Hydrogel Encapsulation of Lactobacillus casei by Block Charge Modified Pectin and Improved Gastric and Storage Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. PME Activity and Pectin Modification
2.2. Characterization of 35 mLMP
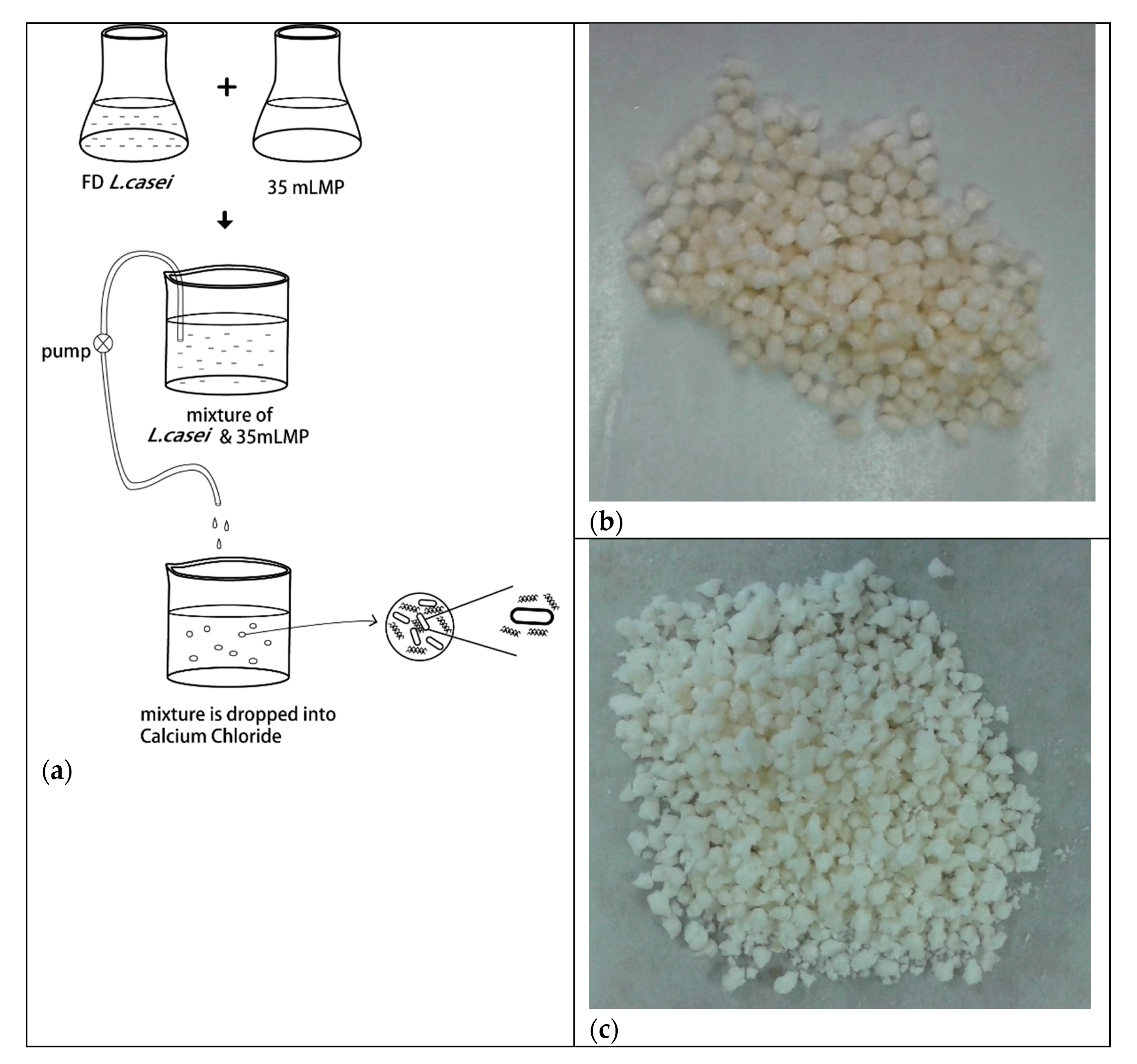
2.3. Encapsulation of L. casei W8
2.4. Stability and Release of Probiotic In Vitro
2.5. Statistical Analysis
3. Results
3.1. Charge Modification and Characterization of Pectin
3.2. Encapsulation and Simulated Gastrointestinal Release
3.3. Stability Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. BioMed Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ju, Z.; Zuo, T. Time for Food: The Impact of Diet on Gut Microbiota and Human Health. Nutrition 2018, 51, 80–85. [Google Scholar] [CrossRef]
- Ramos, P.E.; Cerqueira, M.A.; Teixeira, J.A.; Vicente, A.A. Physiological Protection of Probiotic Microcapsules by Coatings. Crit. Rev. Food Sci. Nutr. 2018, 58, 1864–1877. [Google Scholar] [CrossRef] [PubMed]
- Simões, L.D.S.; Madalena, D.; Pinheiro, A.C.; Teixeira, J.A.; Vicente, A.A.; Ramos, Ó.L. Micro-and Nano Bio-Based Delivery Systems for Food Applications: In Vitro Behavior. Adv. Colloid Interface Sci. 2017, 243, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of Probiotics for Gastrointestinal Delivery. J. Control. Release 2012, 162, 56–67. [Google Scholar] [CrossRef]
- Bajracharya, P.; Islam, M.A.; Jiang, T.; Kang, S.-K.; Choi, Y.-J.; Cho, C.-S. Effect of Microencapsulation of Lactobacillus Salivarus 29 into Alginate/Chitosan/Alginate Microcapsules on Viability and Cytokine Induction. J. Microencapsul. 2012, 29, 429–436. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Lević, S.; Petrović, T.; Ivanović, S.; Nedović, V.; Kourkoutas, Y. Encapsulation of Lactobacillus Casei Atcc 393 in Alginate Capsules for Probiotic Fermented Milk Production. LWT-Food Sci. Technol. 2019, 116, 9. [Google Scholar] [CrossRef]
- Ventura, I.; Jammal, J.; Bianco-Peled, H. Insights into the Nanostructure of Low-Methoxyl Pectin-Calcium Gels. Carbohydr. Polym. 2013, 97, 650–658. [Google Scholar] [CrossRef]
- Sriamornsak, P. Application of Pectin in Oral Drug Delivery. Expert Opin. Drug Deliv. 2011, 8, 1009–1023. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, F.; Han, D.; Zhao, Y.; Liu, Z.; Lei, H.; Song, Y.; Huang, X.; Li, X.; Ma, A.; et al. Preparation and Optimization of Soy Protein Isolate-High Methoxy Pectin Microcapsules Loaded with Lactobacillus Delbrueckii. Int. J. Food Sci. Technol. 2014, 49, 1287–1293. [Google Scholar] [CrossRef]
- Jantzen, M.; Göpel, A.; Beermann, C. Direct Spray Drying and Microencapsulation of Probiotic Lactobacillus Reuteri from Slurry Fermentation with Whey. J. Appl. Microbiol. 2013, 115, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Duongthingoc, D.; George, P.; Katopo, L.; Gorczyca, E.; Kasapis, S. Effect of Whey Protein Agglomeration on Spray Dried Microcapsules Containing Saccharomyces Boulardii. Food Chem. 2013, 141, 1782–1788. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Q.; Liu, C.-M.; Gong, J. Issues Deserve Attention in Encapsulating Probiotics: Critical Review of Existing Literature. Crit. Rev. Food Sci. Nutr. 2015, 57, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Kwiecień, I.; Kwiecień, M. Application of Polysaccharide-Based Hydrogels as Probiotic Delivery Systems. Gels 2018, 4, 47. [Google Scholar] [CrossRef]
- Maxwell, E.G.; Belshaw, N.J.; Waldron, K.W.; Morris, V.J. Pectin-an Emerging New Bioactive Food Polysaccharide. Trends Food Sci. Technol. 2012, 24, 64–73. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a Versatile Polysaccharide Present in Plant Cell Walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Wicker, L.; Kim, Y.; Kim, M.-J.; Thirkield, B.; Lin, Z.; Jung, J. Pectin as a Bioactive Polysaccharide-Extracting Tailored Function from Less. Food Hydrocoll. 2014, 42, 251–259. [Google Scholar] [CrossRef]
- Islamova, Z.I.; Ogai, D.K.; Abramenko, O.I.; Lim, A.L.; Abduazimov, B.B.; Malikova, M.K.; Rakhmanberdyeva, R.K.; Khushbaktova, Z.A.; Syrov, V.N. Comparative Assessment of the Prebiotic Activity of Some Pectin Polysaccharides. Pharm. Chem. J. 2017, 51, 288–291. [Google Scholar] [CrossRef]
- Sandoval-Castilla, O.; Lobato-Calleros, C.; García-Galindo, H.; Alvarez-Ramírez, J.; Vernon-Carter, E. Textural Properties of Alginate-Pectin Beads and Survivability of Entrapped Lb. Casei in Simulated Gastrointestinal Conditions and in Yoghurt. Food Res. Int. 2010, 43, 111–117. [Google Scholar] [CrossRef]
- Bourgeois, S.; Laham, A.; Besnard, M.; Andremont, A.; Fattal, E. In Vitro and in Vivo Evaluation of Pectin Beads for the Colon Delivery of Beta-Lactamases. J. Drug Target. 2005, 13, 277–284. [Google Scholar] [CrossRef]
- Oehme, A.; Valotis, A.; Krammer, G.; Zimmermann, I.; Schreier, P. Preparation and Characterization of Shellac-Coated Anthocyanin Pectin Beads as Dietary Colonic Delivery System. Mol. Nutr. Food Res. 2011, 55, S75–S85. [Google Scholar] [CrossRef] [PubMed]
- Celus, M.; Kyomugasho, C.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Influence of Pectin Structural Properties on Interactions with Divalent Cations and Its Associated Functionalities. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1576–1594. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.L.; Kent, L.M.; Ralet-Renard, M.-C.; Cameron, R.G.; Williams, M.A.K. A Tale of Two Pectins: Diverse Fine Structures Can Result from Identical Processive Pme Treatments on Similar High Dm Substrates. Carbohydr. Polym. 2017, 168, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.G.; Knox, J.P.; Mikkelsen, J.D. Pectin: New Insights into an Old Polymer Are Starting to Gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Willats, W.G.; Orfila, C.; Limberg, G.; Buchholt, H.C.; van Alebeek, G.J.W.; Voragen, A.G.; Knox, J.P. Modulation of the Degree and Pattern of Methyl-Esterification of Pectic Homogalacturonan in Plant Cell Walls-Implications for Pectin Methyl Esterase Action, Matrix Properties, and Cell Adhesion. J. Biol. Chem. 2001, 276, 19404–19413. [Google Scholar] [CrossRef]
- Hunter, J.; Thomas, A.; De Haseth, J.; Wicker, L. Valencia Orange Pectinmethylesterase Modified Pectin Characterized by Fourier Transform Infrared Spectroscopy, Charge Fractionation and Gelling. J. Food Qual. 2006, 29, 479–491. [Google Scholar] [CrossRef]
- Lee, H.; Rivner, J.; Urbauer, J.L.; Garti, N.; Wicker, L. De-Esterification Pattern of Valencia Orange Pectinmethylesterases and Characterization of Modified Pectins. J. Sci. Food Agric. 2008, 88, 2102–2110. [Google Scholar] [CrossRef]
- Cameron, R.G.; Kim, Y.; Galant, A.L.; Luzio, G.A.; Tzen, J.T. Pectin Homogalacturonans: Nanostructural Characterization of Methylesterified Domains. Food Hydrocoll. 2015, 47, 184–190. [Google Scholar] [CrossRef]
- Jung, J.; Arnold, R.D.; Wicker, L. Pectin and Charge Modified Pectin Hydrogel Beads as a Colon-Targeted Drug Delivery Carrier. Colloids Surf. B Biointerfaces 2013, 104, 116–121. [Google Scholar] [CrossRef]
- Ackerley, J.; Corredig, M.; Wicker, L. Clarification of Citrus Juice Is Influenced by Specific Activity of Thermolabile Pectinmethylesterase and Inactive Pme-Pectin Complexes. J. Food Sci. 2002, 67, 2529–2533. [Google Scholar] [CrossRef]
- Kim, Y.; Teng, Q.; Wicker, L. Action Pattern of Valencia Orange Pme De-Esterification of High Methoxyl Pectin and Characterization of Modified Pectins. Carbohydr. Res. 2005, 340, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Wicker, L. Valencia Pme Isozymes Create Charge Modified Pectins with Distinct Calcium Sensitivity and Rheological Properties. Food Hydrocoll. 2009, 23, 957–963. [Google Scholar] [CrossRef]
- Jung, J.; Wicker, L. Laccase Mediated Conjugation of Heat Treated Beta-Lactoglobulin and Sugar Beet Pectin. Carbohydr. Polym. 2012, 89, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Shamekhi, F.; Shuhaimi, M.; Ariff, A.; Manap, Y.A. Cell Viability of Microencapsulated Bifidobacterium Animalis Subsp. Lactis under Freeze-Drying, Storage and Gastrointestinal Tract Simulation Conditions. Folia Microbiol. 2012, 58, 91–101. [Google Scholar] [CrossRef] [PubMed]
- De Castro-Cislaghi, F.P.; Silva, C.D.R.E.; Fritzen-Freire, C.B.; Lorenz, J.G.; Sant’Anna, E.S. Bifidobacterium Bb-12 Microencapsulated by Spray Drying with Whey: Survival under Simulated Gastrointestinal Conditions, Tolerance to Nacl, and Viability During Storage. J. Food Eng. 2012, 113, 186–193. [Google Scholar] [CrossRef]
- Hotchkiss, A.T.; Savary, B.J.; Cameron, R.G.; Chau, H.K.; Brouillette, J.; Luzio, G.A.; Fishman, M.L. Enzymatic Modification of Pectin to Increase Its Calcium Sensitivity While Preserving Its Molecular Weight. J. Agric. Food Chem. 2002, 50, 2931–2937. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Li, X.-Y.; Liu, B.-J.; Meng, X.-H. Microencapsulation of Lactobacillus Bulgaricus and Survival Assays under Simulated Gastrointestinal Conditions. J. Funct. Foods 2017, 29, 248–255. [Google Scholar] [CrossRef]
- Zdunek, A.; Pieczywek, P.M.; Cybulska, J. The Primary, Secondary, and Structures of Higher Levels of Pectin Polysaccharides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1101–1117. [Google Scholar] [CrossRef]
- Luca, L.; Oroian, M. Influence of Different Prebiotics on Viability of Lactobacillus Casei, Lactobacillus Plantarum and Lactobacillus Rhamnosus Encapsulated in Alginate Microcapsules. Foods 2021, 10, 710. [Google Scholar] [CrossRef]
- Xu, M.; Gagné-Bourque, F.; Dumont, M.-J.; Jabaji, S. Encapsulation of Lactobacillus Casei Atcc 393 Cells and Evaluation of Their Survival after Freeze-Drying, Storage and under Gastrointestinal Conditions. J. Food Eng. 2016, 168, 52–59. [Google Scholar] [CrossRef]
- Yan, W.; Jia, X.; Zhang, Q.; Chen, H.; Zhu, Q.; Yin, L. Interpenetrating Polymer Network Hydrogels of Soy Protein Isolate and Sugar Beet Pectin as a Potential Carrier for Probiotics. Food Hydrocoll. 2020, 113, 106453. [Google Scholar] [CrossRef]
- Dafe, A.; Etemadi, H.; Dilmaghani, A.; Mahdavinia, G.R. Investigation of Pectin/Starch Hydrogel as a Carrier for Oral Delivery of Probiotic Bacteria. Int. J. Biol. Macromol. 2017, 97, 536–543. [Google Scholar] [CrossRef]
- Bepeyeva, A.; De Barros, J.M.; Albadran, H.; Kakimov, A.K.; Kakimova, Z.K.; Charalampopoulos, D.; Khutoryanskiy, V.V. Encapsulation of Lactobacillus Casei into Calcium Pectinate-Chitosan Beads for Enteric Delivery. J. Food Sci. 2017, 82, 2954–2959. [Google Scholar] [CrossRef]
- Lee, S.; Kirkland, R.; Grunewald, Z.I.; Sun, Q.; Wicker, L.; De La Serre, C.B. Beneficial Effects of Non-Encapsulated or Encapsulated Probiotic Supplementation on Microbiota Composition, Intestinal Barrier Functions, Inflammatory Profiles, and Glucose Tolerance in High Fat Fed Rats. Nutrients 2019, 11, 1975. [Google Scholar] [CrossRef] [PubMed]
| Sample | ζ-Potential (mV) | Particle Size (nm) | Molecular Weight (kDa) | Polydispersity (Mw/Mn) | |
|---|---|---|---|---|---|
| Trial 1 1 | 35 mLMP 2 | −37 ± 1.5 | 585 ± 21 | 143 ± 2.0 | 1.7 ± 3.0 |
| 72 HMP control 3 | −27 ± 0.1 | 614 ± 14 | 177 ± 2.0 | 1.8 ± 2.9 | |
| Trial 2 | 35 mLMP 2 | −36 ± 0.8 | 546 ± 11 | 141 ± 2.2 | 1.7 ± 3.3 |
| 72 HMP control 3 | −31 ± 1.0 | 577 ± 11 | 171 ± 2.1 | 2.0 ± 3.3 |
| ORIGINAL | SGF 2 H | SBF 20 MIN | SIF 3 H | SGF-SBF-SIF |
|---|---|---|---|---|
| Lactobacillus casei W8 CONTROL | ||||
| 11.24 A ± 0.81 | 4.23 A ± 0.25 | 0.89 A ± 0.44 | 11.65 A ± 0.22 | 1.23 A ± 0.32 |
| WET Lactobacillus casei W8 BEADS | ||||
| 12.94 A ± 0.12 | 10.86 B ± 0.10 | 9.90 B ± 0.09 | 12.47 A ± 0.08 | 10.89 B ± 0.32 |
| Freeze-Dried Lactobacillus casei W8 Beads—No Skim Milk | ||||
| 7.81 B ± 0.24 | 7.30 C ± 0.01 | 7.12 C ± 0.89 | 7.37 B ± 0.69 | 6.94 C ± 0.96 |
| Freeze-Dried Lactobacillus casei W8 Beads—With Skim Milk | ||||
| 10.16 A ± 0.54 | 9.26 D ± 0.07 D | 9.78 B ± 0.32 | 12.00 A ± 0.24 | 9.03 D ± 0.82 |
| SAMPLE | 0 DAY | 7 DAYS | 14 DAYS | 28 DAYS |
|---|---|---|---|---|
| L. casei W8 | 12.91 B ± 0.009 | 2.32 D ± 0.025 | ND | ND |
| Skim Milk, L. casei W8 | 11.95 C ± 0.008 | 10.87 B ± 0.078 | 6.64 B ± 0.031 | ND |
| mLMP, L. casei W8 | 11.80 D ± 0018 | 4.32 C ± 0.006 | 3.11 C ± 0.026 | ND |
| Skim Milk, mLMP, L. casei W8 | 12.99 A ± 0.008 | 11.94 A ± 0.008 | 10.45 A ± 0.096 | 10.39 ± 0.057 |
| 4 °C | ||||
| Day | L. casei W8 | Skim milk, L. casei W8 | mLMP, L. casei W8 | Skim milk, mLMP, L. casei W8 |
| 0 | 11.92 A ± 0.002 | 11.94 A ± 0.008 | 7.82 A ± 0.005 | 12.98 A ± 0.010 |
| 7 | 11.81 A ± 0.004 | 11.92 A ± 0.003 | 7.48 C ± 0.032 | 12.89 A ± 0.011 |
| 14 | 11.50 B ± 0.015 | 10.68 B ± 0.010 | 7.33 C ± 0.028 | 11.82 B ± 0.704 |
| 28 | 11.48 B ± 0.013 | 9.18 C ± 0.722 | 7.63 B ± 0.020 | 11.52 B ± 0.028 |
| 42 | 10.98 C ± 0.111 | 8.21 D ± 0.058 | 7.61 B ± 0.008 | 10.48 C ± 0.017 |
| Room Temperature | ||||
| Day | L. casei W8 | Skim milk, L. casei W8 | mLMP, L. casei W8 | Skim milk, mLMP, L. casei W8 |
| 0 | 11.92 A ± 0.002 | 11.94 A ± 0.008 | 7.82 A ± 0.005 | 12.98 A ± 0.010 |
| 7 | 2.91 B ± 0.012 | 11.94 A ± 0.000 | 7.83 A ± 0.020 | 12.99 A ± 0.006 |
| 14 | 0.89 C ± 0.269 | 1.10 B ± 0.213 | 6.51 B ± 0.019 | 6.03 B ± 0.029 |
| 28 | ND | ND | 6.38 B ± 0.085 | 2.59 C ± 0.042 |
| 42 | ND | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Wicker, L. Hydrogel Encapsulation of Lactobacillus casei by Block Charge Modified Pectin and Improved Gastric and Storage Stability. Foods 2021, 10, 1337. https://doi.org/10.3390/foods10061337
Sun Q, Wicker L. Hydrogel Encapsulation of Lactobacillus casei by Block Charge Modified Pectin and Improved Gastric and Storage Stability. Foods. 2021; 10(6):1337. https://doi.org/10.3390/foods10061337
Chicago/Turabian StyleSun, Qingshen, and Louise Wicker. 2021. "Hydrogel Encapsulation of Lactobacillus casei by Block Charge Modified Pectin and Improved Gastric and Storage Stability" Foods 10, no. 6: 1337. https://doi.org/10.3390/foods10061337
APA StyleSun, Q., & Wicker, L. (2021). Hydrogel Encapsulation of Lactobacillus casei by Block Charge Modified Pectin and Improved Gastric and Storage Stability. Foods, 10(6), 1337. https://doi.org/10.3390/foods10061337






