Encapsulation of Anthocyanins from Cornelian Cherry Fruits Using Heated or Non-Heated Soy Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Obtaining the Cornelian Cherry Powder (CCP)
2.3. Obtaining the Microcapsule Powders
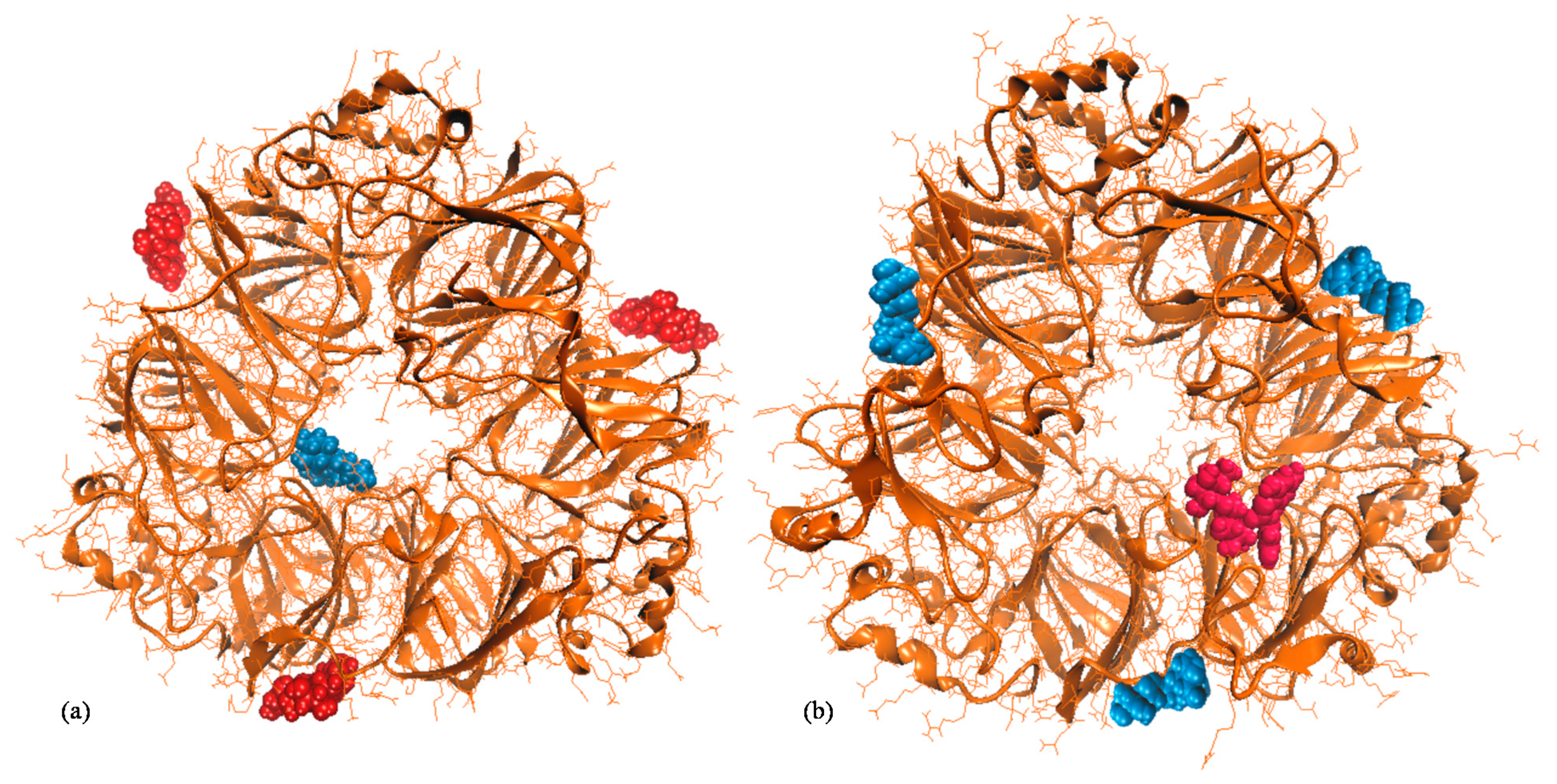
2.4. Modeling the Interactions between Main Soy Proteins and Anthocyanin Molecules
2.5. Characterization of the Powders
2.6. Confocal Microscopy Analysis
2.7. In Vitro Digestibility
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties of the Powders
3.2. RE and EE
3.3. Color Coordinates
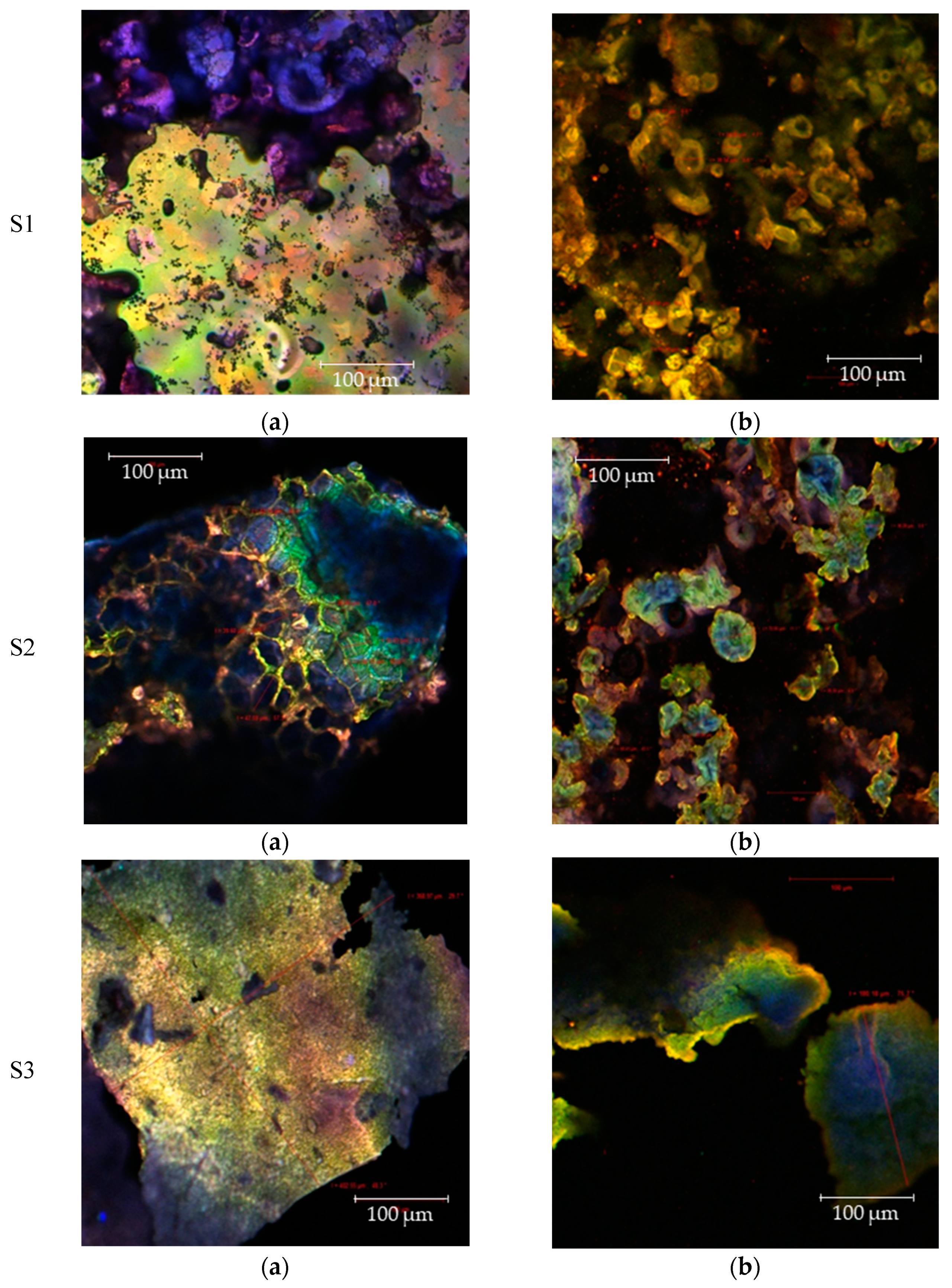
3.4. Microstructure
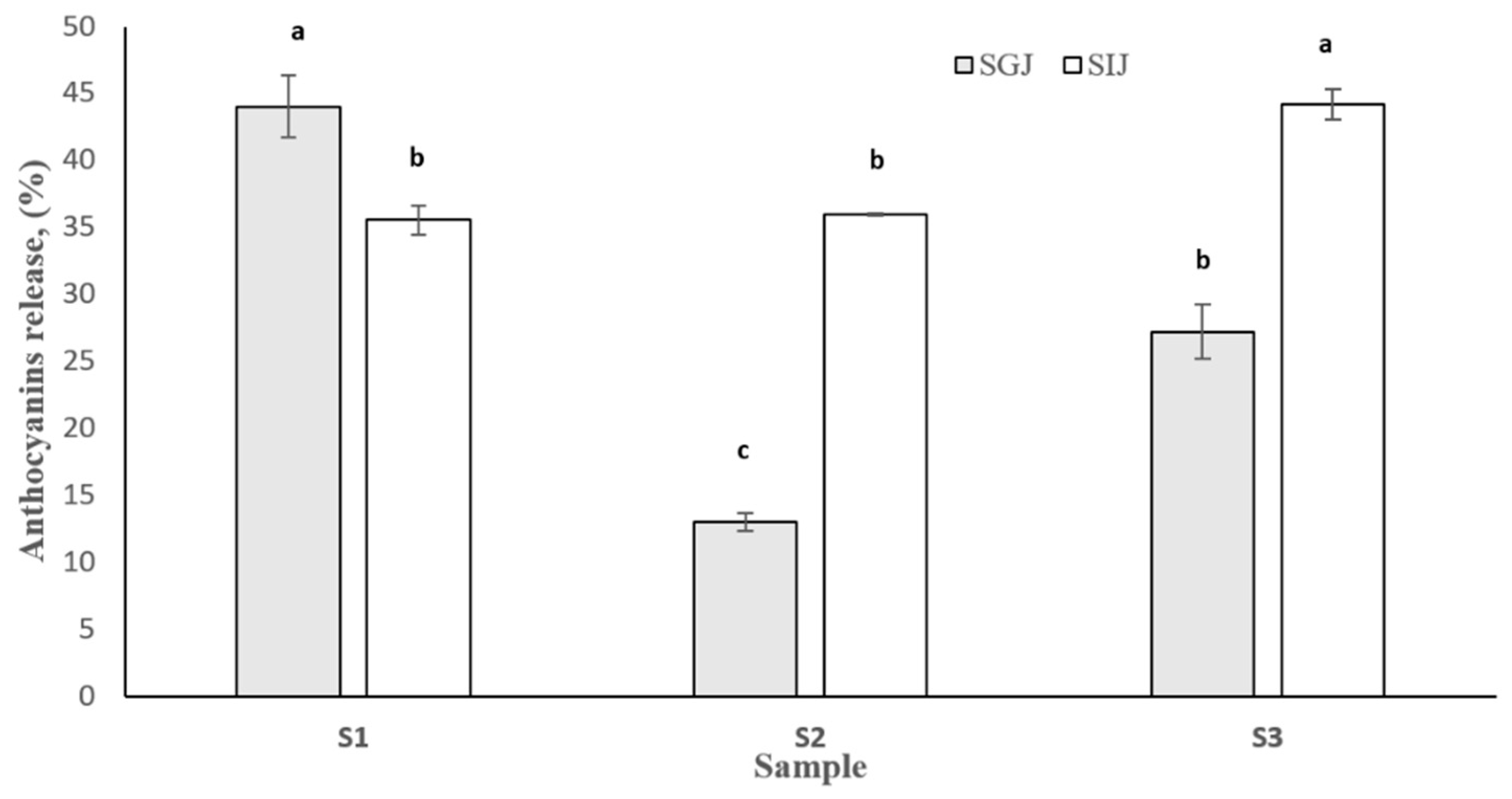
3.5. In Vitro Digestibility
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bayram, H.M.; Ozturkcan, S.A. Bioactive components and biological properties of cornelian cherry (Cornus mas L.): A comprehensive review. J. Funct. Foods 2020, 75, 104252. [Google Scholar] [CrossRef]
- Kazimierski, M.; Regula, J.; Molska, M. Cornelian cherry (Cornus mas L.)—Characteristics, nutritional and pro-health properties. Acta Sci. Pol. Technol. Aliment. 2019, 18, 5–12. [Google Scholar]
- Szczepaniak, O.M.; Kobus-Cisowska, J.; Kusek, W.; Przeor, M. Functional properties of Cornelian cherry (Cornus mas L.): A comprehensive review. Eur. Food Res. Technol. 2019, 245, 2071–2087. [Google Scholar] [CrossRef]
- Dumitrașcu, L.; Enachi, E.; Stănciuc, N.; Aprodu, I. Optimization of ultrasound assisted extraction of phenolic compounds from cornelian cherry fruits using response surface methodology. CYTA J. Food 2019, 17, 814–823. [Google Scholar] [CrossRef]
- Adamenko, K.; Kawa-Rygielska, J.; Kucharska, A.Z. Characteristics of cornelian cherry sour non-alcoholic beers brewed with the special yeast Saccharomycodes ludwigii. Food Chem. 2020, 312, 125968. [Google Scholar] [CrossRef] [PubMed]
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Prorok, P.; Piórecki, N. Physicochemical and antioxidative properties of Cornelian cherry beer. Food Chem. 2019, 281, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, I.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Bekatorou, A.; Plessas, S. Biocatalysis and agricultural biotechnology production of a potentially synbiotic fermented Cornelian cherry (Cornus mas L.) beverage using Lactobacillus paracasei K5 immobilized on wheat bran. Biocatal. Agric. Biotechnol. 2019, 17, 347–351. [Google Scholar] [CrossRef]
- Dinda, B.; Kyriakopoulos, A.M.; Dinda, S.; Zoumpourlis, V.; Thomaidis, N.S.; Velegraki, A.; Dinda, M. Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J. Ethnopharmacol. 2016, 193, 670–690. [Google Scholar] [CrossRef] [PubMed]
- Turker, A.U.; Yildirim, A.B.; Karakas, F.P. Antibacterial and antitumor activities of some wild fruits grown in Turkey. Biotechnol. Biotechnol. Equip. 2012, 26, 2765–2772. [Google Scholar] [CrossRef]
- Kyriakopoulos, A.M.; Dinda, B. Cornus mas (Linnaeus) novel devised medicinal preparations: Bactericidal effect against Staphylococcus aureus and Pseudomonas aeruginosa. Molecules 2015, 20, 11202–11218. [Google Scholar] [CrossRef]
- Milenković-Andjelković, A.S.; Andjelković, M.Z.; Radovanović, A.N.; Radovanović, B.C.; Nikolić, V. Phenol composition, DPPH radical scavenging and anti- microbial activity of cornelian cherry (Cornus mas) fruit and leaf extracts. Hem. Ind. 2015, 69, 331–337. [Google Scholar] [CrossRef]
- Antolak, H.; Czyzowska, A.; Sakač, M.; Mišan, A.; Đuragić, O.; Kregiel, D. Phenolic compounds contained in little-known wild fruits as antiadhesive agents against the beverage-spoiling bacteria Asaia spp. Molecules 2017, 22, 1256. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, A.Z.; Szumny, A.; Sokól-Letowska, A.; Piórecki, N.; Klymenko, S.V. Iridoids and anthocyanins in cornelian cherry (Cornus mas L.) cultivars. J. Food Compost. Anal. 2015, 40, 95–102. [Google Scholar] [CrossRef]
- Souza, P.; Cardinale, A.; Gurak, D.; Damasceno Ferreira Marczak, L. Maltodextrin, pectin and soy protein isolate as carrier agents in the encapsulation of anthocyanins-rich extract from jaboticaba pomace. Food Bioprod. Process. 2017, 102, 186–194. [Google Scholar] [CrossRef]
- Baysan, U.; Bast, Z.; Öznur, N.; Takma, D.K.; Ülkery, E.; Sahin-nadeem, H.; Koç, M. The effect of coating material combination and encapsulation method on propolis powder properties. Powder Technol. 2021, 384, 332–341. [Google Scholar] [CrossRef]
- Wu, Y.; Han, Y.; Tao, Y.; Li, D.; Xie, G.; Show, P.L.; Lee, S.Y. In vitro gastrointestinal digestion and fecal fermentation reveal the effect of different encapsulation materials on the release, degradation and modulation of gut microbiota of blueberry anthocyanin extract. Food Res. Int. 2020, 132, 109098. [Google Scholar] [CrossRef]
- Gani, A.; Jan, R.; Ashwar, B.; Ashraf, Z.; Shah, A.; Gani, A. Encapsulation of saffron and sea buckthorn bioactives: Its utilization for development of low glycemic baked product for growing diabetic population of the world. LWT Food Sci. Technol. 2021, 142, 111035. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation techniques for food bioactive components: A review. Food Bioproc. Tech. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Dumitrascu, L.; Stănciuc, N.; Borda, D.; Neagu, C.; Enachi, E.; Barbu, V.; Aprodu, I. Microencapsulation of bioactive compounds from cornelian cherry fruits using different biopolymers with soy proteins. Food Biosci. 2021, 41, 101032. [Google Scholar] [CrossRef]
- Maruyama, Y.; Maruyama, N.; Mikami, B.; Utsumi, S. Structure of the core region of the soybean β-conglycinin α′ subunit. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, N.; Adachi, M.; Takahashi, K.; Yagasaki, K.; Kohno, M.; Takenaka, Y.; Okuda, E.; Nakagawa, S.; Mikami, B.; Utsumi, S. Crystal structures of recombinant and native soybean β-conglycinin β homotrimers. Eur. J. Biochem. 2001, 268, 3595–3604. [Google Scholar] [CrossRef]
- Adachi, M.; Takenaka, Y.; Gidamis, A.B.; Mikami, B.; Utsumi, S. Crystal structure of soybean proglycinin A1aB1b homotrimer. J. Mol. Biol. 2001, 305, 291–305. [Google Scholar] [CrossRef]
- Dumitrascu, L.; Stănciuc, N.; Grigore-Gurgu, L.; Aprodu, I. Investigation on the interaction of heated soy proteins with anthocyanins from cornelian cherry fruits. Spectrochim. Acta A Mol. Biomol. 2020, 231, 118114. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Schneidman-Duhovny, D.; Inbar, Y.; Nussinov, R.; Wolfson, H.J. PatchDock and SymmDock: Servers for rigid and symmetric docking. Nucleic Acids Res. 2005, 33, W363–W367. [Google Scholar] [CrossRef]
- Saifullah, M.; Yusof, Y.A.; Chin, N.L.; Aziz, M.G. Physicochemical and flow properties of fruit powder and their effect on the dissolution of fast dissolving fruit powder tablets. Powder Technol. 2016, 301, 396–404. [Google Scholar] [CrossRef]
- Dag, D.; Kilercioglu, M.; Oztop, M.H. Physical and chemical characteristics of encapsulated goldenberry (Physalis peruviana L.) juice powder. LWT Food Sci. Technol. 2017, 83, 86–94. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, P.; Wang, J.; Wu, Y.; Han, Y.; Zhou, J. Combining various wall materials for encapsulation of blueberry anthocyanin extracts: Optimization by artificial neural network and genetic algorithm and a comprehensive analysis of anthocyanin powder properties. Powder Technol. 2017, 311, 77–87. [Google Scholar] [CrossRef]
- Jafari, S.M.; Ghalegi, M.; Dehnad, D. Influence of spray drying on water solubility index, apparent density, and anthocyanin content of pomegranate juice powder. Powder Technol. 2017, 311, 59–65. [Google Scholar] [CrossRef]
- Barbosa-Canovas, G.V.; Juliano, P. Physical and Chemical Properties of Food Powders. In Encapsulated and Powdered Foods; Onwulata, C., Ed.; Taylor & Francis, CRC Press: Boca Raton, FL, USA, 2005; pp. 39–74. [Google Scholar]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chavez, J.; Saenz, C. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. Int. J. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Tarone, A.G.; Baú, C.; Cazarin, B.; Roberto, M.; Junior, M. Anthocyanins: New techniques and challenges in microencapsulation. Food Res. Int. 2020, 133, 109092. [Google Scholar] [CrossRef] [PubMed]
- Tumbas Šaponjac, V.; Ćetković, G.; Čanadanović-Brunet, J.; Djilas, S.; Pajin, B.; Petrović, J.; Stajčić, S.; Vulić, J. Encapsulation of sour cherry pomace extract by freeze drying: Characterization and storage stability. Acta Chim. Slov. 2017, 64, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, C.; Gao, X.; Chen, Y.; Santhanam, R.K.; Wang, C.; Xu, L.; Chen, H. Interaction characterization of preheated soy protein isolate with cyanidin- 3- O -glucoside and their effects on the stability of black soybean seed coat anthocyanins extracts. Food Chem. 2019, 271, 266–273. [Google Scholar] [CrossRef]
- Tsali, A.; Goula, A.M. Valorization of grape pomace: Encapsulation and storage stability of its phenolic extract. Powder Technol. 2018, 340, 194–207. [Google Scholar] [CrossRef]

| Sample | BD (kg/m3) | TD (kg/m3) | CI (%) | HR | aw |
|---|---|---|---|---|---|
| S1 | 213.9 ± 5.9 a | 284.7 ± 2.5 a | 24.9 ± 1.9 b | 1.33 ± 0.03 b | 0.17 ± 0.003 a |
| S2 | 105.1 ± 5.2 b | 166.4 ± 3.5 b | 36.8 ± 2.7 a | 1.58 ± 0.06 a | 0.13 ± 0.001 b |
| S3 | 207.5 ± 4.9 a | 280.8 ± 3.3 a | 26.1 ± 1.7 b | 1.35 ± 0.03 b | 0.084 ± 0.007 c |
| Sample | Storage Time, Days | L* | a* | b* | Chroma | Hue Angle |
|---|---|---|---|---|---|---|
| S1 | 0 | 52.08 ± 0.01 aC | 21.49 ± 0.06 aA | −4.73 ± 0.02 bA | 22.00 ± 0.05 aA | 355.52 ± 0.03 aC |
| 30 | 51.44 ± 0.02 bB | 21.13 ± 0.04 bB | −4.39 ± 0.02 aB | 21.58 ± 0.04 aB | 355.25 ± 0.01 bB | |
| S2 | 0 | 53.17 ± 0.12 aB | 21.32 ± 0.11 bA | −6.79 ± 0.01 bC | 22.37 ± 0.11 aA | 356.96 ± 0.02 aA |
| 30 | 46.56 ± 0.49 bC | 29.56 ± 0.05 aA | −2.73 ± 0.07 aA | 29.68 ± 0.04 bA | 349.19 ± 0.3 bC | |
| S3 | 0 | 55.21 ± 0.07 aA | 19.43 ± 0.06 aB | −5.36 ± 0.02 bB | 20.15 ± 0.05 aB | 356.46 ± 0.02 aB |
| 30 | 55.11 ± 0.01 aA | 19.51 ± 0.08 aC | −4.72 ± 0.00 aC | 20.07 ± 0.08 aC | 355.95 ± 0.02 bA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitrașcu, L.; Stănciuc, N.; Aprodu, I. Encapsulation of Anthocyanins from Cornelian Cherry Fruits Using Heated or Non-Heated Soy Proteins. Foods 2021, 10, 1342. https://doi.org/10.3390/foods10061342
Dumitrașcu L, Stănciuc N, Aprodu I. Encapsulation of Anthocyanins from Cornelian Cherry Fruits Using Heated or Non-Heated Soy Proteins. Foods. 2021; 10(6):1342. https://doi.org/10.3390/foods10061342
Chicago/Turabian StyleDumitrașcu, Loredana, Nicoleta Stănciuc, and Iuliana Aprodu. 2021. "Encapsulation of Anthocyanins from Cornelian Cherry Fruits Using Heated or Non-Heated Soy Proteins" Foods 10, no. 6: 1342. https://doi.org/10.3390/foods10061342
APA StyleDumitrașcu, L., Stănciuc, N., & Aprodu, I. (2021). Encapsulation of Anthocyanins from Cornelian Cherry Fruits Using Heated or Non-Heated Soy Proteins. Foods, 10(6), 1342. https://doi.org/10.3390/foods10061342








