Thermal Inactivation Kinetics of Kudzu (Pueraria lobata) Polyphenol Oxidase and the Influence of Food Constituents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PPO Extraction
2.3. PPO Purification
2.4. Constituent Determination
2.5. PPO Activity Assay
2.6. Sample Preparation
2.7. Thermal Processing
2.8. Fluorescence Spectra Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Purification and Characterization of PPO from Kudzu
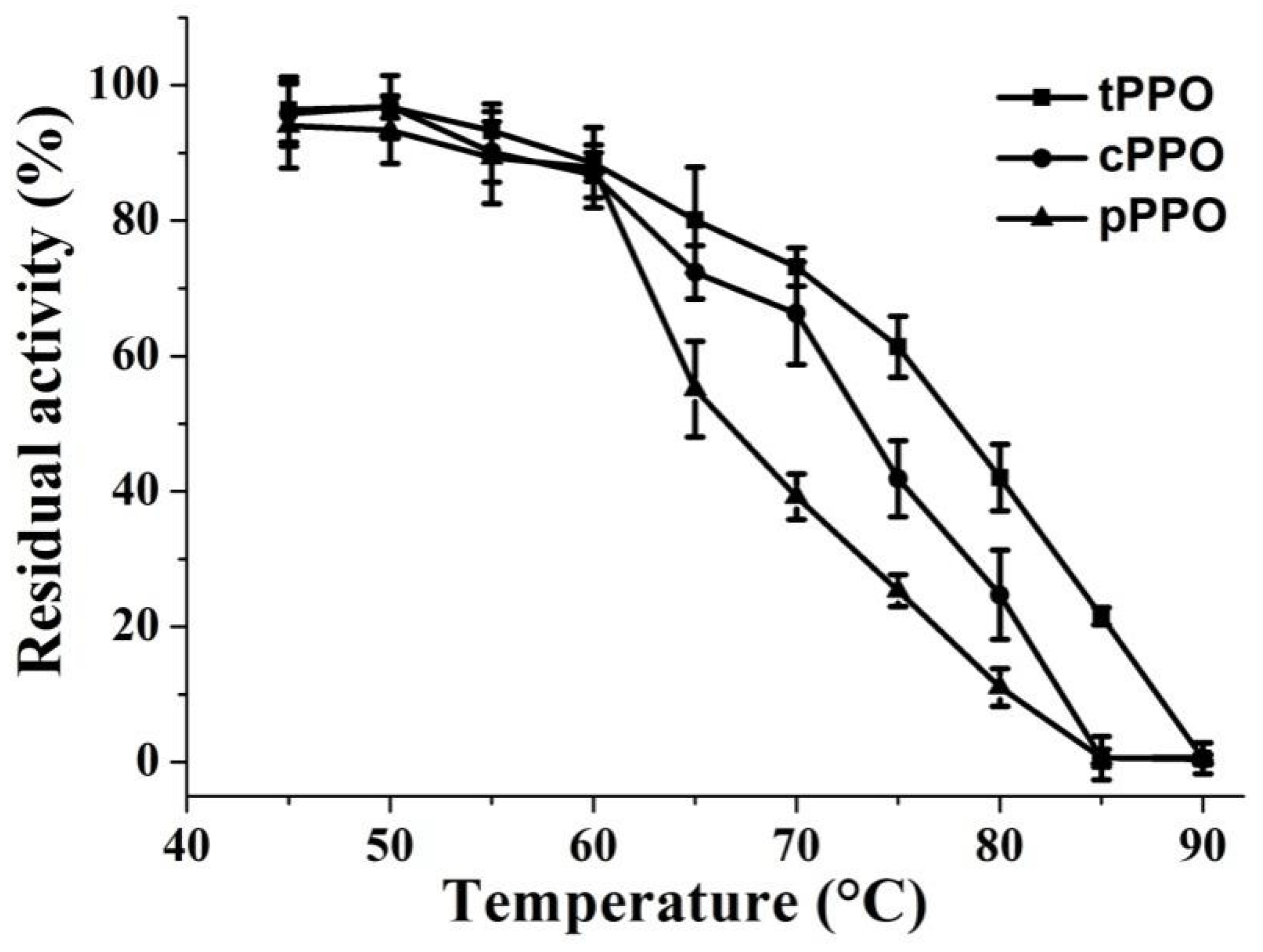
3.2. Thermal Stability of PPO
3.3. Thermal Inactivation Kinetics and Thermodynamics of PPO
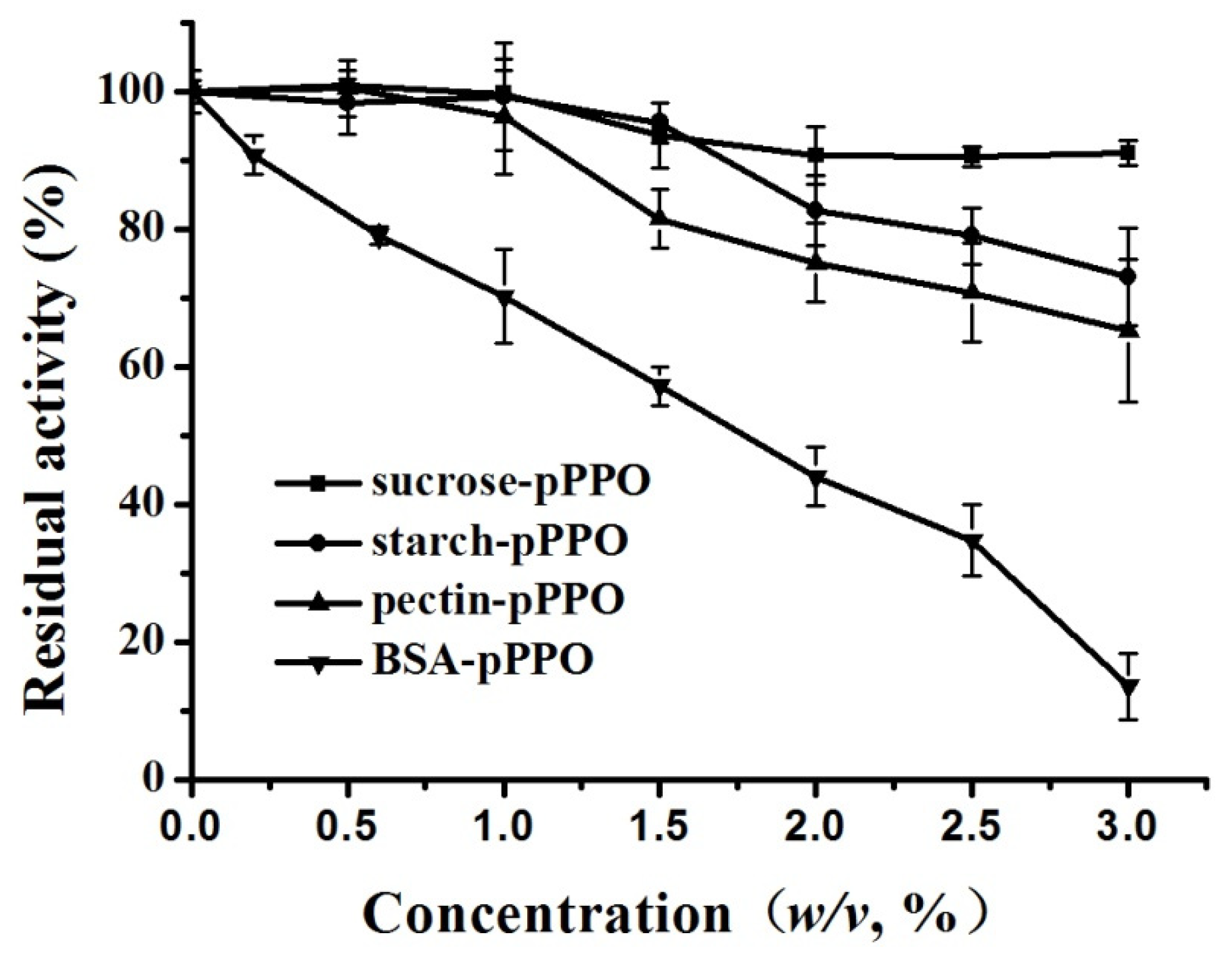
3.4. Effect of Food Constituents on the Activity of PPO
3.5. Effect of Food Constituents on the Thermal Inactivation Kinetics and Thermodynamics of PPO
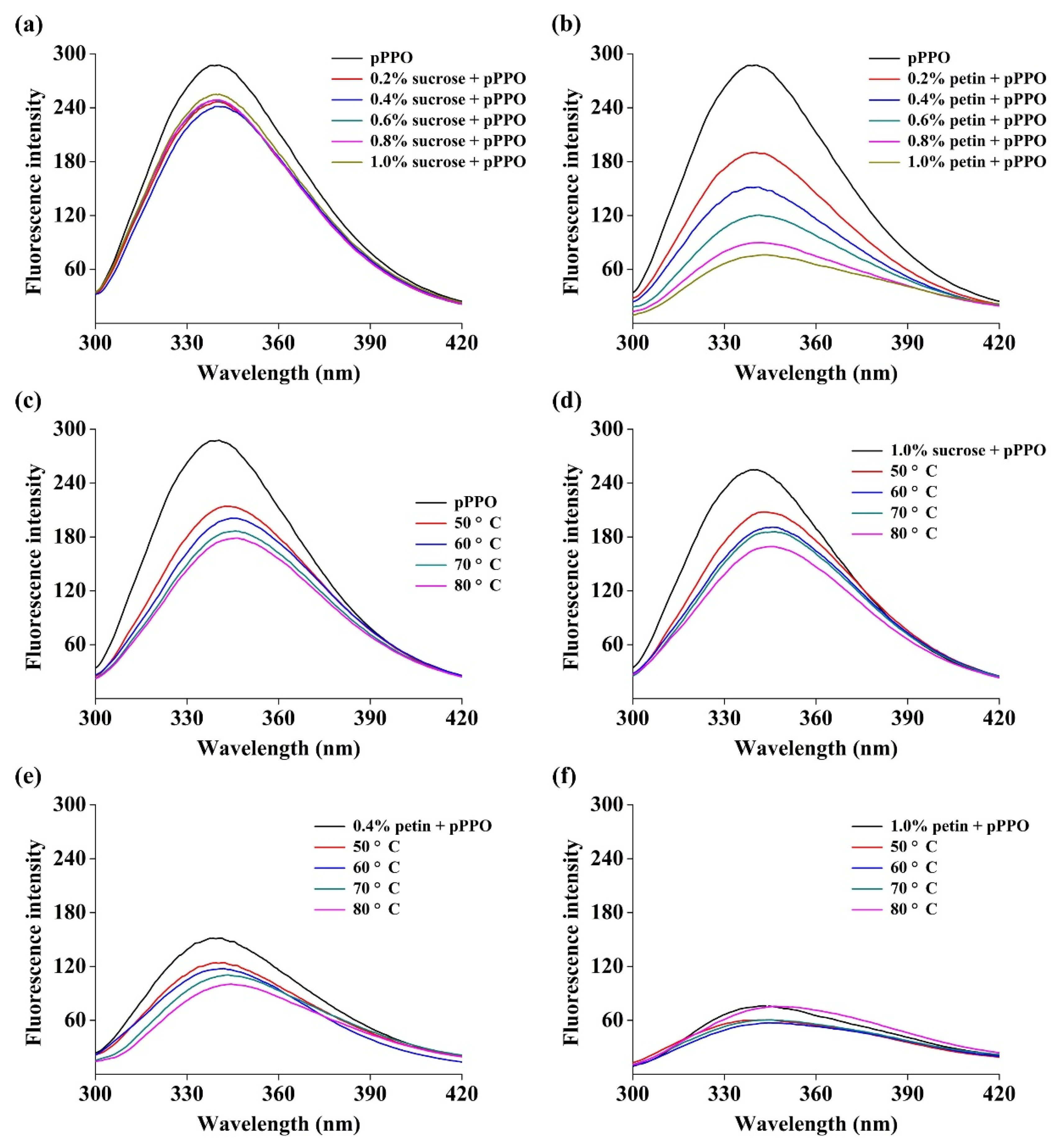
3.6. Effect of Food Constituents on Tertiary Structure of PPO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Q.; Zhang, H.; Xue, D. Enhancement of antioxidant activity of radix puerariae and red yeast rice by mixed fermentation with monascus purpureus. Food Chem. 2017, 226, 89–94. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Z.; Peng, N.; Zhou, J.; Yong, X.; Yuan, H.; Zheng, T. Optimization of ionic liquids-based microwave-assisted hydrolysis of puerarin and daidzein derivatives from radix puerariae lobatae extract. Food Chem. 2018, 256, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, Y.C.; Lee, D.Y. Applications of pueraria lobata in treating diabetics and reducing alcohol drinking. Chin. Herb. Med. 2019, 11, 141–149. [Google Scholar] [CrossRef]
- Hung, P.V.; Morita, N. Chemical compositions, fine structure and physicochemical properties of kudzu (Pueraria lobata) starches from different regions. Food Chem. 2007, 105, 749–755. [Google Scholar] [CrossRef]
- Xu, L.; Shi, W.; Cai, C.; Zhong, W.; Tu, K. Rapid and nondestructive detection of multiple adulterants in kudzu starch by near infrared (NIR) spectroscopy and chemometrics. LWT-Food Sci. Technol. 2015, 61, 590–595. [Google Scholar] [CrossRef]
- Mayer, A.M. Polyphenol oxidases in plants and fungi: Going places? a review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, M.; Dolan, K.D. Characterization of polyphenol oxidase from blueberry (Vaccinium corymbosum L.). Food Chem. 2017, 218, 216–220. [Google Scholar] [CrossRef]
- Gouzi, H.; Depagne, C.; Coradin, T. Kinetics and thermodynamics of the thermal inactivation of polyphenol oxidase in an aqueous extract from Agaricus bisporus. J. Agric. Food Chem. 2012, 60, 500–506. [Google Scholar] [CrossRef]
- Xiong, Z.; Liu, W.; Zhou, L.; Zou, L.; Chen, J. Mushroom (Agaricus bisporus) polyphenoloxidase inhibited by apigenin: Multi-spectroscopic analyses and computational docking simulation. Food Chem. 2016, 203, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, W.; Stockmann, R.; Terefe, N.S. Effect of citric acid and high pressure thermal processing on enzyme activity and related quality attributes of pear puree. Innov. Food Sci. Emerg. 2018, 45, 196–207. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, W.; Terefe, N.S. The inactivation kinetics of soluble and membrane-bound polyphenol oxidase in pear during thermal and high-pressure processing. Food Bioprocess Technol. 2018, 11, 1039–1049. [Google Scholar] [CrossRef]
- Niu, S.; Xu, Z.; Fang, Y.; Zhang, L.; Yang, Y.; Liao, X.; Hu, X. Comparative study on cloudy apple juice qualities from apple slices treated by high pressure carbon dioxide and mild heat. Innov. Food Sci. Emerg. 2010, 11, 91–97. [Google Scholar] [CrossRef]
- Han, Q.Y.; Liu, F.; Li, M.; Wang, K.L.; Ni, Y.Y. Comparison of biochemical properties of membrane-bound and soluble polyphenol oxidase from Granny Smith apple (Malus × domestica Borkh). Food Chem. 2019, 289, 657–663. [Google Scholar] [CrossRef]
- Gong, Z.; Li, D.; Liu, C.; Cheng, A.; Wang, W. Partial purification and characterization of polyphenol oxidase and peroxidase from chestnut kernel. LWT-Food Sci. Technol. 2015, 60, 1095–1099. [Google Scholar] [CrossRef]
- Chutintrasri, B.; Noomhorm, A. Thermal inactivation of polyphenoloxidase in pineapple puree. LWT-Food Sci. Technol. 2006, 39, 492–495. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, T.; Liu, W.; Zou, L.; Liu, C.; Terefe, N.S. Inhibitory effects of organic acids on polyphenol oxidase: From model systems to food systems. Crit. Rev. Food Sci. 2019, 6, 1–28. [Google Scholar] [CrossRef]
- Liu, L.; Cao, S.; Yang, H.; Qi, X. Pectin plays an important role on the kinetics properties of polyphenol oxidase from honeydew peach. Food Chem. 2015, 168, 14–20. [Google Scholar] [CrossRef]
- Ormus, S.; Oulahal, N.; Noel, C.; Degraeve, P.; Gharsallaoui, A. Effect of low methoxyl (LM) pectin complexation on the thermal and proteolytic inactivation of lysozyme: A kinetic study. Food Hydrocoll. 2015, 43, 812–818. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Ling, J.; Liao, X. Effects of high pressure processing on activity and structure of soluble acid invertase in mango pulp, crude extract, purified form and model systems. Food Chem. 2017, 231, 96–104. [Google Scholar] [CrossRef]
- Huang, N.; Cheng, X.; Hu, W.; Pan, S. Inactivation, aggregation, secondary and tertiary structural changes of germin-like protein in satsuma mandarine with high polyphenol oxidase activity induced by ultrasonic processing. Biophys. Chem. 2015, 197, 18–24. [Google Scholar] [CrossRef]
- Chakraborty, S.; Baier, D.; Knorr, D.; Mishra, H.N. High pressure inactivation of polygalacturonase, pectinmethylesterase and polyphenoloxidase in strawberry puree mixed with sugar. Food Bioprod. Process. 2015, 95, 281–291. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, J.H.; Wen, X.; Ni, Y.Y. Purification and structural analysis of membrane-bound polyphenol oxidase from fuji apple. Food Chem. 2015, 183, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Laemli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantization of microgram quantities of prote in utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Terefe, N.S.; Delon, A.; Buckow, R.; Versteeg, C. Blueberry polyphenol oxidase: Characterization and the kinetics of thermal and high pressure activation and inactivation. Food Chem. 2015, 188, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.C.; Cheng, L.H.; Bhat, R.; Rusul, G.; Easa, A.M. Composition, physicochemical properties and thermal inactivation kinetics of polyphenol oxidase and peroxidase from coconut (Cocos nucifera) water obtained from immature, mature and overly-mature coconut. Food Chem. 2014, 142, 121–128. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, T.; Liu, J.P.; Zou, L.Q.; Liu, C.M.; Liu, W. Unfolding and inhibition of polyphenoloxidase induced by acidic pH and mild thermal treatment. Food Bioprocess Tech. 2019, 12, 1907–1916. [Google Scholar] [CrossRef]
- Terefe, N.S.; Yang, Y.H.; Knoerzer, K.; Buckow, R.; Versteeg, C. High pressure and thermal inactivation kinetics of polyphenol oxidase and peroxidase in strawberry puree. Innov. Food Sci. Emerg. 2010, 11, 52–60. [Google Scholar] [CrossRef]
- Chourio, A.M.; Fabiola, S.F.; Zahid, M.; Martinez-Monteagudo, S.I.; Saldaña, M.D.A. Inactivation of peroxidase and polyphenoloxidase in coconut water using pressure-assisted thermal processing. Innov. Food Sci. Emerg. 2018, 49, 41–50. [Google Scholar] [CrossRef]
- Lopes, A.M.; Toralles, R.P.; Rombaldi, C.V. Thermal inactivation of polyphenoloxidase and peroxidase in jubileu clingstone peach and yeast isolated from its spoiled puree. Food Sci. Technol. 2014, 34, 150–156. [Google Scholar] [CrossRef]
- Ji, D.; Oey, I.; Agyei, D. Purification, characterization and thermal inactivation kinetics of β-galactosidase from Lactobacillus leichmannii 313. LWT-Food Sci. Technol. 2019, 116, 108545. [Google Scholar] [CrossRef]
- Isleroglu, H.; Turker, I. Thermal inactivation kinetics of microencapsulated microbial transglutaminase by ultrasonic spray-freeze drying. LWT-Food Sci. Technol. 2019, 101, 653–662. [Google Scholar] [CrossRef]
- Bayarri, M.; Oulahal, N.; Degraeve, P.; Gharsallaoui, A. Properties of lysozyme/low methoxyl (LM) pectin complexes for antimicrobial edible food packaging. J. Food Eng. 2014, 131, 18–25. [Google Scholar] [CrossRef]
- Chisari, M.; Barbagallo, R.N.; Spagna, G. Characterization of polyphenol oxidase and peroxidase and influence on browning of cold stored strawberry fruit. J. Agric. Food Chem. 2007, 55, 3469–3476. [Google Scholar] [CrossRef] [PubMed]
- Matsue, S.; Miyawaki, O. Influence of water activity and aqueous solvent ordering on enzyme kinetics of alcohol dehydrogenase, lysozyme, and β-galactosidase. Enzyme Microb. Technol. 2000, 26, 342–347. [Google Scholar] [CrossRef]
- Jones, O.G.; Decker, E.A.; Mcclements, D.J. Formation of biopolymer particles by thermal treatmentof β-lactoglobulin-pectin complexes. Food Hydrocoll. 2009, 23, 1312–1321. [Google Scholar] [CrossRef]
- Friedman, M.; Bautista, F.F. Inhibition of polyphenol oxidase by thiols in the absence and presence of potato tissue suspensions. J. Agric. Food Chem. 1995, 43, 69–76. [Google Scholar] [CrossRef]
- Kishore, V.; Gowda, S.; Krishna, S.; Sharma, K.; Rashmi, M.; Nishita, K.P. Bovine serum albumin a potential thermostabilizer: A study on α-amylase. J. Appl. Microbiol. 2014, 2, 37–41. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, J.; Hu, X.; Zhi, X.; Liao, X. Alterations in the activity and structure of pectin methylesterase treated by high pressure carbon dioxide. J. Agric. Food Chem. 2009, 57, 1890–1895. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, W.; Zou, L.; Xiong, Z.; Hu, X.; Chen, J. Aggregation and conformational change of mushroom (Agaricus bisporus) polyphenoloxidase subjected to thermal treatment. Food Chem. 2017, 214, 423–431. [Google Scholar] [CrossRef]
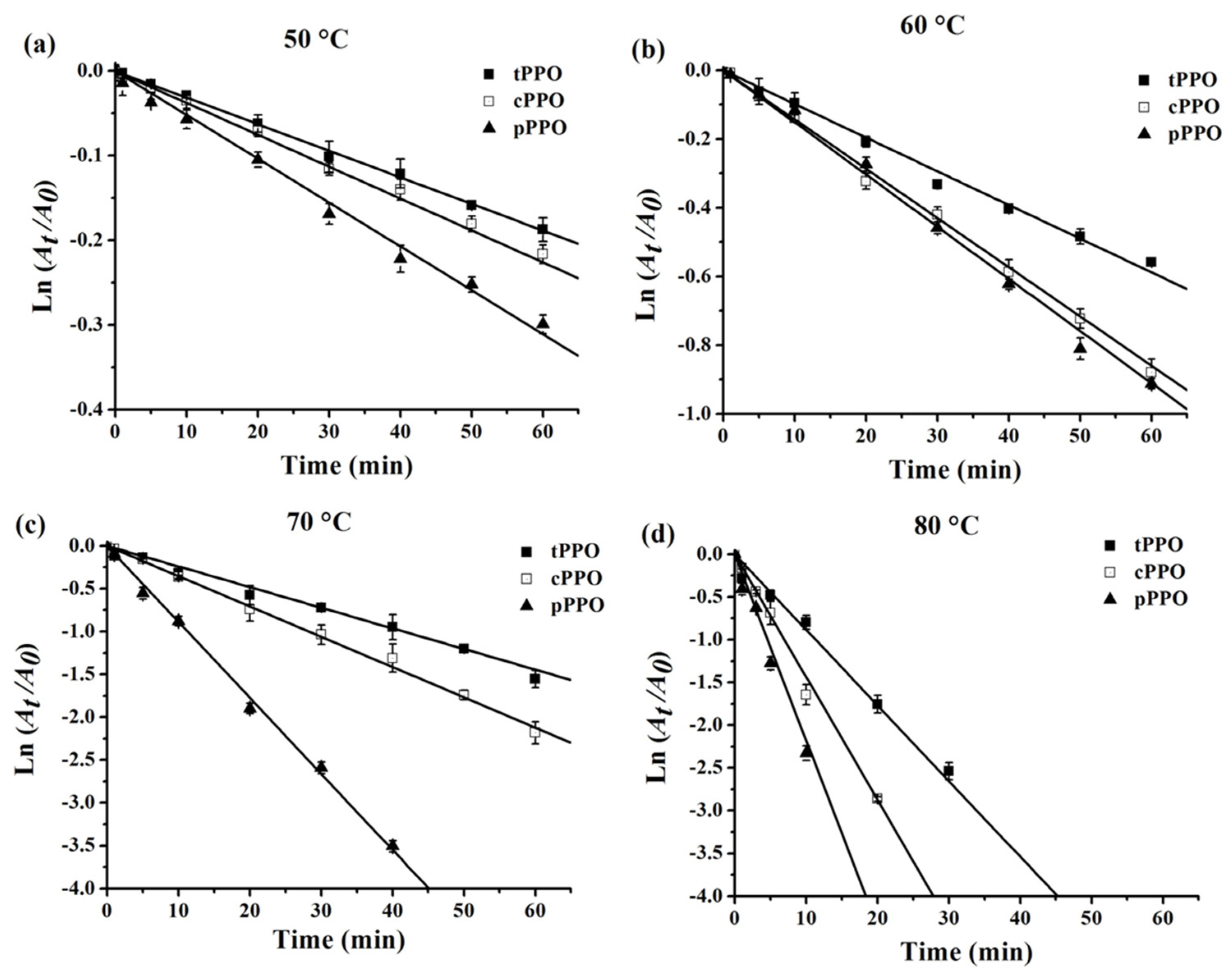

| Enzyme | Temperature (°C) | k (10−2 Min−1) | D min | t1/2 min | Ea (kJ mol−1) | Z (°C) | ∆H (kJ mol−1) | ∆G (kJ mol−1) | ∆S (J mol−1 K−1) |
|---|---|---|---|---|---|---|---|---|---|
| tPPO | 50 | 0.32 ± 0.02 | 729.18 ± 46.66 | 219.50 ± 14.05 | 102.68 ± 2.18 | 21.25 ± 0.46 | 99.99 ± 2.18 c | 83.83 ± 0.17 abc | 50.02 ± 6.28 g |
| 60 | 0.98 ± 0.02 | 235.02 ± 4.80 | 70.75 ± 1.44 | 99.91 ± 2.18 c | 83.37 ± 0.06 de | 49.63 ± 6.38 g | |||
| 70 | 2.50 ± 0.04 | 92.12 ± 1.34 | 27.73 ± 0.40 | 99.82 ± 2.18 c | 83.29 ± 0.04 def | 48.19 ± 6.25 g | |||
| 80 | 8.57 ± 0.34 | 26.90 ± 1.10 | 8.10 ± 0.33 | 99.74 ± 2.18 c | 82.18 ± 0.12 ij | 49.71 ± 6.32 g | |||
| cPPO | 50 | 0.36 ± 0.01 | 639.94 ± 17.78 | 192.64 ± 5.35 | 113.67 ± 0.86 | 19.21 ± 0.15 | 110.98 ± 0.86 b | 83.48 ± 0.07 cde | 77.88 ± 2.41 cde |
| 60 | 1.46 ± 0.05 | 158.20 ± 5.43 | 47.62 ± 1.64 | 110.90 ± 0.86 b | 82.28 ± 0.10 ij | 81.05 ± 2.67 cd | |||
| 70 | 3.50 ± 0.22 | 65.90 ± 4.15 | 19.84 ± 1.25 | 110.82 ± 0.86 b | 82.33 ± 0.18 ij | 80.66 ± 1.99 cd | |||
| 80 | 14.63 ± 0.96 | 15.78 ± 1.05 | 4.75 ± 0.31 | 110.73 ± 0.86 b | 80.62 ± 0.19 l | 85.28 ± 2.91 c | |||
| pPPO | 50 | 0.52 ± 0.02 | 440.54 ± 18.96 | 132.62 ±5.71 | 125.05 ± 0.47 | 17.47 ± 0.09 | 122.36 ± 0.47 a | 82.48 ± 0.12 hi | 112.94 ± 1.52 b |
| 60 | 1.54 ± 0.03 | 149.24 ± 3.08 | 44.93 ± 0.93 | 122.28 ± 0.47 a | 82.12 ± 0.06 ij | 113.73 ± 1.18 b | |||
| 70 | 8.88 ± 0.65 | 26.03 ± 1.85 | 7.84 ± 0.56 | 122.20 ± 0.47 a | 79.68 ± 0.21 m | 120.3 9± 0.79 ab | |||
| 80 | 23.67 ± 1.53 | 9.76 ± 0.63 | 2.94 ± 0.19 | 122.11 ± 0.47 a | 79.20 ± 0.19 n | 121.50 ± 1.83 ab | |||
| Pectin- pPPO | 50 | 0.27 ± 0.01 | 842.66 ± 17.58 | 253.67± 5.29 | 107.53 ± 1.78 | 20.29 ± 0.33 | 104.85 ± 1.78 bc | 84.22± 0.06 a | 58.41 ± 5.02 fg |
| 60 | 0.78 ± 0.04l | 297.02 ±15.93 | 89.41 ± 4.80 | 104.76 ± 1.78 bc | 84.02 ± 0.15 ab | 58.74 ± 5.15 fg | |||
| 70 | 2.35 ± 0.06 | 98.16 ± 2.31 | 29.55 ± 0.70 | 104.68 ± 1.78 bc | 83.47 ± 0.07 cde | 60.06 ± 5.17 efg | |||
| 80 | 8.32 ± 0.49 | 27.73 ±1.63 | 8.35 ± 0.49 | 104.60 ± 1.78 bc | 82.27 ± 0.17 ij | 63.22 ± 5.53 defg | |||
| BSA- pPPO | 50 | 0.34 ± 0.01 | 677.62 ± 19.94 | 203.98 ± 6.00 | 109.79 ± 2.60 | 19.90 ± 0.48 | 107.10 ± 2.60 b | 83.63 ± 0.08 bcd | 66.45 ± 7.16 defg |
| 60 | 0.89 ± 0.04 | 258.11 ± 11.82 | 77.70 ± 3.56 | 107.02 ± 2.60 b | 83.63 ± 0.13 bcd | 66.23 ± 7.29 defg | |||
| 70 | 3.63 ± 0.24 | 63.55 ±4.09 | 19.13 ±1.23 | 106.94 ± 2.60 b | 82.22 ± 0.19 ij | 69.97 ± 7.79 cdef | |||
| 80 | 10.12 ± 0.46 | 22.79 ± 1.06 | 6.86 ± 0.32 | 106.85 ± 2.60 b | 81.70 ± 0.14 k | 71.23 ± 7.72 cdef | |||
| Starch- pPPO | 50 | 0.44 ± 0.01 | 519.62 ± 13.74 | 156.42 ± 4.14 | 110.94 ± 1.23 | 19.64 ± 0.22 | 108.25 ± 1.23 b | 82.92 ± 0.07 fg | 71.73 ± 3.29 cdef |
| 60 | 1.05 ± 0.05 | 218.94 ± 10.49 | 65.91 ± 3.16 | 108.17 ± 1.23 b | 83.17 ± 0.13 ef | 70.77 ± 3.27 cdef | |||
| 70 | 3.84 ± 0.06 | 59.92 ± 0.94 | 18.04 ± 0.28 | 108.09 ± 1.23 b | 82.06 ± 0.04 jk | 73.69 ± 3.58 cdef | |||
| 80 | 14.34 ± 0.31 | 16.06 ± 0.35 | 4.84 ± 0.10 | 108.00 ± 1.23 b | 80.67 ± 0.06 l | 77.39 ± 3.53 cde | |||
| Sucrose- pPPO | 50 | 0.48 ± 0.01 | 483.11 ± 5.89 | 145.43 ± 1.77 | 129.34 ± 3.31 | 17.26 ± 0.25 | 126.65 ± 3.31 a | 82.72 ± 0.03 gh | 124.39 ± 9.45 ab |
| 60 | 1.35 ± 0.02 | 171.00 ± 1.95 | 51.48 ± 0.59 | 126.57 ± 3.31 a | 82.49 ± 0.03 hi | 124.81 ± 9.43 ab | |||
| 70 | 8.59 ± 0.24 | 26.83 ± 0.75 | 8.08 ± 0.23 | 126.49 ± 3.31 a | 79.77 ± 0.08 m | 132.29 ± 9.25 a | |||
| 80 | 21.97 ±1.26 | 10.50 ± 0.59 | 3.16 ± 0.18 | 126.40 ± 3.31 a | 79.42 ± 0.17 mn | 133.04 ± 9.16 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, J.; Liao, T.; Zhou, L.; Zou, L.; Liu, Y.; Zhang, L.; Liu, W. Thermal Inactivation Kinetics of Kudzu (Pueraria lobata) Polyphenol Oxidase and the Influence of Food Constituents. Foods 2021, 10, 1320. https://doi.org/10.3390/foods10061320
Liu J, Zhang J, Liao T, Zhou L, Zou L, Liu Y, Zhang L, Liu W. Thermal Inactivation Kinetics of Kudzu (Pueraria lobata) Polyphenol Oxidase and the Influence of Food Constituents. Foods. 2021; 10(6):1320. https://doi.org/10.3390/foods10061320
Chicago/Turabian StyleLiu, Junping, Jiayan Zhang, Tao Liao, Lei Zhou, Liqiang Zou, Yafei Liu, Li Zhang, and Wei Liu. 2021. "Thermal Inactivation Kinetics of Kudzu (Pueraria lobata) Polyphenol Oxidase and the Influence of Food Constituents" Foods 10, no. 6: 1320. https://doi.org/10.3390/foods10061320
APA StyleLiu, J., Zhang, J., Liao, T., Zhou, L., Zou, L., Liu, Y., Zhang, L., & Liu, W. (2021). Thermal Inactivation Kinetics of Kudzu (Pueraria lobata) Polyphenol Oxidase and the Influence of Food Constituents. Foods, 10(6), 1320. https://doi.org/10.3390/foods10061320





