Natural Food Polysaccharides Ameliorate Inflammatory Bowel Disease and Its Mechanisms
Abstract
1. Introduction
2. Polysaccharides and Their Impact on Inflammatory Bowel Diseases
2.1. Polysaccharides from Hericium Erinaceus
2.2. Sarcodon aspratus Polysaccharides
2.3. Dictyophora indusiata Polysaccharide
2.4. Flammuliana velutipes Polysaccharids
2.5. Pleurotus eryngii Polysaccharides
2.6. Gracilaria Polysaccharides
2.7. Blidingia minima Polysaccharides
2.8. Arctium lappa L. Polysaccharides
2.9. Morinda citrifolia L. Polysaccharides
2.10. Astragalus membranaceus Polysaccharides
2.11. Dendrobium officinale Polysaccharides
2.12. Lycium barbarum Polysaccharides
2.13. Codonopsis pilosula Polysaccharides
2.14. The Polysaccharides from Purple Sweet Potato
2.15. Others
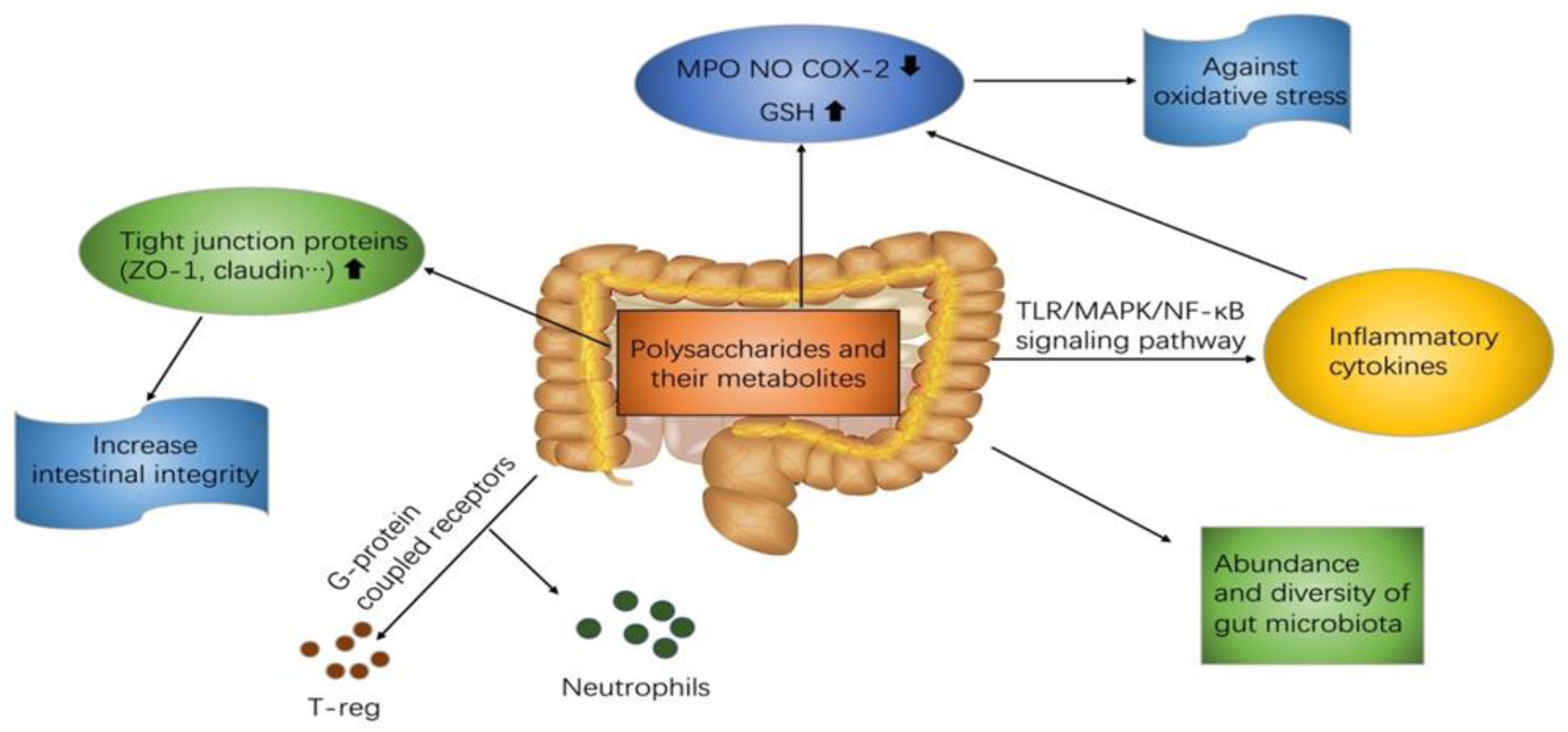
3. Mechanisms
3.1. TLRs-MAPK/NF-κB Mediated Signal Transduction Pathway
3.2. G-Protein-Coupled Receptors
3.3. Increase Intestinal Integrity via Up-regulating Tight Junction Proteins
3.4. Regulation of Oxidative Stress
4. Future Outlooks and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Popivanova, B.K.; Kitamura, K.; Wu, Y.; Kondo, T.; Kagaya, T.; Kaneko, S.; Oshima, M.; Fujii, C.; Mukaida, N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Investig. 2008, 118, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Elinav, E.; Thaiss, C.A.; Flavell, R.A. Analysis of microbiota alterations in inflammasome-deficient mice. Methods Mol. Biol. 2013, 1040, 185–194. [Google Scholar] [CrossRef]
- Mar, J.S.; Nagalingam, N.A.; Song, Y.; Onizawa, M.; Lee, J.W.; Lynch, S.V. Amelioration of DSS-induced murine colitis by VSL#3 supplementation is primarily associated with changes in ileal microbiota composition. Gut Microbes 2014, 5, 494–503. [Google Scholar] [CrossRef]
- Kaur, M.; Dalal, R.L.; Shaffer, S.; Schwartz, D.A.; Rubin, D.T. Inpatient Management of Inflammatory Bowel Disease-Related Complications. Clin. Gastroenterol. Hepatol. 2020, 18, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Chen, X.; Xu, R.; Dong, H.; Yang, F.; Wang, Y.; Zhang, Z.; Ju, J. Polysaccharides from natural resources exhibit great potential in the treatment of ulcerative colitis: A review. Carbohydr. Polym. 2021, 254, 117189. [Google Scholar] [CrossRef]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Chen, M.; Pei, Y.; Sun, P. In intro antioxidant activities of different sulfated polysaccharides from chlorophytan seaweeds Ulva fasciata. Int. J. Biol. Macromol. 2013, 59, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell. Longev. 2017, 2017, 1–18. [Google Scholar] [CrossRef]
- Wang, M.; Gao, Y.; Xu, D.; Gao, Q. A polysaccharide from cultured mycelium of Hericium erinaceus and its anti-chronic atrophic gastritis activity. Int. J. Biol. Macromol. 2015, 81, 656–661. [Google Scholar] [CrossRef]
- Shao, S.; Wang, D.; Zheng, W.; Li, X.; Zhang, H.; Zhao, D.; Wang, M. A unique polysaccharide from Hericium erinaceus mycelium ameliorates acetic acid-induced ulcerative colitis rats by modulating the composition of the gut microbiota, short chain fatty acids levels and GPR41/43 respectors. Int. Immunopharmacol. 2019, 71, 411–422. [Google Scholar] [CrossRef]
- Song, Q.; Wang, Y.; Huang, L.; Shen, M.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res. Int. 2021, 140, 109858. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Jiang, H.; Cai, C.; Li, G.; Hao, J.; Yu, G. Marine polysaccharides attenuate metabolic syndrome by fermentation products and altering gut microbiota: An overview. Carbohydr. Polym. 2018, 195, 601–612. [Google Scholar] [CrossRef]
- Pryde, S.E.; Duncan, S.H.; Hold, G.L.; Stewart, C.S.; Flint, H.J. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 2002, 217, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Geng, Y.; Du, Y.; Li, W.; Lu, Z.M.; Xu, H.Y.; Xu, G.H.; Shi, J.S.; Xu, Z.H. Polysaccharide of Hericium erinaceus attenuates colitis in C57BL/6 mice via regulation of oxidative stress, inflammation-related signaling pathways and modulating the composition of the gut microbiota. J. Nutr. Biochem. 2018, 57, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Y.R. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: Updated experimental and clinical evidence. Exp. Biol. Med. 2012, 237, 474–480. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yang, L.L.; Lee, T.J.F. Oroxylin A inhibition of lipopolysaccharide-induced iNOS and COX-2 gene expression via suppression of nuclear factor-κB activation. Biochem. Pharmacol. 2000, 59, 1445–1457. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Yan, C.; Zhang, C.; Pan, W.; Zhang, W.; Lu, Y.; Chen, L.; Chen, Y. Sarcodon aspratus polysaccharides ameliorated obesity-induced metabolic disorders and modulated gut microbiota dysbiosis in mice fed a high-fat diet. Food Funct. 2020, 11, 2588–2602. [Google Scholar] [CrossRef]
- Liao, W.; Luo, Z.; Liu, D.; Ning, Z.; Yang, J.; Ren, J. Structure characterization of a novel polysaccharide from Dictyophora indusiata and its macrophage immunomodulatory activities. J. Agric. Food Chem. 2015, 63, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, S.; Joseph, T.P.; Aliya, S.; Song, S.; Saleem, M.Z.; Nisar, M.A.; Wang, Y.; Meyiah, A.; Ma, Y.; Xin, Y. Attenuation of DSS induced colitis by Dictyophora indusiata polysaccharide (DIP) via modulation of gut microbiota and inflammatory related signaling pathways. J. Funct. Foods 2020, 64, 103641. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, X.; Yan, M.; Chen, X.; Kang, M.; Teng, L.; Wu, X.; Chen, J.; Deng, C. Protective effect and mechanism of polysaccharide from Dictyophora indusiata on dextran sodium sulfate-induced colitis in C57BL/6 mice. Int. J. Biol. Macromol. 2019, 140, 973–984. [Google Scholar] [CrossRef]
- Mills, C.D. Anatomy of a discovery: m1 and m2 macrophages. Front. Immunol. 2015, 6, 212. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, Y.; Shi, M.; Xu, X.; Zhao, Y.; Wu, X.; Zhang, Y. Gegen Qinlian decoction alleviates experimental colitis via suppressing TLR4/NF-κB signaling and enhancing antioxidant effect. Phytomedicine 2016, 23, 1012–1020. [Google Scholar] [CrossRef]
- Kanwal, S.; Joseph, T.P.; Owusu, L.; Xiaomeng, R.; Meiqi, L.; Yi, X. A Polysaccharide Isolated from Dictyophora indusiata Promotes Recovery from Antibiotic-Driven Intestinal Dysbiosis and Improves Gut Epithelial Barrier Function in a Mouse Model. Nutrients 2018, 10, 1003. [Google Scholar] [CrossRef]
- Antonopoulos, D.A.; Huse, S.M.; Morrison, H.G.; Schmidt, T.M.; Sogin, M.L.; Young, V.B. Reproducible community dynamics of the gastrointestinal microbiota following antibiotic perturbation. Infect. Immun. 2009, 77, 2367–2375. [Google Scholar] [CrossRef]
- Shao, P.; Chen, X.; Sun, P. Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassum horneri. Carbohydr. Polym. 2014, 105, 260–269. [Google Scholar] [CrossRef]
- Kronman, M.P.; Zaoutis, T.E.; Haynes, K.; Feng, R.; Coffin, S.E. Antibiotic exposure and IBD development among children: A population-based cohort study. Pediatrics 2012, 130, e794–e803. [Google Scholar] [CrossRef]
- Besednova, N.N.; Zaporozhets, T.S.; Kuznetsova, T.A.; Makarenkova, I.D.; Kryzhanovsky, S.P.; Fedyanina, L.N.; Ermakova, S.P. Extracts and Marine Algae Polysaccharides in Therapy and Prevention of Inflammatory Diseases of the Intestine. Mar. Drugs 2020, 18, 289. [Google Scholar] [CrossRef]
- Kodama, N.; Murata, Y.; Nanba, H. Administration of a Polysaccharide from Grifola frondosa Stimulates Immune Function of Normal Mice. J. Med. Food 2004, 7, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Hoo, P.C.; Tan, L.T.; Pusparajah, P.; Khan, T.M.; Lee, L.H.; Goh, B.H.; Chan, K.G. Golden Needle Mushroom: A Culinary Medicine with Evidenced-Based Biological Activities and Health Promoting Properties. Front. Pharmacol. 2016, 7, 474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yuan, S.; Ye, J.; Wang, X.; Zhang, X.; Shen, J.; Yuan, M.; Liao, W. Polysaccharide from Flammuliana velutipes improves colitis via regulation of colonic microbial dysbiosis and inflammatory responses. Int. J. Biol. Macromol. 2020, 149, 1252–1261. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Morampudi, V.; Dalwadi, U.; Bhinder, G.; Sham, H.P.; Gill, S.K.; Chan, J.; Bergstrom, K.S.; Huang, T.; Ma, C.; Jacobson, K.; et al. The goblet cell-derived mediator RELM-beta drives spontaneous colitis in Muc2-deficient mice by promoting commensal microbial dysbiosis. Mucosal Immunol. 2016, 9, 1218–1233. [Google Scholar] [CrossRef]
- Li, S.; Shah, N.P. Anti-inflammatory and anti-proliferative activities of natural and sulphonated polysaccharides from Pleurotus eryngii. J. Funct. Foods 2016, 23, 80–86. [Google Scholar] [CrossRef]
- Xu, D.; Wang, H.; Zheng, W.; Gao, Y.; Wang, M.; Zhang, Y.; Gao, Q. Charaterization and immunomodulatory activities of polysaccharide isolated from Pleurotus eryngii. Int. J. Biol. Macromol. 2016, 92, 30–36. [Google Scholar] [CrossRef]
- Brand, S.; Beigel, F.; Olszak, T.; Zitzmann, K.; Eichhorst, S.T.; Otte, J.M.; Diepolder, H.; Marquardt, A.; Jagla, W.; Popp, A.; et al. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G827–G838. [Google Scholar] [CrossRef]
- Ma, G.; Kimatu, B.M.; Zhao, L.; Yang, W.; Pei, F.; Hu, Q. In vivo fermentation of a Pleurotus eryngii polysaccharide and its effects on fecal microbiota composition and immune response. Food Funct. 2017, 8, 1810–1821. [Google Scholar] [CrossRef]
- Gericke, M.; Heinze, T. Homogeneous tosylation of agarose as an approach toward novel functional polysaccharide materials. Carbohydr. Polym. 2015, 127, 236–245. [Google Scholar] [CrossRef]
- Alencar, P.O.C.; Lima, G.C.; Barros, F.C.N.; Costa, L.E.C.; Ribeiro, C.V.P.E.; Sousa, W.M.; Sombra, V.G.; Abreu, C.M.W.S.; Abreu, E.S.; Pontes, E.O.B.; et al. A novel antioxidant sulfated polysaccharide from the algae Gracilaria caudata: In vitro and in vivo activities. Food Hydrocoll. 2019, 90, 28–34. [Google Scholar] [CrossRef]
- Dutra, N.L.S.; de Brito, T.V.; de Aguiar Magalhães, D.; Sousa, S.G.; Batista, J.A.; Pereira, C.M.C.; dos Santos Ferreira, J.; da Rocha Rodrigues, L.; do Nascimento Lima, J.V.; de Albuquerque, I.F.; et al. Sulfated polysaccharide extracted from seaweed Gracilaria caudata attenuates acetic acid-induced ulcerative colitis. Food Hydrocoll. 2021, 111, 106221. [Google Scholar] [CrossRef]
- Han, R.; Wang, L.; Zhao, Z.; You, L.; Pedisić, S.; Kulikouskaya, V.; Lin, Z. Polysaccharide from Gracilaria Lemaneiformis prevents colitis in Balb/c mice via enhancing intestinal barrier function and attenuating intestinal inflammation. Food Hydrocoll. 2020, 109, 106048. [Google Scholar] [CrossRef]
- Jiao, L.; Li, X.; Li, T.; Jiang, P.; Zhang, L.; Wu, M.; Zhang, L. Characterization and anti-tumor activity of alkali-extracted polysaccharide from Enteromorpha intestinalis. Int. Immunopharmacol. 2009, 9, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Li, Y.; Zhang, X.; Wang, Z. Potent anti-inflammatory activity of polysaccharides extracted from Blidingia minima and their effect in a mouse model of inflammatory bowel disease. J. Funct. Foods 2019, 61, 103494. [Google Scholar] [CrossRef]
- Shi, L. Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, N.; Kan, J.; Zhang, X.; Wu, X.; Sun, R.; Tang, S.; Liu, J.; Qian, C.; Jin, C. Structural characterization of water-soluble polysaccharide from Arctium lappa and its effects on colitis mice. Carbohydr. Polym. 2019, 213, 89–99. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.-H.; Luo, J.-P.; Zhang, Q.; Lu, J. The Structure-Activity Relationship and Molecular Mechanism of Anti-tumor Polysaccharide Isolated from Dendrobium Nobile Lindl. Curr. Top. Nutraceutical Res. 2019, 17, 153–163. [Google Scholar]
- Zhang, N.; Wang, Y.; Kan, J.; Wu, X.; Zhang, X.; Tang, S.; Sun, R.; Liu, J.; Qian, C.; Jin, C. In vivo and in vitro anti-inflammatory effects of water-soluble polysaccharide from Arctium lappa. Int. J. Biol. Macromol. 2019, 135, 717–724. [Google Scholar] [CrossRef]
- Zou, M.; Chen, Y.; Sun-Waterhouse, D.; Zhang, Y.; Li, F. Immunomodulatory acidic polysaccharides from Zizyphus jujuba cv. Huizao: Insights into their chemical characteristics and modes of action. Food Chem. 2018, 258, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, N.; Kan, J.; Sun, R.; Tang, S.; Wang, Z.; Chen, M.; Liu, J.; Jin, C. Anti-inflammatory activity of alkali-soluble polysaccharides from Arctium lappa L. and its effect on gut microbiota of mice with inflammation. Int. J. Biol. Macromol. 2020, 154, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Osaka, T.; Moriyama, E.; Date, Y.; Kikuchi, J.; Tsuneda, S. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiol. Rep. 2015, 3, e12327. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Sun, J.; Liu, J.; Chen, H.; Kan, J.; Qian, C.; Zhang, N.; Jin, C. Structural characterization of a water-soluble purple sweet potato polysaccharide and its effect on intestinal inflammation in mice. J. Funct. Foods 2019, 61, 103502. [Google Scholar] [CrossRef]
- Powell, N.; Walker, A.W.; Stolarczyk, E.; Canavan, J.B.; Gokmen, M.R.; Marks, E.; Jackson, I.; Hashim, A.; Curtis, M.A.; Jenner, R.G.; et al. The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells. Immunity 2012, 37, 674–684. [Google Scholar] [CrossRef]
- Rahat, M.A.; Hemmerlein, B. Macrophage-tumor cell interactions regulate the function of nitric oxide. Front. Physiol. 2013, 4, 144. [Google Scholar] [CrossRef]
- Tham, C.S.; Whitaker, J.; Luo, L.; Webb, M. Inhibition of microglial fatty acid amide hydrolase modulates LPS stimulated release of inflammatory mediators. FEBS Lett. 2007, 581, 2899–2904. [Google Scholar] [CrossRef]
- Meng, Z.; Yan, C.; Deng, Q.; Gao, D.F.; Niu, X.L. Curcumin inhibits LPS-induced inflammation in rat vascular smooth muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways. Acta Pharmacol. Sin. 2013, 34, 901–911. [Google Scholar] [CrossRef]
- Ahmad, A.N.; Mat Daud, Z.A.; Ismail, A. Review on potential therapeutic effect of Morinda citrifolia L. Curr. Opin. Food Sci. 2016, 8, 62–67. [Google Scholar] [CrossRef]
- Jin, M.; Wang, Y.; Yang, X.; Yin, H.; Nie, S.; Wu, X. Structure characterization of a polysaccharide extracted from noni (Morinda citrifolia L.) and its protective effect against DSS-induced bowel disease in mice. Food Hydrocoll. 2019, 90, 189–197. [Google Scholar] [CrossRef]
- Han, Y.; Son, S.J.; Akhalaia, M.; Platonov, A.; Son, H.J.; Lee, K.H.; Yun, Y.S.; Song, J.Y. Modulation of radiation-induced disturbances of antioxidant defense systems by ginsan. Evid. Based Complementary Altern. Med. 2005, 2, 529–536. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, Y.; Tian, Z.; Liu, F.; Shi, Y.; Liu, Y.; Xia, P. Astragalus polysaccharides protect against dextran sulfate sodium-induced colitis by inhibiting NF-kB activation. Int. J. Biol. Macromol. 2017, 98, 723–729. [Google Scholar] [CrossRef]
- Guo, L.; Qi, J.; Du, D.; Liu, Y.; Jiang, X. Current advances of Dendrobium officinale polysaccharides in dermatology: A literature review. Pharm. Biol. 2020, 58, 664–673. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.; Liu, J.; Zheng, Z.; Li, Q.; Wang, H.; Chen, Z.; Wang, K. Identification of the core active structure of a Dendrobium officinale polysaccharide and its protective effect against dextran sulfate sodium-induced colitis via alleviating gut microbiota dysbiosis. Food Res. Int. 2020, 137, 109641. [Google Scholar] [CrossRef]
- Yilmaz, B.; Juillerat, P.; Oyas, O.; Ramon, C.; Bravo, F.D.; Franc, Y.; Fournier, N.; Michetti, P.; Mueller, C.; Geuking, M.; et al. Microbial network disturbances in relapsing refractory Crohn’s disease. Nat. Med. 2019, 25, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Song, M.; Gu, M.; Ren, D.; Zhu, X.; Cao, X.; Li, F.; Wang, W.; Cai, X.; Yuan, B.; et al. Dietary Intake of Whole Strawberry Inhibited Colonic Inflammation in Dextran-Sulfate-Sodium-Treated Mice via Restoring Immune Homeostasis and Alleviating Gut Microbiota Dysbiosis. J. Agric. Food Chem. 2019, 67, 9168–9177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, X.; Ran, S.; Wang, K. Purification, structural elucidation and anti-inflammatory activity in vitro of polysaccharides from Smilax china L. Int. J. Biol. Macromol. 2019, 139, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-T.; Guo, H.; Lin, S.; Lam, S.-C.; Zhao, L.; Lin, D.-R.; Qin, W. Review of the structural characterization, quality evaluation, and industrial application of Lycium barbarum polysaccharides. Trends Food Sci. Technol. 2018, 79, 171–183. [Google Scholar] [CrossRef]
- Xie, J.H.; Jin, M.L.; Morris, G.A.; Zha, X.Q.; Chen, H.Q.; Yi, Y.; Li, J.E.; Wang, Z.J.; Gao, J.; Nie, S.P.; et al. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. 1), S60–S84. [Google Scholar] [CrossRef]
- Kang, Y.; Xue, Y.; Du, M.; Zhu, M.J. Preventive effects of Goji berry on dextran-sulfate-sodium-induced colitis in mice. J. Nutr. Biochem. 2017, 40, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.J.; Lv, Q.Q.; Zhang, B.; Chen, H.Q. Structural characterization and hepatoprotective activities of polysaccharides from the leaves of Toona sinensis (A. Juss) Roem. Carbohydr. Polym. 2019, 212, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Metzemaekers, M.; Gouwy, M.; Proost, P. Neutrophil chemoattractant receptors in health and disease: Double-edged swords. Cell. Mol. Immunol. 2020, 17, 433–450. [Google Scholar] [CrossRef]
- Tang, S.; Liu, W.; Zhao, Q.; Li, K.; Zhu, J.; Yao, W.; Gao, X. Combination of polysaccharides from Astragalus membranaceus and Codonopsis pilosula ameliorated mice colitis and underlying mechanisms. J. Ethnopharmacol. 2021, 264, 113280. [Google Scholar] [CrossRef]
- Sugimoto, K.; Ogawa, A.; Mizoguchi, E.; Shimomura, Y.; Andoh, A.; Bhan, A.K.; Blumberg, R.S.; Xavier, R.J.; Mizoguchi, A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Investig. 2008, 118, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Neufert, C.; Pickert, G.; Zheng, Y.; Wittkopf, N.; Warntjen, M.; Nikolaev, A.; Ouyang, W.; Neurath, M.F.; Becker, C. Activation of epithelial STAT3 regulates intestinal homeostasis. Cell Cycle 2010, 9, 652–655. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, B.; Tang, C.; Gou, Y.; Chen, H.; Wang, Y.; Jin, C.; Liu, J.; Niu, F.; Kan, J.; et al. Characterization, antioxidant activity and hepatoprotective effect of purple sweet potato polysaccharides. Int. J. Biol. Macromol. 2018, 115, 69–76. [Google Scholar] [CrossRef]
- Chen, H.; Sun, J.; Liu, J.; Gou, Y.; Zhang, X.; Wu, X.; Sun, R.; Tang, S.; Kan, J.; Qian, C.; et al. Structural characterization and anti-inflammatory activity of alkali-soluble polysaccharides from purple sweet potato. Int. J. Biol. Macromol. 2019, 131, 484–494. [Google Scholar] [CrossRef]
- Sun, J.; Chen, H.; Kan, J.; Gou, Y.; Liu, J.; Zhang, X.; Wu, X.; Tang, S.; Sun, R.; Qian, C.; et al. Anti-inflammatory properties and gut microbiota modulation of an alkali-soluble polysaccharide from purple sweet potato in DSS-induced colitis mice. Int. J. Biol. Macromol. 2020, 153, 708–722. [Google Scholar] [CrossRef]
- Wu, Q.; Qu, H.; Jia, J.; Kuang, C.; Wen, Y.; Yan, H.; Gui, Z. Characterization, antioxidant and antitumor activities of polysaccharides from purple sweet potato. Carbohydr. Polym. 2015, 132, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.B.; Jiang, H. A review on the structure-function relationship aspect of polysaccharides from tea materials. Crit. Rev. Food Sci. Nutr. 2015, 55, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, G.; Chen, C.; Zeng, X.; Ye, H. Prebiotics effects in vitro of polysaccharides from tea flowers on gut microbiota of healthy persons and patients with inflammatory bowel disease. Int. J. Biol. Macromol. 2020, 158, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Zhang, X.; Liu, X.; Li, Y.; Tan, Q.; Dan, Q.; Yuan, T.; Liu, X.; Liu, R.H.; Liu, Z. Ficus carica polysaccharide attenuates DSS-induced ulcerative colitis in C57BL/6 mice. Food Funct. 2020, 11, 6666–6679. [Google Scholar] [CrossRef]
- Meng, X.L.; Li, S.; Qin, C.B.; Zhu, Z.X.; Hu, W.P.; Yang, L.P.; Lu, R.H.; Li, W.J.; Nie, G.X. Intestinal microbiota and lipid metabolism responses in the common carp (Cyprinus carpio L.) following copper exposure. Ecotoxicol. Environ. Saf. 2018, 160, 257–264. [Google Scholar] [CrossRef]
- Li, L.F.; Liu, H.B.; Zhang, Q.W.; Li, Z.P.; Wong, T.L.; Fung, H.Y.; Zhang, J.X.; Bai, S.P.; Lu, A.P.; Han, Q.B. Comprehensive comparison of polysaccharides from Ganoderma lucidum and G. sinense: Chemical, antitumor, immunomodulating and gut-microbiota modulatory properties. Sci. Rep. 2018, 8, 6172. [Google Scholar] [CrossRef]
- Kobayashi, M.; Mikami, D.; Kimura, H.; Kamiyama, K.; Morikawa, Y.; Yokoi, S.; Kasuno, K.; Takahashi, N.; Taniguchi, T.; Iwano, M. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-alpha-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem. Biophys. Res. Commun. 2017, 486, 499–505. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, T.; Huang, B.; Luo, M.; Chen, Z.; Zhao, Z.; Wang, J.; Leung, D.; Yang, X.; Chan, K.W.; et al. Excessive deubiquitination of NLRP3-R779C variant contributes to very-early-onset inflammatory bowel disease development. J. Allergy Clin. Immunol. 2021, 147, 267–279. [Google Scholar] [CrossRef]
- Huang, L.; Shen, M.; Morris, G.A.; Xie, J. Sulfated polysaccharides: Immunomodulation and signaling mechanisms. Trends Food Sci. Technol. 2019, 92, 1–11. [Google Scholar] [CrossRef]
- Phull, A.R.; Majid, M.; Haq, I.U.; Khan, M.R.; Kim, S.J. In vitro and in vivo evaluation of anti-arthritic, antioxidant efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol. 2017, 97, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Gaynor, R.B. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J. Clin. Investig. 2001, 107, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Hamouda, N.; Utsumi, D.; Matsumoto, K.; Amagase, K.; Kato, S. G protein-coupled receptor 35 contributes to mucosal repair in mice via migration of colonic epithelial cells. Pharmacol. Res. 2017, 123, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Utsumi, D.; Matsumoto, K. G protein-coupled receptor 40 activation ameliorates dextran sulfate sodium-induced colitis in mice via the upregulation of glucagon-likepeptide-2. J. Pharmacol. Sci. 2019, 140, 144–152. [Google Scholar] [CrossRef]
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 2826–2832. [Google Scholar] [CrossRef]
- Lee, H.A.; Lee, D.Y.; Cho, H.M.; Kim, S.Y.; Iwasaki, Y.; Kim, I.K. Histone deacetylase inhibition attenuates transcriptional activity of mineralocorticoid receptor through its acetylation and prevents development of hypertension. Circ. Res. 2013, 112, 1004–1012. [Google Scholar] [CrossRef]
- DeWire, S.M.; Ahn, S.; Lefkowitz, R.J.; Shenoy, S.K. β-Arrestins and cell signaling. Annu. Rev. Physiol. 2007, 69, 483–510. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Chen, S.; Wang, Y.; Zeng, Y.; Ma, Y.; Hu, Y.; Zhang, H.; Sun, S.; Wu, X.; Meng, G.; et al. β-arrestin1 is critical for the full activation of NLRP3 and NLRC4 inflammasomes. J. Immunol. 2015, 194, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Higashimura, Y.; Tanaka, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Niki, E.; Naito, Y. Trans-unsaturated fatty acid activates NLRP3 inflammasome in macrophages and exacerbates intestinal inflammation in mice. Biochem. Biophys. Res. Commun. 2020, 529, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Chen, S.; Chen, J.; Lin, J.; Xiong, Q.; Yang, Y.; Yuan, J.; Zhou, L.; He, L.; Hou, S.; et al. Therapeutic roles of polysaccharides from Dendrobium officinale on colitis and its underlying mechanisms. Carbohydr. Polym. 2018, 185, 159–168. [Google Scholar] [CrossRef]
- Johansson, M.E.; Larsson, J.M.; Hansson, G.C. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4659–4665. [Google Scholar] [CrossRef]
- Mees, S.T.; Mennigen, R.; Spieker, T.; Rijcken, E.; Senninger, N.; Haier, J.; Bruewer, M. Expression of tight and adherens junction proteins in ulcerative colitis associated colorectal carcinoma: Upregulation of claudin-1, claudin-3, claudin-4, and β-catenin. Int. J. Colorectal Dis. 2009, 24, 361–368. [Google Scholar] [CrossRef]
- Marchiando, A.M.; Shen, L.; Graham, W.V.; Weber, C.R.; Schwarz, B.T.; Austin, J.R.; Raleigh, D.R.; Guan, Y.; Watson, A.J.M.; Montrose, M.H.; et al. Caveolin-1–dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J. Cell Biol. 2010, 189, 111–126. [Google Scholar] [CrossRef]
- Wang, F.; Graham, W.V.; Wang, Y.; Witkowski, E.D.; Schwarz, B.T.; Turner, J.R. Interferon-γ and Tumor Necrosis Factor-α Synergize to Induce Intestinal Epithelial Barrier Dysfunction by Up-Regulating Myosin Light Chain Kinase Expression. Am. J. Pathol. 2005, 166, 409–419. [Google Scholar] [CrossRef]
- Cao, W.; Vrees, M.D.; Potenti, F.M.; Harnett, K.M.; Fiocchi, C.; Pricolo, V.E. Interleukin 1β-induced production of H2O2 contributes to reduced sigmoid colonic circular smooth muscle contractility in ulcerative colitis. J. Pharmacol. Exp. Ther. 2004, 311, 60–70. [Google Scholar] [CrossRef]
- Masaki, T.; Kishiki, T.; Kojima, K.; Asou, N.; Beniya, A.; Matsuoka, H. Recent trends (2016–2017) in the treatment of inflammatory bowel disease. Ann. Gastroenterol. Surg. 2018, 2, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Scharl, M.; Paul, G.; Barrett, K.E.; McCole, D.F. AMP-activated protein kinase mediates the interferon-gamma-induced decrease in intestinal epithelial barrier function. J. Biol. Chem. 2009, 284, 27952–27963. [Google Scholar] [CrossRef]
- Kucharzik, T.; Walsh, S.V.; Chen, J.; Parkos, C.A.; Nusrat, A. Neutrophil Transmigration in Inflammatory Bowel Disease Is Associated with Differential Expression of Epithelial Intercellular Junction Proteins. Am. J. Pathol. 2001, 159, 2001–2009. [Google Scholar] [CrossRef]
- Ye, D.; Ma, I.; Ma, T.Y. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G496–G504. [Google Scholar] [CrossRef]
- Knutson, C.G.; Mangerich, A.; Zeng, Y.; Raczynski, A.R.; Liberman, R.G.; Kang, P.; Ye, W.; Prestwich, E.G.; Lu, K.; Wishnok, J.S.; et al. Chemical and cytokine features of innate immunity characterize serum and tissue profiles in inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 2013, 110, E2332–E2341. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Alemany-Cosme, E.; Saez-Gonzalez, E.; Moret, I.; Mateos, B.; Iborra, M.; Nos, P.; Sandoval, J.; Beltran, B. Oxidative Stress in the Pathogenesis of Crohn’s Disease and the Interconnection with Immunological Response, Microbiota, External Environmental Factors, and Epigenetics. Antioxidants 2021, 10, 64. [Google Scholar] [CrossRef]

| Name | Source | Origin | Molecular Weight (kDa) | Model | Targets | Reference |
|---|---|---|---|---|---|---|
| EP-1 | Hericium erinaceus | Mushroom | 3.1 | Acetic acid-induced mice | Recover the level of Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria | [17] |
| HECP | Hericium erinaceus | Mushroom | 87 | DSS-induced colitis mice | Decrease expression of pro-inflammatory cytokines (IL-6, IL-1β, TNF-α), COX-2, iNOS | [18] |
| HCP | Sarcodon aspratus | Mushroom | 670 | RAW264.7 cells | Increase the expression of NO, NOS, cytokines and phagocytic activity Stimulate TLR4-MAPK-NFkB signaling pathway | [21] |
| DP1 | Dictyophora indusiata | Mushroom | 1132 | RAW264.7 cells | Increase the level of Bacteroidaceae and Enterobacteriaceae Reduce the pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IFN-γ), MPO, NO and enhance anti-inflammatory cytokines (IL-4, IL-10), T-SOD; Inhibit CD86 Increase tight junction proteins including claudin-1, occludin, and ZO-1; and expression of Bcl-2 and Bax | [22,23,24] |
| FVP | Flammuliana velutipes | Mushroom | dozens to hundreds | DSS-induced colitis mice | Decrease the level of MPO, NO, and increase SOD Raise the ratio of Firmicutes/Bacteroidetes and enhance the abundance of Lachnospiraceae | [34] |
| PEP | Pleurotus eryngii | [34] | 426 | C57BL/6 mice | Increase the abundance of Porphyromonadaceae, Rikenellaceae, Bacteroidaceae, and Lactobacillaceae Decrease SCFA production | [41] |
| EPA-1 | Pleurotus eryngii | Mushroom | 99.7 | RAW 264.7 cells | Increase the production of pro-inflammatory cytokines TNF-α, IL-1, and IL-6 Increase expression level of phosphorylated p38, ERK, JNK, and NF-κB | [43] |
| SP | Graciliaria lemaneiformis | Seaweed | DSS-induced colitis mice | Inhibit activation of MPO Repress intestinal endotoxin and lipopolysaccharide-binding protein production Decrease the expression of TNF-α, IL-6, IL-1β | [44] | |
| BMP | Blidingia minima | Seaweed | DSS-induced mice | Decrease the expression of MPO and EPO Increase the expression of IL-10 and decrease the production L-1β and TNF-α, NF-κB, IκB-α, and AKT | [46] | |
| ALP-1 | Arctium lappa L. | Chinese herbs | 5.12 | Systemic inflammatory mice | Decrease the IL-1β, IL-6, TNF-α; increase IL-10 and Ig A; Increase the abundance of Lactobacillaceae, Lachnospiraceae, and Ruminococcaceae and inhibit the abundance of Bacteroides and Staphylococcus | [45,46] |
| ASPP | Arctium lappa L. | Chinese herbs | 120 | LPS-induced inflammatory cell | Increase the expression of IL-10 and decrease MPO Increase the abundance of Firmicutes, Alistipes, Odoribacter, and the Firmicutes/Bacteroides ratio | [52] |
| NFP | Morinda citrifolia | Chinese herbs | 456 | DSS-induced mice | Improve the tight junction proteins production including zonula, occludens-1, and occludins | [60] |
| APS | Astragalus membranaceus | Chinese herbs | DSS-induced mice | Down-regulating production of MPO, NF-κB, and pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) | [62] | |
| DOP/EDOP | Dendrobium officinale | Chinese herbs | DSS-induced mice | Inhibit NLRP3 inflammasome and β-arrestin1 Decrease the expression of pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α; Activate GPR41/43 signaling pathway Increase the abundance of Bacteroides, Lactobacillus plus Ruminococcaceae, but down-regulate the levels of Proteobacteria and Akkermansia | [64] | |
| LBP | Goji berry | Chinese herbs | 2.1−6.5 × 103 | Inhibit the expression of CXCL1, MCP-1, COX-2, and IL-6 Increase the abundance of Akkermansia, Lactobacillus, and Prevotellaceae Increase the expression level of TGF-β and sIgA | [70] | |
| CREP | Codonopssi pilosula | Chinese herbs | 2.0 × 103/7.3 | DSS-induced mice | Down-regulation of TJ proteins including Occludin, ZO-1, claudins, and MUC-2 Up-regulation of IL-22 | [73] |
| WPSPP-1 | Purple sweet potato | Plant | 103 | DSS-induced mice | Increase the expression of pro-inflammatory cytokines IL-10 and decrease the expression of IL-1β, TNF-α, IL-6. Promote the level of SOD and T-AOC, and inhibit the production of MDA | [54] |
| ASPP | Purple sweet potato | Plant | DSS-induced mice | Inhibit the pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α Increase the production of SCFAs | [78] | |
| TFPS | Camellia sinensis L. | Plant | 4.4/31 | Human stool samples | Increase the abundance of Escherichia/Shigella, Enterococcus, Collinsella, Lactobacillus, and Bifidobacterium Decrease the abundance of Enterobacter, Streptococcus, Bacteroides, Clostridium XlVa, Megasphaera, Roseburia, Granulicatella, Akkermansia, and Fusobacterium | [81] |
| FCPS | Ficus carica | Plant | 98.9 | DSS-treated mice | Decrease expression of IL-1β, iNOS, TNF-α, MCP1, IL-6, and COX-2 Decrease the abundance of Esherichia and Clostridium Increase the abundance of Prevotella, Bacteroides, Butyricicoccus, and Coprococcus | [83] |
| Mechanism Involved | Source of Polysaccharides | Results | Reference |
|---|---|---|---|
| MAPK transduction signaling pathway | Ganoderma lucidum and G. sinense/Pleurotus eryngii | Increase the phosphorylation level of ERK, JNK, and p38 in macrophage cells | [38,84] |
| Dictyophora indusiata | Decrease the phosphorylation level of ERK and pro-inflammatory cytokines in DSS-induced mice | [27] | |
| NF-κB signaling pathway | Blidingia minima/H. erinaceus/Arctium lappa L./Purple sweet potato | Restore phosphorylation level of NF-κB, IκB-α, and AKT in colitis mice model | [18,46,53] |
| G-protein-coupled receptors | Hericium Erinaceus | Increase the expression of GPR41/43 in acetic acid-induced colitis mice | [85] |
| Dendrobium officinaleon | Inhibit β-arrestin1and NLRP3 inflammasome signaling pathway in DSS-induced mice | [86] | |
| Increase intestinal integrity via upregulate tight junction proteins | Ficus carica | Increase the expression of light junction protein claudin-1 and decrease the expression of TNF-α and IL-1β in DSS-induced mice | [82] |
| Dictyophora indusiata | Increase the expression of claudin-1, occludin, and ZO-1 in DSS-induced mice | [27] | |
| Regulation of oxidative stress | Graciliaria lemaneiformis/Flammuliana velutipes | Decrease the expression of MPO and NO in DSS-induced mice | [34,44] |
| Hericium Erinaceus | Decrease the expression of COX-2 in acetic acid-induced mice | [14] | |
| Graciliaria caudata | Restoration of GSH and decrease of MDA in acetic acid-induced mice | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhu, H.; Wang, X.; Yu, Y.; Xie, J. Natural Food Polysaccharides Ameliorate Inflammatory Bowel Disease and Its Mechanisms. Foods 2021, 10, 1288. https://doi.org/10.3390/foods10061288
Wang Y, Zhu H, Wang X, Yu Y, Xie J. Natural Food Polysaccharides Ameliorate Inflammatory Bowel Disease and Its Mechanisms. Foods. 2021; 10(6):1288. https://doi.org/10.3390/foods10061288
Chicago/Turabian StyleWang, Yikun, Haibin Zhu, Xiaoji Wang, Yue Yu, and Jianhua Xie. 2021. "Natural Food Polysaccharides Ameliorate Inflammatory Bowel Disease and Its Mechanisms" Foods 10, no. 6: 1288. https://doi.org/10.3390/foods10061288
APA StyleWang, Y., Zhu, H., Wang, X., Yu, Y., & Xie, J. (2021). Natural Food Polysaccharides Ameliorate Inflammatory Bowel Disease and Its Mechanisms. Foods, 10(6), 1288. https://doi.org/10.3390/foods10061288







