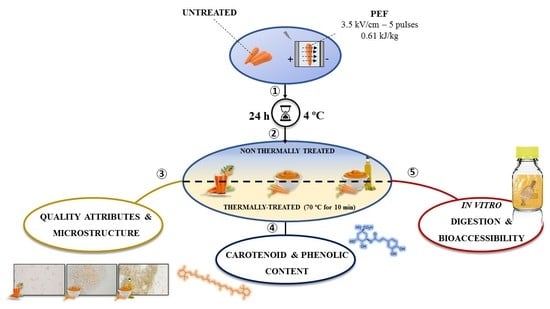
Applying Pulsed Electric Fields to Whole Carrots Enhances the Bioaccessibility of Carotenoid and Phenolic Compounds in Derived Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Carrot Samples
2.3. Pulsed Electric Fields (PEF) Treatments
2.4. Preparation of Carrot Derived Products
2.5. Evaluation of Quality Attributes
2.6. Particle Size Distribution
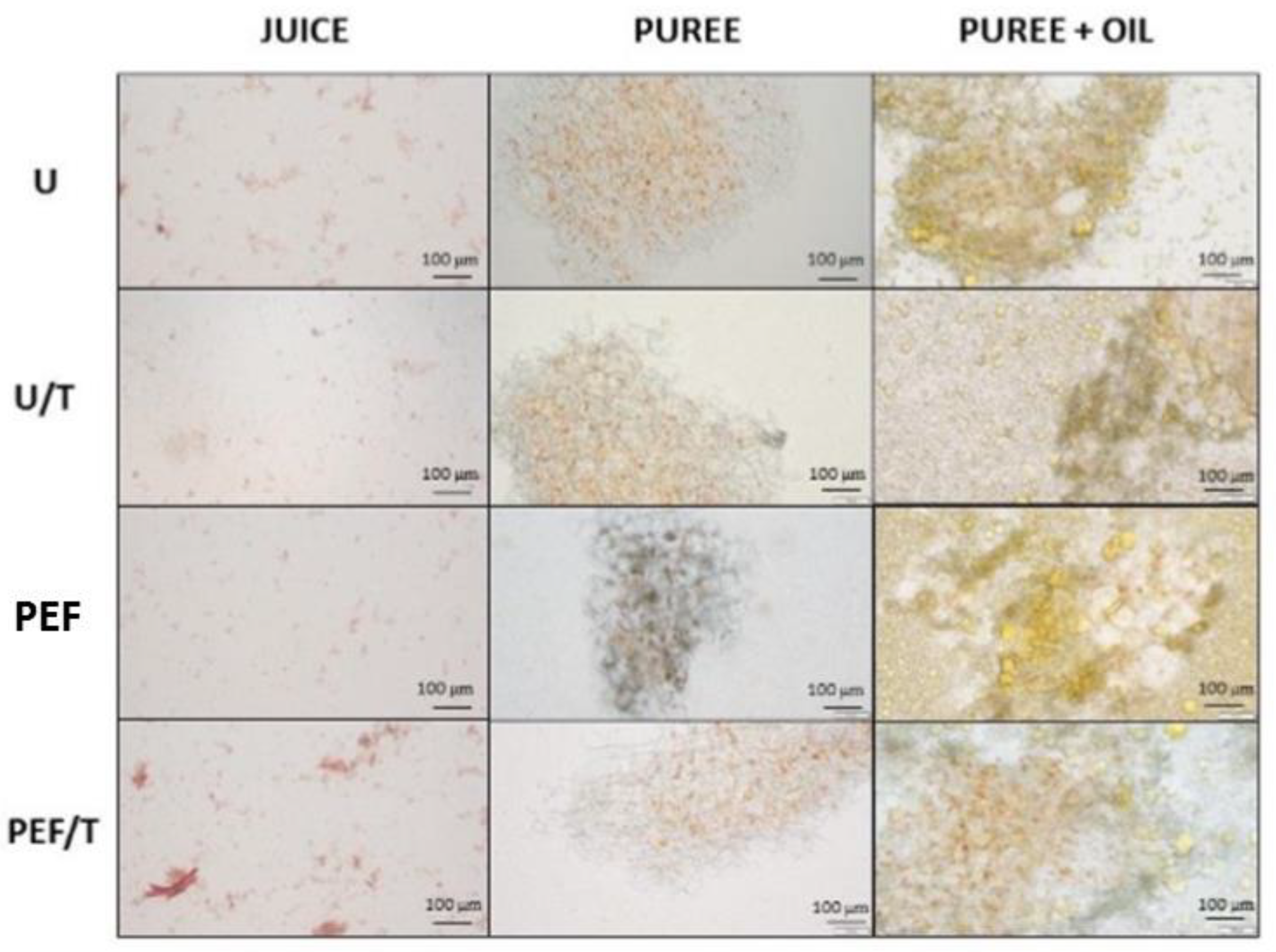
2.7. Microstructure
2.8. In Vitro Digestion
2.9. Carotenoids Determination
2.9.1. Carotenoids Extraction
2.9.2. Identification and Quantification of Carotenoids by HPLC-DAD
2.10. Phenolic Compounds Determination
2.10.1. Phenolic Compounds Extraction
2.10.2. Identification and Quantification of Phenolic Compounds by Ultra-Performance™ Liquid Chromatography (UPLC-MS/MS)
2.11. Bioaccessibility Calculation
2.12. Statistical Analysis
3. Results
3.1. Quality Attributes
3.2. Particle Size Distribution
3.3. Microstructure
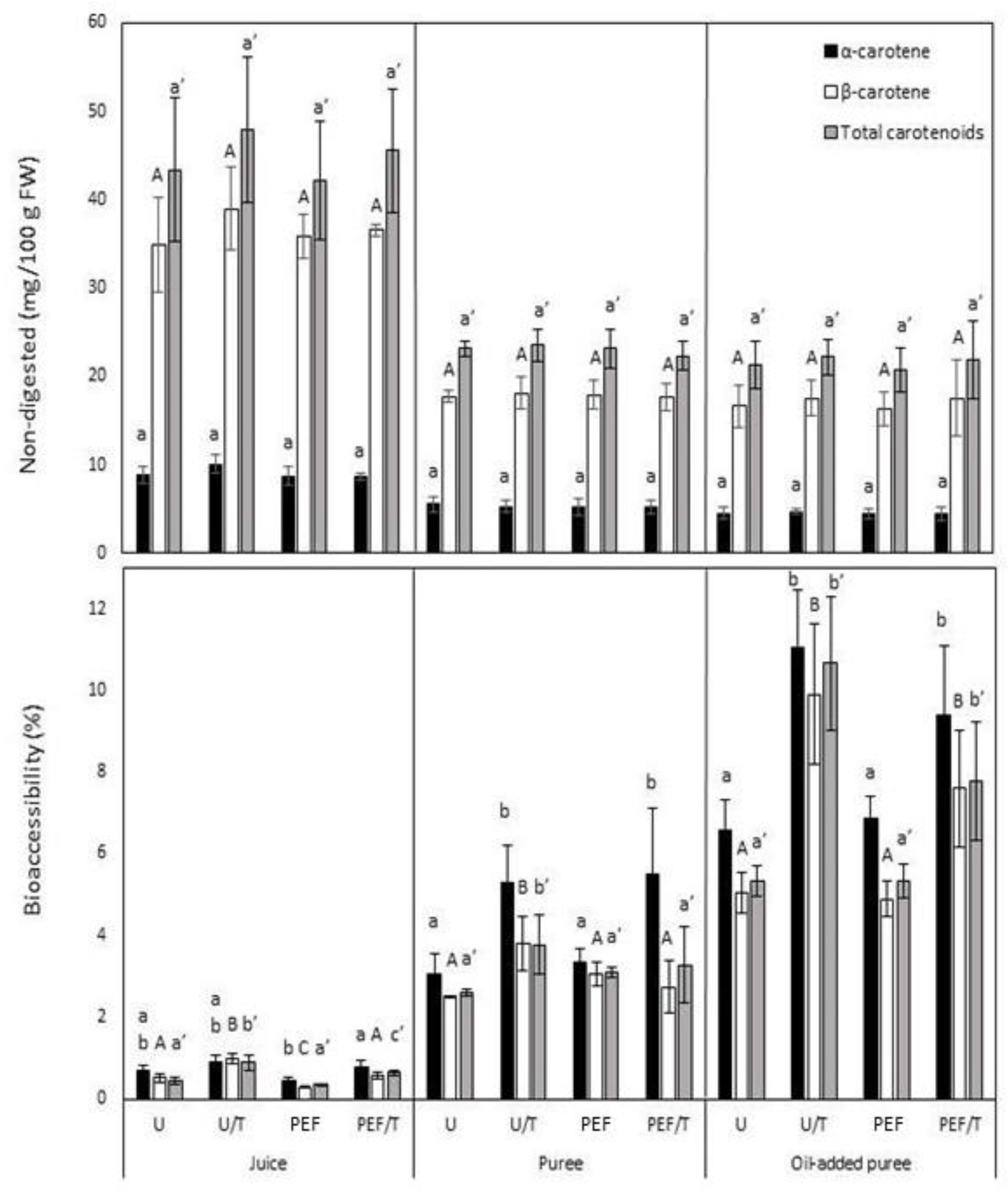
3.4. Carotenoid Content
3.5. Carotenoid Bioaccessibility
3.6. Phenolic Content
3.7. Phenolic Bioaccessibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Cvejić, J.H.; Krstonošić, M.A.; Bursać, M.; Miljić, U. Polyphenols; Academic Press: Cambridge, MA, USA, 2017; ISBN 9780128052570. [Google Scholar]
- Granado-Lorencio, F.; Olmedilla-Alonso, B.; Herrero-Barbudo, C.; Blanco-Navarro, I.; Pérez-Sacristán, B.; Blázquez-García, S. In vitro bioaccessibility of carotenoids and tocopherols from fruits and vegetables. Food Chem. 2007, 102, 641–648. [Google Scholar] [CrossRef]
- Van Buggenhout, S.; Alminger, M.; Lemmens, L.; Colle, I.; Knockaert, G.; Moelants, K.; Van Loey, A.; Hendrickx, M. In vitro approaches to estimate the effect of food processing on carotenoid bioavailability need thorough understanding of process induced microstructural changes. Trends Food Sci. Technol. 2010, 21, 607–618. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Knockaert, G.; Lemmens, L.; Van Buggenhout, S.; Hendrickx, M.; Van Loey, A. Changes in β-carotene bioaccessibility and concentration during processing of carrot puree. Food Chem. 2012, 133, 60–67. [Google Scholar] [CrossRef]
- Lemmens, L.; Van Buggenhout, S.; Van Loey, A.M.; Hendrickx, M.E. Particle size reduction leading to cell wall rupture is more important for the β-carotene bioaccessibility of raw compared to thermally processed carrots. J. Agric. Food Chem. 2010, 58, 12769–12776. [Google Scholar] [CrossRef] [PubMed]
- Hedrén, E.; Diaz, V.; Svanberg, U. Estimation of carotenoid accessibility from carrots determined by an in vitro digestion method. Eur. J. Clin. Nutr. 2002, 56, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Hornero-Méndez, D.; Mínguez-Mosquera, M.I. Bioaccessibility of carotenes from carrots: Effect of cooking and addition of oil. Innov. Food Sci. Emerg. Technol. 2007, 8, 407–412. [Google Scholar] [CrossRef]
- Palmero, P.; Lemmens, L.; Hendrickx, M.; Van Loey, A. Role of carotenoid type on the effect of thermal processing on bioaccessibility. Food Chem. 2014, 157, 275–282. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Tao, Y.; Zeng, M.; Zhang, S.; Tao, G.; Qin, F.; Chen, J. High pressure homogenization processing, thermal treatment and milk matrix affect in vitro bioaccessibility of phenolics in apple, grape and orange juice to different extents. Food Chem. 2016, 200, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Tao, Y.; Qie, X.; Zeng, M.; Qin, F.; Chen, J.; He, Z. Effects of high-pressure homogenization, thermal processing, and milk matrix on the in vitro bioaccessibility of phenolic compounds in pomelo and kiwi juices. J. Funct. Foods 2020, 64, 103633. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; Ancos, B.; Sánchez-Moreno, C.; Cano, M.P.; Elez-Martínez, P.; Martín-Belloso, O. Impact of food matrix and processing on the in vitro bioaccessibility of vitamin C, phenolic compounds, and hydrophilic antioxidant activity from fruit juice-based beverages. J. Funct. Foods 2015, 14, 33–43. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Cuéllar-Villarreal, M.d.R.; Welti-Chanes, J.; Cisneros-Zevallos, L.; Ramos-Parra, P.A.; Hernández-Brenes, C. Nonthermal processing technologies as elicitors to induce the biosynthesis and accumulation of nutraceuticals in plant foods. Trends Food Sci. Technol. 2017, 60, 80–87. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Enhancing hydroxycinnamic acids and flavan-3-ol contents by pulsed electric fields without affecting quality attributes of apple. Food Res. Int. 2019, 121, 433–440. [Google Scholar] [CrossRef]
- López-Gámez, G.; Elez-Martínez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Enhancing phenolic content in carrots by pulsed electric fields during post-treatment time: Effects on cell viability and quality attributes. Innov. Food Sci. Emerg. Technol. 2020, 59, 102252. [Google Scholar] [CrossRef]
- Bot, F.; Verkerk, R.; Mastwijk, H.; Anese, M.; Fogliano, V.; Capuano, E. The effect of pulsed electric fields on carotenoids bioaccessibility: The role of tomato matrix. Food Chem. 2018, 240, 415–421. [Google Scholar] [CrossRef]
- Jayathunge, K.G.L.R.; Stratakos, A.C.; Cregenzán-Albertia, O.; Grant, I.R.; Lyng, J.; Koidis, A. Enhancing the lycopene in vitro bioaccessibility of tomato juice synergistically applying thermal and non-thermal processing technologies. Food Chem. 2017, 221, 698–705. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Influence of pulsed electric fields processing on the bioaccessible and non-bioaccessible fractions of apple phenolic compounds. J. Funct. Foods 2019, 59, 206–214. [Google Scholar] [CrossRef]
- López-Gámez, G.; Elez-Martínez, P.; Quiles-Chuliá, A.; Martín-Belloso, O.; Hernando-Hernando, I.; Soliva-Fortuny, R. Effect of pulsed electric fields on carotenoid and phenolic bioaccessibility and their relationship with carrot structure. Food Funct. 2021, 12, 2772–2783. [Google Scholar] [CrossRef]
- González-Casado, S.; Martín-Belloso, O.; Elez-Martínez, P.; Soliva-Fortuny, R. Application of pulsed electric fields to tomato fruit for enhancing the bioaccessibility of carotenoids in derived products. Food Funct. 2018, 9, 2282–2289. [Google Scholar] [CrossRef]
- Wiktor, A.; Sledz, M.; Nowacka, M.; Rybak, K.; Chudoba, T.; Lojkowski, W.; Witrowa-Rajchert, D. The impact of pulsed electric field treatment on selected bioactive compound content and color of plant tissue. Innov. Food Sci. Emerg. Technol. 2015, 30, 69–78. [Google Scholar] [CrossRef]
- Balogh, T.; Smout, C.; Nguyen, B.L.; Van Loey, A.M.; Hendrickx, M.E. Thermal and high-pressure inactivation kinetics of carrot pectinmethylesterase: From model system to real foods. Innov. Food Sci. Emerg. Technol. 2004, 5, 429–436. [Google Scholar] [CrossRef]
- Soysal, Ç.; Söylemez, Z. Kinetics and inactivation of carrot peroxidase by heat treatment. J. Food Eng. 2005, 68, 349–356. [Google Scholar] [CrossRef]
- Houben, K.; Kermani, Z.J.; Van Buggenhout, S.; Van Loey, A.M.; Hendrickx, M.E. Thermal and high-pressure stability of pectin-converting enzymes in broccoli and carrot purée: Towards the creation of specific endogenous enzyme populations through processing. Food Bioprocess Technol. 2014, 7, 1713–1724. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrì, F.; Boutrou, R.; Corredig, F.M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Eriksen, J.N.; Luu, A.Y.; Dragsted, L.O.; Arrigoni, E. Adaption of an in vitro digestion method to screen carotenoid liberation and in vitro accessibility from differently processed spinach preparations. Food Chem. 2017, 224, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Svelander, C. In Vitro Bioaccessibility of Carotenes. Influence of Microstructure in Tomato and Carrot as Modified by Processing; Chalmers University of Technology: Göteborg, Sweden, 2011. [Google Scholar]
- Sadler, G.; Davis, J.; Dezman, D. Rapid extraction of lycopene and β-Carotene from reconstituted tomato paste and pink grapefruit homogenates. J. Food Sci. 1990, 55, 1460–1461. [Google Scholar] [CrossRef]
- Cortés, C.; Esteve, M.J.; Frígola, A.; Torregrosa, F. Identification and quantification of carotenoids including geometrical isomers in fruit and vegetable juices by liquid chromatography with ultraviolet-diode array detection. J. Agric. Food Chem. 2004, 52, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Esteve, M.J.; Frígola, A. High pressure treatment effect on physicochemical and nutritional properties of fluid foods during storage: A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 307–322. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Hossain, M.B.; Brunton, N.; Lyng, J.; Valverde, J.; Rai, D.K. Pulsed electric fields pre-treatment of carrot purees to enhance their polyacetylene and sugar contents. Innov. Food Sci. Emerg. Technol. 2014, 23, 79–86. [Google Scholar] [CrossRef]
- Xiang, B.; Sundararajan, S.; Solval, K.M.; Espinoza-Rodezno, L.; Aryana, K.; Sathivel, S. Effects of pulsed electric fields on physicochemical properties and microbial inactivation of carrot juice. J. Food Process. Preserv. 2013, 38, 1556–1564. [Google Scholar] [CrossRef]
- Yan, B.; Martínez-Monteagudo, S.I.; Cooperstone, J.L.; Riedl, K.M.; Schwartz, S.J.; Balasubramaniam, V.M. Impact of Thermal and Pressure-Based Technologies on Carotenoid Retention and Quality Attributes in Tomato Juice. Food Bioprocess Technol. 2017, 10, 808–818. [Google Scholar] [CrossRef]
- Kebede, B.T.; Grauwet, T.; Palmers, S.; Vervoort, L.; Carle, R.; Hendrickx, M.; Van Loey, A. Effect of high pressure high temperature processing on the volatile fraction of differently coloured carrots. Food Chem. 2014, 153, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Mutsokoti, L.; Panozzo, A.; Van Loey, A.; Hendrickx, M. Carotenoid transfer to oil during thermal processing of low fat carrot and tomato particle based suspensions. Food Res. Int. 2016, 86, 64–73. [Google Scholar] [CrossRef][Green Version]
- Knockaert, G.; Pulissery, S.K.; Lemmens, L.; Van Buggenhout, S.; Hendrickx, M.; Van Loey, A. Carrot β-carotene degradation and isomerization kinetics during thermal processing in the presence of oil. J. Agric. Food Chem. 2012, 60, 10312–10319. [Google Scholar] [CrossRef] [PubMed]
- Rybak, K.; Samborska, K.; Jedlinska, A.; Parniakov, O.; Nowacka, M.; Witrowa-Rajchert, D.; Wiktor, A. The impact of pulsed electric field pretreatment of bell pepper on the selected properties of spray dried juice. Innov. Food Sci. Emerg. Technol. 2020, 65, 102446. [Google Scholar] [CrossRef]
- Li, N.; Feng, Z.; Niu, Y.; Yu, L.L. Structural, rheological and functional properties of modified soluble dietary fiber from tomato peels. Food Hydrocoll. 2018, 77, 557–565. [Google Scholar] [CrossRef]
- Velázquez-Estrada, R.M.; Hernández-Herrero, M.M.; Guamis-López, B.; Roig-Saguès, A.X. Influence of ultra-high pressure homogenisation on physicochemical and sensorial properties of orange juice in comparison with conventional thermal processing. Int. J. Food Sci. Technol. 2019, 54, 1858–1864. [Google Scholar] [CrossRef]
- Stinco, C.M.; Sentandreu, E.; Mapelli-Brahm, P.; Navarro, J.L.; Vicario, I.M.; Meléndez-Martínez, A.J. Influence of high pressure homogenization and pasteurization on the in vitro bioaccessibility of carotenoids and flavonoids in orange juice. Food Chem. 2020, 331, 127259. [Google Scholar] [CrossRef]
- Greve, L.C.; McArdle, R.N.; Gohlke, J.R.; Labavitch, J.M. Impact of Heating on Carrot Firmness: Changes in Cell Wall Components. J. Agric. Food Chem. 1994, 42, 2900–2906. [Google Scholar] [CrossRef]
- Marx, M.; Stuparic, M.; Schieber, A.; Carle, R. Effects of thermal processing on trans-cis-isomerization of β-carotene in carrot juices and carotene-containing preparations. Food Chem. 2003, 83, 609–617. [Google Scholar] [CrossRef]
- Galindo, F.G.; Dejmek, P.; Lundgren, K.; Rasmusson, A.G.; Vicente, A.; Moritz, T. Metabolomic evaluation of pulsed electric field-induced stress on potato tissue. Planta 2009, 230, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Blenkinsop, R.W.; Yada, R.Y.; Marangoni, A.G. Metabolic Control of Low-Temperature Sweetening in Potato Tubers during Postharvest Storage. In Horticultural Reviews; John Wiley & Sons: Hoboken, NJ, USA, 2004; Volume 30, ISBN 9780471354208. [Google Scholar]
- Wiktor, A.; Gondek, E.; Jakubczyk, E.; Dadan, M.; Nowacka, M.; Rybak, K.; Witrowa-Rajchert, D. Acoustic and mechanical properties of carrot tissue treated by pulsed electric field, ultrasound and combination of both. J. Food Eng. 2018, 238, 12–21. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Van Buggenhout, S.; Palmero, P.; Hendrickx, M.; Loey, A. Van Investigating the role of pectin in carrot cell wall changes during thermal processing: A microscopic approach. Innov. Food Sci. Emerg. Technol. 2014, 24, 113–120. [Google Scholar] [CrossRef]
- Augusto, P.E.D.; Ibarz, A.; Cristianini, M. Effect of high pressure homogenization (HPH) on the rheological properties of tomato juice: Time-dependent and steady-state shear. J. Food Eng. 2012, 111, 570–579. [Google Scholar] [CrossRef]
- Li, Q.; Li, T.; Liu, C.; Chen, J.; Zhang, R.; Zhang, Z.; Dai, T.; Julian, D. Potential physicochemical basis of Mediterranean diet effect: Ability of emulsified olive oil to increase carotenoid bioaccessibility in raw and cooked tomatoes. Food Res. Int. 2016, 89, 320–329. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Zou, L.; Xiao, H.; Zhang, G.; Decker, E.A.; McClements, D.J. Enhancing Nutraceutical Bioavailability from Raw and Cooked Vegetables Using Excipient Emulsions: Influence of Lipid Type on Carotenoid Bioaccessibility from Carrots. J. Agric. Food Chem. 2015, 63, 10508–10517. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Mezger, D.; Schimpf, F.; Steingass, C.B.; Carle, R. Influence of chromoplast morphology on carotenoid bioaccessibility of carrot, mango, papaya, and tomato. Food Chem. 2012, 135, 2736–2742. [Google Scholar] [CrossRef] [PubMed]
- Knockaert, G.; De Roeck, A.; Lemmens, L.; Van Buggenhout, S.; Hendrickx, M.; Van Loey, A. Effect of thermal and high pressure processes on structural and health-related properties of carrots (Daucus carota). Food Chem. 2011, 125, 903–912. [Google Scholar] [CrossRef]
- Panozzo, A.; Lemmens, L.; Van Loey, A.; Manzocco, L.; Nicoli, M.C.; Hendrickx, M. Microstructure and bioaccessibility of different carotenoid species as affected by high pressure homogenisation: A case study on differently coloured tomatoes. Food Chem. 2013, 141, 4094–4100. [Google Scholar] [CrossRef] [PubMed]
- Morales-De La Peña, M.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Changes on phenolic and carotenoid composition of high intensity pulsed electric field and thermally treated fruit juice-soymilk beverages during refrigerated storage. Food Chem. 2011, 129, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Park, S.J.; Cho, Y.H.; Park, J. Effects of combined treatment of high hydrostatic pressure and mild heat on the quality of carrot juice. J. Food Sci. 2001, 66, 1355–1360. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.; Da Pieve, S.; Butler, F.; Downey, G. Effect of thermal and high pressure processing on antioxidant activity and instrumental colour of tomato and carrot purées. Innov. Food Sci. Emerg. Technol. 2009, 10, 16–22. [Google Scholar] [CrossRef]
- Blanquet-Diot, S.; Soufi, M.; Rambeau, M.; Rock, E.; Alric, M. Digestive stability of xanthophylls exceeds that of carotenes as studied in a dynamic in vitro gastrointestinal system. J. Nutr. 2009, 139, 876–883. [Google Scholar] [CrossRef]
- Faulks, R.M.; Southon, S. Challenges to understanding and measuring carotenoid bioavailability. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2005, 1740, 95–100. [Google Scholar] [CrossRef]
- Gence, L.; Servent, A.; Poucheret, P.; Hiol, A.; Dhuique-Mayer, C. Pectin structure and particle size modify carotenoid bioaccessibility and uptake by Caco-2 cells in citrus juices: Vs. concentrates. Food Funct. 2018, 9, 3523–3531. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Paulino, M.; Stinco, C.M.; Mapelli-Brahm, P.; Wang, X.D. Study of the time-course of cis/trans (Z/E) isomerization of lycopene, phytoene, and phytofluene from tomato. J. Agric. Food Chem. 2014, 62, 12399–12406. [Google Scholar] [CrossRef]
- Lemmens, L.; Van Buggenhout, S.; Oey, I.; Van Loey, A.; Hendrickx, M. Towards a better understanding of the relationship between the β-carotene in vitro bio-accessibility and pectin structural changes: A case study on carrots. Food Res. Int. 2009, 42, 1323–1330. [Google Scholar] [CrossRef]
- Palmero, P.; Panozzo, A.; Colle, I.; Chigwedere, C.; Hendrickx, M.; Van Loey, A. Role of structural barriers for carotenoid bioaccessibility upon high pressure homogenization. Food Chem. 2016, 199, 423–432. [Google Scholar] [CrossRef]
- Huo, T.; Ferruzzi, M.G.; Schwartz, S.J.; Failla, M.L. Impact of fatty acyl composition and quantity of triglycerides on bioaccessibility of dietary carotenoids. J. Agric. Food Chem. 2007, 55, 8950–8957. [Google Scholar] [CrossRef]
- Palmero, P.; Panozzo, A.; Simatupang, D.; Hendrickx, M.; Van Loey, A. Lycopene and β-carotene transfer to oil and micellar phases during in vitro digestion of tomato and red carrot based-fractions. Food Res. Int. 2014, 64, 831–838. [Google Scholar] [CrossRef]
- Becerra-Moreno, A.; Redondo-Gil, M.; Benavides, J.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Combined effect of water loss and wounding stress on gene activation of metabolic pathways associated with phenolic biosynthesis in carrot. Front. Plant Sci. 2015, 6, 837. [Google Scholar] [CrossRef] [PubMed]
- Quitão-Teixeira, L.J.; Odriozola-Serrano, I.; Soliva-Fortuny, R.; Mota-Ramos, A.; Martín-Belloso, O. Comparative study on antioxidant properties of carrot juice stabilised by high-intensity pulsed electric fields or heat treatments. J. Sci. Food Agric. 2009, 89, 2636–2642. [Google Scholar] [CrossRef]
- Szczepańska, J.; Barba, F.J.; Skąpska, S.; Marszałek, K. High pressure processing of carrot juice: Effect of static and multi-pulsed pressure on the polyphenolic profile, oxidoreductases activity and colour. Food Chem. 2020, 307, 125549. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, 6–15. [Google Scholar] [CrossRef] [PubMed]
- López-Gámez, G.; Elez-Martínez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Pulsed electric fields affect endogenous enzyme activities, respiration and biosynthesis of phenolic compounds in carrots. Postharvest Biol. Technol. 2020, 168, 111284. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
| Time (min) | Acetic Acid (0.2% v/v) (%) | Acetonitrile (%) |
|---|---|---|
| 0 | 95 | 5 |
| 5 | 90 | 10 |
| 10 | 87.6 | 12.4 |
| 18 | 72 | 28 |
| 21 | 15 | 85 |
| 23 | 0 | 100 |
| 25.5 | 0 | 100 |
| 27 | 95 | 5 |
| 30 | 95 | 5 |
| Product | Treatment | L* | a* | b* | ΔE | pH | TSS (%) | D [4, 3] (μm) | D [3, 2] (μm) |
|---|---|---|---|---|---|---|---|---|---|
| Puree | U | 41.3 ± 0.3 a,A | 11.0 ± 0.3 a,A | 24.4 ± 0.2 a,A | - | 6.4 ± 0.1 a,b,c,A | 3.6 ± 0.3 a,A | 596 ± 12 a,A | 207 ± 48 a,A |
| PEF | 40.5 ± 0.1 a,A | 10.3 ± 0.1 a,A | 23.1 ± 0.2 a,A | 1.6 ± 0.2 a,A | 6.5 ± 0.1 b,A | 3.4 ± 0.2 a,A | 589 ± 16 a,A | 183 ± 34 a,A | |
| U/T | 42.0 ± 0.2 a,A | 10.4 ± 0.5 a,A | 24.3 ± 0.5 a,A | 1.3 ± 0.1 a,A | 6.1 ± 0.1 d,A | 3.7 ± 0.0 a,A | 608 ± 11 a,A | 472 ± 20 b,A | |
| PEF/T | 41.0 ± 0.4 a,A | 9.9 ± 0.5 a,A | 23.1 ± 0.8 a,A | 1.8 ± 0.7 a,A | 6.3 ± 0.0 c,A | 3.5 ± 0.3 a,A | 601 ± 12 a,A | 431 ± 53 b,A | |
| Oil-added puree | U | 56.0 ± 1.7 a′,B | 15.2 ± 0.7 a′,B | 44.8 ± 1.9 a′,B | - | 6.3 ± 0.0 a′,A | 4.0 ± 0.6 a′,A | 449 ± 41 a′,B | 15 ± 3 a′,B |
| PEF | 55.7 ± 0.3 a′,B | 14.5 ± 0.5 a′,b′,B | 45.6 ± 3.9 a′,B | 3.3 ± 2.4 a′A | 6.4 ± 0.1 a′,A | 3.8 ± 0.4 a′,A | 422 ± 15 a′,B | 13.8 ± 2.0 a′,B | |
| U/T | 55.3 ± 0.7 a′,B | 13.8 ± 0.4 b′,B | 45.6 ± 3.9 a′,B | 3.6 ± 1.9 a′,B | 6.0 ± 0.0 b′,A | 4.2 ± 0.4 a′,A | 460 ± 30 a′,B | 20 ± 4 a′,B | |
| PEF/T | 55.2 ± 0.7 a′,B | 12.4 ± 0.9 d′,B | 47 ± 3 a′,B | 4.7 ± 2.1 a′,B | 6.1 ± 0.0 c′,B | 3.9 ± 0.1 a′,A | 408 ± 39 a′,B | 16 ± 4 a′,B | |
| Juice | U | 43.0 ± 0.5 a″ | 15.8 ± 0.7 a″,b″ | 29.2 ± 0.7 a″ | - | 6.2 ± 0.1 a″ | 8.2 ± 0.5 a″ | 487 ± 43 a″ | 66 ± 17 a″,b″ |
| PEF | 41.3 ± 0.3 a″ | 13.4 ± 0.7 a″,b″ | 26.9 ± 0.7 b″ | 3.8 ± 1.9 a″ | 6.2 ± 0.1 a″ | 8.2 ± 06 a″ | 499 ± 4 a″ | 74.3 ± 0.9 a″ | |
| U/T | 40.7 ± 2.3 a″ | 18.6 ± 1.6 c″ | 28.1 ± 1.3 a″,b″ | 4.8 ± 1.7 a″ | 6.1 ± 0.0 a″ | 7.7 ± 0.4 a″ | 359 ± 48 b″ | 32 ± 2 b″ | |
| PEF/T | 41.7 ± 0.3 a″ | 17.9 ± 0.6 c″ | 27.7 ± 0.7 a″,b″ | 2.86 ± 0.2 a″ | 6.3 ± 0.2 a″ | 7.5 ± 0.2 a″ | 408 ± 61 b″ | 46 ± 3 b″ |
| Phenolic Compounds | Puree | Oil-Added Puree | Juice | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| U | PEF | U/T | PEF/T | U | PEF | U/T | PEF/T | U | PEF | U/T | PEF/T | |
| Coumaric acid | 5.05 ± 0.21 a | 7.17 ± 1.82 b | 2.91 ± 0.50 c | 1.03 ± 0.24 d | 4.33 ± 0.58 A | 2.84 ± 0.30 B,C | 1.67 ± 0.04 C | 2.16 ± 0.71 C | 0.70 ± 0.07 a′ | 0.79 ± 0.04 a′ | 0.71 ± 0.13 a′ | 0.75 ± 0.05 a′ |
| Coumaroylquinic acid | 17 ± 8 a | 8 ± 3 a,b | 9.0 ± 2.5 a,b | 5.9 ± 1.23 b | 3.22 ± 0.60 A | 3.6 ± 0.74 A | 6.2 ± 2.3 A | 10 ± 4 A | 15 ± 7 a′ | 6.01 ± 0.37 b′ | 19 ± 3 a′ | 6.41 ± 0.48 b′ |
| Coumaric acid and its derivates | 22 ± 8 a | 15 ± 5 a,b | 12 ± 3 a,b | 6.9 ± 1.5 b | 7.6 ± 0.8 A | 6.4 ± 1.0 A | 7.9 ± 2.2 A | 12 ± 4 A | 15 ± 7 a′ | 6.80 ± 0.36 b′ | 19 ± 4 a′ | 7.2 ± 0.5 b′ |
| Caffeic acid | 1.49 ± 0.92 a | 0.83 ± 0.13 a | 0.92 ± 0.30 a | 0.42 ± 0.07 b | 1.72 ± 0.64 A | 0.61 ± 0.12 B | 0.51 ± 0.24 B | 0.39 ± 0.07 B | 1.3 ± 0.2 a′ | 0.90 ± 0.11 a′ | 2.47 ± 0.34 b′ | 1.13 ± 0.10 a′ |
| Caffeic acid arab/xiloside | nd 1,a | nd 1,a | nd a | nd a | nd 1,A | nd 1,A | nd 1,A | nd 1,A | 0.12 ± 0.03 a′ | 0.06 ± 0.01 a′ | 0.29 ± 0.06 b′ | 0.14 ± 0.02 a′ |
| Caffeoylshikimic acid | nd 1,a | nd 1,a | nd 1,a | nd 1,a | nd 1,A | nd 1,A | nd 1,A | nd 1,A | 0.06 ± 0.02 a′ | 0.02 ± 0.01 b′ | 0.05 ± 0.01 a′ | 0.02 ± 0.003 b′ |
| 3-caffeoylquinic acid | nd 1,a | nd 1,a | 0.18 ± 0.08 b | 0.23 ± 0.02 b | nd 1,A | nd 1,A | nd 1,A | 0.08 ± 0.01 A | 0.21 ± 0.02 a′ | 0.14 ± 0.02 a′ | 0.97 ± 0.11 b′ | 0.57 ± 0.09 c′ |
| 5-caffeoylquinic acid | 32 ± 17 a | 15.1 ± 1.0 a | 106 ± 23 b | 85 ± 13 b | 67 ± 35 A | 65 ± 20 A | 79 ± 6 A | 59 ± 19 A | 264 ± 47 a′ | 83 ± 7 b′ | 264 ± 32 a′ | 92 ± 7 c′ |
| 4-caffeoylquinic acid | nd 1,a | nd 1,a | 1.73 ± 0.66 b | 1.62 ± 0.09 b | 0.17 ± 0.10 A | 0.30 ± 0.12 A | 0.8 ± 0.1 B | 0.65 ± 0.09 B | 0.76 ± 0.14 a′ | 0.34 ± 0.07 a′ | 7.66 ± 1.15 b′ | 3.4 ± 0.3 c′ |
| Dicaffeoylferuoylquinic acid | nd 1,a | nd 1,a | 0.25 ± 0.10 b | 0.21 ± 0.03 b | nd 1,A | nd 1,A | nd 1,A | nd 1,A | 0.31 ± 0.06 a′ | 0.06 ± 0.01 b′ | 0.55 ± 0.07 c′ | 0.07 ± 0.02 b′ |
| Caffeoylferuoylquinic acid | 0.35 ± 0.10 a | 0.38 ± 0.07 a | 0.29 ± 0.11 a | 0.35 ± 0.08 a | 0.24 ± 0.05 A | 0.14 ± 0.02 A | 0.23 ± 0.09 A | 0.18 ± 0.05 A | 0.48 ± 0.02 a′ | 0.56 ± 0.05 b′ | 0.43 ± 0.06 a′ | 0.62 ± 0.06 b′ |
| Caffeic acid arabinoside glucoside | 0.06 ± 0.02 a | 0.1 ± 0.02 a | 0.07 ± 0.01 a | 0.11 ± 0.02 a | 0.05 ± 0.002 A | 0.07 ± 0.02 A | 0.05 ± 0.02 A | 0.07 ± 0.01 A | 0.12 ± 0.06 a′ | 0.18 ± 0.01 b′ | 0.09 ± 0.02 a′ | 0.22 ± 0.03 b′ |
| Caffeic acid Glu Acetyl glucoside | 0.84 ± 0.46 a | 0.7 ± 0.19 a | 0.8 ± 0.5 a | 0.78 ± 0.07 a | 0.5 ± 0.04 A | 1.0 ± 0.35 A | 0.8 ± 0.14 A | 1.04 ± 0.13 A | 3.8 ± 0.2 a′ | 4.32 ± 0.24 a′,b′ | 3.34 ± 0.57 a′ | 4.7 ± 0.4 b′ |
| Caffeic acid and its derivates | 35 ± 17 a | 17.2 ± 1.3 a | 111 ± 23 b | 89 ± 13 b | 69 ± 35 A | 67 ± 20 A | 81 ± 5 A | 62 ± 19 A | 271 ± 48 a′ | 90 ± 7 b′ | 280 ± 34 a′ | 103 ± 7 c′ |
| Ferulic acid | 0.32 ± 0.07 a | 0.40 ± 0.15 a | 0.35 ± 0.14 a | 0.40 ± 0.02 a | 0.32 ± 0.03 A | 0.2 ± 0.02 A | 0.54 ± 0.18 A | 0.49 ± 0.05 A | 1.21 ± 0.12 a′ | 1.69 ± 0.14 a′ | 2.90 ± 0.55 b′ | 2.64 ± 0.19 b′ |
| Isoferulic acid | 0.08 ± 0.04 a | 0.10 ± 0.03 a | 0.07 ± 0.03 a | 0.08 ± 0.02 a | 0.12 ± 0.04 A | 0.06 ± 0.02 A | 0.09 ± 0.03 A | 0.10 ± 0.02 A | 0.20 ± 0.03 a′ | 0.09 ± 0.02 b′ | 0.18 ± 0.05 a′,b′ | 0.11 ± 0.02 b′ |
| 3-feruloylquinic acid | nd 1,a | nd 1,a | 0.17 ± 0.03 b | 0.13 ± 0.02 b | 0.11 ± 0.06 A | 0.13 ± 0.02 A | 0.16 ± 0.03 A | 0.12 ± 0.03 A | 0.94 ± 0.12 a′ | 0.45 ± 0.10 b′ | 0.9 ± 0.12 a′ | 0.67 ± 0.05 b′ |
| 5-feruloylquinic acid | 1.34 ± 0.41 a | 1.26 ± 0.05 a | 2.49 ± 0.46 a | 2.43 ± 0.19 a | 1.07 ± 0.16 A | 1.18 ± 0.1 A | 1.35 ± 0.27 A | 1.48 ± 0.47 A | 13.15 ± 1.92 a′ | 8.90 ± 0.37 b′ | 9.73 ± 1.20 b′ | 9.2 ± 0.8 b′ |
| 4-feruloylquinic acid | 0.11 ± 0.01 a | 0.14 ± 0.01 a | 0.33 ± 0.06 a | 0.3 ± 0.04 a | 0.13 ± 0.0 A | 0.1 ± 0.09 A | 0.18 ± 0.04 A | 0.17 ± 0.02 A | 1.74 ± 0.25 a′ | 1.25 ± 0.04 b′ | 1.62 ± 0.28 a′ | 1.56 ± 0.11 a′ |
| Ferulic acid glucoside | nd 1,a | nd 1,a | 0.05 ± 0.03 a | 0.05 ± 0.004 a | nd 1,A | nd 1,A | nd 1,A | 0.49 ± 0.25 B | 0.32 ± 0.03 a′ | 0.25 ± 0.02 a′ | 0.29 ± 0.06 a′ | 0.19 ± 0.01 a′ |
| Ferulic acid coumaroyl glucoside | 2.02 ± 0.50 a | 1.46 ± 0.21 a | 1.72 ± 0.43 a | 1.41 ± 0.19 a | 1.52 ± 0.24 A | 1.44 ± 0.84 A | 2.15 ± 0.48 A | 1.32 ± 0.02 A | 3.72 ± 0.35 a′ | 3.44 ± 0.21 a′ | 2.48 ± 0.42 b′ | 1.65 ± 0.08 c′ |
| Ferulic acid caffeoyl glucoside | 0.09 ± 0.03 a | 0.07 ± 0.03 a | 0.18 ± 0.12 a | 0.14 ± 0.08 a | 0.06 ± 0.03 A | nd 1,A | 0.08 ± 0.03 A | 0.07 ± 0.03 A | 8.56 ± 0.61 a′ | 4.31 ± 0.31 b′ | 7.3 ± 1.0 a′,c′ | 6.95 ± 0.53 c′ |
| Feruloylquinic acid derivative | 0.85 ± 0.18 a | 0.8 ± 0.1 a | 0.68 ± 0.33 a | 0.83 ± 0.10 a | 0.43 ± 0.15 A | 0.4 ± 0.02 A | 0.43 ± 0.01 A | 0.42 ± 0.05 A | 2.92 ± 0.25 a′ | 4.25 ± 0.10 b′ | 2.52 ± 0.42 a′ | 2.42 ± 0.23 a′ |
| Feruloylquinic acid derivative (2) | 0.2 ± 0.08 a | 0.1 ± 0.02 a | 0.23 ± 0.10 a | 0.11 ± 0.01 a | 0.09 ± 0.03 A | 0.11 ± 0.01 A | 0.12 ± 0.01 A | 0.11 ± 0.02 A | 1.20 ± 0.11 a′ | 1.34 ± 0.05 a′ | 0.9 ± 0.12 b′ | 1.34 ± 0.1 a′ |
| Ferulic acid and its derivatives | 5.09 ± 0.12 a | 4.4 ± 0.3 a | 6.3 ± 1.3 a | 5.9 ± 0.3 a | 3.9 ± 0.4 A | 3.7 ± 0.7 A | 5.1 ± 0.8 A | 4.8 ± 0.8 A | 36 ± 4 a′ | 26.8 ± 1.0 b′ | 30 ± 4 b′ | 27.8 ± 1.9 b′ |
| Total phenolic compounds | 62 ± 23 a | 37 ± 5 a | 129 ± 25 b | 101 ± 14 b | 81 ± 36 A | 77 ± 20 A | 94 ± 6 A | 79 ± 24 A | 322 ± 56 a′ | 124 ± 8 b′ | 329 ± 40 a′ | 138 ± 9 b′ |
| Phenolic Compounds | Puree | Oil-Added Puree | Juice | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| U | PEF | U/T | PEF/T | U | PEF | U/T | PEF/T | U | PEF | U/T | PEF/T | |
| Coumaric acid | 91 ± 11 a | 56 ± 12 b | 53 ± 10 b | 100 ± 0 a | 63 ± 4 A | 99.4 ± 1.1 B | 79 ± 7 A | 77 ± 28 A | 100 ± 0 a′ | 100 ± 0 a′ | 47 ± 7 b′ | 45.1 ± 1.1 b′ |
| Coumaroylquinic acid | 80 ± 20 a | 100 ± 0 b | 100 ± 0 b | 100 ± 0 b | 84 ± 14 A | 97.4 ± 2.2 B | 42.7 ± 0.4 C | 26.3 ± 2.2 D | 42 ± 4 a′ | 35.9 ± 2.1 a′ | 25 ± 4 b′ | 50 ± 4 c′ |
| Coumaric acid and its derivatives | 84 ± 18 a | 96.2 ± 6.5 a | 100 ± 0 a | 100 ± 0 a | 74 ± 8 A | 100 ± 0 B | 48.8 ± 0.1 C | 31.2 ± 0.2 D | 56 ± 6 a′ | 60 ± 3 a′ | 25 ± 4 b′ | 50 ± 4 a′ |
| Caffeic acid | 0 a | 0 a | 52 ± 32 b | 24 ± 4 b | 0 A | 0 A | 43 ± 13 B | 24 ± 5 C | 0 a′ | 0 a′ | 47 ± 7 b′ | 34 ± 5 b′ |
| Caffeic acid arab/xiloside | 0* a | 0* a | 0 a | 0 a | 0 *,A | 0 *,A | 0 *,A | 84.4 ± 18.6 B | 0 a′ | 0 a′ | 23 ± 7 b′ | 55 ± 9 c′ |
| Caffeoylshikimic acid | 0 a | 0 a | 0 a | 0 a | 0 A | 0 A | 0 A | 0 A | 0 a′ | 0 a′ | 0 a′ | 0 a′ |
| 3-caffeoylquinic acid | 0 *,a | 0 *,A | 100 ± 0 b | 100 ± 0 b | 0 *,A | 0 *,A | 0 *,A | 0 *,A | 0 a′ | 0 a′ | 0 a′ | 0 a′ |
| 5-caffeoylquinic acid | 15 ± 9 a | 56 ± 10 b | 22 ± 4 a | 12 ± 4 a | 16 ± 11 A | 8.4 ± 1.6 B | 29 ± 3 A | 28 ± 11 A | 0 a′ | 0 a′ | 26.1 ± 1.7 b′ | 23 ± 5 b′ |
| 4-caffeoylquinic acid | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 66 ± 6 b | 100 ± 0 A | 73 ± 35 B | 100 ± 0 A | 100 ± 0 A | 10.6 ± 1.8 a′ | 19 ± 5 a′ | 93 ± 6 b′ | 84 ± 14 b′ |
| Dicaffeoylferuoylquinic acid | 0 a | 0 a | 0 a | 0 a | 0 A | 0 A | 0 A | 0 A | 0 a′ | 0 a′ | 18 ± 5 b′ | 0 a′ |
| Caffeoylferuoylquinic acid | 39 ± 5 a | 69 ± 16 b | 46 ± 14 a | 66 ± 18 a,b | 48 ± 17 A | 48 ± 12 A | 36 ± 12 A | 25.9 ± 1.7 A | 67 ± 5 a′ | 47 ± 1.7 a′,b′ | 43 ± 13 a′,b′ | 28.7 ± 1.8 b′ |
| Caffeic acid arabinoside glucoside | 73 ± 27 a | 55.2 ± 2.4 a | 62 ± 6 a | 48.0 ± 2.4 a | 84 ± 27 A | 42 ± 28 B | 100 ± 0 A | 71 ± 22 A | 74 ± 29 a′ | 57 ± 3 a′ | 86 ± 8 a′ | 35.8 ± 1.9 a′ |
| Caffeic acid Glu acetyl glucoside | 89 ± 19 a | 100 ± 0 a | 99.1 ± 1.5 a | 100 ± 0 a | 100 ± 0 A | 100 ± 0 A | 100 ± 0 A | 100 ± 0 A | 55 ± 3 a′ | 45 ± 7 a′ | 35 ± 9 a′ | 31.9 ± 0.9 a′ |
| Caffeic acid and its derivatives | 19 ± 9 a | 66 ± 9 b | 26 ± 5 a | 16 ± 4 a | 20 ± 13 A,B | 11.7 ± 2.3 B | 33 ± 3 A | 33 ± 12 A | 1.3 ± 0.2 a′ | 3.4 ± 0.4 a′ | 29.1 ± 1.9 b′ | 27 ± 5 b′ |
| Ferulic acid | 99 ± 0.7 a | 100 ± 0 a | 76 ± 24 b | 99.2 ± 1.3 a | 100 ± 0 A | 100 ± 0 A | 100 ± 0 A | 95.1 ± 8.5 A | 100 ± 0 a′ | 100 ± 0 a′ | 68 ± 15 b′ | 45 ± 6 c′ |
| Isoferulic acid | 100 ± 0 a | 100 ± 0 a | 98 ± 4 a | 100 ± 0 a | 100 ± 0 A | 100 ± 0 A | 100 ± 0 A | 100 ± 0 A | 70 ± 16 a′ | 100 ± 0 b′ | 91.3 ± 15 b′ | 99.2 ± 1.3 b′ |
| 3-feruloylquinic acid | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 93.8 ± 11 A,B | 81 ± 19 B | 100 ± 0 A | 100 ± 0 A | 79 ± 11 a′ | 100 ± 0 a′ | 98 ± 4 a′ | 98.0 ± 2.0 a′ |
| 5-feruloylquinic acid | 81 ± 17 a | 99.6 ± 0.7 b | 95 ± 5 a,b | 96 ± 4 a,b | 100 ± 0 A | 91 ± 9 A | 100 ± 0 A | 100 ± 0 A | 40 ± 6 a′ | 43 ± 3 a′ | 56 ± 9 b′ | 35 ± 5 a′ |
| 4-feruloylquinic acid | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 A | 100 ± 0 A | 100 ± 0 A | 99.5 ± 0.8 A | 86 ± 12 a′,b′ | 83 ± 7 a′,b′ | 94 ± 5 b′ | 66 ± 6 c′ |
| Ferulic acid glucoside | 56 ± 4 a | 99.1 ± 1.5 b | 100 ± 0 b | 91 ± 15 b | 0 A | 0 A | 60.5 ± 35 B | 8 ± 3 C | 34.7 ± 0.9 a′ | 38 ± 6 a′ | 36 ± 8 a′ | 37 ± 7 a′ |
| Ferulic acid coumaroyl glucoside | 71 ± 19 a | 100 ± 0 a | 76 ± 20 a | 94 ± 12 a | 81 ± 24 A | 57 ± 37 A | 78 ± 22 A | 82 ± 24 A | 44 ± 8 a′ | 41.2 ± 1.7 a′ | 99 ± 3 b′ | 57.9 ± 1.9 a′ |
| Ferulic acid caffeoyl glucoside | 38 ± 15 a | 65 ± 3 b | 100 ± 0 c | 92 ± 14 c | 71 ± 31 A | 92 ± 14 A | 61 ± 14 A | 78 ± 19 A | 36 ± 3 a′ | 43.3 ± 0.9 a′ | 37 ± 7 a′ | 24 ± 7 a′ |
| Feruloylquinic acid derivative | 46 ± 11 a | 89 ± 9 b | 89 ± 19 b | 93 ± 12 b | 73 ± 27 A | 78 ± 10 A | 68 ± 14 A | 78 ± 9 A | 46 ± 7 a′ | 33 ± 3 a′ | 41 ± 14 a′ | 33.5 ± 0.3 a′ |
| Feruloylquinic acid derivative (2) | 18 ± 7 a | 57 ± 12 b | 53 ± 24 b | 89 ± 19 c | 77 ± 21 A | 42 ± 17 B | 39 ± 17 B | 38 ± 10 B | 38.3 ± 2.4 a′ | 33.6 ± 1.2 a′ | 31 ± 5 a′ | 19 ± 4 b′ |
| Ferulic acid and its derivatives | 77 ± 4 a | 100 ± 0 b | 97 ± 5 b | 100 ± 0 b | 98 ± 3 A | 89 ± 12 A | 100 ± 0 A | 100 ± 0 A | 45 ± 4 a′ | 50.0 ± 1.8 a′,b′ | 60 ± 10 b′ | 37 ± 5 a′ |
| Total phenolic compounds | 52 ± 14 a | 100 ± 0 b | 49 ± 8 a | 48 ± 6 a | 31 ± 15 A | 24 ± 5 A | 40 ± 4 A | 40 ± 17 A | 16.1 ± 2.5 a′ | 27.9 ± 2.0 a′ | 34 ± 3 a′ | 33 ± 2 a′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Gámez, G.; Elez-Martínez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Applying Pulsed Electric Fields to Whole Carrots Enhances the Bioaccessibility of Carotenoid and Phenolic Compounds in Derived Products. Foods 2021, 10, 1321. https://doi.org/10.3390/foods10061321
López-Gámez G, Elez-Martínez P, Martín-Belloso O, Soliva-Fortuny R. Applying Pulsed Electric Fields to Whole Carrots Enhances the Bioaccessibility of Carotenoid and Phenolic Compounds in Derived Products. Foods. 2021; 10(6):1321. https://doi.org/10.3390/foods10061321
Chicago/Turabian StyleLópez-Gámez, Gloria, Pedro Elez-Martínez, Olga Martín-Belloso, and Robert Soliva-Fortuny. 2021. "Applying Pulsed Electric Fields to Whole Carrots Enhances the Bioaccessibility of Carotenoid and Phenolic Compounds in Derived Products" Foods 10, no. 6: 1321. https://doi.org/10.3390/foods10061321
APA StyleLópez-Gámez, G., Elez-Martínez, P., Martín-Belloso, O., & Soliva-Fortuny, R. (2021). Applying Pulsed Electric Fields to Whole Carrots Enhances the Bioaccessibility of Carotenoid and Phenolic Compounds in Derived Products. Foods, 10(6), 1321. https://doi.org/10.3390/foods10061321