Primary Processing and Storage Affect the Dominant Microbiota of Fresh and Chill-Stored Sea Bass Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Provision and Storage of Sea Bass
2.2. Rejection Time
2.3. Microbiological Analysis
2.4. Identification of Bacteria Isolated from the Sea Bass Products
2.4.1. Isolation of Colonies and DNA Extraction
2.4.2. HRM Analysis
2.4.3. Sequencing
2.5. Statistical Analysis
3. Results
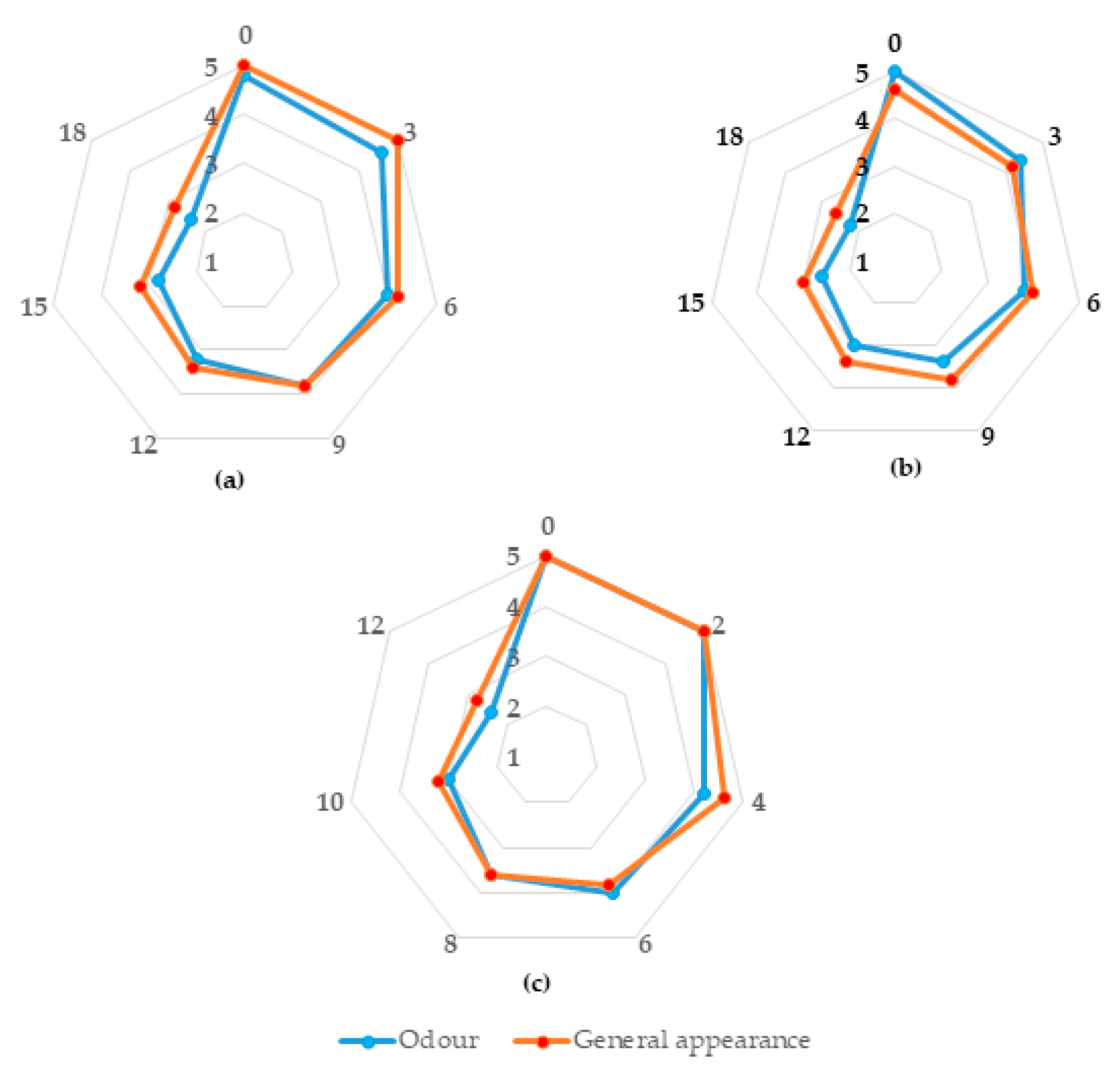
3.1. Shelf-Life of Sea Bass Products
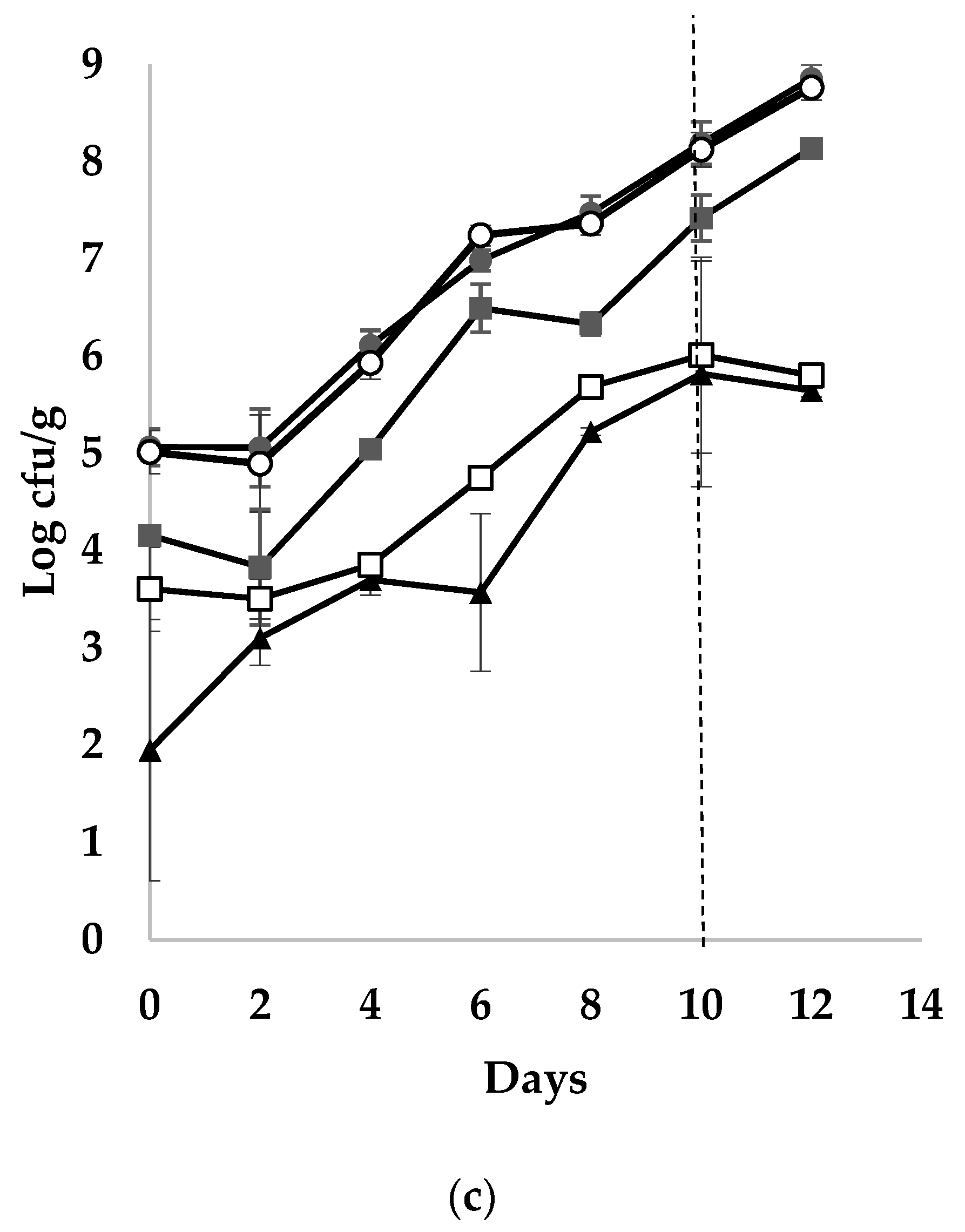
3.2. Microbial Populations
3.3. Bacterial Communities of Fresh and Chill-Stored Sea Bass Products
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock—A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K.; et al. The Future of Aquatic Protein: Implications for Protein Sources in Aquaculture Diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018. [Google Scholar]
- Food and Agriculture Organization (FAO). Fisheries and Aquaculture Department. 2020. Available online: http://www.fao.org/fishery/countrysector/naso_greece/en (accessed on 20 October 2020).
- Parlapani, F.F. Microbial Diversity of Seafood. Curr. Opin. Food Sci. 2021, 37, 45–51. [Google Scholar] [CrossRef]
- Gram, L.; Huss, H.H. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef]
- Boziaris, I.S.; Parlapani, F.F. Specific Spoilage Organisms (SSO) in Fish. In Microbiological Quality of Food: Foodborne Spoilers; Bevilacqua, A., Corbo, M.R., Sinigaglia, M., Sykes, R., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 60–98. [Google Scholar]
- Liu, Y.; Singh, P.; Mustapha, A. High-resolution melt curve PCR assay for specific detection of E. coli O157:H7 in beef. Food Control 2018, 86, 275–282. [Google Scholar] [CrossRef]
- Omiccioli, E.; Amagliani, G.; Brandi, G.; Magnani, M. A new platform for Real-Time PCR detection of Salmonella spp., Listeria monocytogenes and Escherichia coli O157 in milk. Food Microbiol. 2009, 26, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Parlapani, F.F.; Syropoulou, F.; Tsiartsafis, A.; Ekonomou, S.; Madesis, P.; Exadactylos, A.; Boziaris, I.S. HRM analysis as a tool to facilitate identification of bacteria from mussels during storage at 4 °C. Food Microbiol. 2020, 85, 103304. [Google Scholar] [CrossRef] [PubMed]
- Tamburro, M.; Ripabelli, G. High Resolution Melting as a rapid, reliable, accurate and cost-effective emerging tool for genotyping pathogenic bacteria and enhancing molecular epidemiological surveillance: A comprehensive review of the literature. Ann. Ig. 2017, 29, 293–316. [Google Scholar]
- Syropoulou, F.; Parlapani, F.F.; Bosmali, I.; Madesis, P.; Boziaris, I.S. HRM and 16S rRNA gene sequencing reveal the cultivable microbiota of the European seabass during ice storage. Int. J. Food Microbiol. 2020, 327, 108658. [Google Scholar] [CrossRef]
- Howgate, P.; Johnston, A.; Whittle, K.J. Multilingual Guide to EC Freshness Grades for Fishery Products; Marine Laboratory, Scottish Office of Agriculture, Environment and Fisheries Department: Aberdeen, UK, 1992. [Google Scholar]
- Gram, L.; Trolle, G.; Huss, H.H. Detection of specific spoilage bacteria from fish stored at low (0oC) and high (20oC) temperatures. Int. J. Food Microbiol. 1987, 4, 65–72. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. Available online: https://pubmed.ncbi.nlm.nih.gov/22933715/ (accessed on 25 October 2020). [CrossRef]
- Basic Local Alignment Search Tool (BLAST). Available online: http://www.ncbi.nlm.nih.gov/BLAST/ (accessed on 25 October 2020).
- geneious. Available online: http://www.geneious.com (accessed on 25 October 2020).
- Parlapani, F.F.; Verdos, G.I.; Haroutounian, S.A.; Boziaris, I.S. The dynamics of Pseudomonas and volatilome during the spoilage of gutted sea bream stored at 2 °C. Food Control 2015, 55, 257–265. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Kormas, K.A.; Boziaris, I.S. Microbiological changes, shelf-life and identification of initial and spoilage microbiota of sea bream fillets stored under various conditions using 16S rRNA gene analysis. J. Sci. Food Agric. 2015, 95, 2386–2394. [Google Scholar] [CrossRef] [PubMed]
- Tryfinopoulou, P.; Tsakalidou, E.; Nychas, G.-J.E. Characterization of Pseudomonas spp. associated with spoilage of gilt-head sea bream stored under various conditions. Appl. Environ. Microbiol. 2002, 68, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Boulares, M.; Mankai, M.; Aouadhi, C.; Olfa, B.M.; Hassouna, M. Characterisation and identification of spoilage psychotrophic Gram-negative bacteria originating from Tunisian fresh fish. Ann. Microbiol. 2013, 63, 733–744. [Google Scholar] [CrossRef]
- Duan, S.; Zhou, X.; Xiao, H.; Miao, J.; Zhao, L. Characterization of Bacterial Microbiota in Tilapia Fillets under Different Storage Temperatures. J. Food Sci. 2019, 84, 1487–1493. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Nychas, G.-J.E. Application of a systematic experimental procedure to develop a microbial model for rapid fish shelf-life predictions. Int. J. Food Microbiol. 2000, 60, 171–184. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Boziaris, I.S. Monitoring of spoilage and determination of microbial communities based on 16S rRNA gene sequence analysis of whole sea bream stored at various temperatures. LWT-Food Sci. Technol. 2016, 66, 553–559. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Giannakourou, M.C.; Taoukis, P.S.; Nychas, G.-J.E. Application of shelf life decision system (SLDS) to marine cultured fish quality. Int. J. Food Microbiol. 2002, 73, 375–382. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Haroutounian, S.A.; Nychas, G.-J.E.; Boziaris, I.S. Microbiological spoilage and volatiles production of gutted European sea bass stored under air and commercial modified atmosphere package at 2 °C. Food Microbiol. 2015, 50, 44–53. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Chouliara, I.; Badeka, A.; Savvaidis, I.N.; Kontominas, M.G. Effect of gutting on microbiological, chemical and sensory properties of aquacultured sea bass (Dicentrarchus labrax) stored in ice. Food Microbiol. 2003, 20, 411–420. [Google Scholar] [CrossRef]
- Taliadourou, D.; Papadopoulos, V.; Domvridou, E.; Savvaidis, I.N.; Kontominas, M.G. Microbiological, chemical and sensory changes of whole and filleted Mediterranean aquacultured sea bass (Dicentrarchus labrax) stored in ice. J. Sci. Food Agric. 2003, 83, 1373–1379. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Nychas, G.-J.E. Chemical and sensory changes associated with microbial flora of Mediterranean Boque (Boops boops) stored aerobically at 0, 3, 7 and 10 °C. Appl. Environ. Microbiol. 1999, 65, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Parlapani, F.F.; Malouchos, A.; Haroutounian, S.A.; Boziaris, I.S. Microbial and non-microbial origin volatile organic compounds produced on model fish substrate inoculated or not with gilt-head sea bream spoilage bacteria. LWT Food Sci. Technol. 2017, 78, 54–62. [Google Scholar] [CrossRef]
- Alvarez-Ordóñez, A.; Leong, D.; Morgan, C.A.; Hill, C.; Gahan, C.G.; Jordan, K. Occurrence, Persistence, and Virulence Potential of Listeria ivanovii in Foods and Food Processing Environments in the Republic of Ireland. BioMed Res. Int. 2015, 350526. [Google Scholar] [CrossRef]
- Pellegrino, R.; Crandall, P.G.; Seo, H.-S. Hand washing and disgust response to handling different food stimuli between two different cultures. Food Res. Int. 2015, 76 Pt 2, 301–308. [Google Scholar] [CrossRef]
- Yap, M.; Chau, M.L.; Hartantyo, S.H.P.; Oh, J.Q.; Aung, K.T.; Gutiérrez, R.A.; Ng, L.C. Microbial Quality and Safety of Sushi Prepared with Gloved or Bare Hands: Food Handlers’ Impact on Retail Food Hygiene and Safety. J. Food Prot. 2019, 82, 615–622. [Google Scholar] [CrossRef] [PubMed]


| Groups | Isolates | Peaks (°C) | Phylotypes | Closest Relatives | Identity (%) | GenBank Number * |
|---|---|---|---|---|---|---|
| 1 | 51 | 85.3 ± 0.1 | SBS-FS1 | Pseudomonas gessardii | 100 | MT626825 |
| 2 | 23 | 85.25 ± 0.05 | SBS-FS2 | Pseudomonas putida | 100 | MT626824 |
| 3 | 21 | 85.3 ± 0.1 | SBS-FS3 | Pseudomonas sp. | 99.75 | MT626826 |
| 4 | 10 | 85.9 ± 0.1 | SBS-FS4 | Pseudomonas sp. | 99.75 | MT507079 |
| 5 | 6 | 85.5 ± 0.1 | SBS-FS5 | Pseudomonas sp. | 100 | MT585910 |
| 6 | 24 | 87.2 ± 0.1 | SBS-FS6 | Shewanella baltica | 100 | MT516290 |
| 7 | 4 | 86.8 ± 0.1 | SBS-FS7 | Shewanella putrefaciens | 99.75 | AB205575 |
| 8 | 3 | 86.8 | SBS-FS8 | Shewanella putrefaciens | 99.75 | MN865784 |
| 9 | 10 | 86.6 ± 0.1 | SBS-FS9 | Aeromonas hydrophila | 100 | MT416424 |
| 10 | 2 | 87.4 | SBS-FS10 | Bacillus paralicheniformis | 99.74 | MT645610 |
| 11 | 4 | 86.95 ± 0.05 | SBS-FS11 | Serratia grimesii | 100 | MN826574 |
| 12 | 2 | 86.8 | SBS-FS12 | Serratia fonticola | 100 | CP054160 |
| 13 | 2 | 87.9 | SBS-FS13 | Klebsiella oxytoca | 100 | MN967236 |
| 14 | 14 | 85.2 ± 0.1 | SBS-FS14 | Pseudomonas fluorescens | 99.75 | MT624739 |
| 15 | 4 | 86.4 ± 0.1 | SBS-FS15 | Stenotrophomonas maltophilia | 100 | CP040432 |
| 16 | 4 | 85.1 ± 0.1 | SBS-FS16 | Pseudomonas fragi | 99.75 | MT631986 |
| 17 | 6 | 85.7 ± 0.1 | SBS-FS17 | Staphylococcus warneri | 100 | MT642942 |
| 18 | 6 | 85.9 ± 0.1 | SBS-FS18 | Rouxiella sp. | 100 | MK590241 |
| 19 | 4 | 87.8 ± 0.1 | SBS-FS19 | Enterobacter ludwigii | 99.75 | MT507089 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syropoulou, F.; Parlapani, F.F.; Kakasis, S.; Nychas, G.-J.E.; Boziaris, I.S. Primary Processing and Storage Affect the Dominant Microbiota of Fresh and Chill-Stored Sea Bass Products. Foods 2021, 10, 671. https://doi.org/10.3390/foods10030671
Syropoulou F, Parlapani FF, Kakasis S, Nychas G-JE, Boziaris IS. Primary Processing and Storage Affect the Dominant Microbiota of Fresh and Chill-Stored Sea Bass Products. Foods. 2021; 10(3):671. https://doi.org/10.3390/foods10030671
Chicago/Turabian StyleSyropoulou, Faidra, Foteini F. Parlapani, Stefanos Kakasis, George-John E. Nychas, and Ioannis S. Boziaris. 2021. "Primary Processing and Storage Affect the Dominant Microbiota of Fresh and Chill-Stored Sea Bass Products" Foods 10, no. 3: 671. https://doi.org/10.3390/foods10030671
APA StyleSyropoulou, F., Parlapani, F. F., Kakasis, S., Nychas, G.-J. E., & Boziaris, I. S. (2021). Primary Processing and Storage Affect the Dominant Microbiota of Fresh and Chill-Stored Sea Bass Products. Foods, 10(3), 671. https://doi.org/10.3390/foods10030671








