Preservation of the Antioxidant Capacity of Resveratrol via Encapsulation in Niosomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Niosomes
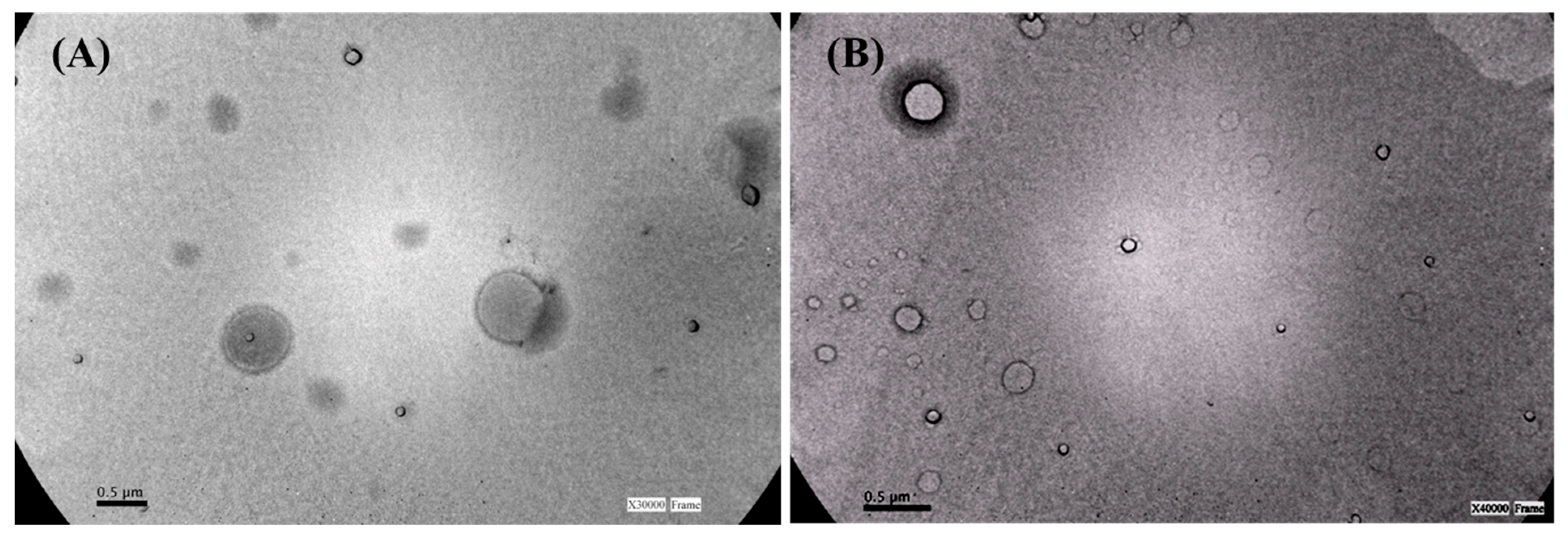
2.3. Niosomes Size and Morphology
2.4. Encapsulation Efficiency
2.5. In Vitro Release Experiments
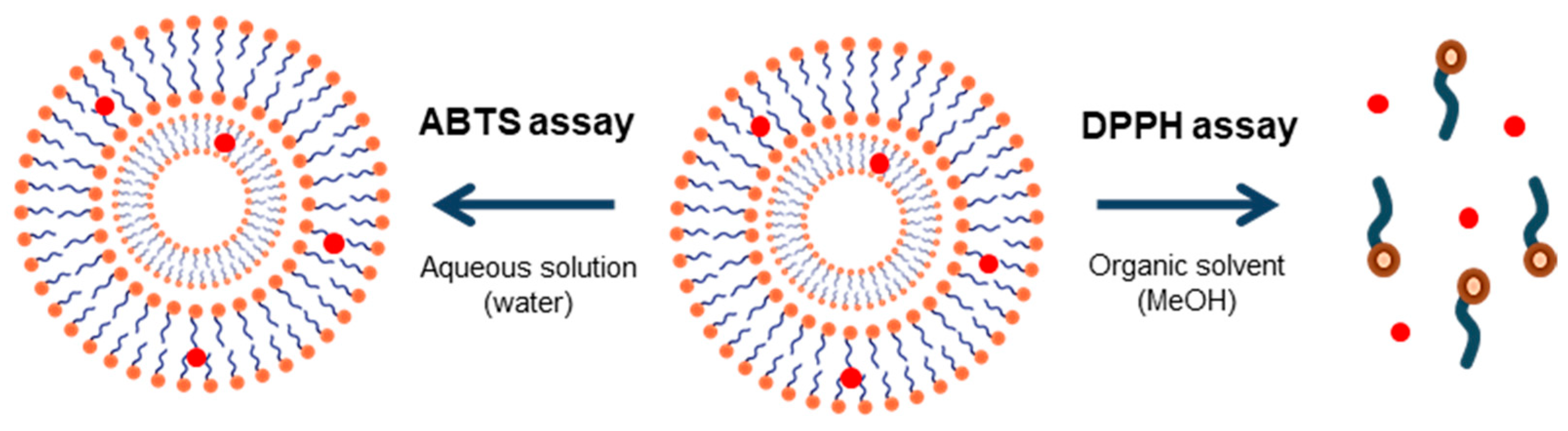
2.6. Antioxidant Activity
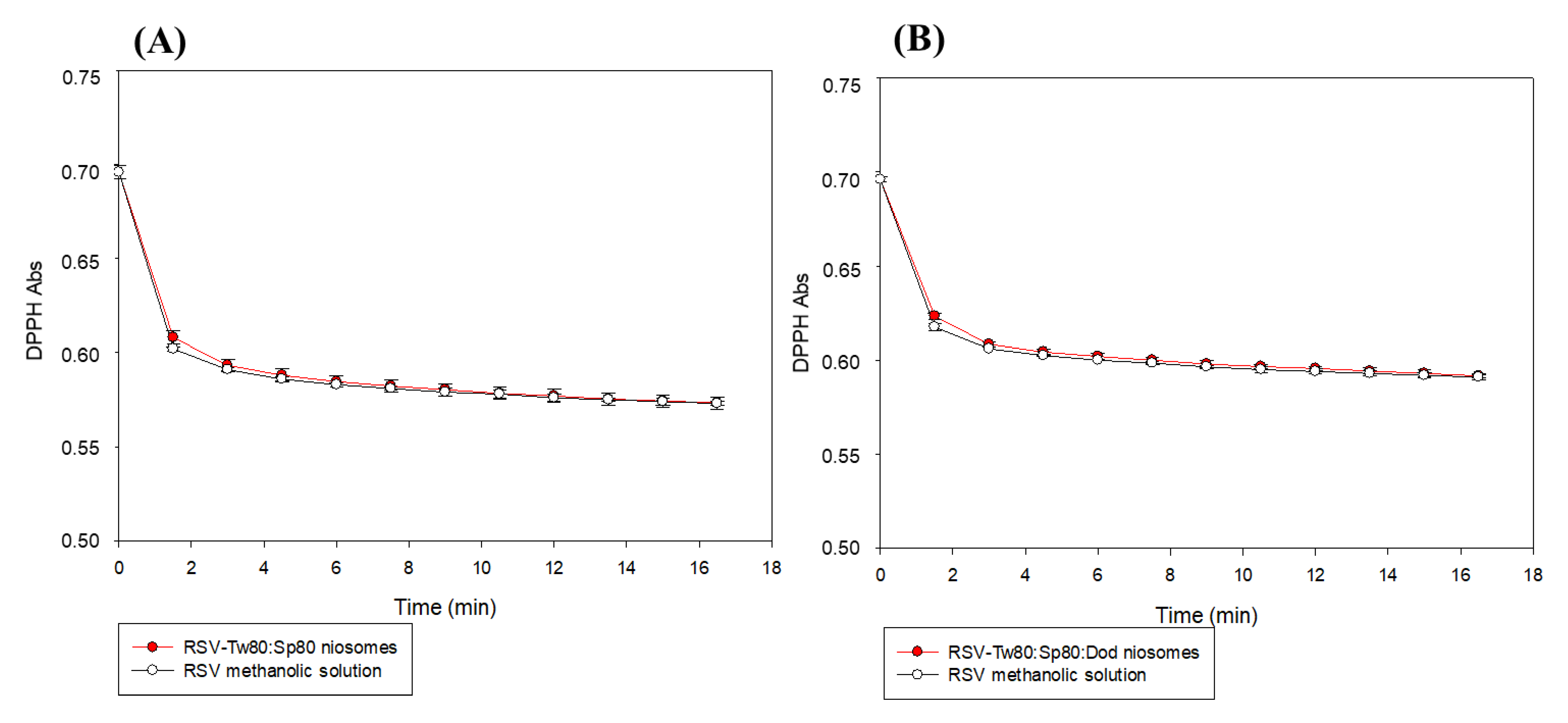
2.6.1. DPPH Test
2.6.2. ABTS Test
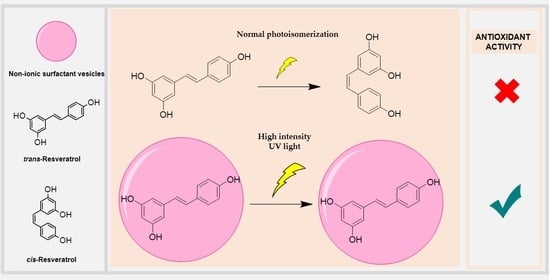
2.7. Stability of Trans-RSV against Photoisomerization Induced by UV Light
2.8. Statistical Analysis
3. Results and Discussion
3.1. Niosomes Size and Size Distribution
3.2. Encapsulation Efficiency
3.3. In Vitro Release of RSV
3.4. Antioxidant Activity
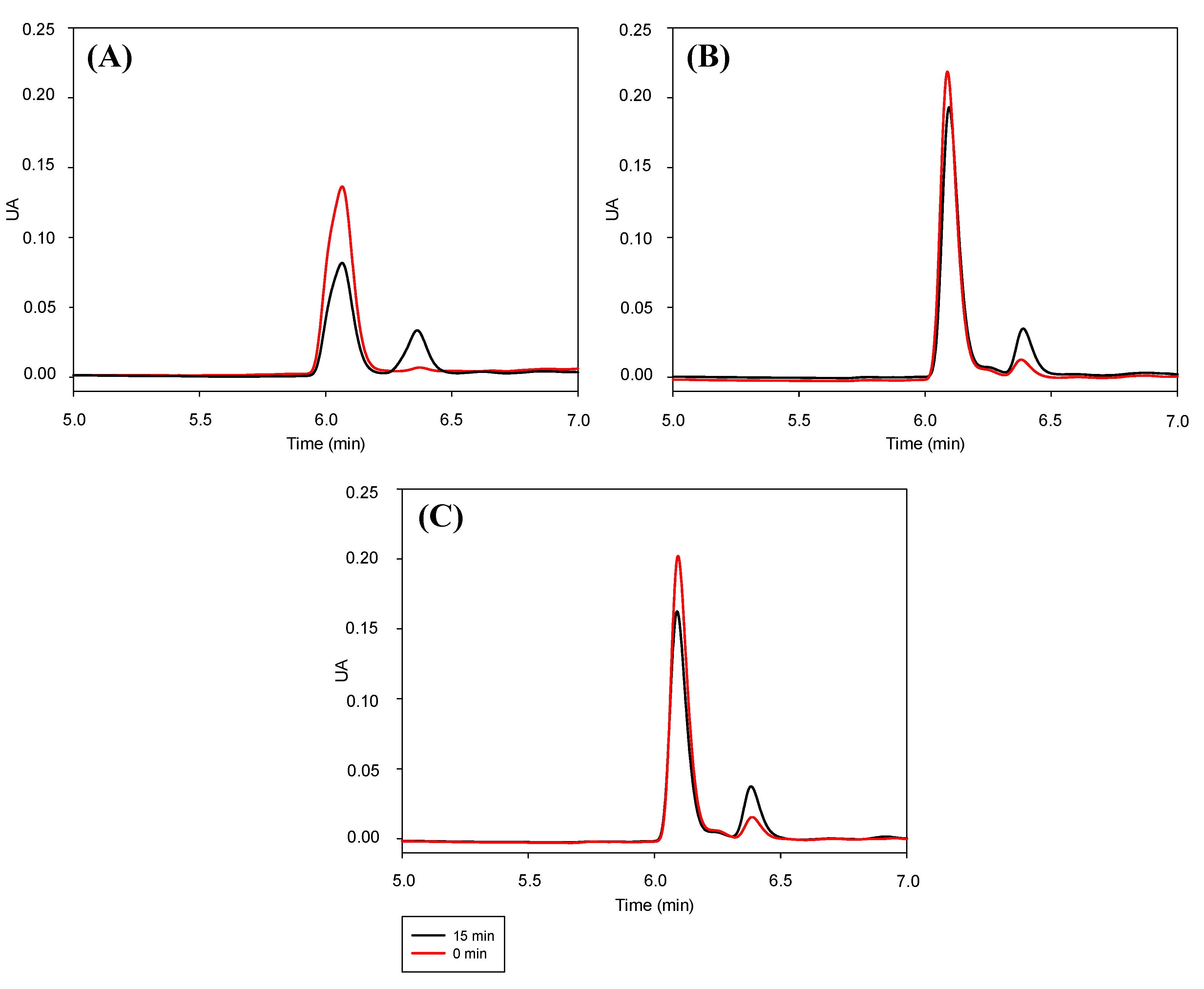
3.5. Stability of Trans-RSV against Photoisomerization Induced by UV Light
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Summerlin, N.; Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Popat, A. Resveratrol nanoformulations: Challenges and opportunities. Int. J. Pharm. 2015, 479, 282–290. [Google Scholar] [CrossRef]
- Mei, Y.Z.; Liu, R.X.; Wang, D.P.; Wang, X.; Dai, C.C. Biocatalysis and biotransformation of resveratrol in microorganisms. Biotechnol. Lett. 2015, 37, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Magdalena, A.; Pop, A.; Cimpeanu, C.; Turcus, V. Nanoencapsulation techniques for compounds and products with antioxidant and antimicrobial activity—A critical view. Eur. J. Med. Chem. 2018, 157, 1326–1345. [Google Scholar]
- Yang, X.; Xu, S.; Qian, Y.; Xiao, Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain Behav. Immun. 2017, 64, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An overview of its anti-cancer mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.; Lieben Louis, X.; Thandapilly, S.J.; Movahed, A.; Zieroth, S.; Netticadan, T. Potential of resveratrol in the treatment of heart failure. Life Sci. 2014, 95, 63–71. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; McClements, D.J. Nutraceutical delivery systems: Resveratrol encapsulation in grape seed oil nanoemulsions formed by spontaneous emulsification. Food Chem. 2015, 167, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Delmas, D.; Cornebise, C.; Courtaut, F.; Xiao, J.; Aires, V. New highlights of resveratrol: A review of properties against ocular diseases. Int. J. Mol. Sci. 2021, 22, 1295. [Google Scholar] [CrossRef]
- Arora, D.; Jaglan, S. Nanocarriers for resveratrol delivery. In Nanoscience in Food and Agriculture 5; Sustainable Agriculture Reviews; Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2017; Volume 26, pp. 123–138. [Google Scholar]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007, 224, 274–283. [Google Scholar] [CrossRef]
- Gleeson, J.P.; Ryan, S.M.; Brayden, D.J. Oral delivery strategies for nutraceuticals: Delivery vehicles and absorption enhancers. Trends Food Sci. Technol. 2016, 53, 90–101. [Google Scholar] [CrossRef]
- Montsko, G.; Nikfardjam, M.S.P.; Szabo, Z.; Boddi, K.; Lorand, T.; Ohmacht, R.; Mark, L. Determination of products derived from trans-resveratrol UV photoisomerisation by means of HPLC-APCI-MS. J. Photochem. Photobiol. A Chem. 2008, 196, 44–50. [Google Scholar] [CrossRef]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Tatarczak-Michalewska, M.; Blicharska, E. Characterization of the cis/trans isomerization of Resveratrol by High-Performance Liquid Chromatography. Anal. Lett. 2017, 50, 294–303. [Google Scholar] [CrossRef]
- Pinto, M.d.C.; García-Barrado, J.A.; Macías, P. Oxidation of resveratrol catalyzed by soybean lipoxygenase. J. Agric. Food Chem. 2003, 51, 1653–1657. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Zheng, L.; Cui, X.; Huang, H.; Geng, X.; Xie, X. Regioselective acylation of resveratrol catalyzed by lipase under microwave. Green Chem. Lett. Rev. 2018, 11, 312–317. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; McClements, D.J. Resveratrol encapsulation: Designing delivery systems to overcome solubility, stability and bioavailability issues. Trends Food Sci. Technol. 2014, 38, 88–103. [Google Scholar] [CrossRef]
- Machado, N.D.; Fernández, M.A.; Díaz, D.D. Recent strategies in Resveratrol delivery systems. ChemPlusChem 2019, 84, 951–973. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.F.; Zhou, J.; Hu, X.; Cong, Z.Q.; Liu, C.Y.; Pan, R.; Le, C.Q.; Liu, X.M.; Liao, Y.H. Improving oral bioavailability of resveratrol by a UDP-glucuronosyltransferase inhibitory excipient-based self-microemulsion. Eur. J. Pharm. Sci. 2018, 114, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.; Laca, A.; Rea, F.; Iglesias, O.; Rayner, M.; Gutiérrez, G. O/W emulsions stabilized by OSA-modified starch granules versus non-ionic surfactant: Stability, rheological behaviour and resveratrol encapsulation. J. Food Eng. 2018, 22, 207–217. [Google Scholar] [CrossRef]
- Wang, J.; Shi, A.; Agyei, D.; Wang, Q. Formulation of water-in-oil-in-water (W/O/W) emulsions containing trans-resveratrol. RSC Adv. 2017, 7, 35917–35927. [Google Scholar] [CrossRef]
- Khan, M.A.; Chen, L.; Liang, L. Improvement in storage stability and resveratrol retention by fabrication of hollow zein-chitosan composite particles. Food Hydrocoll. 2021, 113, 106477. [Google Scholar] [CrossRef]
- Naserifar, M.; Hosseinzadeh, H.; Abnous, K.; Mohammadi, M.; Taghdisig, S.M.; Ramezani, M.; Alibolandi, M. Oral delivery of folate-targeted resveratrol-loaded nanoparticles for inflammatory bowel disease therapy in rats. Life Sci. 2020, 262, 118555. [Google Scholar] [CrossRef]
- Negi, P.; Aggarwal, M.; Sharma, G.; Rathore, C.; Sharma, G.; Singh, B.; Katare, O.P. Niosome-based hydrogel of resveratrol for topical applications: An effective therapy for pain related disorder(s). Biomed. Pharmacother. 2017, 88, 480–487. [Google Scholar] [CrossRef]
- Qiu, C.; McClements, D.J.; Jin, Z.; Qin, Y.; Hu, Y.; Xu, X.; Wang, J. Resveratrol-loaded core-shell nanostructured delivery systems: Cyclodextrin-based metal-organic nanocapsules prepared by ionic gelation. Food Chem. 2020, 317, 126328. [Google Scholar] [CrossRef]
- Singh, S.K.; Makadia, V.; Sharma, S.; Rashid, M.; Shahi, S.; Mishra, P.R.; Wahajuddin, M.; Gayen, J.R. Preparation and in-vitro/in-vivo characterization of trans-resveratrol nanocrystals for oral administration. Drug Deliv. Transl. Res. 2017, 7, 395–407. [Google Scholar] [CrossRef]
- Emami, S.; Azadmard-Damirchi, S.; Peighambardoust, S.H.; Valizadeh, H.; Hesari, J. Liposomes as carrier vehicles for functional compounds in food sector. J. Exp. Nanosci. 2016, 11, 737–759. [Google Scholar] [CrossRef]
- Schlich, M.; Lai, F.; Pireddu, R.; Pini, E.; Ailuno, G.; Fadda, A.M.; Valenti, D.; Sinico, C. Resveratrol proniosomes as a convenient nanoingredient for functional food. Food Chem. 2020, 310, 125950. [Google Scholar] [CrossRef]
- Marianecci, C.; Petralito, S.; Rinaldi, F.; Hanieh, P.N.; Carafa, M. Some recent advances on liposomal and niosomal vesicular carriers. J. Drug Deliv. Sci. Technol. 2016, 32, 256–269. [Google Scholar] [CrossRef]
- Marianecci, C.; Di, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Esposito, S.; Carafa, M. Niosomes from 80s to present: The state of the art. Adv. Coll. Interface Sci. 2014, 205, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, G.N.; Parakh, S.R.; Devraj, R.; Apte, S.S.; Rao, B.R.; Rambhau, D. Release studies on niosomes containing fatty alcohols as bilayer stabilizers instead of cholesterol. J. Coll. Interface Sci. 2002, 251, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Uchegbu, I.F.; Florence, A.T. Non-ionic surfactant vesicles (niosomes): Physical and pharmaceutical chemistry. Adv. Colloid Interface Sci. 1995, 58, 1–55. [Google Scholar] [CrossRef]
- Pando, D.; Beltrán, M.; Gerone, I.; Matos, M.; Pazos, C. Resveratrol entrapped niosomes as yoghurt additive. Food Chem. 2015, 170, 281–287. [Google Scholar] [CrossRef]
- Davies, S.; Vidor Contri, R.; Stanisciaski Guterres, S.; Raffin Pohlmann, A.; Kulkamp Guerreiro, I. Simultaneous nanoencapsulation of lipoic acid and resveratrol with improved antioxidant properties for the skin. Colloids Surf. B 2020, 192, 111023. [Google Scholar] [CrossRef]
- Machado, N.D.; Fernández, M.A.; Häring, M.; Saldías, C.; Díaz, D.D. Niosomes encapsulated in biohydrogels for tunable delivery of phytoalexin resveratrol. RSC Adv. 2019, 9, 7601–7609. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Gao, L.; Zhang, Y.; Yi, J. Improved chemical stability and cellular antioxidant activity of resveratrol in zein nanoparticle with bovine serum albumin-caffeic acid conjugate. Food Chem. 2018, 261, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Cong, Z.; Liu, Z.; Ma, X.; Xu, M.; Tian, Y.; Zhang, Z.; Xu, B.; Zhang, J.; Tang, Z. Improvement of the solubility, photostability, antioxidant activity and UVB photoprotection of trans-resveratrol by essential oil based microemulsions for topical application. J. Drug Deliv. Sci. Technol. 2018, 48, 346–354. [Google Scholar] [CrossRef]
- Machado, N.D.; Silva, O.F.; de Rossi, R.H.; Fernández, M.A. Cyclodextrin modified niosomes to encapsulate hydrophilic compounds. RSC Adv. 2018, 8, 29909–29916. [Google Scholar] [CrossRef]
- Pando, D.; Gutiérrez, G.; Coca, J.; Pazos, C. Preparation and characterization of niosomes containing resveratrol. J. Food Eng. 2013, 117, 227–234. [Google Scholar] [CrossRef]
- Mehta, S.K.; Jindal, N. Formulation of Tyloxapol niosomes for encapsulation, stabilization and dissolution of anti-tubercular drugs. Colloids Surf. B 2013, 101, 434–441. [Google Scholar] [CrossRef]
- Tavano, L.; Muzzalupo, R.; Picci, N.; de Cindio, B. Co-encapsulation of antioxidants into niosomal carriers: Gastrointestinal release studies for nutraceutical applications. Colloids Surf. B 2014, 114, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Vankayala, J.S.; Battula, S.N.; Kandasamy, R.; Mariya, G.A.; Franklin, M.E.E.; Pushpadass, H.A.; Naik, L.N. Surfactants and fatty alcohol based novel nanovesicles for resveratrol: Process optimization, characterization and evaluation of functional properties in RAW 264.7 macrophage cells. J. Mol. Liq. 2018, 261, 387–396. [Google Scholar] [CrossRef]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Brenner, D.E. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: Safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef]
- López-Nicolás, J.M.; García-Carmona, F. Aggregation state and pKa values of (E)-Resveratrol as determined by fluorescence spectroscopy and UV-Visible absorption. J. Agric. Food Chem. 2008, 56, 7600–7605. [Google Scholar] [CrossRef] [PubMed]
- Smoliga, J.M.; Blanchard, O. Enhancing the delivery of resveratrol in humans: If low bioavailability is the problem, what is the solution? Molecules 2014, 19, 17154–17172. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Comp. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Feng, J.; Lin, C.; Wang, H.; Liu, S. Gemini dodecyl O-glucoside-based vesicles as nanocarriers for catechin laurate. J. Funct. Foods 2017, 32, 256–265. [Google Scholar] [CrossRef]
- Doppalapudi, S.; Mahira, S.; Khan, W. Development and in vitro assessment of psoralen and resveratrol co-loaded ultradeformable liposomes for the treatment of vitiligo. J. Photochem. Photob. B Biol. 2017, 174, 44–57. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Muntean, C. Ultraviolet irradiation of trans-resveratrol and HPLC determination of trans-resveratrol and cis-resveratrol in romanian red wines. J. Chromatogr. Sci. 2012, 50, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Koga, C.C.; Andrade, J.E.; Ferruzzi, M.G.; Lee, Y. Stability of trans-Resveratrol encapsulated in a protein matrix produced using spray drying to UV light stress and simulated gastro-intestinal digestion. J. Food Sci. 2016, 81, 292–300. [Google Scholar] [CrossRef]
- Liu, F.; Ma, D.; Luo, X.; Zhang, Z.; He, L.; Gao, Y.; McClements, D.J. Fabrication and characterization of protein-phenolic conjugate nanoparticles for co-delivery of curcumin and resveratrol. Food Hydrocoll. 2018, 79, 450–461. [Google Scholar] [CrossRef]

| Niosome Composition | Diameter (nm) | PDI | EE (%) | % RSV Released Gastric Conditions | % RSV Released Intestinal Conditions | Antioxidant Activity (%) | Chemical Stability (% Trans-Cis Conversion) | |
|---|---|---|---|---|---|---|---|---|
| DPPH | ABTS | |||||||
| Tw80:Sp80 | 267 ± 10 | 0.37 | - | - | - | - | - | - |
| RSV-Tw80:Sp80 | 469 ± 73 | 0.37 | 49 ± 9 | 86 ± 4 | 97 ± 3 | 100 | 75 ± 1 | 13 ± 4 |
| Tw80:Sp80:Dod | 312 ± 16 | 0.36 | - | - | - | - | - | - |
| RSV-Tw80:Sp80:Dod | 420 ± 40 | 0.34 | 57 ± 2 | 47 ± 3 | 94 ± 2 | 100 | 96 ± 2 | 19 ± 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, N.D.; Gutiérrez, G.; Matos, M.; Fernández, M.A. Preservation of the Antioxidant Capacity of Resveratrol via Encapsulation in Niosomes. Foods 2021, 10, 988. https://doi.org/10.3390/foods10050988
Machado ND, Gutiérrez G, Matos M, Fernández MA. Preservation of the Antioxidant Capacity of Resveratrol via Encapsulation in Niosomes. Foods. 2021; 10(5):988. https://doi.org/10.3390/foods10050988
Chicago/Turabian StyleMachado, Noelia D., Gemma Gutiérrez, María Matos, and Mariana A. Fernández. 2021. "Preservation of the Antioxidant Capacity of Resveratrol via Encapsulation in Niosomes" Foods 10, no. 5: 988. https://doi.org/10.3390/foods10050988
APA StyleMachado, N. D., Gutiérrez, G., Matos, M., & Fernández, M. A. (2021). Preservation of the Antioxidant Capacity of Resveratrol via Encapsulation in Niosomes. Foods, 10(5), 988. https://doi.org/10.3390/foods10050988