Cacao Pod Husk Flour as an Ingredient for Reformulating Frankfurters: Effects on Quality Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Cacao Pod Husk Flour (CPHF)
2.2. Frankfurters Elaboration Process and Treatments
2.3. Proximate Composition
2.4. Physicochemical Analysis
2.5. Residual Nitrite Level
2.6. Measurement of Lipid Oxidation: Thiobarbituric Acid Index (TBARS)
2.7. Color, Reflectance Spectra and Reflectance Ratios
2.8. Textural Properties
2.9. Sensory Evaluation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition of Frankfurters
3.2. Physicochemical Analysis. pH and aw
3.3. Residual Nitrite Level
3.4. Lipid Oxidation. Thiobarbituric Acid Index (TBARS)
3.5. Color
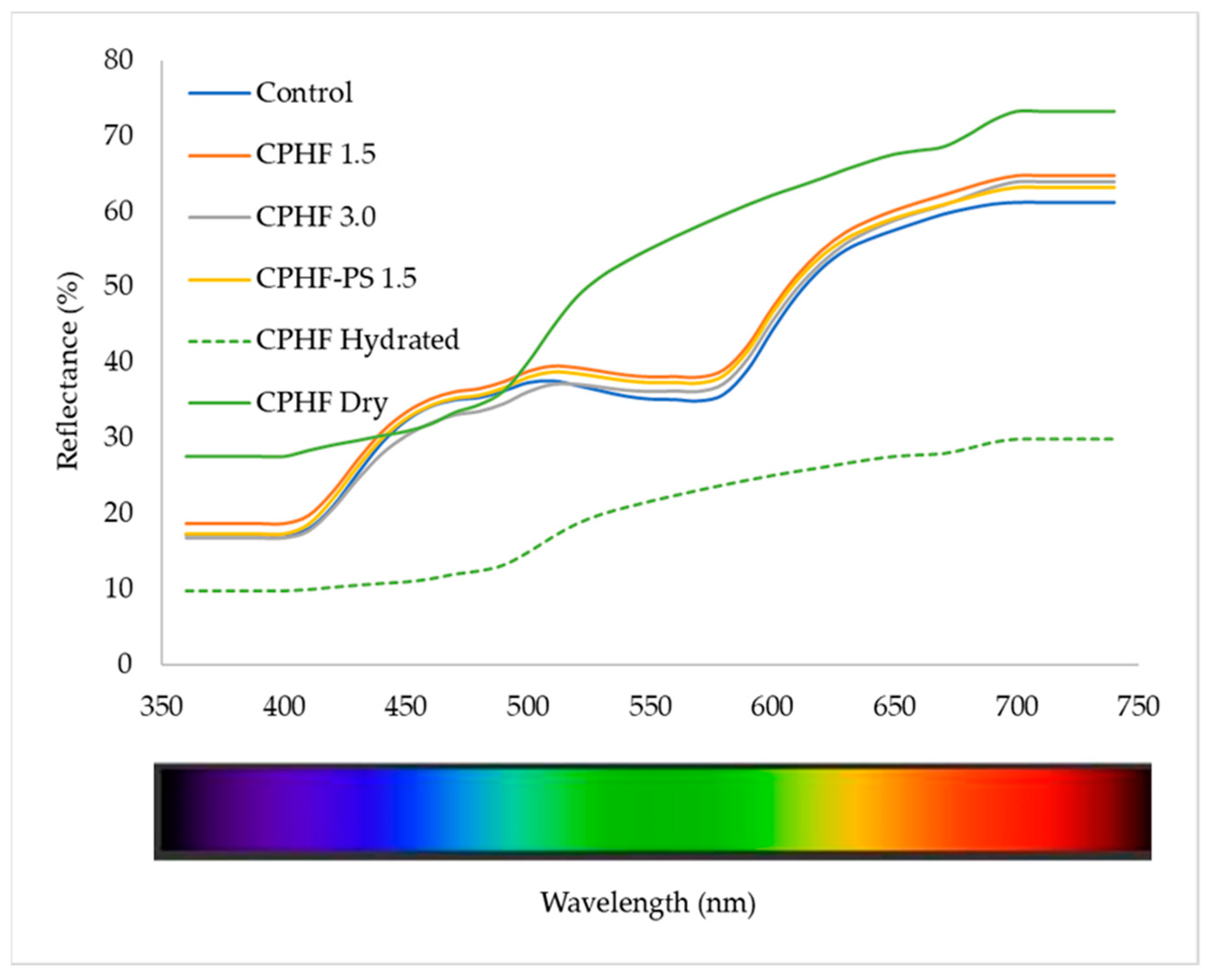
3.5.1. Reflectance Spectra
3.5.2. Reflectance Ratios R630/R580, R650/R570, and R560/R500
3.6. TPA Analysis
3.7. Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández-López, J.; Viuda-Martos, M.; Pérez-Alvarez, J.A. Quinoa and chia products as ingredients for healthier processed meat products: Technological strategies for their application and effects on the final product. Curr. Opin. Food Sci. 2021, 40, 26–32. [Google Scholar] [CrossRef]
- Fernández-López, J.; Viuda-Martos, M.; Sayas-Barberá, M.E.; de Vera Navarro-Rodríguez, C.; Lucas-González, R.; Roldán-Verdú, A.; Botella-Martínez, C.; Pérez-Alvarez, J.A. Chia, Quinoa, and Their Coproducts as Potential Antioxidants for the Meat Industry. Plants 2020, 9, 1359. [Google Scholar] [CrossRef]
- Delgado-Ospina, J.; Lucas-González, R.; Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.Á.; Martuscelli, M.; Chaves-López, C. Bioactive compounds and techno-functional properties of high-fiber co-products of the cacao agro-industrial chain. Heliyon 2021, 7, e06799. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; López-Marcos, M.C.; Fernández-López, J.; Sendra, E.; López-Vargas, J.H.; Pérez-Álvarez, J.A. Role of Fiber in Cardiovascular Diseases: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 240–258. [Google Scholar] [CrossRef]
- Pérez-Álvarez, J.Á.; Botella-Martínez, C.M.; de Vera Navarro-Rodríguez, C.; Sayas-Barberá, E.; Viuda-Martos, M.; Fernández-López, J.; Sánchez-Zapata, E. A Preliminary Study on the Incorporation of Quinoa Flour in Organic Pumpkin Creams: Effect on the Physicochemical Properties. Proceedigs 2021, 70, 71. [Google Scholar]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Föste, M.; Verheyen, C.; Jekle, M.; Becker, T. Fibres of milling and fruit processing by-products in gluten-free bread making: A review of hydration properties, dough formation and quality-improving strategies. Food Chem. 2020, 306, 125451. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.S.; Santos, M.J.M.C.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; da Silva Lannes, S.C.; da Silva, M.V. Natural antioxidants used in meat products: A brief review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Madane, P.; Biswas, S.; Das, A.; Zhang, W.; Lorenzo, J.M. A comprehensive review on antioxidant dietary fibre enriched meat-based functional foods. Trends Food Sci. Technol. 2020, 99, 323–336. [Google Scholar] [CrossRef]
- Chaves-López, C.; Serio, A.; Mazzarrino, G.; Martuscelli, M.; Scarpone, E.; Paparella, A. Control of household mycoflora in fermented sausages using phenolic fractions from olive mill wastewaters. Int. J. Food Microbiol. 2015, 207, 49–56. [Google Scholar] [CrossRef]
- Longato, E.; Meineri, G.; Peiretti, P.G.; Gai, F.; Viuda-Martos, M.; Pérez-Álvarez, J.Á.; Amarowicz, R.; Fernández-López, J. Effects of hazelnut skin addition on the cooking, antioxidant and sensory properties of chicken burgers. J. Food Sci. Technol. 2019, 57, 3329–3336. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Ahlawat, S.S.; Sharma, D.P.; Dabur, R.S. Novel trends in development of dietary fiber rich meat products—A critical review. J. Food Sci. Technol. 2015, 52, 633–647. [Google Scholar] [CrossRef]
- Lu, F.; Rodriguez-Garcia, J.; Van Damme, I.; Westwood, N.J.; Shaw, L.; Robinson, J.S.; Warren, G.; Chatzifragkou, A.; McQueen Mason, S.; Gomez, L.; et al. Valorisation strategies for cocoa pod husk and its fractions. Curr. Opin. Green Sustain. Chem. 2018, 14, 80–88. [Google Scholar] [CrossRef]
- International Cocoa Organization ICCO. Quarterly Bulletin of Cocoa Statistics; No. 4, Cocoa Year 2019/20; International Cocoa Organization ICCO: Abidjan, Côte d’Ivoire, 2020; Volume XLVI. [Google Scholar]
- Campos-Vega, R.; Nieto-Figueroa, K.H.; Oomah, B.D. Cocoa (Theobroma cacao L.) pod husk: Renewable source of bioactive compounds. Trends Food Sci. Technol. 2018, 81, 172–184. [Google Scholar] [CrossRef]
- Yapo, B.M.; Besson, V.; Koubala, B.B.; Koffi, K.L. Adding Value to Cacao Pod Husks as a Potential Antioxidant-Dietary Fiber Source. Am. J. Food Nutr. 2013, 1, 38–46. [Google Scholar] [CrossRef]
- Vriesmann, L.C.; de Mello Castanho Amboni, R.D.; De Oliveira Petkowicz, C.L. Cacao pod husks (Theobroma cacao L.): Composition and hot-water-soluble pectins. Ind. Crop. Prod. 2011, 34, 1173–1181. [Google Scholar] [CrossRef]
- Yusof, F.; Khanahmadi, S.; Amid, A.; Mahmod, S.S. Cocoa pod husk, a new source of hydrolase enzymes for preparation of cross-linked enzyme aggregate. Springerplus 2016, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, J.; Lucas-González, R.; Viuda-Martos, M.; Sayas-Barberá, E.; Navarro, C.; Haros, C.M.; Pérez-Álvarez, J.A. Chia (Salvia hispanica L.) products as ingredients for reformulating frankfurters: Effects on quality properties and shelf-life. Meat Sci. 2019, 156, 139–145. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000; ISBN 0935584544. [Google Scholar]
- International Organization for Standardization. Meat and Meat Products: Determination of Nitrite Content; International Organization for Standardization: Genevè, Switzerland, 1975. [Google Scholar]
- Rosmini, M.R.; Perlo, F.; Pérez-Alvarez, J.A.; Pagán-Moreno, M.J.; Gago-Gago, A.; López-Santoveña, F.; Aranda-Catalá, V. TBA test by an extractive method applied to “Paté. ” Meat Sci. 1996, 42, 103–110. [Google Scholar] [CrossRef]
- AMSA. Meat Color Measurement Guidelines; Association, A.M.S., Ed.; American Meat Science Association: Champaign, IL, USA, 2012; ISBN 8005172672. [Google Scholar]
- Sánchez-Zapata, E.; Fuentes-Zaragoza, E.; de Vera Navarro-Rodríguez, C.; Sayas, E.; Sendra, E.; Fernández-López, J.; Pérez-Alvarez, J.A. Effects of tuna pâté thickness and background on CIEL∗a∗b∗ color parameters and reflectance spectra. Food Control 2011, 22, 1226–1232. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Valadez-Carmona, L.; Mendiola, J.A.; Ibáñez, E.; Villamiel, M. Structural characterisation of pectin obtained from cacao pod husk. Comparison of conventional and subcritical water extraction. Carbohydr. Polym. 2019, 217, 69–78. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernández-López, J.; Sayas-Barbera, E.; Sendra, E.; Navarro, C.; Pérez-Álvarez, J.A. Citrus Co-Products as Technological Strategy to Reduce Residual Nitrite Content in Meat Products. J. Food Sci. 2009, 74, R93–R100. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, M.L.; Bosch-Bosch, N.; Garciá-Mata, M. Monitoring nitrite and nitrate residues in frankfurters during processing and storage. Meat Sci. 1996, 44, 65–73. [Google Scholar] [CrossRef]
- Merino, L.; Darnerud, P.; Toldrá, F.; Ilbäck, N.-G. Time-dependent depletion of nitrite in pork-beef and chicken meat products affects nitrite intake estimation. Food Addit. Contam. Part A 2016, 33, 186–192. [Google Scholar] [CrossRef]
- Martín León, V.; Luzardo, O.P. Evaluation of nitrate contents in regulated and non-regulated leafy vegetables of high consumption in the Canary Islands, Spain: Risk assessment. Food Chem. Toxicol. 2020, 146, 111812. [Google Scholar] [CrossRef]
- Lebrun, S.; Van Nieuwenhuysen, T.; Crèvecoeur, S.; Vanleyssem, R.; Thimister, J.; Denayer, S.; Jeuge, S.; Daube, G.; Clinquart, A.; Fremaux, B. Influence of reduced levels or suppression of sodium nitrite on the outgrowth and toxinogenesis of psychrotrophic Clostridium botulinum Group II type B in cooked ham. Int. J. Food Microbiol. 2020, 334, 108853. [Google Scholar] [CrossRef]
- Garrote, G.; Cruz, J.M.; Moure, A.; Domínguez, H.; Parajó, J.C. Antioxidant activity of byproducts from the hydrolytic processing of selected lignocellulosic materials. Trends Food Sci. Technol. 2004, 15, 191–200. [Google Scholar] [CrossRef]
- Sheard, P.R.; Enser, M.; Wood, J.D.; Nute, G.R.; Gill, B.P.; Richardson, R.I. Shelf life and quality of pork and pork products with raised n-3 PUFA. Meat Sci. 2000, 55, 213–221. [Google Scholar] [CrossRef]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Hernández, B.; Sáenz, C.; Alberdi, C.; Diñeiro, J.M. CIELAB color coordinates versus relative proportions of myoglobin redox forms in the description of fresh meat appearance. J. Food Sci. Technol. 2016, 53, 4159–4167. [Google Scholar] [CrossRef] [PubMed]
- Shimokomaki, M.; Youssef Youssef, E.; Terra, N. Curing. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Finglas, P., Toldra, F., Eds.; Academic Press: Cambridge, MA, USA, 2003; pp. 1702–18708. ISBN 978-0-12-227055-0. [Google Scholar]
- Hernández Salueña, B.; Sáenz Gamasa, C.; Diñeiro Rubial, J.M.; Alberdi Odriozola, C. CIELAB color paths during meat shelf life. Meat Sci. 2019, 157, 107889. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sánchez, A.M.; Sánchez-Zapata, E.; Viuda-Martos, M.; Sendra, E.; Sayas-Barberá, E.; Fernández-López, J.; Pérez-Álvarez, J.Á.; Chaves-López, C. Influencia de la adición de sorbato sobre los cocientes de reflectancia R650/R570, R560/R500 y R630/R580 en productos cárnicos crudo-curados. Opt. Pura y Apl. 2010, 43, 185–191. [Google Scholar]
- Strange, E.D.; Benedict, R.C.; Gugger, R.E.; Metzger, V.G.; Swift, C.E. Simplified Methodology for Measuring Meat Color. J. Food Sci. 1974, 39, 988–992. [Google Scholar] [CrossRef]
- Sánchez-Zapata, E.; Zunino, V.; Pérez-Alvarez, J.A.; Fernández-López, J. Effect of tiger nut fibre addition on the quality and safety of a dry-cured pork sausage (“Chorizo”) during the dry-curing process. Meat Sci. 2013, 95, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.G.; Cadena, R.S.; Walter, E.H.M.; Mortazavian, A.M.; Granato, D.; Faria, J.A.F.; Bolini, H.M.A. Sensory analysis: Relevance for prebiotic, probiotic, and synbiotic product development. Compr. Rev. Food Sci. Food Saf. 2010, 9, 358–373. [Google Scholar] [CrossRef]
- Biswas, A.K.; Kumar, V.; Bhosle, S.; Sahoo, J.; Chatli, M.K. Dietary fibers as functional ingredients in meat products and their role in human health. Int. J. Livest. Prod. 2011, 2, 45–54. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Khalid, M.; Younis, K. Interaction study of dietary fibers (pectin and cellulose) with meat proteins using bioinformatics analysis: An In-Silico study. LWT 2020, 119, 108889. [Google Scholar] [CrossRef]
- Martínez, J.A.; Melgosa, M.; Pérez, M.M.; Hita, E.; Negueruela, A.I. Note. Visual and Instrumental Color Evaluation in Red Wines. Food Sci. Technol. Int. 2001, 7, 439–444. [Google Scholar] [CrossRef]
- Cook, S.L.; Woods, S.; Methven, L.; Parker, J.K.; Khutoryanskiy, V. Mucoadhesive polysaccharides modulate sodium retention, release and taste perception. Food Chem. 2018, 240, 482–489. [Google Scholar] [CrossRef]
- Sun, C.; Zhou, X.; Hu, Z.; Lu, W.; Zhao, Y.; Fang, Y. Food and salt structure design for salt reducing. Innov. Food Sci. Emerg. Technol. 2020, 67, 102570. [Google Scholar] [CrossRef]
- Pintado, T.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Strategies for incorporation of chia (Salvia hispanica L.) in frankfurters as a health-promoting ingredient. Meat Sci. 2016, 114, 75–84. [Google Scholar] [CrossRef] [PubMed]

| Treatment | CPHF (%) | PS (%) |
|---|---|---|
| Control | 0 | 3.0 |
| CPHF 1.5 * | 1.5 | 0 |
| CPHF 3.0 | 3.0 | 0 |
| CPHF-PS 1.5 | 1.5 | 1.5 |
| CPHF | Frankfurters | |||||
|---|---|---|---|---|---|---|
| Control | CPHF 1.5 | CPHF 3.0 | CPHF-PS 1.5 | Sig. | ||
| Protein (g/100 g) | 4.85 ± 0.01 | 12.82 ± 0.39 a | 13.76 ± 0.31 ab | 14.32 ± 0.61 b | 13.89 ± 0.19 b | * |
| Lipid (g/100 g) | 0.77 ± 0.15 | 24.43 ± 0.11 c | 20.72 ± 0.46 b | 16.09 ± 0.10 a | 20.01 ± 1.35 b | ** |
| Total dietary fiber (g/100 g) | 37.4 ± 1.0 | 0.06 ± 0.17 a | 0.49 ± 0.08 b | 0.96 ± 0.19 c | 0.52 ± 0.09 b | * |
| Carbohydrates (g/100 g) | 49.7 ± 1.0 | 6.4 ± 0.4 c | 4.8 ± 0.4 b | 5.8 ± 0.6 c | 3.1 ± 1.3 a | * |
| Moisture (g/100 g) | 1.08 ± 0.20 | 53.29 ± 0.41 a | 57.43 ± 0.06 b | 60.02 ± 0.21 c | 59.84 ± 0.21 c | ** |
| Ash (g/100 g) | 7.3 ± 0.1 | 2.91 ± 0.03 b | 2.75 ± 0.18 ab | 2.80 ± 0.01 b | 2.57 ± 0.09 a | * |
| pH | 5.47 ± 0.03 | 6.30 ± 0.03 b | 6.36 ± 0.04 b | 6.38 ± 0.06 b | 6.09 ± 0.06 a | ** |
| aw | 0.317 ± 0.015 | 0.941 ± 0.020 | 0.946 ± 0.010 | 0.949 ± 0.008 | 0.954 ± 0.009 | n.s. |
| Residual nitrite level (mg NaNO2)/kg | 47.51 ± 1.82 b | 41.18 ± 1.21 a | 47.70 ± 0.23 b | 36.46 ± 4.62 a | * | |
| TBA * (mg MDA/kg product) | 0.18 ± 0.03 a | 0.25 ± 0.04 b | 0.37 ± 0.09 c | 0.28 ± 0.07 bc | * | |
| L* | a* | b* | C* | hab | ΔE* | |
|---|---|---|---|---|---|---|
| Cocoa pod husk | ||||||
| Dry | 78.92 ± 1.24 | 4.02 ± 0.35 | 25.23 ± 0.46 | 25.55 ± 0.50 | 80.96 ± 0.59 | |
| Hydrated | 53.25 ± 0.68 | 4.56 ± 0.69 | 22.95 ± 1.63 | 23.40 ± 1.74 | 78.81 ± 0.87 | |
| Frankfurters | ||||||
| Control | 69.32 ± 0.69 | 6.42 ± 0.15 ab | 10.19 ± 0.16 a | 12.05 ± 0.17 a | 57.79 ± 0.58 a | - |
| CPHF 1.5 | 70.63 ± 0.83 | 6.23 ± 0.37 ab | 11.25 ± 0.18 b | 12.87 ± 0.25 b | 61.05 ± 1.45 b | 1.82 ± 0.59 ab |
| CPHF 3.0 | 69.25 ± 0.86 | 6.53 ± 0.19 b | 12.31 ± 0.41 c | 13.94 ± 0.39 c | 62.05 ± 0.99 b | 2.28 ± 0.35 a |
| CPHF-PS 1.5 | 69.74 ± 0.14 | 6.04 ± 0.08 a | 11.46 ± 0.15 b | 12.96 ± 0.17 b | 62.22 ± 0.08 b | 1.40 ± 0.08 b |
| Significance | n.s. | * | * | * | ** | * |
| Treatments | R630/R580 | R650/R570 | R560/R500 |
|---|---|---|---|
| Control | 1.53 ± 0.01 b | 1.64 ± 0.02 b | 0.94 ± 0.00 b |
| CPHF 1.5 | 1.47 ± 0.03 a | 1.58 ± 0.04 a | 0.98 ± 0.01 a |
| CPHF 3.0 | 1.48 ± 0.02 a | 1.61 ± 0.02 a | 1.00 ± 0.00 a |
| CPHF-PS 1.5 | 1.47 ± 0.01 a | 1.58 ± 0.01 a | 0.98 ± 0.00 a |
| Significance | * | * | * |
| Parameter | Control | CPHF 1.5 | CPHF 3.0 | CPHF-PS 1.5 | Sign. |
|---|---|---|---|---|---|
| Hardness (N) | 81.82 ± 8.29 a | 94.63 ± 15.07 b | 86.30 ± 8.15 ab | 85.60 ± 8.16 ab | ** |
| Adhesiveness | 0.20 ± 0.04 a | 1.07 ± 0.12 c | 0.72 ± 0.24 b | 0.62 ± 0.15 b | * |
| Springiness (mm) | 0.29 ± 0.02 b | 0.24 ± 0.02 a | 0.27 ± 0.01 b | 0.28 ± 0.02 b | * |
| Cohesiveness | 0.76 ± 0.01 b | 0.75 ± 0.04 b | 0.69 ± 0.11 a | 0.79 ± 0.04 b | * |
| Gumminess (N) | 61.83 ± 6.09 ab | 70.90 ± 8.07 b | 58.90 ± 4.94 a | 65.60 ± 3.54 b | * |
| Chewiness (N mm) | 17.86 ± 2.11 | 17.05 ± 2.90 | 15.98 ± 1.60 | 17.58 ± 1.81 | n.s. |
| Resilience | 0.42 ± 0.01 | 0.43 ± 0.03 | 0.37 ± 0.07 | 0.39 ± 0.08 | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Ospina, J.; Martuscelli, M.; Grande-Tovar, C.D.; Lucas-González, R.; Molina-Hernandez, J.B.; Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.Á.; Chaves-López, C. Cacao Pod Husk Flour as an Ingredient for Reformulating Frankfurters: Effects on Quality Properties. Foods 2021, 10, 1243. https://doi.org/10.3390/foods10061243
Delgado-Ospina J, Martuscelli M, Grande-Tovar CD, Lucas-González R, Molina-Hernandez JB, Viuda-Martos M, Fernández-López J, Pérez-Álvarez JÁ, Chaves-López C. Cacao Pod Husk Flour as an Ingredient for Reformulating Frankfurters: Effects on Quality Properties. Foods. 2021; 10(6):1243. https://doi.org/10.3390/foods10061243
Chicago/Turabian StyleDelgado-Ospina, Johannes, Maria Martuscelli, Carlos David Grande-Tovar, Raquel Lucas-González, Junior Bernardo Molina-Hernandez, Manuel Viuda-Martos, Juana Fernández-López, José Ángel Pérez-Álvarez, and Clemencia Chaves-López. 2021. "Cacao Pod Husk Flour as an Ingredient for Reformulating Frankfurters: Effects on Quality Properties" Foods 10, no. 6: 1243. https://doi.org/10.3390/foods10061243
APA StyleDelgado-Ospina, J., Martuscelli, M., Grande-Tovar, C. D., Lucas-González, R., Molina-Hernandez, J. B., Viuda-Martos, M., Fernández-López, J., Pérez-Álvarez, J. Á., & Chaves-López, C. (2021). Cacao Pod Husk Flour as an Ingredient for Reformulating Frankfurters: Effects on Quality Properties. Foods, 10(6), 1243. https://doi.org/10.3390/foods10061243












