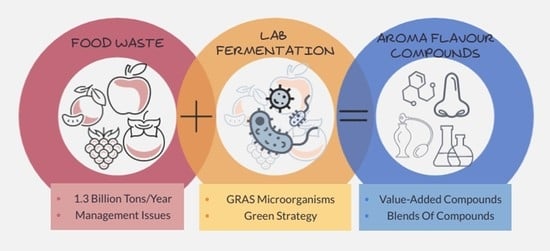
Fermentation of Agri-Food Waste: A Promising Route for the Production of Aroma Compounds
Abstract
:1. Are We Sure They Are Really Waste?
2. Agri-Food Waste: A Rising Problem or a Valuable Resource?
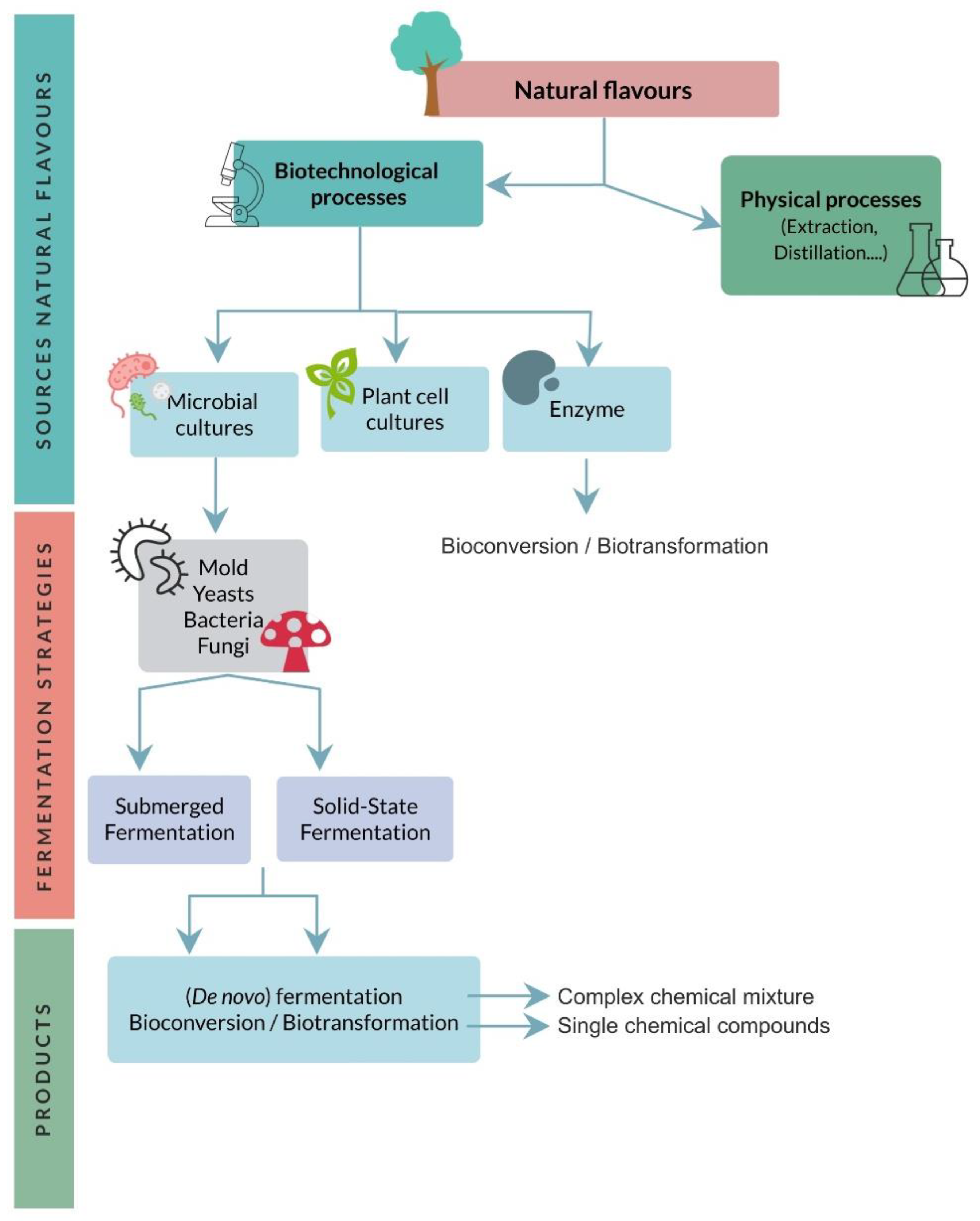
3. Repurposing Agri-Food Waste by Solid-State Fermentation for the Production of Aroma Compounds
4. Lactic Acid Bacteria: Biological Resources for Volatile Compound Production
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Food Wastage Footprint. Impact on Natural Resources; Summary Report; FAO: Rome, Italy, 2013; ISBN 978925107752. [Google Scholar]
- FAO. Global Initiative on Food Loss and Waste Reduction; FAO: Rome, Italy, 2015. [Google Scholar]
- Take Action for the Sustainable Development Goals—United Nations Sustainable Development. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 28 January 2021).
- Barba, F.J.; Putnik, P.; Bursać Kovačević, D.; Poojary, M.M.; Roohinejad, S.; Lorenzo, J.M.; Koubaa, M. Impact of conventional and non-conventional processing on prickly pear (Opuntia spp.) and their derived products: From preservation of beverages to valorization of by-products. Trends Food Sci. Technol. 2017, 67, 260–270. [Google Scholar] [CrossRef]
- Clementz, A.; Torresi, P.A.; Molli, J.S.; Cardell, D.; Mammarella, E.; Yori, J.C. Novel method for valorization of by-products from carrot discards. LWT 2019, 100, 374–380. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of by-products from olive oil industry and added-value applications for innovative functional foods. Food Res. Int. 2020, 137, 109683. [Google Scholar] [CrossRef] [PubMed]
- Hartikainen, H.; Mogensen, L.; Svanes, E.; Franke, U. Food waste quantification in primary production—The Nordic countries as a case study. Waste Manag. 2018, 71, 502–511. [Google Scholar] [CrossRef]
- Galanakis, C. Food Waste Valorization Opportunities for Different Food Industries; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128164495. [Google Scholar]
- Fidelis, M.; de Moura, C.; Kabbas Junior, T.; Pap, N.; Mattila, P.; Mäkinen, S.; Putnik, P.; Bursać Kovačević, D.; Tian, Y.; Yang, B.; et al. Fruit Seeds as Sources of Bioactive Compounds: Sustainable Production of High Value-Added Ingredients from By-Products within Circular Economy. Molecules 2019, 24, 3854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Girotto, F.; Alibardi, L.; Cossu, R. Food waste generation and industrial uses: A review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef]
- Pfaltzgraff, L.A.; De Bruyn, M.; Cooper, E.C.; Budarin, V.; Clark, J.H. Food waste biomass: A resource for high-value chemicals. Green Chem. 2013, 15, 307–314. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, A.; Artola, A.; Font, X.; Gea, T.; Barrena, R.; Gabriel, D.; Sánchez-Monedero, M.Á.; Roig, A.; Cayuela, M.L.; Mondini, C. Greenhouse gas emissions from organic waste composting. Environ. Chem. Lett. 2015, 13, 223–238. [Google Scholar] [CrossRef] [Green Version]
- Bhuyan, N.; Narzari, R.; Gogoi, L.; Bordoloi, N.; Hiloidhari, M.; Palsaniya, D.R.; Deb, U.; Gogoi, N.; Kataki, R. Valorization of agricultural wastes for multidimensional use. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 41–78. [Google Scholar]
- Carmona-Cabello, M.; Garcia, I.L.; Leiva-Candia, D.; Dorado, M.P. Valorization of food waste based on its composition through the concept of biorefinery. Curr. Opin. Green Sustain. Chem. 2018, 14, 67–79. [Google Scholar] [CrossRef]
- Sydney, E.B.; de Carvalho, J.C.; Letti, L.A.J.; Magalhães, A.I.; Karp, S.G.; Martinez-Burgos, W.J.; de Souza Candeo, E.; Rodrigues, C.; de SouzaVandenberghe, L.P.; Neto, C.J.D.; et al. Current developments and challenges of green technologies for the valorization of liquid, solid, and gaseous wastes from sugarcane ethanol production. J. Hazard. Mater. 2021, 404, 124059. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Mamun, A.A.; Volk, J. Physical, chemical and surface properties of wheat husk, rye husk and soft wood and their polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 480–488. [Google Scholar] [CrossRef]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.A.; Kenny, J.M. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef] [Green Version]
- Masood, F.; Yasin, T.; Hameed, A. Polyhydroxyalkanoates—What are the uses? Current challenges and perspectives. Crit. Rev. Biotechnol. 2015, 35, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Naqash, F.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gani, A. Emerging concepts in the nutraceutical and functional properties of pectin—A Review. Carbohydr. Polym. 2017, 168, 227–239. [Google Scholar] [CrossRef] [PubMed]
- De Moraes Crizel, T.; Jablonski, A.; de Oliveira Rios, A.; Rech, R.; Flôres, S.H. Dietary fiber from orange byproducts as a potential fat replacer. LWT Food Sci. Technol. 2013, 53, 9–14. [Google Scholar] [CrossRef]
- Klavins, L.; Kviesis, J.; Nakurte, I.; Klavins, M. Berry press residues as a valuable source of polyphenolics: Extraction optimisation and analysis. LWT 2018, 93, 583–591. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [Green Version]
- Pereira, G.V.D.M.; Neto, D.P.D.C.; Junqueira, A.C.D.O.; Karp, S.G.; Letti, L.A.J.; Júnior, A.I.M.; Soccol, C.R. A Review of Selection Criteria for Starter Culture Development in the Food Fermentation Industry. Food Rev. Int. 2020, 36, 135–167. [Google Scholar] [CrossRef]
- Tlais, A.Z.A.; Fiorino, G.M.; Polo, A.; Filannino, P.; Di Cagno, R. High-Value Compounds in Fruit, Vegetable and Cereal Byproducts: An Overview of Potential Sustainable Reuse and Exploitation. Molecules 2020, 25, 2987. [Google Scholar] [CrossRef] [PubMed]
- Yazid, N.A.; Barrena, R.; Komilis, D.; Sánchez, A. Solid-state fermentation as a novel paradigm for organic waste valorization: A review. Sustainability 2017, 9, 1–28. [Google Scholar]
- Ali, H.; Zulkali, M. Utilization of Agro-Residual Ligno-Cellulosic Substances by Using Solid State Fermentation: A Review. Hrvat. Časopis Prehrambenu Tehnol. Biotehnol. Nutr. 2011, 6, 5–12. [Google Scholar]
- Ben Akacha, N.; Gargouri, M. Microbial and enzymatic technologies used for the production of natural aroma compounds: Synthesis, recovery modeling, and bioprocesses. Food Bioprod. Process. 2015, 94, 675–706. [Google Scholar] [CrossRef]
- Arora, S.; Rani, R.; Ghosh, S. Bioreactors in solid state fermentation technology: Design, applications and engineering aspects. J. Biotechnol. 2018, 269, 16–34. [Google Scholar] [CrossRef]
- Singhania, R.R.; Kumar Patel, A.; Soccol, C.R.; Pandey, A. Recent advances in solid-state fermentation. Biochem. Eng. J. 2009, 44, 13–18. [Google Scholar] [CrossRef]
- Cerda, A.; Artola, A.; Barrena, R.; Font, X.; Gea, T.; Sánchez, A. Innovative Production of Bioproducts from Organic Waste Through Solid-State Fermentation. Front. Sustain. Food Syst. 2019, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Couto, S.R.; Sanromán, M.Á. Application of solid-state fermentation to food industry—A review. J. Food Eng. 2006, 76, 291–302. [Google Scholar] [CrossRef]
- Lizardi-Jiménez, M.A.; Hernández-Martínez, R. Solid state fermentation (SSF): Diversity of applications to valorize waste and biomass. 3 Biotech. 2017, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Mussatto, S.I.; Martínez-avila, G.; Montañez-saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Orzua, M.C.; Mussatto, S.I.; Contreras-Esquivel, J.C.; Rodriguez, R.; De La Garza, H.; Teixeira, J.A.; Aguilar, C.N. Exploitation of agro industrial wastes as immobilization carrier for solid-state fermentation. Ind. Crops Prod. 2009, 30, 24–27. [Google Scholar] [CrossRef] [Green Version]
- Sales, A.; Paulino, B.N.; Pastore, G.M.; Bicas, J.L. Biogeneration of aroma compounds. Curr. Opin. Food Sci. 2018, 19, 77–84. [Google Scholar] [CrossRef]
- de Oliveira Felipe, L.; de Oliveira, A.M.; Bicas, J.L. Bioaromas—Perspectives for sustainable development. Trends Food Sci. Technol. 2017, 62, 141–153. [Google Scholar] [CrossRef]
- Pessôa, M.G.; Vespermann, K.A.C.; Paulino, B.N.; Barcelos, M.C.S.; Pastore, G.M.; Molina, G. Newly isolated microorganisms with potential application in biotechnology. Biotechnol. Adv. 2019, 37, 319–339. [Google Scholar] [CrossRef]
- Longo, M.A.; Sanromán, M.A. Production of food aroma compounds: Microbial and enzymatic methodologies. Food Technol. Biotechnol. 2006, 44, 335–353. [Google Scholar]
- Kumar, A.; Gudiukaite, R.; Gricajeva, A.; Sadauskas, M.; Malunavicius, V.; Kamyab, H.; Sharma, S.; Sharma, T.; Pant, D. Microbial lipolytic enzymes—Promising energy-efficient biocatalysts in bioremediation. Energy 2020, 192, 116674. [Google Scholar] [CrossRef]
- Kumari, A.; Ahmad, R.; Negi, S.; Khare, S.K. Biodegradation of waste grease by Penicillium chrysogenum for production of fatty acid. Bioresour. Technol. 2017, 226, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Zelena, K.; Krings, U.; Berger, R.G. Functional expression of a valencene dioxygenase from Pleurotus sapidus in E. coli. Bioresour. Technol. 2012, 108, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Zamzuri, N.A.; Abd-Aziz, S. Biovanillin from agro wastes as an alternative food flavour. J. Sci. Food Agric. 2013, 93, 429–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, P.L.; Hassan, O. Bioconversion of ferulic acid attained from pineapple peels and pineapple crown leaves into vanillic acid and vanillin by Aspergillus niger I-1472. BMC Chem. 2020, 14, 1–11. [Google Scholar] [CrossRef]
- Carroll, A.L.; Desai, S.H.; Atsumi, S. Microbial production of scent and flavor compounds. Curr. Opin. Biotechnol. 2016, 37, 8–15. [Google Scholar] [CrossRef]
- Boccia, F.; Covino, D.; Sarnacchiaro, P. Genetically modified food versus knowledge and fear: A Noumenic approach for consumer behaviour. Food Res. Int. 2018, 111, 682–688. [Google Scholar] [CrossRef]
- Soares, M.; Christen, P.; Pandey, A.; Soccol, C.R. Fruity flavour production by Ceratocystis fimbriata grown on coffee husk in solid-state fermentation. Process. Biochem. 2000, 35, 857–861. [Google Scholar] [CrossRef]
- Aggelopoulos, T.; Katsieris, K.; Bekatorou, A.; Pandey, A.; Banat, I.M.; Koutinas, A.A. Solid state fermentation of food waste mixtures for single cell protein, aroma volatiles and fat production. Food Chem. 2014, 145, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Quilter, M.G.; Hurley, J.C.; Lynch, F.J.; Murphy, M.G. The Production of Isoamyl Acetate from Amyl Alcohol by Saccharomyces cerevisiae. J. Inst. Brew. 2003, 109, 34–40. [Google Scholar] [CrossRef]
- Bramorski, A.; Soccol, C.R.; Christen, P.; Revah, S. Fruity aroma production by Ceratocystis fimbriata in solid cultures from agro-industrial wastes. Rev. Microbiol. 1998, 29, 208–212. [Google Scholar] [CrossRef]
- Shetty, K.; Sarkar, D. Functional Foods and Biotechnology: Biotransformation and Analysis of Functional Foods and Ingredients; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Martínez, O.; Sánchez, A.; Font, X.; Barrena, R. Valorization of sugarcane bagasse and sugar beet molasses using Kluyveromyces marxianus for producing value-added aroma compounds via solid-state fermentation. J. Clean. Prod. 2017, 158, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Martínez, O.; Sánchez, A.; Font, X.; Barrena, R. Enhancing the bioproduction of value-added aroma compounds via solid-state fermentation of sugarcane bagasse and sugar beet molasses: Operational strategies and scaling-up of the process. Bioresour. Technol. 2018, 263, 136–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, S.C.; Vandenberghe, L.P.S.; Pereira, B.M.P.; Gago, F.D.; Rizzolo, J.A.; Pandey, A.; Soccol, C.R.; Medeiros, A.B.P. Improving fruity aroma production by fungi in SSF using citric pulp. Food Res. Int. 2009, 42, 484–486. [Google Scholar] [CrossRef]
- Medeiros, A.B.P.; Pandey, A.; Vandenberghe, L.P.S.; Pastore, G.M.; Soccol, C.R. Production and recovery of aroma compounds produced by solid-state fermentation using different adsorbents. Food Technol. Biotechnol. 2006, 44, 47–51. [Google Scholar]
- Christen, P.; Meza, J.C.; Revah, S. Fruity aroma production in solid state fermentation by Ceratocystis fimbriata: Influence of the substrate type and the presence of precursors. Mycol. Res. 1997, 101, 911–919. [Google Scholar] [CrossRef]
- Christen, P.; Bramorski, A.; Revah, S.; Soccol, C.R. Characterization of volatile compounds produced by Rhizopus strains grown on agro-industrial solid wastes. Bioresour. Technol. 2000, 71, 211–215. [Google Scholar] [CrossRef]
- De Aráujo, Á.A.; Pastore, G.M.; Berger, R.G. Production of Coconut Aroma by Fungi Cultivation in Solid-State Fermentation. In Applied Biochemistry and Biotechnology; Finkelstein, M., McMillan, J., Davison, B.H., Eds.; Humana Press: Totowa, NJ, USA, 2002; pp. 747–751. ISBN 9781461266211. [Google Scholar]
- Fadel, H.H.M.; Mahmoud, M.G.; Asker, M.M.S.; Lotfy, S.N. Characterization and evaluation of coconut aroma produced by Trichoderma viride EMCC-107 in solid state fermentation on sugarcane bagasse. Electron. J. Biotechnol. 2015, 18, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Da Penha, M.P.; da Rocha Leão, M.H.M.; Leite, S.G.F. Sugarcane bagasse as support for the production of coconut aroma by solid state fermentation (SSF). BioResources 2012, 7, 2366–2375. [Google Scholar] [CrossRef] [Green Version]
- Ladeira, N.C.; Peixoto, V.J.; Penha, M.P.; de Paula Barros, E.B.; Leite, S.G.F. Optimization of 6-pentyl-α-pyrone production by solid state fermentation using sugarcane bagasse as residue. BioResources 2010, 5, 2297–2306. [Google Scholar]
- Medeiros, A.B.; Pandey, A.; Freitas, R.J.; Christen, P.; Soccol, C.R. Optimization of the production of aroma compounds by Kluyveromyces marxianus in solid-state fermentation using factorial design and response surface methodology. Biochem. Eng. J. 2000, 6, 33–39. [Google Scholar] [CrossRef]
- Zheng, L.; Zheng, P.; Sun, Z.; Bai, Y.; Wang, J.; Guo, X. Production of vanillin from waste residue of rice bran oil by Aspergillus niger and Pycnoporus cinnabarinus. Bioresour. Technol. 2007, 98, 1115–1119. [Google Scholar] [CrossRef]
- Rodríguez Madrera, R.; Pando Bedriñana, R.; Suárez Valles, B. Production and characterization of aroma compounds from apple pomace by solid-state fermentation with selected yeasts. LWT Food Sci. Technol. 2015, 64, 1342–1353. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. 2-Phenylethanol (Rose Aroma) Production Potential of an Isolated Pichia kudriavzevii Through Solid-State Fermentation. Process. Biochem. 2020, 93, 94–103. [Google Scholar] [CrossRef]
- Mantzouridou, F.T.; Paraskevopoulou, A.; Lalou, S. Yeast flavour production by solid state fermentation of orange peel waste. Biochem. Eng. J. 2015, 101, 1–8. [Google Scholar] [CrossRef]
- Marella, E.R.; Dahlin, J.; Dam, M.I.; ter Horst, J.; Christensen, H.B.; Sudarsan, S.; Wang, G.; Holkenbrink, C.; Borodina, I. A single-host fermentation process for the production of flavor lactones from non-hydroxylated fatty acids. Metab. Eng. 2019, 61, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Cirlini, M.; Levante, A.; Dall’Asta, C.; Galaverna, G.; Lazzi, C. Volatile profile of elderberry juice: Effect of lactic acid fermentation using L. plantarum, L. rhamnosus and L. casei strains. Food Res. Int. 2018, 105, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Willrodt, C.; Hoschek, A.; Bühler, B.; Schmid, A.; Julsing, M.K. Decoupling production from growth by magnesium sulfate limitation boosts de novo limonene production. Biotechnol. Bioeng. 2015, 113, 1305–1314. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. Fed-Batch and Sequential-Batch Approaches to Enhance the Bioproduction of 2-Phenylethanol and 2-Phenethyl Acetate in Solid-State Fermentation Residue-Based Systems. J. Agric. Food Chem. 2019, 67, 3389–3399. [Google Scholar] [CrossRef]
- Xie, H.; Ma, Q.; Wei, D.Z.; Wang, F.Q. Transcriptomic analysis of Aspergillus niger strains reveals the mechanism underlying high citric acid productivity. Bioresour. Bioprocess. 2018, 5, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Sharma, P.; Singh, J.; Singh, S.; Nain, L. Prospecting the Potential of Agroresidues as Substrate for Microbial Flavor Production. Front. Sustain. Food Syst. 2020, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Try, S.; Voilley, A.; Chunhieng, T.; De-Coninck, J.; Waché, Y. Aroma compounds production by solid state fermentation, importance of in situ gas-phase recovery systems. Appl. Microbiol. Biotechnol. 2018, 102, 7239–7255. [Google Scholar] [CrossRef]
- Berger, R.G. Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 9783540493389. [Google Scholar]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Mazzoli, R.; Bosco, F.; Mizrahi, I.; Bayer, E.A.; Pessione, E. Towards lactic acid bacteria-based biorefineries. Biotechnol. Adv. 2014, 32, 1216–1236. [Google Scholar] [CrossRef]
- Spaggiari, M.; Ricci, A.; Calani, L.; Bresciani, L.; Neviani, E.; Dall’Asta, C.; Lazzi, C.; Galaverna, G. Solid state lactic acid fermentation: A strategy to improve wheat bran functionality. LWT 2020, 118, 108668. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Guido, A.; Liberatore, C.M.; Ganino, T.; Lazzi, C.; Chiancone, B. From byproduct to resource: Fermented apple pomace as beer flavoring. Foods 2019, 8, 309. [Google Scholar] [CrossRef] [Green Version]
- Ricci, A.; Marrella, M.; Hadj Saadoun, J.; Bernini, V.; Godani, F.; Dameno, F.; Neviani, E.; Lazzi, C. Development of Lactic Acid-Fermented Tomato Products. Microorganisms 2020, 8, 1192. [Google Scholar] [CrossRef]
- Martelli, F.; Cirlini, M.; Lazzi, C.; Neviani, E.; Bernini, V. Solid-State Fermentation of Arthrospira platensis to Implement New Food Products: Evaluation of Stabilization Treatments and Bacterial Growth on the Volatile Fraction. Foods 2021, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, V.G.; Kavitha, S.; Obulisamy, P.K.; Banu, J.R. Production of fine chemicals from food wastes. In Food Waste to Valuable Resources; Elsevier: Amsterdam, The Netherlands, 2020; pp. 163–188. [Google Scholar]
- Smid, E.J.; Kleerebezem, M. Production of Aroma Compounds in Lactic Fermentations. Annu. Rev. Food Sci. Technol. 2014, 5, 313–326. [Google Scholar] [CrossRef] [PubMed]
- García-Quintáns, N.; Repizo, G.; Martín, M.; Magni, C.; López, P. Activation of the diacetyl/acetoin pathway in Lactococcus lactis subsp. lactis bv. diacetylactis CRL264 by acidic growth. Appl. Environ. Microbiol. 2008, 74, 1988–1996. [Google Scholar] [CrossRef] [Green Version]
- Papagianni, M. Metabolic engineering of lactic acid bacteria for the production of industrially important compounds. Comput. Struct. Biotechnol. J. 2012, 3, e201210003. [Google Scholar] [CrossRef] [Green Version]
- Hugenholtz, J.; Kleerebezem, M.; Starrenburg, M.; Delcour, J.; De Vos, W.; Hols, P. Lactococcus lactis as a Cell Factory for High-Level Diacetyl Production. Appl. Environ. Microbl. 2000, 66, 4112–4114. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Kong, J.; Zhang, L.; Zhang, C.; Hu, S. Fine Tuning of the Lactate and Diacetyl Production through Promoter Engineering in Lactococcus lactis. PLoS ONE 2012, 7, e36296. [Google Scholar]
- Flahaut, N.A.L.; de Vos, W.M. Systems biology and metabolic engineering of lactic acid bacteria for improved fermented foods. In Advances in Fermented Foods and Beverages: Improving Quality, Technologies and Health Benefits; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 177–196. ISBN 9781782420248. [Google Scholar]
- Bancalari, E.; Montanari, C.; Levante, A.; Alinovi, M.; Neviani, E.; Gardini, F.; Gatti, M. Lactobacillus paracasei 4341 as adjunct culture to enhance flavor in short ripened Caciotta-type cheese. Food Res. Int. 2020, 135, 109284. [Google Scholar] [CrossRef]
- Levante, A.; Bancalari, E.; Tambassi, M.; Lazzi, C.; Neviani, E. Phenotypic Diversity of Lactobacillus casei group isolates as a selection criterion for use as secondary adjunct starters. Microorganisms 2020, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Lu, Y.; Yu, H.; Chen, Z.; Tian, H. Influence of 4 lactic acid bacteria on the flavor profile of fermented apple juice. Food Biosci. 2019, 27, 30–36. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Lactic acid fermentation drives the optimal volatile flavor-aroma profile of pomegranate juice. Int. J. Food Microbiol. 2017, 248, 56–62. [Google Scholar] [CrossRef]
- Nsogning Dongmo, S.; Sacher, B.; Kollmannsberger, H.; Becker, T. Key volatile aroma compounds of lactic acid fermented malt based beverages—Impact of lactic acid bacteria strains. Food Chem. 2017, 229, 565–573. [Google Scholar] [CrossRef]
- Szutowska, J. Functional properties of lactic acid bacteria in fermented fruit and vegetable juices: A systematic literature review. Eur. Food Res. Technol. 2020, 246, 357–372. [Google Scholar] [CrossRef]
- Ali Abdel-Rahman, M.; Tashiro, Y.; Sonomoto, K. Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: Overview and limits. J. Biotechnol. 2011, 156, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, G.; Yu, X.; Chen, H.; Sun, Y.; Chen, G. Pretreatment of corn stover by solid acid for D-lactic acid fermentation. Bioresour. Technol. 2017, 239, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Tarraran, L.; Mazzoli, R. Alternative strategies for lignocellulose fermentation through lactic acid bacteria: The state of the art and perspectives. FEMS Microbiol. Lett. 2018, 365, 126. [Google Scholar] [CrossRef]
- Escamilla-Hurtado, M.L.; Valdés-Martínez, S.E.; Soriano-Santos, J.; Gómez-Pliego, R.; Verde-Calvo, J.R.; Reyes-Dorantes, A.; Tomasini-Campocosio, A. Effect of culture conditions on production of butter flavor compounds by Pediococcus pentosaceus and Lactobacillus acidophilus in semisolid maize-based cultures. Int. J. Food Microbiol. 2005, 105, 305–316. [Google Scholar] [CrossRef]
| Agri-Food Waste | Pretreatment(s) | Aroma | Reference | |
|---|---|---|---|---|
| Mold | ||||
| Ceratocystis fimbriata | Citrus pulp + 25% sugarcane molasses (+50% soya bran) | Drying, milling, sieving | Fruity aroma | [56] |
| Coffee husks (+glucose) | Milling, steam treatment | Pineapple aroma (acetaldehyde, ethanol, isopropanol, ethyl acetate) | [49] | |
| Coffee husks | Drying, milling, sieving, sterilization | Fruity flavor | [57] | |
| Cassava bagasse, apple pomace, amaranth, soybean | Drying, milling, sieving, sterilization | Fruity aroma (+ amaranth and + banana aroma) | [52] | |
| Rhizopus oryzae | Wheat bran, cassava bagasse, sugarcane bagasse | Milling, sieving, sterilization. For sugar cane bagasse: preliminary washing | Fruity aroma (strong banana aroma) | [58] |
| Cassava bagasse, apple pomace, soybean, amaranth, soybean oil | Grinding, drying, sterilization | Acetaldehyde, Ethanol, 1-Propanol, Ethyl acetate, Ethyl propionate, 3-Methyl butanol | [59] | |
| Trichoderma viride | Sugarcane bagasse | N.d. | Coconut aroma, 6-pentyl-α-pyrone | [60] |
| Drying, milling | Coconut aroma, 6-pentyl-α-pyrone, from δ-Octalactone to Dodecalactone | [61] | ||
| Trichoderma harzianum | Sugarcane bagasse | Drying, milling | 6-Pentyl-α-pyrone | [62,63] |
| Kluyveromyces marxianus | Apple pomace, cassava bagasse, sugar cane bagasse, sunflower seeds, giant palm | Drying, milling, sieving, sterilization | Ethanol, ethyl acetate | [64] |
| Sugarcane bagasse + sugar beet molasses | Drying, milling, pH adjustment | Fruity aroma (43% alcohol, 35% esters) | [54] | |
| Drying, milling, pH adjustment | Fruity aroma | [55] | ||
| Aspergilius niger, Penicilium cinnabarium | Rice brain oil residue (+ferulic acid) | Water-ethanl extraction, pH adjustment, filter sterilization | Vanillin | [65] |
| Hanseniaspora velbyensis and uvarum, Saccharomyces cerevisiae | Apple peels | Drying, homogenization | 132 volatile compounds | [66] |
| Yeasts | ||||
| Pichia kudriavzevii | Sugarcane bagasse + l-phenylalanine | Drying, milling, pH adjustment | Rose aroma | [67] |
| Saccharomyces cerevisiae | Citrus peels | Slicing, grinding | Isoamylacetate, ethyl dodecanoate, ethyl decanoate, ethyl hexanoate | [68] |
| Yarrowia lipolytica (engineered) | Fatty feedstock | N.d. | Coconut like flavor (γ-dodecalactone, ẟ-decalactone) | [69] |
| Kefir (symbiotic yeasts and bacteria) | Food industrial wastes (cheese whey, molasses, brewer’s spent grains, malt spent rootlets, orange and potato pulp) | Blending | Ɛ-pinene | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadj Saadoun, J.; Bertani, G.; Levante, A.; Vezzosi, F.; Ricci, A.; Bernini, V.; Lazzi, C. Fermentation of Agri-Food Waste: A Promising Route for the Production of Aroma Compounds. Foods 2021, 10, 707. https://doi.org/10.3390/foods10040707
Hadj Saadoun J, Bertani G, Levante A, Vezzosi F, Ricci A, Bernini V, Lazzi C. Fermentation of Agri-Food Waste: A Promising Route for the Production of Aroma Compounds. Foods. 2021; 10(4):707. https://doi.org/10.3390/foods10040707
Chicago/Turabian StyleHadj Saadoun, Jasmine, Gaia Bertani, Alessia Levante, Fabio Vezzosi, Annalisa Ricci, Valentina Bernini, and Camilla Lazzi. 2021. "Fermentation of Agri-Food Waste: A Promising Route for the Production of Aroma Compounds" Foods 10, no. 4: 707. https://doi.org/10.3390/foods10040707
APA StyleHadj Saadoun, J., Bertani, G., Levante, A., Vezzosi, F., Ricci, A., Bernini, V., & Lazzi, C. (2021). Fermentation of Agri-Food Waste: A Promising Route for the Production of Aroma Compounds. Foods, 10(4), 707. https://doi.org/10.3390/foods10040707