The Combined Use of Lachancea thermotolerans and Lactiplantibacillus plantarum (former Lactobacillus plantarum) in Wine Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Inoculum Preparation
2.2. Vinification
2.3. Chemical-Parameter Measurements
2.4. Statistical Analyses
3. Results and Discussion
3.1. Fermentation Kinetics
3.2. Ethanol
3.3. l-Lactic Acid
3.4. Malic Acid
3.5. pH
3.6. Acetic Acid
3.7. Glycerol
3.8. Color Intensity
3.9. Biogenic Amines
3.10. Volatile Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Implications of new research and technologies for malolactic fermentation in wine. Appl. Microbiol. Biotechnol. 2014, 98, 8111–8132. [Google Scholar] [CrossRef]
- Pinto, L.; Baruzzi, F.; Cocolin, L.; Malfeito-Ferreira, M. Emerging technologies to control Brettanomyces spp. in wine: Recent advances and future trends. Trends Food Sci. Technol. 2020, 99, 88–100. [Google Scholar] [CrossRef]
- Varela, J.; Varela, C. Microbiological strategies to produce beer and wine with reduced ethanol concentration. Curr. Opin. Biotechnol. 2019, 56, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Benito, S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 6775–6790. [Google Scholar] [CrossRef] [PubMed]
- Porter, T.J.; Divol, B.; Setati, M.E. Lachancea yeast species: Origin, biochemical characteristics and oenological significance. Food Res. Int. 2019, 119, 378–389. [Google Scholar] [CrossRef]
- Vilela, A. Use of nonconventional yeasts for modulating wine acidity. Fermentation 2019, 5, 27. [Google Scholar] [CrossRef]
- Fairbairn, S.; Engelbrecht, L.; Setati, M.E.; du Toit, M.; Bauer, F.F.; Divol, B.; Rossouw, D. Combinatorial analysis of population dynamics, metabolite levels and malolactic fermentation in Saccharomyces cerevisiae/Lachancea thermotolerans mixed fermentations. Food Microbiol. 2021, 96, 103712. [Google Scholar] [CrossRef]
- Capozzi, V.; Tufariello, M.; De Simone, N.; Fragasso, M.; Grieco, F. Biodiversity of Oenological Lactic Acid Bacteria: Species- and Strain-Dependent Plus/Minus Effects on Wine Quality and Safety. Fermentation 2021, 7, 24. [Google Scholar] [CrossRef]
- Hranilovic, A.; Gambetta, J.M.; Schmidtke, L.; Boss, P.K.; Grbin, P.R.; Masneuf-Pomarede, I.; Bely, M.; Albertin, W.; Jiranek, V. Oenological traits of Lachancea thermotolerans show signs of domestication and allopatric differentiation. Sci. Rep. 2018, 8, 1–3. [Google Scholar] [CrossRef]
- Hranilovic, A.; Albertin, W.; Capone, D.L.; Gallo, A.; Grbin, P.R.; Danner, L.; Bastian, S.E.P.; Masneuf-Pomarede, I.; Coulon, J.; Bely, M.; et al. Impact of Lachancea thermotolerans on chemical composition and sensory profiles of Merlot wines. Food Chem. 2021, 349, 129015. [Google Scholar] [CrossRef]
- Escribano, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine aromatic compound production and fermentative behaviour within different non-Saccharomyces species and clones. J. Appl. Microbiol. 2018, 124, 1521–1531. [Google Scholar] [CrossRef]
- Dutraive, O.; Benito, S.; Fritsch, S.; Beisert, B.; Patz, C.-D.; Rauhut, D. Effect of Sequential Inoculation with Non-Saccharomyces and Saccharomyces Yeasts on Riesling Wine Chemical Composition. Fermentation 2019, 5, 79. [Google Scholar] [CrossRef]
- Borren, E.; Tian, B. The Important Contribution of Non-Saccharomyces Yeasts to the Aroma Complexity of Wine: A Review. Foods 2020, 10, 13. [Google Scholar] [CrossRef]
- Vilela, A. Lachancea thermotolerans, the Non-Saccharomyces Yeast that Reduces the Volatile Acidity of Wines. Fermentation 2018, 4, 56. [Google Scholar] [CrossRef]
- Hranilovic, A.; Li, S.; Boss, P.K.; Bindon, K.; Ristic, R.; Grbin, P.R.; Van der Westhuizen, T.; Jiranek, V. Chemical and sensory profiling of Shiraz wines co-fermented with commercial non-Saccharomyces inocula. Aust. J. Grape Wine Res. 2018, 24, 166–180. [Google Scholar] [CrossRef]
- Shekhawat, K.; Porter, T.J.; Bauer, F.F.; Setati, M.E. Employing oxygen pulses to modulate Lachancea thermotolerans–Saccharomyces cerevisiae Chardonnay fermentations. Ann. Microbiol. 2018, 68, 93–102. [Google Scholar] [CrossRef]
- Benito, S. Combined use of Lachancea thermotolerans and Schizosaccharomyces pombe in winemaking: A review. Microorganisms 2020, 8, 655. [Google Scholar] [CrossRef] [PubMed]
- Kapsopoulou, K.; Mourtzini, A.; Anthoulas, M.; Nerantzis, E. Biological acidification during grape must fermentation using mixed cultures of Kluyveromyces thermotolerans and Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2007, 23, 735–739. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef]
- Ciani, M.; Beco, L.; Comitini, F. Fermentation behaviour and metabolic interactions of multistarter wine yeast fermentations. Int. J. Food Microbiol. 2006, 108, 239–245. [Google Scholar] [CrossRef]
- Benito, A.; Calderón, F.; Benito, S. Combined use of S. pombe and L. thermotolerans in winemaking. Beneficial effects determined through the study of wines’ analytical characteristics. Molecules 2016, 21, 1744. [Google Scholar] [CrossRef]
- Chen, K.; Escott, C.; Loira, I.; del Fresno, J.M.; Morata, A.; Tesfaye, W.; Calderon, F.; Suárez-Lepe, J.A.; Han, S.; Benito, S. Use of non-Saccharomyces yeasts and oenological tannin in red winemaking: Influence on colour, aroma and sensorial properties of young wines. Food Microbiol. 2018, 69, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Use of non-Saccharomyces wine yeasts as novel sources of mannoproteins in wine. Food Microbiol. 2014, 43, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Navascués, E.; Marquina, D.; Santos, A.; Calderón, F.; Benito, S. Outlining the influence of non-conventional yeasts in wine ageing over lees. Yeast 2016, 33, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Snyman, C.; Mekoue Nguela, J.; Sieczkowski, N.; Marangon, M.; Divol, B. Optimised Extraction and Preliminary Characterisation of Mannoproteins from Non-Saccharomyces Wine Yeasts. Foods 2021, 10, 924. [Google Scholar] [CrossRef]
- Roudil, L.; Russo, P.; Berbegal, C.; Albertin, W.; Spano, G.; Capozzi, V. Non-Saccharomyces Commercial Starter Cultures: Scientific Trends, Recent Patents and Innovation in the Wine Sector. Recent Pat. Food. Nutr. Agric. 2019, 11, 27–39. [Google Scholar] [CrossRef]
- Du Toit, M.; Engelbrecht, L.; Lerm, E.; Krieger-Weber, S. Lactobacillus: The Next Generation of Malolactic Fermentation Starter Cultures-an Overview. Food Bioprocess Technol. 2011, 4, 876–906. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Costello, P.J.; Chambers, P.J. Emerging trends in the application of malolactic fermentation. Aust. J. Grape Wine Res. 2015, 21, 663–669. [Google Scholar] [CrossRef]
- Mendes Ferreira, A.; Mendes-Faia, A. The Role of Yeasts and Lactic Acid Bacteria on the Metabolism of Organic Acids during Winemaking. Foods 2020, 9, 1231. [Google Scholar] [CrossRef]
- Pardo, I.; Ferrer, S. Yeast-Bacteria Coinoculation. In Red Wine Technology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 99–114. ISBN 9780128144008. [Google Scholar]
- Petruzzi, L.; Capozzi, V.; Berbegal, C.; Corbo, M.R.; Bevilacqua, A.; Spano, G.; Sinigaglia, M. Microbial resources and enological significance: Opportunities and benefits. Front. Microbiol. 2017, 8, 995. [Google Scholar] [CrossRef]
- Krieger-Weber, S.; Heras, J.M.; Suarez, C. beverages Lactobacillus plantarum, a New Biological Tool to Control Malolactic Fermentation: A Review and an Outlook. Beverages 2020, 6, 23. [Google Scholar] [CrossRef]
- Virdis, C.; Sumby, K.; Bartowsky, E.; Jiranek, V. Lactic Acid Bacteria in Wine: Technological Advances and Evaluation of Their Functional Role. Front. Microbiol. 2021, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.Y.; du Toit, M. Evaluating the Influence of Malolactic Fermentation Inoculation Practices and Ageing on Lees on Biogenic Amine Production in Wine. Food Bioprocess Technol. 2013, 6, 198–206. [Google Scholar] [CrossRef]
- Benito, S. The Management of Compounds that Influence Human Health in Modern Winemaking from an HACCP Point of View. Fermentation 2019, 5, 33. [Google Scholar] [CrossRef]
- Vilela, A. The importance of yeasts on fermentation quality and human health-promoting compounds. Fermentation 2019, 5, 46. [Google Scholar] [CrossRef]
- Scansani, S.; Rauhut, D.; Brezina, S.; Semmler, H.; Benito, S. The impact of chitosan on the chemical composition of wines fermented with Schizosaccharomyces pombe and Saccharomyces cerevisiae. Foods 2020, 9, 1423. [Google Scholar] [CrossRef]
- Du Plessis, H.; Du Toit, M.; Nieuwoudt, H.; Van der Rijst, M.; Hoff, J.; Jolly, N. Modulation of wine flavor using Hanseniaspora uvarum in combination with different Saccharomyces cerevisiae, lactic acid bacteria strains and malolactic fermentation strategies. Fermentation 2019, 5, 64. [Google Scholar] [CrossRef]
- Tufariello, M.; Capozzi, V.; Spano, G.; Cantele, G.; Venerito, P.; Mita, G.; Grieco, F. Microorganisms Effect of Co-Inoculation of Candida zemplinina, Saccharomyces cerevisiae and Lactobacillus plantarum for the Industrial Production of Negroamaro Wine in Apulia (Southern Italy). Microorganisms 2020, 8, 726. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Englezos, V.; Capozzi, V.; Pollon, M.; Río Segade, S.; Rantsiou, K.; Spano, G.; Cocolin, L. Effect of mixed fermentations with Starmerella bacillaris and Saccharomyces cerevisiae on management of malolactic fermentation. Food Res. Int. 2020, 134, 109246. [Google Scholar] [CrossRef]
- Du Plessis, H.W.; du Toit, M.; Hoff, J.W.; Hart, R.S.; Ndimba, B.K.; Jolly, N.P. Characterisation of non-Saccharomyces yeasts using different methodologies and evaluation of their compatibility with malolactic fermentation. South African J. Enol. Vitic. 2017, 38, 46–63. [Google Scholar] [CrossRef]
- Balmaseda, A.; Bordons, A.; Reguant, C.; Bautista-Gallego, J. Non-Saccharomyces in Wine: Effect Upon Oenococcus oeni and Malolactic Fermentation. Front. Microbiol. 2018, 9, 534. [Google Scholar] [CrossRef] [PubMed]
- Masneuf-Pomarède, I.; Bely, M.; Marullo, P.; Lonvaud-Funel, A.; Dubourdieu, D. Reassessment of phenotypic traits for Saccharomyces bayanus var. uvarum wine yeast strains. Int. J. Food Microbiol. 2010, 139, 79–86. [Google Scholar] [CrossRef]
- Eglinton, J.M.; McWilliam, S.J.; Fogarty, M.W.; Francis, I.L.; Kwiatkowski, M.J.; Høj, P.B.; Henschke, P.A. The effect of Saccharomyces bayanus-mediated fermentation on the chemical composition and aroma profile of Chardonnay wine. Aust. J. Grape Wine Res. 2000, 6, 190–196. [Google Scholar] [CrossRef]
- Sipiczki, M. Taxonomic and physiological diversity of Saccharomyces bayanus. Biodivers. Biotechnol. Wine Yeasts 2002, 53–69. [Google Scholar]
- Abrahamse, C.E.; Bartowsky, E.J. Timing of malolactic fermentation inoculation in Shiraz grape must and wine: Influence on chemical composition. World J. Microbiol. Biotechnol. 2012, 28, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, H.; du Toit, M.; Nieuwoudt, H.; van der Rijst, M.; Kidd, M.; Jolly, N. Effect of Saccharomyces, Non-Saccharomyces Yeasts and Malolactic Fermentation Strategies on Fermentation Kinetics and Flavor of Shiraz Wines. Fermentation 2017, 3, 64. [Google Scholar] [CrossRef]
- Iorizzo, M.; Testa, B.; Lombardi, S.J.; García-Ruiz, A.; Muñoz-González, C.; Bartolomé, B.; Moreno-Arribas, M.V. Selection and technological potential of Lactobacillus plantarum bacteria suitable for wine malolactic fermentation and grape aroma release. LWT Food Sci. Technol. 2016, 73, 557–566. [Google Scholar] [CrossRef]
- Minnaar, P.P.; du Plessis, H.W.; Paulsen, V.; Ntushelo, N.; Jolly, N.P.; du Toit, M. Saccharomyces cerevisiae, Non-Saccharomyces Yeasts and Lactic Acid Bacteria in Sequential Fermentations: Effect on Phenolics and Sensory Attributes of South African Syrah Wines. South African J. Enol. Vitic. 2017, 38, 237–244. [Google Scholar] [CrossRef]
- Minnaar, P.P.; du Plessis, H.W.; Jolly, N.P.; van der Rijst, M.; du Toit, M. Non-Saccharomyces yeast and lactic acid bacteria in Co-inoculated fermentations with two Saccharomyces cerevisiae yeast strains: A strategy to improve the phenolic content of Syrah wine. Food Chem. X 2019, 4, 100070. [Google Scholar] [CrossRef]
- Brizuela, N.S.; Bravo-Ferrada, B.M.; Pozo-Bayón, M.Á.; Semorile, L.; Elizabeth Tymczyszyn, E. Changes in the volatile profile of Pinot noir wines caused by Patagonian Lactobacillus plantarum and Oenococcus oeni strains. Food Res. Int. 2018, 106, 22–28. [Google Scholar] [CrossRef] [PubMed]
- G-Alegría, E.; López, I.; Ruiz, J.I.; Sáenz, J.; Fernández, E.; Zarazaga, M.; Dizy, M.; Torres, C.; Ruiz-Larrea, F. High tolerance of wild Lactobacillus plantarum and Oenococcus oeni strains to lyophilisation and stress environmental conditions of acid pH and ethanol. FEMS Microbiol. Lett. 2004, 230, 53–61. [Google Scholar] [CrossRef]
- Lucio, O.; Pardo, I.; Krieger-Weber, S.; Heras, J.M.; Ferrer, S. Selection of Lactobacillus strains to induce biological acidification in low acidity wines. LWT Food Sci. Technol. 2016, 73, 334–341. [Google Scholar] [CrossRef]
- Lucio, O.; Pardo, I.; Heras, J.M.; Krieger, S.; Ferrer, S. Influence of yeast strains on managing wine acidity using Lactobacillus plantarum. Food Control 2018, 92, 471–478. [Google Scholar] [CrossRef]
- Blanco, P.; Rabuñal, E.; Neira, N.; Castrillo, D. Dynamic of Lachancea thermotolerans Population in Monoculture and Mixed Fermentations: Impact on Wine Characteristics. Beverages 2020, 6, 36. [Google Scholar] [CrossRef]
- Lasik-Kurdyś, M.; Majcher, M.; Nowak, J. Effects of Different Techniques of Malolactic Fermentation Induction on Diacetyl Metabolism and Biosynthesis of Selected Aromatic Esters in Cool-Climate Grape Wines. Molecules 2018, 23, 2549. [Google Scholar] [CrossRef]
- Knoll, C.; Fritsch, S.; Schnell, S.; Grossmann, M.; Krieger-Weber, S.; du Toit, M.; Rauhut, D. Impact of different malolactic fermentation inoculation scenarios on Riesling wine aroma. World J. Microbiol. Biotechnol. 2012, 28, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Bordeu, C.A.O.E. Pre-alcoholic fermentation acidification of red grape must using Lactobacillus plantarum. Antonie Van Leeuwenhoek 2015, 108, 1469–1475. [Google Scholar]
- Englezos, V.; Cachón, D.C.; Rantsiou, K.; Blanco, P.; Petrozziello, M.; Pollon, M.; Giacosa, S.; Río Segade, S.; Rolle, L.; Cocolin, L. Effect of mixed species alcoholic fermentation on growth and malolactic activity of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2019, 103, 7687–7702. [Google Scholar] [CrossRef]
- Muñoz, V.; Beccaria, B.; Abreo, E. Simultaneous and successive inoculations of yeasts and lactic acid bacteria on the fermentation of an unsulfited Tannat grape must. Braz. J. Microbiol. 2014, 45, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Mylona, A.E.; Del Fresno, J.M.; Palomero, F.; Loira, I.; Bañuelos, M.A.; Morata, A.; Calderón, F.; Benito, S.; Suárez-Lepe, J.A. Use of Schizosaccharomyces strains for wine fermentation-Effect on the wine composition and food safety. Int. J. Food Microbiol. 2016, 232, 63–72. [Google Scholar] [CrossRef]
- Burns, T.R.; Osborne, J.P. Loss of pinot noir wine color and polymeric pigment after malolactic fermentation and potential causes. Am. J. Enol. Vitic. 2015, 66, 130–137. [Google Scholar] [CrossRef]
- Dev, A.; Anu-Appaiah, K.A. Yeast-bacterial interactions during malolactic inoculations affecting anthocyanin adsorption and content in shiraz wine. Am. J. Enol. Vitic. 2020, 71, 105–113. [Google Scholar] [CrossRef]
- Devi, A.; Konerira Aiyappaa, A.; Waterhouse, A.L. Adsorption and biotransformation of anthocyanin glucosides and quercetin glycosides by Oenococcus oeni and Lactobacillus plantarum in model wine solution. J. Sci. Food Agric. 2020, 100, 2110–2120. [Google Scholar] [CrossRef]
- Sumby, K.M.; Bartle, L.; Grbin, P.R.; Jiranek, V. Measures to improve wine malolactic fermentation. Appl. Microbiol. Biotechnol. 2019, 103, 2033–2051. [Google Scholar] [CrossRef]
- Capozzi, V.; Russo, P.; Ladero, V.; Fernández, M.; Fiocco, D.; Alvarez, M.A.; Grieco, F.; Spano, G. Biogenic Amines Degradation by Lactobacillus plantarum: Toward a Potential Application in Wine. Front. Microbiol. 2012, 3, 122. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Cañas, P.M.I.; Pérez-Martín, F.; Romero, E.G.; Prieto, S.S.; Herreros, M.d.l.L.P. Influence of inoculation time of an autochthonous selected malolactic bacterium on volatile and sensory profile of Tempranillo and Merlot wines. Int. J. Food Microbiol. 2012, 156, 245–254. [Google Scholar] [CrossRef]
- Tristezza, M.; di Feo, L.; Tufariello, M.; Grieco, F.; Capozzi, V.; Spano, G.; Mita, G. Simultaneous inoculation of yeasts and lactic acid bacteria: Effects on fermentation dynamics and chemical composition of Negroamaro wine. LWT 2016, 66, 406–412. [Google Scholar] [CrossRef]
- Gardoni, E.; Benito, S.; Scansani, S.; Brezina, S.; Fritsch, S.; Rauhut, D. Biological deacidification strategie for white wine. South Afr. J. Enol. Vitic. 2021. accepted. [Google Scholar]
- Osborne, J.P.; Mira De Orduña, R.; Pilone, G.J.; Liu, S.Q. Acetaldehyde metabolism by wine lactic acid bacteria. FEMS Microbiol. Lett. 2000, 191, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascués, E.; Calderón, F.; Benito, A.; Rauhut, D.; Santos, A.; et al. Effects on varietal aromas during wine making: A review of the impact of varietal aromas on the flavor of wine. Appl. Microbiol. Biotechnol. 2019, 103, 7425–7450. [Google Scholar] [CrossRef] [PubMed]
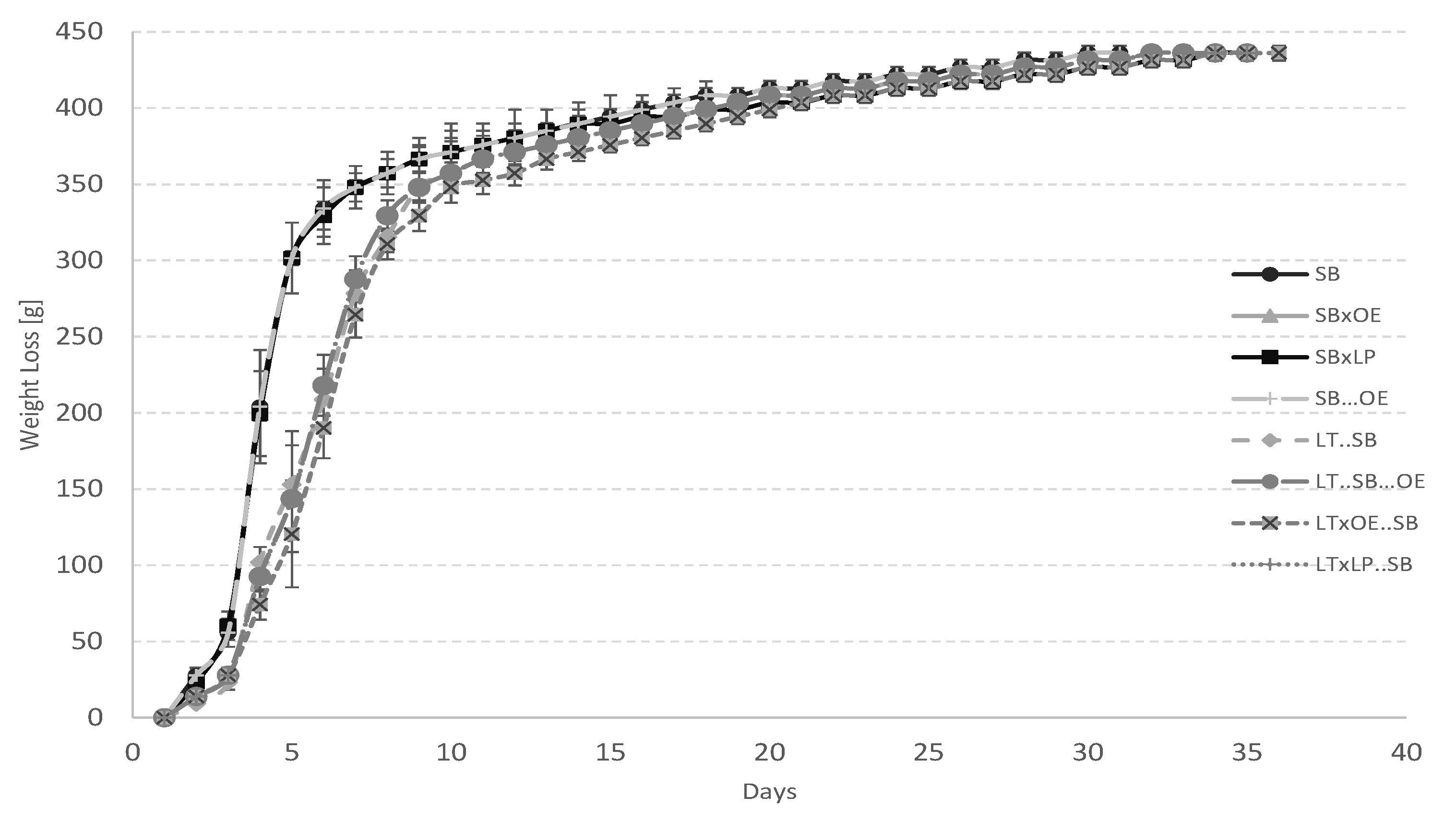
| SB | Inoculation of the must with S. bayanus (106 CFU/mL) alone. |
| SB × OE | Inoculation of the must with S. bayanus (106 CFU/mL) followed by O. oeni (106 CFU/mL) 24 h later. |
| SB × LP | Inoculation of the must with S. bayanus (106 CFU/mL) followed by L. plantarum (106 CFU/mL) 24 h later. |
| SB…OE | Inoculation of the must with S. bayanus (106 CFU/mL) followed by O. oeni (106 CFU/mL) after alcoholic fermentation. |
| LT..SB | Inoculation of the must with L. thermotolerans (106 CFU/mL) followed by S. bayanus (106 CFU/mL) 96 h later. |
| LT..SB…OE | Inoculation of the must with L. thermotolerans (106 CFU/mL) followed by S. bayanus (106 CFU/mL) 96 h later, followed by O. oeni (106 CFU/mL) after alcoholic fermentation. |
| LT × OE..SB | Inoculation of the must with S. bayanus (106 CFU/mL), followed by O. oeni (106 CFU/mL) 24 h later and followed by S. bayanus (106 CFU/mL) 72 h later. |
| LT × LP..SB | Inoculation of the must with L. thermotolerans (106 CFU/mL) followed by L. plantarum (106 CFU/mL) 24 h later, followed by S. bayanus (106 CFU/mL) 72 h later. |
| SB | SB × OE | SB × LP | SB…OE | LT..SB | LT..SB…OE | LT × OE..SB | LT × LP..SB | |
|---|---|---|---|---|---|---|---|---|
| l-Lactic acid (g/L) | 0.01 ± 0.01 a | 0.52 ± 0.06 b | 0.42 ± 0.04 b | 0.49 ± 0.03 b | 2.44 ± 0.05 c | 2.85 ± 0.09 d | 2.91 ± 0.12 d | 2.88 ± 0.10 d |
| l-Malic acid (g/L) | 0.74 ± 0.03 c | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.65 ± 0.04 b | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a |
| Acetic acid (g/L) | 0.42 ± 0.02 a | 0.58 ± 0.04 b | 0.44 ± 0.02 a | 0.51 ± 0.03 b | 0.40 ± 0.03 a | 0.48 ± 0.03 ab | 0.53 ± 0.05 b | 0.43 ± 0.04 a |
| pH | 3.91 ± 0.01 c | 3.97 ± 0.01 d | 3.96 ± 0.01 d | 3.98 ± 0.01 d | 3.67 ± 0.01 a | 3.74 ± 0.02 b | 3.75 ± 0.03 b | 3.72 ± 0.02 b |
| Ethanol (% v/v) | 15.24 ± 0.11 c | 14.91 ± 0.20 b | 15.11 ± 0.18 c | 15.17 ± 0.20 c | 14.81 ± 0.22 b | 14.78 ± 0.24 b | 14.55 ± 0.21 a | 14.80 ± 0.19 b |
| Residual sugar (g/L) | 1.34 ± 0.21 b | 0.11 ± 0.06 a | 1.55 ± 0.31 b | 0.09 ± 0.05 a | 1.62 ± 0.29 b | 0.13 ± 0.11 a | 0.13 ± 0.09 a | 1.68 ± 0.31 b |
| Glycerol (g/L) | 8.21 ± 0.12 a | 8.24 ± 0.25 ab | 8.16 ± 0.19 a | 8.19 ± 0.21 a | 8.72 ± 0.22 b | 8.76 ± 0.25 b | 8.78 ± 0.27 b | 8.71 ± 0.21 b |
| SB | SB × OE | SB × LP | SB…OE | LT..SB | LT..SB…OE | LT × OE..SB | LT × LP..SB | |
|---|---|---|---|---|---|---|---|---|
| 420 nm | 3.56 ± 0.06 b | 3.59 ± 0.08 b | 3.55 ± 0.05 b | 3.11 ± 0.08 a | 3.68 ± 0.04 b | 3.52 ± 0.07 a | 3.70 ± 0.10 b | 3.64 ± 0.07 b |
| 520 nm | 4.52 ± 0.05 b | 4.49 ± 0.06 b | 4.54 ± 0.06 b | 3.96 ± 0.08 a | 5.26 ± 0.06 c | 4.58 ± 0.06 b | 5.29 ± 0.11 c | 5.25 ± 0.07 c |
| 620 nm | 1.38 ± 0.02 b | 1.36 ± 0.03 b | 1.36 ± 0.03 b | 1.17 ± 0.03 a | 1.59 ± 0.03 c | 1.42 ± 0.03 b | 1.55 ± 0.05 c | 1.61 ± 0.06 c |
| CI | 9.46 ± 0.08 b | 9.43 ± 0.10 b | 9.45 ± 0.08 b | 8.25 ± 0.12 a | 10.54 ± 0.08 c | 9.51 ± 0.10 b | 10.52 ± 0.16 c | 10.50 ± 0.11 c |
| SB | SB × OE | SB × LP | SB…OE | LT..SB | LT..SB…OE | LT × OE..SB | LT × LP..SB | |
|---|---|---|---|---|---|---|---|---|
| Histamine (mg/L) | 0.11 ± 0.01 a | 0.10 ± 0.02 a | 0.12 ± 0.02 a | 0.11 ± 0.02 a | 0.13 ± 0.02 a | 0.11 ± 0.02 a | 0.12 ± 0.02 a | 0.12 ± 0.02 a |
| Tiramine (mg/L) | 0.08 ± 0.01 a | 0.08 ± 0.02 a | 0.07 ± 0.02 a | 0.07 ± 0.02 a | 0.09 ± 0.02 a | 0.08 ± 0.02 a | 0.08 ± 0.02 a | 0.07 ± 0.02 a |
| Phenylethylamine (mg/L) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Putrescine (mg/L) | 0.15 ± 0.01 a | 0.15 ± 0.02 a | 0.14 ± 0.02 a | 0.14 ± 0.02 a | 0.15 ± 0.02 a | 0.13 ± 0.02 a | 0.15 ± 0.02 a | 0.14 ± 0.02 a |
| Cadaverine (mg/L) | 0.19 ± 0.01 a | 0.18 ± 0.02 a | 0.19 ± 0.02 a | 0.18 ± 0.02 a | 0.18 ± 0.02 a | 0.17 ± 0.02 a | 0.18 ± 0.02 a | 0.17 ± 0.02 a |
| Compounds (mg/L) | SB | SB × OE | SB × LP | SB…OE | LT..SB | LT..SB…OE | LT × OE…SB | LT × LP..SB |
|---|---|---|---|---|---|---|---|---|
| Acetaldehyde | 14.33 ± 0.21 d | 13.22 ± 0.26 c | 14.15 ± 0.24 c | 2.16 ± 0.25 a | 12.11 ± 0.41 b | 1.99 ± 0.27 a | 11.93 ± 0.62 b | 12.31 ± 0.33 b |
| Ethyl lactate | 4.36 ± 0.31 a | 62.31 ± 18.05 bc | 52.23 ± 12.12 b | 47.42 ± 8.46 b | 79.31 ± 11.23 c | 116.31 ± 12.43 d | 99.27 ± 21.53 cd | 89.76 ± 14.62 cd |
| Ethyl acetate | 24.63 ± 2.22 a | 40.92 ± 4.89 c | 25.45 ± 3.78 a | 33.62 ± 3.11 b | 24.15 ± 2.06 a | 33.97 ± 3.86 b | 43.77 ± 5.12 c | 26.12 ± 3.88 a |
| Diacetyl | 1.99 ± 0.11 a | 3.95 ± 0.26 b | 2.03 ± 0.15 a | 5.92 ± 0.46 c | 1.95 ± 0.12 a | 5.58 ± 0.51 c | 4.02 ± 0.25 b | 1.97 ± 0.15 a |
| Isoamyl acetate | 5.26 ± 0.33 a | 5.86 ± 0.48 ab | 5.84 ± 0.52 ab | 5.95 ± 0.49 ab | 6.19 ± 0.43 b | 6.29 ± 0.56 b | 5.98 ± 0.58 ab | 6.16 ± 0.49 b |
| 1-Propanol | 26.72 ± 1.99 b | 27.92 ± 2.21 b | 26.15 ± 2.09 b | 28.03 ± 2.11 b | 19.12 ± 2.16 a | 20.41 ± 2.24 a | 21.73 ± 2.45 a | 20.26 ± 2.55 a |
| Isobutanol | 15.28 ± 1.11 a | 16.42 ± 1.67 a | 15.26 ± 1.79 a | 17.14 ± 1.49 a | 14.86 ± 1.28 a | 15.02 ± 1.33 a | 15.46 ± 1.79 a | 15.21 ± 1.76 a |
| 1-Butanol | 7.38 ± 0.74 a | 7.27 ± 0.76 a | 7.16 ± 0.79 a | 7.04 ± 0.84 a | 7.84 ± 0.88 a | 7.14 ± 0.94 a | 7.13 ± 0.86 a | 7.56 ± 0.84 a |
| 2-Methyl-butanol | 36.83 ± 1.54 b | 23.64 ± 1.65 a | 24.94 ± 1.69 a | 25.86 ± 1.63 a | 27.41 ± 1.82 a | 23.85 ± 1.76 a | 24.98 ± 1.55 a | 26.14 ± 1.93 a |
| 3-Methyl-butanol | 85.67 ± 16.72 a | 84.79 ± 17.92 a | 73.41 ± 19.56 a | 81.71 ± 18.41 a | 82.33 ± 18.91 a | 79.52 ± 21.81 a | 83.62 ± 21.13 a | 83.97 ± 20.73 a |
| Hexanol | 1.87 ± 0.22 b | 1.89 ± 0.28 b | 1.65 ± 0.33 a | 1.86 ± 0.25 b | 1.91 ± 0.32 b | 1.89 ± 0.29 b | 1.88 ± 0.29 b | 1.68 ± 0.39 a |
| 2-Phenyl-ethanol | 19.41 ± 1.35 a | 20.55 ± 1.82 a | 18.36 ± 1.79 a | 21.47 ± 1.56 a | 29.32 ± 2.19 b | 26.87 ± 2.37 b | 27.86 ± 2.93 b | 27.15 ± 2.88 b |
| 2-Phenyl ethyl acetate | 3.21 ± 0.24 a | 3.36 ± 0.29 a | 3.08 ± 0.27 a | 3.41 ± 0.32 a | 5.63 ± 0.34 c | 4.62 ± 0.46 b | 4.85 ± 0.52 bc | 5.35 ± 0.41 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbina, Á.; Calderón, F.; Benito, S. The Combined Use of Lachancea thermotolerans and Lactiplantibacillus plantarum (former Lactobacillus plantarum) in Wine Technology. Foods 2021, 10, 1356. https://doi.org/10.3390/foods10061356
Urbina Á, Calderón F, Benito S. The Combined Use of Lachancea thermotolerans and Lactiplantibacillus plantarum (former Lactobacillus plantarum) in Wine Technology. Foods. 2021; 10(6):1356. https://doi.org/10.3390/foods10061356
Chicago/Turabian StyleUrbina, Ángel, Fernando Calderón, and Santiago Benito. 2021. "The Combined Use of Lachancea thermotolerans and Lactiplantibacillus plantarum (former Lactobacillus plantarum) in Wine Technology" Foods 10, no. 6: 1356. https://doi.org/10.3390/foods10061356
APA StyleUrbina, Á., Calderón, F., & Benito, S. (2021). The Combined Use of Lachancea thermotolerans and Lactiplantibacillus plantarum (former Lactobacillus plantarum) in Wine Technology. Foods, 10(6), 1356. https://doi.org/10.3390/foods10061356






