Biowaste as a Potential Source of Bioactive Compounds—A Case Study of Raspberry Fruit Pomace
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Sample Preparation
2.2. Determination of the Total Phenolic, Flavonoid, and Anthocyanin Content
2.3. Antioxidant Assays
2.4. Determination of Polyphenolic Content
2.5. Statistical Analysis
3. Results and Discussion
3.1. The Total Phenolic, Flavonoid, and Anthocyanin Content of RFP
3.2. Antioxidant Activity of RFP
3.3. The Polyphenolic Content of RFP
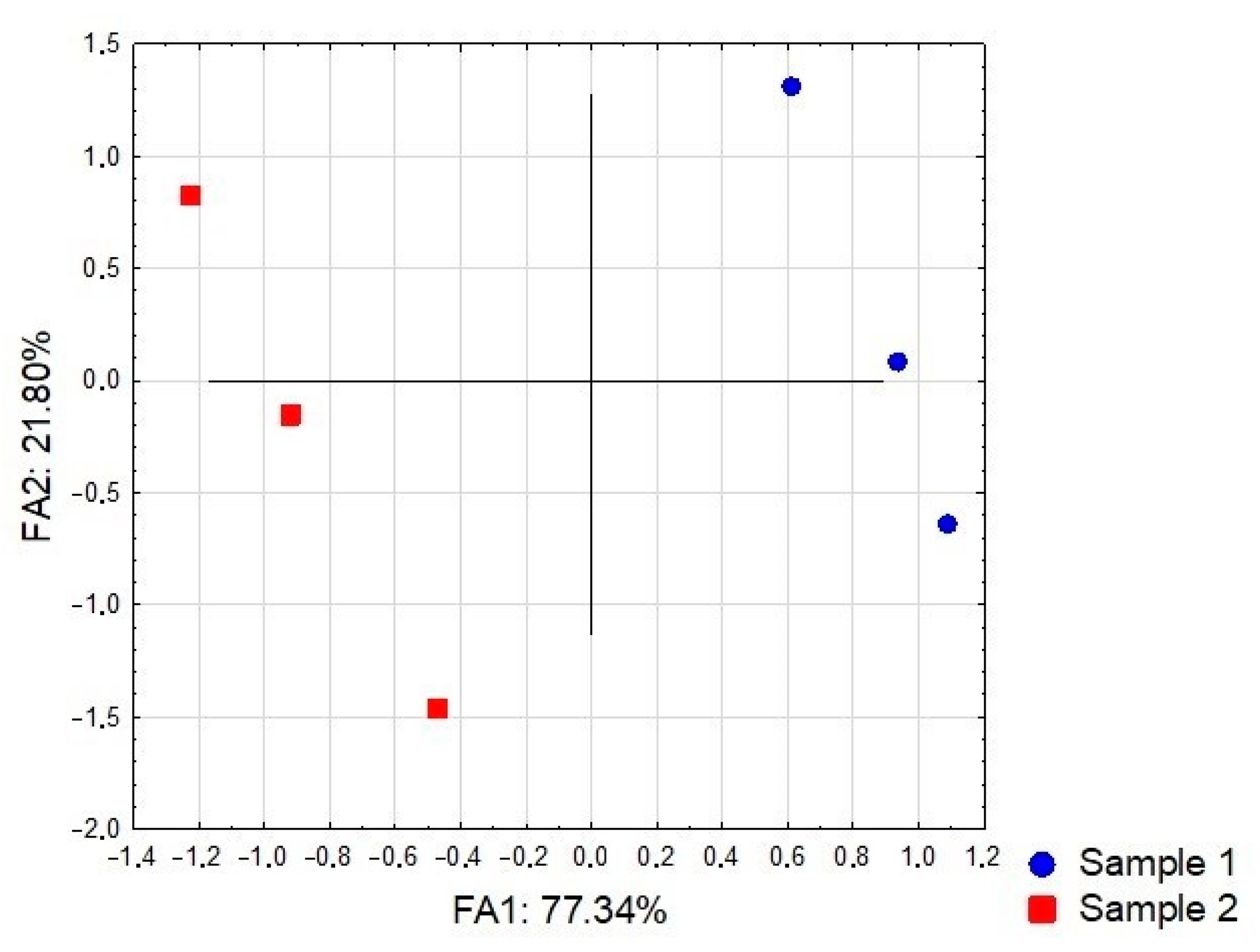
3.4. A Multivariate Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Münzel, T.; Daiber, A. Environmental Stressors and Their Impact on Health and Disease with Focus on Oxidative Stress. Antioxid. Redox Signal. 2018, 28, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Lodovici, M.; Bigagli, E. Oxidative stress and air pollution exposure. J. Toxicol. 2011, 2011, 487074. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- St. Angelo, A.; Vercellotti, D.J.; Jacks, T.; Legendre, M.M. Lipid oxidation in foods. Crit. Rev. Food Sci. Nutr. 1996, 36, 175–224. [Google Scholar] [CrossRef]
- Hosseini, B.; Berthon, B.S.; Saedisomeolia, A.; Starkey, M.R.; Collison, A.; Wark, P.A.B.; Wood, L.G. Effects of fruit and vegetable consumption on inflammatory biomarkers and immune cell populations: A systematic literature review and meta-analysis. Am. J. Clin. Nutr. 2018, 108, 136–155. [Google Scholar] [CrossRef]
- Bagatolli, P.C.D.; Cipriani, D.C.; Mariano, L.N.B.; Correa, M.; Wagner, T.M.; Noldin, V.F.; Cecknel Filho, V.; Niero, R. Phytochemical, Antioxidant and Anticancer Activities of Extracts of Seven Fruits Found in the Southern Brazilian Flora. Indian J. Pharm. Sci. 2016, 78, 34–40. [Google Scholar]
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2017, 58, 1–11. [Google Scholar] [CrossRef]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of funciomal compounds—Recent development. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Alok Sagar, N.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Comp. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Đilas, S.; Čanadanović-Brunet, J.; Ćetković, G. By-products of fruits processing as a source of phytochemicals. Chem. Ind. Chem. Eng. Q. 2009, 15, 191–202. [Google Scholar] [CrossRef]
- Shcieber, A. Side Streams of Plant Food Processing as a Source of Valuable Compounds: Selected Examples. Annu. Rev. Food Sci. Technol. 2017, 28, 97–112. [Google Scholar] [CrossRef]
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro-Food Byproducts as a New Source of Natural Food Additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef]
- Rao, A.V.; Snyder, D.M. Raspberries and human health: A review. J. Agric. Food Chem. 2010, 58, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Vara, A.L.; Pinela, J.; Dias, M.I.; Petrović, J.; Nogueira, A.; Soković, M.; Ferreira, I.C.F.R.; Barros, L. Compositional Features of the “Kweli” Red Raspberry and Its Antioxidant and Antimicrobial Activities. Foods 2020, 9, 1522. [Google Scholar] [CrossRef]
- East Fruit. Available online: https://east-fruit.com/en/news/fresh-raspberries-the-fastest-growing-export-segment-of-polish-horticulture/ (accessed on 7 August 2020).
- Tridge. Available online: https://www.tridge.com/intelligences/raspberry/ME/production (accessed on 7 August 2020).
- Brodowska, A.J. Raspberry pomace—Composition, properties and application. J. Biol. Res. 2017, 7, 86–96. [Google Scholar]
- Čandanović Brunet, J.; Vulić, J.; Ćebović, T.; Ćetković, G.; Čandanović, V.; Djilas, S.; Tumbaš Šaponjac, V. Phenolic Profile, Antiradical and Antitumour Evaluation of Raspberries Pomace Extract from Serbia. Iran. J. Pharm Res. 2017, 16, 142–152. [Google Scholar]
- Šarić, B.; Mišan, A.; Mandić, A.; Nedeljković, N.; Pojić, M.; Pestorić, M.; Đilas, S. Valorisation of raspberry and blueberry pomace through the formulation of value-added gluten-free cookies. J. Food Sci. Technol. 2016, 53, 1140–1150. [Google Scholar] [CrossRef]
- Ispiryan, A.; Viškelis, J. Valorisation of Raspberries By-Products for Food and Pharmaceutical Industries. Adv. Agric. Hortic. Entomol. 2019, 1, 1–6. [Google Scholar]
- Juranic, Z.; Zizak, Z.; Tasic, S.; Petrovic, S.; Nidzovic, S.; Leposavic, A.; Stanojkovic, T. Antiproliferative action of water extracts of seeds or pulp of five different raspberry cultivars. Food Chem. 2005, 93, 39–45. [Google Scholar] [CrossRef]
- Badin, E.E.; Rossi, Y.E.; Montenegro, M.A.; Ibarz, A.; Ribotta, P.D.; Lespinard, A.R. Thermal processing of raspberry pulp: Effect on the color and bioactive compounds. Food Bioprod. Process 2020, 124, 469–477. [Google Scholar] [CrossRef]
- Janković, M.; Zlatković, B.; Bukvić, B.; Stevanović, S.; Vukosavljević, P. Freeze-drying of raspberry pulp and extracted raspberry juice. J. Sci. Agric. Res. 2007, 68, 17–23. [Google Scholar]
- Khan, M.K.; Paniwnyk, L.; Hassan, S. Polyphenols as Natural Antioxidants: Sources, Extraction and Applications in Food, Cosmetics and Drugs. In Plant Based “Green Chemistry 2.0”. Green Chemistry and Sustainable Technology; Li, Y., Chemat, F., Eds.; Springer: Singapore, 2019; pp. 197–235. [Google Scholar] [CrossRef]
- Hidalgo, G.I.; Almajano, M.P. Red Fruits: Extraction of Antioxidants, Phenolic Content, and Radical Scavenging Determination: A Review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; ElSohly, M.A.; Khan, I.A. Assessment of Total Phenolic and Flavonoid Content, Antioxidant Properties, and Yield of Aeroponically and Conventionally Grown Leafy Vegetables and Fruit Crops: A Comparative Study. Evid. Based Complement. Alternat. Med. 2014, 2014, 253875. [Google Scholar] [CrossRef] [PubMed]
- Guisti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. In Current Protocols in Food Analytical Chemistry; Wiley Publishing: Hoboken, NJ, USA, 2001; pp. F1.2.1–F1.2.13. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Brand Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Tech. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Blois, M. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Salaj, N.; Kladar, N.; Srđenović Čonić, B.; Jeremić, K.; Barjaktarović, J.; Hitl, M.; Gavrić, N.; Božin, B. Stabilization of sunflower and olive oils with savory (Satureja kitaibelii, Lamiaceae). J. Food Nutr. Res. 2020, 59, 259–271. [Google Scholar]
- Zhou, B.; Wu, Z.; Li, X.; Zhang, J.; Hu, X. Analysis of ellagic acid in pomegranate rinds by capillary electrophoresis and high-performance liquid chromatography. Phytochem. Anal. 2008, 19, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Vulić, J.; Velićanski, A.; Ćetejević Simin, D.; Tumbas Šaponjac, V.; Djilas, S.; Cvetković, D.; Markov, S. Antioxidant, antiproliferative and antimicrobial activity of freeze-dried raspberry. Acta Period. Technol. 2014, 45, 99–116. [Google Scholar] [CrossRef]
- Zorzi, M.; Gai, F.; Medana, C.; Aigotti, R.; Morello, S.; Peiretti, P.G. Bioactive Compounds and Antioxidant Capacity of Small Berries. Foods 2018, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Kostecka-Gugała, A.; Ledwożyw-Smoleń, I.; Augustynowicz, J.; Wyżgolik, G.; Kruczek, M.; Kaszycki, P. Antioxidant properties of fruits of raspberry and blackberry grown in central Europe. Open Chem. 2015, 13, 1313–1325. [Google Scholar] [CrossRef]
- Četojević Simin, D.; Ranitović, A.; Cvetković, D.; Markov, S.; Vinčić, M.; Djilas, S. Bioactivity of blackberry (Rubus fruticosus L.) pomace: Polyphenol content, radical scavenging, antimicrobial and antitumor activity. Acta Period. Technol. 2017, 48, 63–76. [Google Scholar] [CrossRef]
- Gavrilas, S.; Stanescu, D.M. Improving the Antioxidant Properties of Wild Raspberry Juice by Enzymatic Treatments. Rev. Chim. 2016, 67, 219–222. [Google Scholar]
- Azofeifa, G.; Quesada, S.; Perez, A.M. Effect of the microfiltration process on antioxidant activity and lipid peroxidation protection capacity of blackberry juice. Rev. Bras. Farmacogn. 2011, 21, 829–834. [Google Scholar] [CrossRef]
- Konić Ristić, A.; Šavikin, K.; Zdunić, G.; Janković, T.; Juranic, Z.; Menković, N.; Stanković, I. Biological activity and chemical composition of different berry juices. Food Chem. 2011, 125, 1412–1417. [Google Scholar] [CrossRef]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, rubus, and ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef]
- Plessi, M.; Bertelli, D.; Albasini, A. Distribution of metals and phenolic compounds as a criterion to evaluate variety of berries and related jams. Food Chem. 2007, 100, 419–427. [Google Scholar] [CrossRef]
- Kabtni, S.; Sdouga, D.; Bettaib Rebey, I.; Save, M.; Trifi Farah, N.; Fauconnier, M.L.; Marghali, S. Influence of climate variation on phenolic composition and antioxidant capacity of Medicago minima populations. Sci. Rep. 2020, 10, 8293. [Google Scholar] [CrossRef]
- Alam, Z.; Morales, H.; Roncal, J. Environmental conditions affect phenolic content and antioxidant capacity of leaves and fruit in wild partridgeberry (Vaccinium vitis-idaea). Botany 2016, 94, 509–521. [Google Scholar] [CrossRef]
- IRO Raspberry. Available online: https://www.internationalraspberry.net/ (accessed on 7 August 2020).
- Fierascu, R.C.; Sieniawska, I.; Ortan, A.; Fierascu, I.; Xiao, J. Fruits By-Products—A Source of Valuable Active Principles. A Short Review. Front. Bioeng. Biotechnol. 2020, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Singh Pansear, P.; Bandhu Bera, M. Comparative Study on the Extraction and Quantification of Polyphenols from Citrus Peels Using Maceration and Ultrasonic Technique. Curr. Res. Nutr. Food Sci. 2019, 7, 678–685. [Google Scholar] [CrossRef]
- Putnik, P.; Bursać Kovačević, D.; Radojičin, M.; Dragović Uzelac, M. Influence of Acidity and Extraction Time on the Recovery of Flavonoids from Grape Skin Pomace Optimized by Response Surface Methodology. Chem. Biochem. Eng. Q. 2016, 30, 455–464. [Google Scholar] [CrossRef]
- Baraniak, B.; Szymanowska, U. Antioxidant and Potentially Anti-Inflammatory Activity of Anthocyanin Fractions from Pomace Obtained from Enzymatically Treated Raspberries. Antioxidants 2019, 8, 299. [Google Scholar] [CrossRef]
- Ermeeva, N.; Makarova, N.; Zhidikova, E.; Maximova, V.; Lesova, E. Ultrasonic and microwave activation of raspberry extract: Antioxidant and anti-carcinogenic properties. Foods Raw Mater. 2019, 7, 237–264. [Google Scholar] [CrossRef]
- Szeto, Y.T.; Tomlinson, B.; Benzie, I.F.F. Total antioxidant and ascorbic acid content of fresh fruits and vegetables:implications for dietary planning and food preservation. Br. J. Nutr. 2002, 87, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Koss Mikołajczyk, I.; Kusznierewicz, B.; Bartoszek, A. The Relationship between Phytochemical Composition and Biological Activities of Differently Pigmented Varieties of Berry Fruits; Comparison between Embedded in Food Matrix and Isolated Anthocyanins. Foods 2019, 8, 646. [Google Scholar] [CrossRef]
- Xue, H.; Tan, J.; Li, Q.; Tang, J.; Cai, X. Optimization Ultrasound-Assisted Deep Eutectic Solvent Extraction of Anthocyanins from Raspberry Using Response Surface Methodology Coupled with Genetic Algorithm. Foods 2020, 9, 1409. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Qureshi, M.N.; Stecher, G.; Bonn, G.K. Quantification of polyphenolic compounds and flavonoids in Achillea millefolium and Equisetum arvense. Pak. J. Pharm. Sci. 2016, 29, 1519–1523. [Google Scholar]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect of human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Daniel, E.M.; Krupnick, A.S.; Heur, Y.H.; Blinzer, J.A.; Nims, R.W.; Stoner, G.D. Extraction, stability, and quantitation of ellagic acid in various fruits and nuts. J. Food Compos. Anal. 1989, 2, 338–349. [Google Scholar] [CrossRef]
- Muthukumaran, S.; Tranchant, C.; Shi, J.; Ye, X.; Xue, S.J. Ellagic acid in strawberry (Fragaria spp.): Biological, technological stability and human health aspects. Food Qual. Saf. 2017, 1, 227–252. [Google Scholar] [CrossRef]

| Type of Extraction | TPC (mg/L GAE) | TFC (mg/g QE) | TAC (mg/L C3G) |
|---|---|---|---|
| UAE | 27.79 ± 1.25 | 8.02 ± 0.31 | 7.13 ± 0.43 |
| CM | 8.49 ± 1.45 | 3.16 ± 0.35 | 4.87 ± 0.38 |
| Species and Sample Type | TPC | TFC | TAC |
|---|---|---|---|
| Rubus idaeus L. | |||
| Freeze-dried fruit [36] | 2209.86 ± 70.32 mg GAE/100 g GAE | 831.87 ± 12.61 mg/100 g RE | 144.55 ± 0.39 mg/100 g C3G |
| Rubus fruticosus | |||
| Lyophilized fruit [37] | N/A | 6.54–24.00 mg/g RE | 0.57–2.10 mg/L C3G |
| Rubus idaeus L. | |||
| Fruit | 234 ± 5.1 mg/100 g GAE | N/A | 68.00 mg/100 g C3G |
| Pomace [19] | 633.7 mg/100 g GAE | 591.65 mg/100 g RE | 65.21 mg/100 g C3G |
| Rubus idaeus L. | |||
| Freeze-dried fresh fruit [38] | 169 ± 4–2494 ± 77 mg/100 g GAE | N/A | 10.56 ± 1.73–113.6 ± 7.7 mg/100 g C3G |
| Rubus fruticosus | |||
| Fruit pomace [39] | 7.97 ± 0.31–88.28 ±3.48 g/kg GAE | 4.11 ± 0.20–45.51 ± 2.16 g/kg RE | 1.14 ± 0.04–12.61 ± 0.48 g/kg C3G |
| Rubus fruitcosus Juice [40] | N/A | 1.11 ± 0.04–1.20 ± 0.02 QE g/L−1 | 0.09 ± 0.01–0.12 ± 0.02 g/L |
| Rubus fruticosus | |||
| Juice [41] | 24.9 ± 1.0 mg/g GAE | N/A | 5.6 ± 0.3 mg/g C3G |
| Rubus idaeus L. | |||
| Juice [42] | 164.4 ± 5.1 mg/100 g GAE | N/A | 0.08 ± 0.01% C3G |
| Vaccinium spp. | 171 ± 12–961 ± 15 mg/100 g GA | 34 ± 1.0–515 ± 3.6 mg/100 g C3G | |
| Rubus spp. | 126 ± 0.3–1079 ±34 mg/100 g GAE | N/A | 52 ± 0.6–627 ± 8.3 mg/100 g C3G |
| Ribes spp. | 191 ± 17–1790 ± 5 mg/100 g GAE | 14 ± 0.4–411 ± 12 mg/100 g C3G | |
| Fruit [43] | |||
| Rubus idaeus L. | |||
| Frozen fruit | 0.251–0.321 g/100 g GAE | N/A | 0.016–0.078 g/100 g C3G |
| Jam [44] | 0.218–0.361 g/100 g GAE | 0.007–0.021 g/100 g C3G |
| Type of Extraction | FRAP (µmol/L) | DPPH | ||
|---|---|---|---|---|
| AAE (µmol/mL vit C Eq) | IC50 (µL/mL) | Trolox Eq (µmol/100 g Trolox Eq) | ||
| UAE | 1002.72 ± 12.20 | 969.71 ± 8.2 | 20.00 ± 2.02 | 567.00 ± 4.56 |
| CM | 772.73 ± 10.50 | 931.80 ± 7.9 | 37.40 ± 2.43 | 361.27 ± 5.65 |
| Species and Sample Type | DPPH | FRAP |
|---|---|---|
| Rubus idaeus L. Pomace [51] | IC50 = 8.15–12.92 mg/mL FW | N/A |
| Rubus fruticosus L. Pomace [39] | 1.03 ± 0.03–2.12 ± 0.07 mmol TEAC g−1 | N/A |
| Rubus idaeus L. Fruit extract [52] | EC50 = 31.5 mg/cm3 | 10.08 mmol Fe2+/kg RM |
| Various fresh fruits [53] | N/A | 1460–15,940 μmol/kg FW |
| µg/g Extract | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Extraction | Galic Acid | Caffeic Acid | p-Coumaric Acid | |||||||||
| Met 1 | Met 2 | Met 1 | Met 2 | Met 1 | Met 2 | |||||||
| x | u | x | u | x | u | x | u | x | u | x | u | |
| UAE | 8.75 | 1.31 | 7.92 | 1.19 | 19.17 | 0.96 | 17.36 | 0.87 | 0.56 | 0.06 | 0.51 | 0.05 |
| CM | 6.36 | 0.95 | 5.69 | 0.85 | 9.95 | 0.50 | 8.90 | 0.45 | 0.95 | 0.09 | 0.85 | 0.08 |
| Type of Extraction | Quercetin | Chlorogenic Acid | Ellagic Acid | |||||||||
| Met 1 | Met 2 | Met 1 | Met 2 | Met 1 | Met 2 | |||||||
| x | u | x | u | x | u | x | u | x | u | x | u | |
| UAE | 0.06 | 0.00 | 0.06 | 0.00 | 3.56 | 0.18 | 3.22 | 0.16 | 105.52 | 4.22 | 95.59 | 3.82 |
| CM | 1.27 | 0.02 | 0.24 | 0.02 | 1.55 | 0.08 | 1.38 | 0.07 | 55.00 | 2.2 | 49.15 | 1.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krivokapić, S.; Vlaović, M.; Damjanović Vratnica, B.; Perović, A.; Perović, S. Biowaste as a Potential Source of Bioactive Compounds—A Case Study of Raspberry Fruit Pomace. Foods 2021, 10, 706. https://doi.org/10.3390/foods10040706
Krivokapić S, Vlaović M, Damjanović Vratnica B, Perović A, Perović S. Biowaste as a Potential Source of Bioactive Compounds—A Case Study of Raspberry Fruit Pomace. Foods. 2021; 10(4):706. https://doi.org/10.3390/foods10040706
Chicago/Turabian StyleKrivokapić, Slađana, Milorad Vlaović, Biljana Damjanović Vratnica, Andrej Perović, and Svetlana Perović. 2021. "Biowaste as a Potential Source of Bioactive Compounds—A Case Study of Raspberry Fruit Pomace" Foods 10, no. 4: 706. https://doi.org/10.3390/foods10040706
APA StyleKrivokapić, S., Vlaović, M., Damjanović Vratnica, B., Perović, A., & Perović, S. (2021). Biowaste as a Potential Source of Bioactive Compounds—A Case Study of Raspberry Fruit Pomace. Foods, 10(4), 706. https://doi.org/10.3390/foods10040706






