflectance spectroscopy to predict starch pasting properties in whole grain barley. Food Biophys. 2013, 8, 256–261. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, B.; Wei, Y.; Zhang, B. Effects of grown origin, genotype, harvest year, and their interactions of wheat kernels on near infrared spectral fingerprints for geographical traceability. Food Chem. 2014, 152, 316–322. [Google Scholar] [CrossRef] [PubMed]
- McGoverin, C.M.; Engelbrecht, P.; Geladi, P.; Manley, M. Characterisation of non-viable whole barley, wheat and sorghum grains using near-infrared hyperspectral data and chemometrics. Anal. Bioanal. Chem. 2011, 401, 2283. [Google Scholar] [CrossRef] [PubMed]
- Sohn, M.; Himmelsbach, D.S.; Barton, F.E.; Griffey, C.A.; Brooks, W.; Hicks, K.B. Near-infrared analysis of whole kernel barley: Comparison of three spectrometers. Appl. Spectrosc. 2008, 62, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Federal Ministry of Food and Agriculture (BMEL)—Besondere Ernte- und Qualitätsermittlung 2018. Available online: https://www.bmel-statistik.de/fileadmin/daten/EQB-1002000-2018.pdf (accessed on 23 May 2021).
- Chaves, M.M. Effects of water deficits on carbon assimilation. J. Exp. Bot. 1991, 42, 1–16. [Google Scholar] [CrossRef]
- Högy, P.; Poll, C.; Marhan, S.; Kandeler, E.; Fangmeier, A. Impacts of temperature increase and change in precipitation pattern on crop yield and yield quality of barley. Food Chem. 2013, 136, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Kebede, A.; Kang, M.S.; Bekele, E. Advances in mechanisms of drought tolerance in crops, with emphasis on barley. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: London, UK, 2019; Volume 156, pp. 265–314. [Google Scholar] [CrossRef]
- Ishitani, M.; Nakamura, T.; Han, S.Y.; Takabe, T. Expression of the betaine aldehyde dehydrogenase gene in barley in response to osmotic stress and abscisic acid. Plant Mol. Biol. 1995, 27, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Corol, D.I.; Ravel, C.; Raksegi, M.; Bedo, Z.; Charmet, G.; Beale, M.H.; Shewry, P.R.; Ward, J.L. Effects of genotype and environment on the contents of betaine, choline, and trigonelline in cereal grains. J. Agric. Food Chem. 2012, 60, 5471–5481. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bindereif, S.G.; Rüll, F.; Kolb, P.; Köberle, L.; Willms, H.; Steidele, S.; Schwarzinger, S.; Gebauer, G. Impact of Global Climate Change on the European Barley Market Requires Novel Multi-Method Approaches to Preserve Crop Quality and Authenticity. Foods 2021, 10, 1592. https://doi.org/10.3390/foods10071592
Bindereif SG, Rüll F, Kolb P, Köberle L, Willms H, Steidele S, Schwarzinger S, Gebauer G. Impact of Global Climate Change on the European Barley Market Requires Novel Multi-Method Approaches to Preserve Crop Quality and Authenticity. Foods. 2021; 10(7):1592. https://doi.org/10.3390/foods10071592
Chicago/Turabian StyleBindereif, Stefan G., Felix Rüll, Peter Kolb, Lucas Köberle, Holger Willms, Simon Steidele, Stephan Schwarzinger, and Gerhard Gebauer. 2021. "Impact of Global Climate Change on the European Barley Market Requires Novel Multi-Method Approaches to Preserve Crop Quality and Authenticity" Foods 10, no. 7: 1592. https://doi.org/10.3390/foods10071592
APA StyleBindereif, S. G., Rüll, F., Kolb, P., Köberle, L., Willms, H., Steidele, S., Schwarzinger, S., & Gebauer, G. (2021). Impact of Global Climate Change on the European Barley Market Requires Novel Multi-Method Approaches to Preserve Crop Quality and Authenticity. Foods, 10(7), 1592. https://doi.org/10.3390/foods10071592




