Characterization of Coffee Silver Skin as Potential Food-Safe Ingredient
Abstract
1. Introduction
2. Materials and Methods
2.1. Coffee Silver Skin
2.2. Physico-Chemical, Color, and Compositional Analyses
2.2.1. Acrylamide Content
2.2.2. Water-Holding and Oil-Holding Capacity (WHC, OHC)
2.3. Polyphenol Extraction, Total Polyphenol Content (TPC), and Phenolic Profile Determination in CSS
2.4. Melanoidin Determination of CSS
2.5. Radical-Scavenging Activity (TEAC) of CSS
2.6. Mineral Determination of CSS
2.7. Volatile Compounds in CSS
2.8. Statistical Analysis
3. Results
3.1. pH, aW, and Color of CSS
3.2. Characteristics of CSS Composition
3.3. Water-Holding and Oil-Holding Capacity
3.4. Polyphenols, Melanoidin, and Radical-Scavenging Activity
3.5. Minerals in CSS
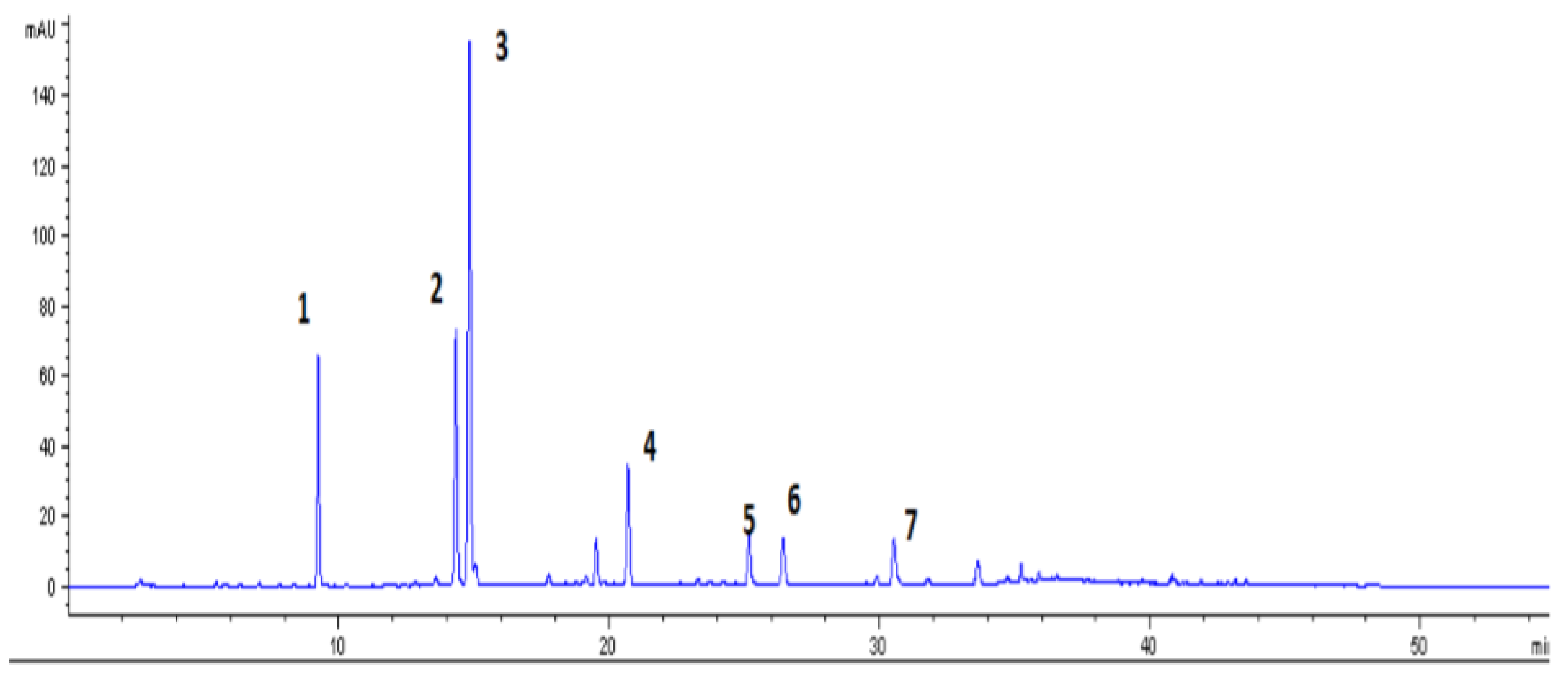
3.6. Volatile Organic Compounds (VOCs) in CSS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bahadur, K.C.; Dias, G.M.; Veeramani, A.; Swanton, C.J.; Fraser, D.; Steinke, D.; Lee, E.; Wittman, H.; Farber, J.M.; Dunfield, K.; et al. When too much isn’t enough: Does current food production meet global nutritional needs? PLoS ONE 2018, 13, e0205683. [Google Scholar]
- Bessada, S.M.F.; Alves, R.C.; Oliveira, M.B.P.P. Coffee Silverskin: A Review on Potential Cosmetic Applications. Cosmetics 2018, 5, 5. [Google Scholar] [CrossRef]
- Balentić, J.P.; Ačkar, Đ.; Jokić, S.; Jozinović, A.; Babić, J.; Miličević, B.; Šubarić, D.; Pavlovic, N. Cocoa Shell: A By-Product with Great Potential for Wide Application. Molecules 2018, 23, 1404. [Google Scholar] [CrossRef]
- Klingel, T.; Kremer, J.I.; Vera Gottstein, V.; Rajcic de Rezende, T.; Schwarz, S.; Lachenmeier, D.W. A Review of Coffee By-Products Including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union. Foods 2020, 9, 655. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–48. [Google Scholar] [CrossRef]
- Jannissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry byproducts: A review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Alves, R.C.; Rodrigues, F.; Nunes, M.A.; Vinha, A.F.; Oliveira, M.B.P.P. State of the art in coffee processing by-products (chapter 1). In Handbook of Coffee Processing By-Products; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 1–26. [Google Scholar]
- Gobena Gemechu, F. Embracing nutritional qualities, biological activities and technological properties of coffee byproducts in functional food formulation. Trends Food Sci. Technol. 2020, 104, 235–261. [Google Scholar] [CrossRef]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Costa, C.S.G.; Costa, M.A.; Nunes, A.A.; Almeida, A.; Santos-Silva Oliveira, M.B.P.P. Nutritional, chemical and antioxidant/pro-oxidant profiles of silverskin, a coffee roasting by-product. Food Chem. 2018, 30, 28–35. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Garcia, N.A.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Escobar, F.V.; Blanch, G.P.; San Andres, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef]
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Machado, S.; Costa, A.S.; Pimentel, F.; Oliveira, M.B.; Alves, R.C. A study on the protein fraction of coffee silverskin: Protein/non-protein nitrogen and free and total amino acid profiles. Food Chem. 2020, 326, 126940. [Google Scholar] [CrossRef]
- Pourfarzad, A.; Mahdavian-Mehr, H.; Sedaghat, N. Coffee silverskin as a source of dietary fiber in bread-making: Optimization of chemical treatment using response surface methodology. LWT-Food Sci. Technol. 2013, 50, 599–606. [Google Scholar] [CrossRef]
- Bertolino, M.; Barbosa-Pereira, L.; Ghirardello, D.; Botta, C.; Rolle, L.; Guglielmetti, A.; Borotto, C.; Dalla Vecchia, S.; Zeppa, G. Coffee silverskin as nutraceutical ingredient in yogurt: Its effect on functional properties and its bioaccessibility. J. Sci. Food Agric. 2019, 99, 4267–4275. [Google Scholar] [CrossRef] [PubMed]
- García-Serna, E.; Martinez–Saez, N.; Mesias, M.; Morales, F.J.; del Castillo, M.D. Use of Coffee Silverskin and Stevia to Improve the Formulation of Biscuits. Pol. J. Food Nutr. Sci. 2014, 64, 243–251. [Google Scholar] [CrossRef]
- Santos Ribeiro, V.; Leitão, A.E.; Cochicho Ramalho, J.; Cebola Lidon, F. Chemical characterization and antioxidant properties of a new coffee blend with cocoa, coffee silverskin and green coffee minimally processed. Food Res. Int. 2014, 16, 39–47. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; Ullate, M.; Martin-Cabrejas, M.A.; Martorell, P.; Genovés, S.; Ramon, D.; del Castillo, M.D. A novel antioxidant beverage for body weight control based on coffee silverskin. Food Chem. 2014, 150, 227–234. [Google Scholar] [CrossRef]
- Walker, J.M.; Mennella, I.; Ferracane, R.; Tagliamonte, S.; Holik, A.K.; Hölz, K.; Somoza, M.M.; Somoza, V.; Fogliano, V.; Vitaglione, P. Melanoidins from coffee and bread differently influence energy intake: A randomized controlled trial of food intake and gut-brain axis response. J. Funct. Food 2020, 72, 104063. [Google Scholar] [CrossRef]
- European Union. European Union Commission implementing regulation (EU) 2017/2468 of 20 December 2017 laying down administrative and scientific requirements concerning traditional foods from third countries in accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. Off. J. Eur. Union 2017, L351, 55–63. [Google Scholar]
- European Union. European Union Commission implementing regulation (EU) 2017/2469 of 20 December 2017 laying down administrative and scientific requirements for applications referred to in Article 10 of Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. Off. J. Eur. Union 2017, L351, 64–71. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, Methods 2009.01, and 2011.25, 19th ed.; AOAC International: Rockville, MD, USA, 2012. [Google Scholar]
- Folch, J.; Lees, M.; Sloane-Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess. Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Di Mattia, C.D.; Martuscelli, M.; Sacchetti, G.; Scheirlinck, I.; Beheydt, B.; Mastrocola, D.; Pittia, P. Effect of Fermentation and Drying on Procyanidins, Antiradical Activity and Reducing Properties of Cocoa Beans. Food Bioprocess. Technol. 2013, 6, 3420–3432. [Google Scholar] [CrossRef]
- Horžić, D.; Komes, D.; Belščak, A.; Kovačević Ganić, K.; Iveković, D.; Karlović, D. The composition of polyphenols and methylxanthines in teas and herbal infusions. Food Chem. 2009, 115, 441–448. [Google Scholar] [CrossRef]
- Rivero-Perez, M.; Perez-Magarino, S.; Gonzalez-San, J. Role of melanoidins in sweet wines. Anal. Chim. Acta 2012, 458, 169–175. [Google Scholar] [CrossRef]
- Delgado-Ospina, J.; Di Mattia, C.D.; Paparella, A.; Mastrocola, D.; Martuscelli, M.; Chaves-Lopez, C. Effect of Fermentation, Drying and Roasting on Biogenic Amines and Other Biocompounds in Colombian Criollo Cocoa Beans and Shells. Foods 2020, 9, 520. [Google Scholar] [CrossRef]
- UNI EN 13657:2002. Characterization of waste—Digestion for subsequent determination of aqua regia soluble portion of elements.
- Qi, J.; Wang, H.H.; Zhou, G.H.; Xu, X.L.; Li, X.; Bai, Y.; Yu, X. Evaluation of the taste-active and volatile compounds in stewed meat from the Chinese yellow-feather chicken breed. Int. J. Food Prop. 2017, 20, S2579–S2595. [Google Scholar] [CrossRef]
- Tores de la Cruz, S.; Iriondo-DeHond, A.; Herrera, T.; Lopez-Tofiño, Y.; Galvez-Robleño, C.; Prodanov, M.; Velazquez-Escobar, F.; Abalo, R.; Del Castillo, M.D. An Assessment of the Bioactivity of Coffee Silverskin Melanoidins. Foods 2019, 8, 68. [Google Scholar] [CrossRef]
- Mathlouthi, M. Water content, water activity, water structure and the stability of foodstuffs. Food Control 2001, 12, 409–417. [Google Scholar] [CrossRef]
- Sacchetti, G.; Di Mattia, C.D.; Pittia, P.; Mastrocola, D. Effect of roasting degree, equivalent thermal effect and coffee type on the radical scavenging activity of coffee brews and their phenolic fraction. J. Food Eng. 2009, 90, 74–80. [Google Scholar] [CrossRef]
- Gocmen, D.; Sahan, Y.; Yildiz, E.; Coskun, M.; Aroufai, I.A. Use of coffee silverskin to improve the functional properties of cookies. J. Food Sci Technol. 2019. [Google Scholar] [CrossRef]
- Ateş, G.; Elmacı, Y. Coffee silverskin as fat replacer in cake formulations and its effect on physical, chemical and sensory attributes of cakes. LWT 2018, 90, 519–525. [Google Scholar] [CrossRef]
- Ateş, G.; Elmacı, Y. Physical, chemical and sensory characteristics of fiber-enriched cakes prepared with coffee silverskin as wheat flour substitution. J. Food Meas. Charact. 2019. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. Review on utilization and composition of coffee silverskin. Food Res. Int. 2014, 61, 16–22. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, L.; Gutin, B.; Haidong, Z. Total, insoluble, and soluble dietary fiber intake and insulin resistance and blood pressure in adolescents. Eur. J. Clin. Nutr. 2019, 73, 1172–1178. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Blancas-Benítez, F.J.; Sáyago-Ayerdi, S.G. Polyphenols associated with dietary fibers in plant foods: Molecular interactions and bioaccessibility. Curr. Opin. Food Sci. 2017, 13, 84–88. [Google Scholar] [CrossRef]
- Food and Nutrition Board. Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Protein, and Aminoacids; National Academy of Sciences: Washington, DC, USA, 2002. [Google Scholar]
- Prasadi, N.V.P.; Joyce, I.J. Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health. Nutrients 2020, 12, 3045. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables American Society for Nutrition. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Commission regulation (EU) 2017/2158 of 20 November 2017 establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food. Off. J. Eur. Union 2017. Available online: http://data.europa.eu/eli/reg/2017/2158/oj (accessed on 12 May 2021).
- Bagdonaite, K.; Derler, K.; Murkovic, M. Determination of acrylamide during roasting of coffee. J. Agric. Food Chem. 2008, 56, 6081–6086. [Google Scholar] [CrossRef]
- Behrouzian, F.; Amini, A.M.; Alghooneh, A.; Razavi, S.M.A. Characterization of dietary fiber from coffee silverskin: An optimization study using response surface methodology. Bioact. Carbohydr. Diet. Fibre 2016, 8, 58–64. [Google Scholar] [CrossRef]
- Belmiro, R.H.; Artigiani Lima Tribst, A.; Cristianini, M. Application of high pressure homogenization on gums. J. Sci. Food Agric. 2018, 98, 2060–2069. [Google Scholar] [CrossRef]
- Belmiro, R.H.; de Carvalho Oliveira, L.; Geraldi, M.V.; Junior, M.R.; Cristianini, M. Modification of coffee coproducts by-products by dynamic high pressure, acetylation and hydrolysis by cellulase: A potential functional and sustainable food ingredient. Innov. Food Sci. Emerg. Technol. 2021, 68, 10268. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Çelik, E.E.; Gökmen, V. Interactions between free and bound antioxidants under different conditions in food systems. Crit. Rev. Food Sci. Nutr. 2021, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Guglielmetti, A.; Fernandez-Gomez, B.; Zeppa, G.; Del Castillo, M.D. Nutritional quality, potential health promoting properties and sensory perception of an improved gluten-free bread formulation containing inulin, rice protein and bioactive compounds extracted from coffee byproducts. Pol. J. Food Nutr. Sci. 2019, 69, 157–166. [Google Scholar] [CrossRef]
- Mesías, M.; Navarro, M.; Martínez-Saez, N.; Ullate, M.; del Castillo, M.D.; Morales, F.J. Antiglycative and carbonyl trapping properties of the water soluble fraction of coffee silverskin. Food Res. Int. 2014, 62, 1120–1126. [Google Scholar] [CrossRef]
- Bresciani, L.; Calani, L.; Bruni, R.; Brighenti, F.; Del Rio, D. Phenolic composition, caffeine content and antioxidant capacity of coffee silverskin. Food Res. Int. 2014, 61, 196–201. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Esposito, F.; Napolitano, A.; Ritieni, A.; Fogliano, V. Characterization of a New Potential Functional Ingredient: Coffee Silverskin. J. Agric. Food Chem. 2004, 52, 1338–1343. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Alves, R.C.; Costa, A.S.G.; Nunes, M.A.; Oliveira, M.B.P.P. Coffea canephora silverskin from different geographical origins: A comparative study. Sci. Total Environ. 2018, 645, 1021–1028. [Google Scholar] [CrossRef]
- Mesías, M.; Delgado- Andrade, C. Melanoidins as a potential functional food ingredient. Curr. Opin. Food Sci. 2017, 14, 37–42. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Hardisson, A.; Revert, C.; Gonzales-Weler, D.; Rubio, C. Aluminium Exposure Through the Diet. Food Sci. Nutr. 2017, 3, 19. [Google Scholar]
- FAO; WHO. Human Vitamin and Mineral Requirements; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and A (NDA). Scientific Opinion on principles for deriving and applying Dietary Reference Values. EFSA J. 2010, 8, 1–30. [Google Scholar]
- World Health Organization. Guideline: Potassium Intake for Adults and Children. Geneva. 2009. Available online: http://www.who.int/nutrition/publications/guidelines/potassium_intake/en/ (accessed on 2 May 2021).
- Sharma Ashimav, D. Low Nickel Diet in Dermatology. Ind. J. Dermatol. 2013, 58, 240–250. [Google Scholar] [CrossRef]
- Vītola, V.; Ciproviča, I. The Effect of Cocoa Beans Heavy and Trace Elements on Safety and Stability of Confectionery Products. Rural Sustain. Res. 2016, 35, 19–23. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). 2018. Available online: https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2018.5372 (accessed on 2 May 2021).
- European Food Safety Authority (EFSA). On the Evaluation of a New Study Related to the Bioavailability of Aluminium in Food. EFSA J. 2011, 9, 2157. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Update of the risk assessment of Nickel in food and drinking water. EFSA J. 2020, 18, e06268. [Google Scholar] [CrossRef]
- Angeloni, S.; Scortichini, S.; Fiorini, D.; Sagratini, G.; Vittori, S.; Neiens, S.D.; Steinhaus, M.; Zheljazkov, V.D.; Maggi, F.; Caprioli, G. Characterization of Odor-Active Compounds, Polyphenols, and Fatty Acids in Coffee Silverskin. Molecules 2020, 25, 2993. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Cui, C.; Fisk, I.D. Variability of single bean coffee volatile compounds of Arabica and robusta roasted coffees analysed by SPME-GC-MS. Food Res. Int. 2018, 108, 628–640. [Google Scholar] [CrossRef]
- De Toledo, P.A.B.; de Melo, M.M.R.; Pezza, H.R.; Toci, A.T.; Pezza, L.; Silva, C.M. Discriminant analysis for unveiling the origin of roasted coffee samples: A tool for quality control of coffee related products. Food Control 2017, 73, 164–174. [Google Scholar] [CrossRef]
- Yang, N.; Liu, C.; Liu, X.; Kreuzfeldt Degn, T.; Munchow, M.; Fisk, I. Determination of volatile marker compounds of common coffee roast defects. Food Chem. 2016, 15, 206–214. [Google Scholar] [CrossRef]
- Fisk, I.D.; Kettle, A.; Hofmeister, S.; Virdie, A.; Kenny, J.S. Discrimination of roast and ground coffee aroma. Flavour 2012, 1, 14. [Google Scholar] [CrossRef]
- Dos Santos Polidoro, A.; Scapin, E.; Lazzari, E.; Nunes Silva, A.; Loreiro dos Santos, A.; Bastos Caramão, E.; Assis Jacques, R. Valorization of coffee silverskin industrial waste by pyrolysis: From optimization of bio-oil production to chemical characterization by GC × GC/qMS. J. Anal. Appl. Pyrol. 2018, 129, 43–52. [Google Scholar] [CrossRef]
| Time (min) | B Phase (%) |
|---|---|
| 0 | 2 |
| 20 | 32 |
| 30 | 40 |
| 40 | 95 |
| 45 | 5 |
| 52 | 95 |
| 55 | 95 |
| aw | 0.32 ± 0.004 |
| pH | 5.34 ± 0.02 |
| L* | 20.8 ± 2.62 |
| a* | 4.27 ± 0.18 |
| b* | 13.4 ± 0.80 |
| C* | 14.12 ± 0.79 |
| Moisture (%) | 2.78 ± 0.30 |
| Ash (%) | 6.79 ± 0.78 |
| Proteins (%) | 18.15 ± 2.17 |
| Lipids (%) | 2.31 ± 0.50 |
| Fibers (%) | 51.05 ± 0.35 |
| Soluble Fibers (%) | 7.86 ± 1.01 |
| Insoluble Fibers (%) | 48.85 ± 0.83 |
| Acrylamide (μg kg−1) | 720 ± 110 |
| WHC (g water/g CSS) | 4.89 ± 0.36 |
| OHC (g oil/g CSS) | 3.02 ± 0.11 |
| Phenolic Fraction | TPC (mg GAE 100 g−1) | TEAC (mmol Trolox eq 100 g−1) | Chlorogenic Acid (mg g−1) | Caffeic Acid (mg g−1) | Caffeine (mg g−1) |
|---|---|---|---|---|---|
| Soluble | 578 ± 64 | 1.57 ± 0.04 | 0.39 ± 0.02 | 0.31 ± 0.01 | 17.45 ± 0.69 |
| Bound | 467 ± 29 | 1.11 ± 0.07 | 0.18 ± 0.01 | 0.21 ± 0.01 | n.d. |
| Sign. | ** | ** | ** | * | - |
| Minerals | (mg Kg−1) |
|---|---|
| Calcium | 5465 ± 343 |
| Magnesium | 2227 ± 26 |
| Phosphorus | 1462 ± 27 |
| Potassium | 14,600 ± 387 |
| Sodium | 115.85 ± 2.31 |
| Copper | 72.15 ± 4.70 |
| Chromium | 5.55 ± 0.14 |
| Iron | 212.46 ± 6.56 |
| Manganese | 23.13 ± 2.23 |
| Nickel | 3.17 ± 0.23 |
| Zinc | 18.06 ± 1.56 |
| Aluminum | 223.67 ± 16.38 |
| VOCs from CSS | Area % |
|---|---|
| Aldehydes | |
| 3-Furaldehyde | 1.32 |
| Benzaldehyde | 2.51 |
| Butanal, 2-methyl- | 2.95 |
| Butanal, 3-methyl- | 3.19 |
| Heptanal | 1.51 |
| Hexanal | 8.83 |
| Pentanal | 0.81 |
| Propanal, 2-methyl- | 2.20 |
| Alcohols | |
| 1-Methylcyclopropanemethanol | 3.00 |
| 1,3-Butanediol, (S)- | 1.26 |
| 2-Furanmethanol | 0.47 |
| 2-Furanmethanol, acetate | 0.47 |
| 2-Nonen-1-ol | 7.51 |
| 2-Octen-1-ol, (E)- | 0.23 |
| 2-Octyn-1-ol | 0.34 |
| 3-Hexen-1-ol, 2-ethyl- | 0.43 |
| Ketones | |
| 2-Propanone, 1-(acetyloxy)- | 1.11 |
| Nitrogen-containing species | |
| (2-Aziridinylethyl)amine | 1.30 |
| 3-Amino-2-oxazolidinone | 1.98 |
| Carane, 4,5-epoxy-, trans | 1.58 |
| Hydroxyurea | 0.72 |
| O-Methylisourea | 0.43 |
| Propanamide, 2-hydroxy- | 0.32 |
| Pyrazines | |
| 1-Methyl-3-Phenylpiperazine | 0.69 |
| Pyrazine, methyl- | 4.38 |
| Pyrazine, 2-ethyl-6-methyl- | 2.40 |
| Pyrazine, 2-ethyl-3-methyl- | 6.65 |
| Pyrazine, 3-ethyl-2,5-dimethyl- | 1.75 |
| Pyrazole, 3,5-dimethyl-1-allyl- | 0.78 |
| Hydrocarbons | |
| Ethylbenzene | 1.08 |
| Pentane, 1-(2-propenyloxy)- | 1.44 |
| Undecane,1,2-dibromo -2-methyl- | 1.64 |
| Other compounds | |
| 3(2H)-Furanone,dihydro-2 methyl- | 0.85 |
| α-Pinene | 0.27 |
| Furan, 2-pentyl- | 1.13 |
| Methyl glyoxal | 2.0 |
| Pyrimidine, 4-methyl- | 0.57 |
| Pyrimidine, 2-methyl- | 15.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martuscelli, M.; Esposito, L.; Di Mattia, C.D.; Ricci, A.; Mastrocola, D. Characterization of Coffee Silver Skin as Potential Food-Safe Ingredient. Foods 2021, 10, 1367. https://doi.org/10.3390/foods10061367
Martuscelli M, Esposito L, Di Mattia CD, Ricci A, Mastrocola D. Characterization of Coffee Silver Skin as Potential Food-Safe Ingredient. Foods. 2021; 10(6):1367. https://doi.org/10.3390/foods10061367
Chicago/Turabian StyleMartuscelli, Maria, Luigi Esposito, Carla Daniela Di Mattia, Antonella Ricci, and Dino Mastrocola. 2021. "Characterization of Coffee Silver Skin as Potential Food-Safe Ingredient" Foods 10, no. 6: 1367. https://doi.org/10.3390/foods10061367
APA StyleMartuscelli, M., Esposito, L., Di Mattia, C. D., Ricci, A., & Mastrocola, D. (2021). Characterization of Coffee Silver Skin as Potential Food-Safe Ingredient. Foods, 10(6), 1367. https://doi.org/10.3390/foods10061367









