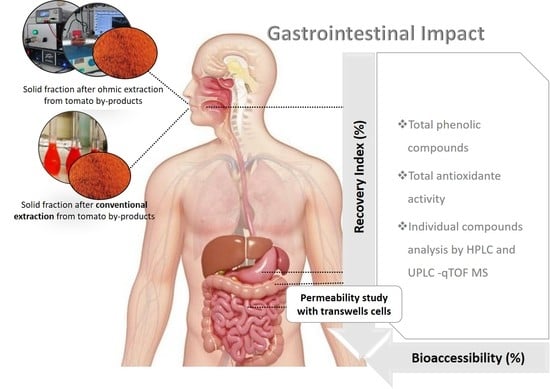
In Vitro Gastrointestinal Digestion Impact on the Bioaccessibility and Antioxidant Capacity of Bioactive Compounds from Tomato Flours Obtained after Conventional and Ohmic Heating Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Tomato Bagasse Flours
2.3. Chemical Composition of TBF—Proximate Composition of Tomato Bagasse Flours
2.4. Bioactive Phytochemicals
2.4.1. Phenolic Compound Quantification: Total, Free, and Bound Profiles
2.4.2. Total Antioxidant Activity and Total Carotenoids Content
2.4.3. Qualitative and Quantitative Profiles of Polyphenols and Carotenoids
HPLC Analysis
UPLC-qTOF MS Analysis
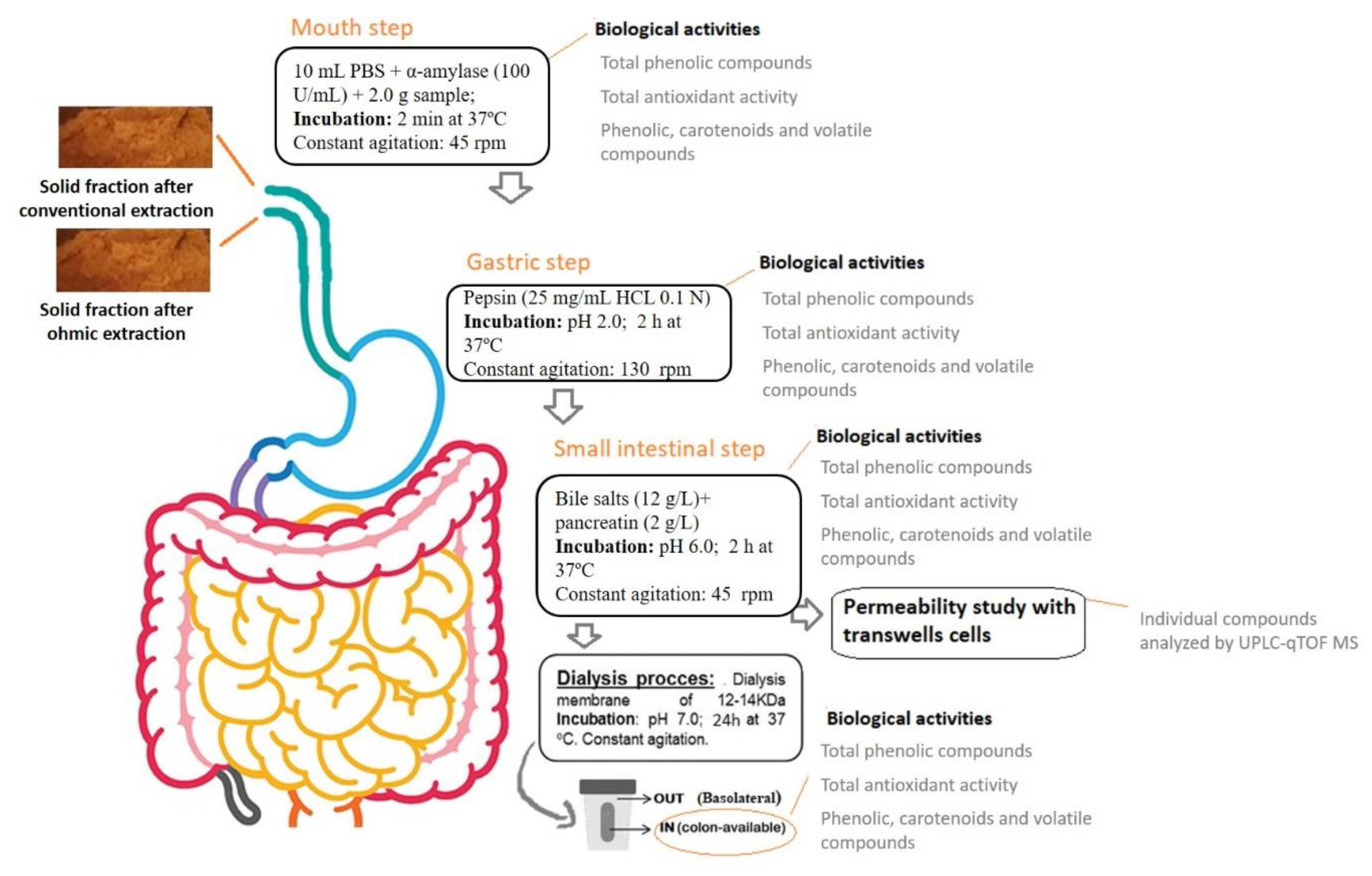
2.5. In Vitro Gastrointestinal Digestion
2.6. Simulated Digestion and Transepithelial Diffusion across Intestinal (Caco-2/HT29-MTX) Cell Layers
2.6.1. Cell Culture
2.6.2. Cell Layer Integrity
2.6.3. Permeability Assay
2.7. Volatile Compounds
2.8. Recovery and Bioaccessibility Indexes of Polyphenolic and Carotenoids Compounds Throughout In Vitro Gastrointestinal Digestion
2.9. Mutagenicity
2.10. Statistical Analysis
3. Results
3.1. Characterization of Solid Fractions Obtained after OH and CONV Extraction of Tomato Bagasse
3.2. Total Phenolic Compounds, and Individual Compounds throughout the Digestive Tract and Antioxidant Activity
3.3. Carotenoid-Derived Aroma Compounds
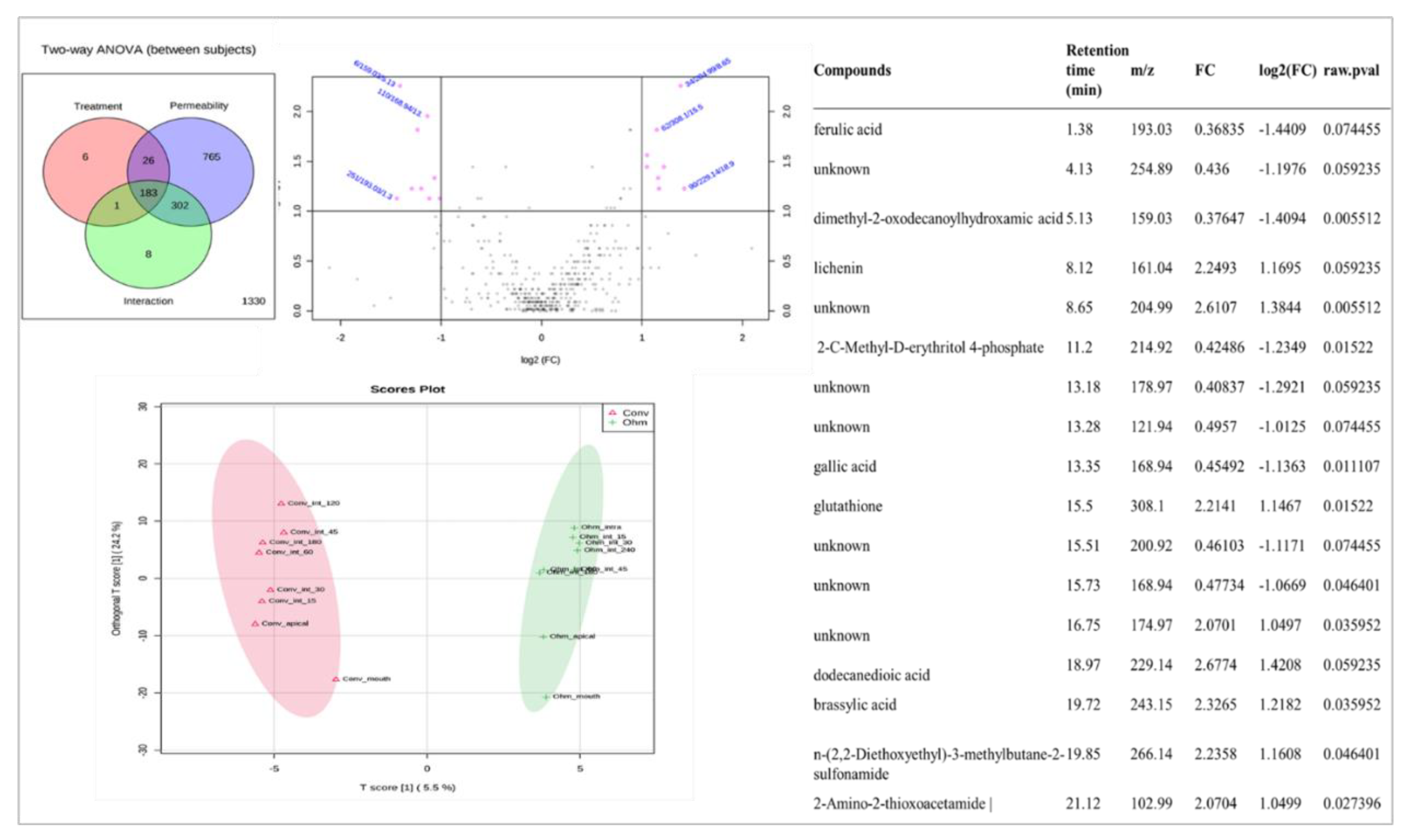
3.4. Metabolomics Analysis—LC-ESI-UHR-QqTOF-MS
3.5. Mutagenicity
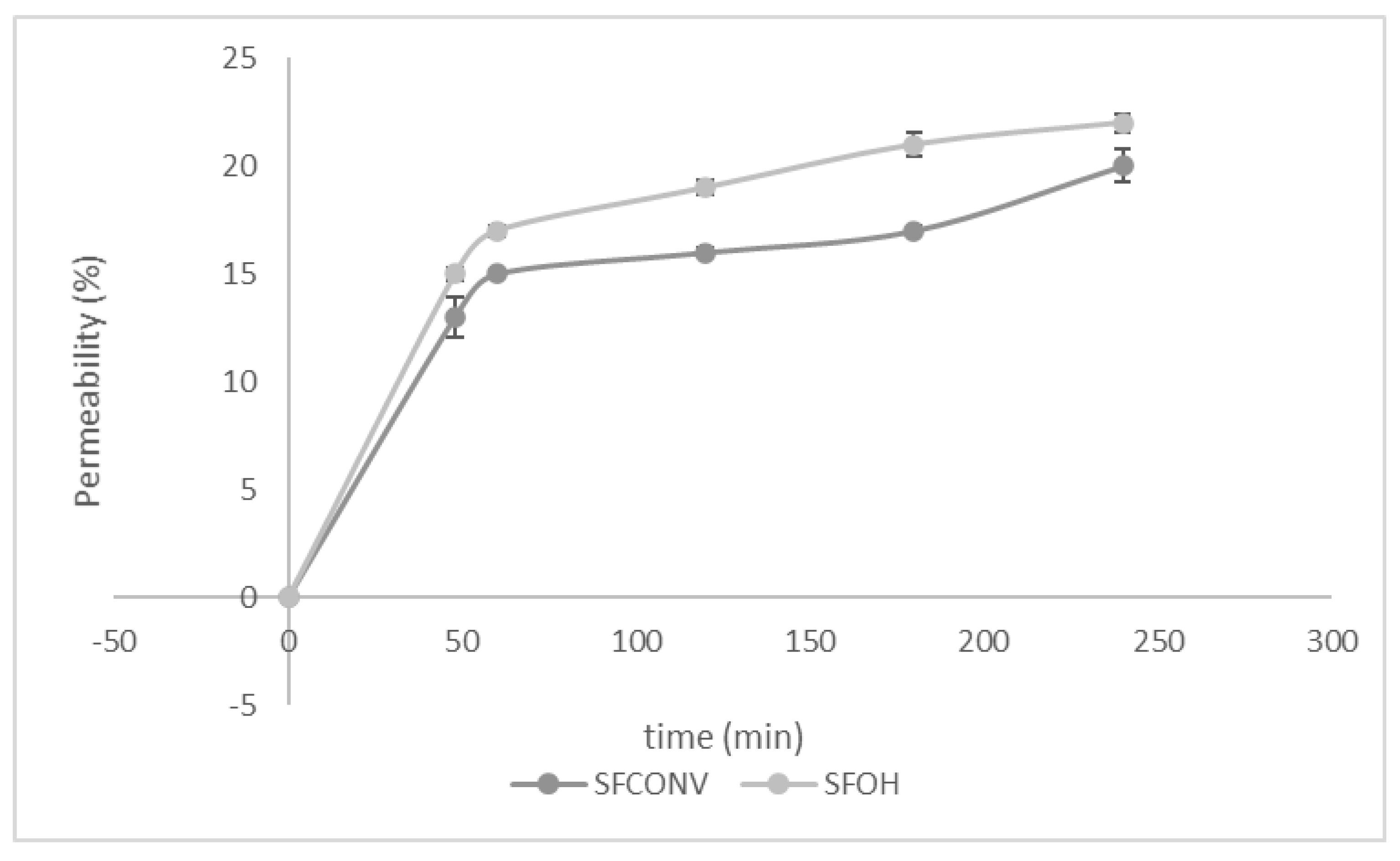
3.6. Permeability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coelho, M.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Extraction of tomato by-products’ bioactive compounds using ohmic technology. Food Bioprod. Process. 2019, 117, 329–339. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QL (accessed on 21 December 2020).
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Periago, M.J. Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- Szabo, K.; Cătoi, A.-F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing by-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Oliveira, A.; Campos, D.; Nunes, J.; Vicente, A.A.; Pintado, M. Simulated digestion of an olive pomace water-soluble ingredient: relationship between the bioaccessibility of compounds and their potential health benefits. Food Funct. 2020, 11, 2238–2254. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability of nutraceuticals: Role of the food matrix, processing conditions, the gastrointestinal tract, and nanodelivery systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 954–994. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cabezuelo, M.T.; Zaragozá, R.; Barber, T.; Viña, J.R. Role of vitamin A in mammary gland development and lactation. Nutrients 2020, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Maurya, V.K.; Singh, J.; Ranjan, V.; Muthukalinnan, G.K.; Bohn, T.; Pareek, S. Factors affecting the fate of β-carotene in the human gastrointestinal tract: A narrative review. Int. J. Vitam. Nutr. Res. 2020, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Moran, N.E.; Mohn, E.S.; Hason, N.; Erdman, J.W., Jr.; Johnson, E.J. Intrinsic and Extrinsic Factors Impacting Absorption, Metabolism, and Health Effects of Dietary Carotenoids. Adv. Nutr. 2018, 9, 465–492. [Google Scholar] [CrossRef]
- Coelho, M.; Pereira, R.; Rodrigues, A.; Teixeira, J.; Pintado, M. The use of emergent technologies to extract added value compounds from grape by-products. Trends Food Sci. Technol. 2020, 106, 182–197. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Oliveira, A.; Coelho, M.; Veiga, M.; Costa, E.M.; Silva, S.; Nunes, J.; Vicente, A.A.; Pintado, M. Are olive pomace powders a safe source of bioactives and nutrients? J. Sci. Food Agric. 2020. [Google Scholar] [CrossRef] [PubMed]
- Monforte, A.R.; Martins, S.I.F.S.; Ferreira, A.C.S. Impact of Phenolic Compounds in Strecker Aldehyde Formation in Wine Model Systems: Target and Untargeted Analysis. J. Agric. Food Chem. 2019, 68, 10281–10286. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Coelho, M.; Alexandre, E.M.C.; Pintado, M. Impact of storage on phytochemicals and milk proteins in peach yoghurt. J. Food Meas. Charact. 2017, 11, 1804–1814. [Google Scholar] [CrossRef]
- Association of Official Analysis Chemists (AOAC). Official Methods of Analysis of AOAC International, 17th ed.; W. Horwitz, AOAC Intl, Ed.; AOAC Int.: Gaithersburg, MD, USA, 2000; ISBN 093558467-6. [Google Scholar]
- Coelho, M.; Silva, S.; Rodríguez-Alcalá, L.M.; Oliveira, A.; Costa, E.M.; Borges, A.; Martins, C.; Rodrigues, A.S.; Pintado, M.M.E. Quercus based coffee-like beverage: effect of roasting process and functional characterization. J. Food Meas. Charact. 2017, 12, 471–479. [Google Scholar] [CrossRef]
- Association of Official Analysis Chemists International (AOAC)Official Method 2011.25 Insoluble, soluble, and total dietary fiber in foods enzymatic-gravimetric-liquid chromatography. In AOAC International; AOAC Int.: Gaithersburg, MD, USA, 2011.
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res. Int. 2011, 44, 2712–2720. [Google Scholar] [CrossRef]
- Xie, P.-J.; Huang, L.-X.; Zhang, C.-H.; Zhang, Y.-L. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure–activity relationships. J. Funct. Foods 2015, 16, 460–471. [Google Scholar] [CrossRef]
- Xue, D.; Farid, M.M. Pulsed electric field extraction of valuable compounds from white button mushroom (Agaricus bisporus). Innov. Food Sci. Emerg. Technol. 2015, 29, 178–186. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Campos, D.A.; Osório, H.; Nerli, B.B.; Pintado, M. Enzymatic soy protein hydrolysis: A tool for biofunctional food ingredient production. Food Chem. X 2019, 1, 100006. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Rodriguez-Amaya, D.B.; Godoy, H.T. Assessment of the saponification step in the quantitative determination of carotenoids and provitamins A. Food Chem. 1990, 35, 187–195. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Barros, A.S.; Ferreira, A.C.S.; Silva, A.M. Influence of the temperature and oxygen exposure in red Port wine: A kinetic approach. Food Res. Int. 2015, 75, 337–347. [Google Scholar] [CrossRef]
- Oliveira, C.; Ferreira, A.C.; Costa, P.; Guerra, J.; De Pinho, P.G. Effect of Some Viticultural Parameters on the Grape Carotenoid Profile. J. Agric. Food Chem. 2004, 52, 4178–4184. [Google Scholar] [CrossRef] [PubMed]
- Rivera, S.; Vilaró, F.; Canela, R. Determination of carotenoids by liquid chromatography/mass spectrometry: effect of several dopants. Anal. Bioanal. Chem. 2011, 400, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.M.; Baptista, P.; Zuccheri, G.; Madureira, A.R.; Sarmento, B.; Pintado, M.E. Film-nanoparticle composite for enhanced oral delivery of alpha-casozepine. Colloids Surf. B Biointerfaces 2019, 181, 149–157. [Google Scholar] [CrossRef]
- Costa, J.R.; Xavier, M.; Amado, I.R.; Gonçalves, C.; Castro, P.M.; Tonon, R.V.; Cabral, L.M.; Pastrana, L.; Pintado, M.E. Polymeric nanoparticles as oral delivery systems for a grape pomace extract towards the improvement of biological activities. Mater. Sci. Eng. C 2021, 119, 111551. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, P.; Nunes, R.; De Biasio, F.; Spigno, G.; Gorgoglione, D.; Teixeira, J.A.; Rocha, C.M. Influence of thermal and electrical effects of ohmic heating on C-phycocyanin properties and biocompounds recovery from Spirulina platensis. LWT 2020, 128, 109491. [Google Scholar] [CrossRef]
- Pereira, R.N.; Teixeira, J.A.; Vicente, A.A.; Cappato, L.P.; Ferreira, M.V.D.S.; Rocha, R.D.S.; Da Cruz, A.G. Ohmic heating for the dairy industry: a potential technology to develop probiotic dairy foods in association with modifications of whey protein structure. Curr. Opin. Food Sci. 2018, 22, 95–101. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; Martins, A.J.; Ramos, O.L.; Malcata, F.X.; Teixeira, J.A.; Vicente, A.A.; Pereira, R.N. Influence of moderate electric fields on gelation of whey protein isolate. Food Hydrocoll. 2015, 43, 329–339. [Google Scholar] [CrossRef]
- Pereira, R.N.; Rodrigues, R.M.; Ramos, Ó.L.; Malcata, F.X.; Teixeira, J.A.; Vicente, A.A. Production of Whey Protein-Based Aggregates Under Ohmic Heating. Food Bioprocess Technol. 2015, 9, 576–587. [Google Scholar] [CrossRef]
- Pereira, R.N.; Rodrigues, R.M.; Genisheva, Z.; Oliveira, H.; De Freitas, V.; Teixeira, J.A.; Vicente, A.A. Effects of ohmic heating on extraction of food-grade phytochemicals from colored potato. LWT 2016, 74, 493–503. [Google Scholar] [CrossRef]
- Moongngarm, A.; Loypimai, P.; Fitriati, A.; Moontree, T. Ohmic heating assisted extraction improves the concentrations of phytochemicals in rice bran oil and unsaponifiable matter. Int. Food Res. J. 2019, 26, 1389–1396. [Google Scholar]
- Al-Hilphy, A.R.; Al-Musafer, A.M.; Gavahian, M. Pilot-scale ohmic heating-assisted extraction of wheat bran bioactive compounds: Effects of the extract on corn oil stability. Food Res. Int. 2020, 137, 109649. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Jiménez, A.K.; Rangel-Hernández, J.; Morales-Sánchez, E.; Loarca-Piña, G.; Gaytán-Martínez, M. Changes on the phytochemicals profile of instant corn flours obtained by traditional nixtamalization and ohmic heating process. Food Chem. 2019, 276, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Schaich, K.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Goñi, I.; Díaz-Rubio, M.E.; Pérez-Jiménez, J.; Calixto, F.D.S. Towards an updated methodology for measurement of dietary fiber, including associated polyphenols, in food and beverages. Food Res. Int. 2009, 42, 840–846. [Google Scholar] [CrossRef]
- Silva, Y.P.A.; Borba, B.C.; Pereira, V.A.; Reis, M.G.; Caliari, M.; Brooks, M.S.-L.; Ferreira, T.A.P.C. Characterization of tomato processing by-product for use as a potential functional food ingredient: nutritional composition, antioxidant activity and bioactive compounds. Int. J. Food Sci. Nutr. 2018, 70, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.A.; Borba, B.C.; Reis, M.G.; Caliari, M.; Ferreira, T.A.P.C. Tomato industrial waste as potential source of nutrients. In Proceedings of the XXV Congresso Brasileiro de Ciência e Tecnologia de Alimentos: X CIGR Section VI International Technical Symposium, Gramado/RS, Brasil, 24–26 October 2016; pp. 3–8. [Google Scholar]
- Liu, S.; Jia, M.; Chen, J.; Wan, H.; Dong, R.; Nie, S.; Xie, M.; Yu, Q. Removal of bound polyphenols and its effect on antioxidant and prebiotics properties of carrot dietary fiber. Food Hydrocoll. 2019, 93, 284–292. [Google Scholar] [CrossRef]
- Perea-Domínguez, X.P.; Hernández-Gastelum, L.Z.; Olivas-Olguin, H.R.; Espinosa-Alonso, L.G.; Valdez-Morales, M.; Medina-Godoy, S. Phenolic composition of tomato varieties and an industrial tomato by-product: free, conjugated and bound phenolics and antioxidant activity. J. Food Sci. Technol. 2018, 55, 3453–3461. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F. Dietary fibre as a carrier of dietary antioxidants: An essential physiological function. J. Agric. Food Chem. 2011. [Google Scholar] [CrossRef]
- Pavez-Guajardo, C.; Ferreira, S.R.S.; Mazzutti, S.; Guerra-Valle, M.E.; Sáez-Trautmann, G.; Moreno, J. Influence of In Vitro Digestion on Antioxidant Activity of Enriched Apple Snacks with Grape Juice. Foods 2020, 9, 1681. [Google Scholar] [CrossRef] [PubMed]
- Ou-Yang, Z.; Cao, X.; Wei, Y.; Zhang, W.-W.-Q.; Zhao, M.; Duan, J.-A. Pharmacokinetic study of rutin and quercetin in rats after oral administration of total flavones of mulberry leaf extract. Rev. Bras. Farm. 2013, 23, 776–782. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Cömert, E.D.; Gökmen, V. Antioxidants Bound to an Insoluble Food Matrix: Their Analysis, Regeneration Behavior, and Physiological Importance. Compr. Rev. Food Sci. Food Saf. 2017, 16, 382–399. [Google Scholar] [CrossRef] [PubMed]
- Etcheverry, P.; Grusak, M.A.; Fleige, L.E. Application of in vitro bioaccessibility and bioavailability methods for calcium, carotenoids, folate, iron, magnesium, polyphenols, zinc, and vitamins B6, B12, D, and E. Front. Physiol. 2012, 3, 317. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Serrano, J.; Tabernero, M.; Arranz, S.; Díaz-Rubio, M.E.; García-Diz, L.; Goñi, I.; Calixto, F.D.S. Bioavailability of Phenolic Antioxidants Associated with Dietary Fiber: Plasma Antioxidant Capacity After Acute and Long-Term Intake in Humans. Plant Foods Hum. Nutr. 2009, 64, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Mariutti, L.R.; Bragagnolo, N.; Mercadante, A.Z.; Barbosa-Cánovas, G.V.; Orlien, V. Bioaccessibility of bioactive compounds from fruits and vegetables after thermal and nonthermal processing. Trends Food Sci. Technol. 2017, 67, 195–206. [Google Scholar] [CrossRef]
- Dufour, C.; Loonis, M.; Delosière, M.; Buffière, C.; Hafnaoui, N.; Santé-Lhoutellier, V.; Rémond, D. The matrix of fruit & vegetables modulates the gastrointestinal bioaccessibility of polyphenols and their impact on dietary protein digestibility. Food Chem. 2018, 240, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, L.; Nagao, A. Intestinal absorption of dietary carotenoids. Mol. Nutr. Food Res. 2007, 51, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, J.; Ramanan, R.N.; Raghunandan, M.E.; Krishnamurthy, N.P. Chapter 8 – Carotenoids. In Nutraceutical and Functional Food Components; Charis, M.G., Ed.; Academic Press: Chania, Greece, 2017; pp. 259–296. ISBN 9780128052570. [Google Scholar]
- Nagao, A. Bioavailability of Dietary Carotenoids: Intestinal Absorption and Metabolism. Jpn. Agric. Res. Q. 2014, 48, 385–391. [Google Scholar] [CrossRef]
- Shi, J.; Le Maguer, M. Lycopene in Tomatoes: Chemical and Physical Properties Affected by Food Processing. Crit. Rev. Food Sci. Nutr. 2000, 40, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, C.; Zhang, R.; Gao, Y.; McClements, D.J. Controlling the potential gastrointestinal fate of β-carotene emulsions using interfacial engineering: Impact of coating lipid droplets with polyphenol-protein-carbohydrate conjugate. Food Chem. 2017, 221, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E. Absorption of Vitamin A and Carotenoids by the Enterocyte: Focus on Transport Proteins. Nutrients 2013, 5, 3563–3581. [Google Scholar] [CrossRef]
- Said, H.M. Physiology of the Gastrointestinal Tract, 6th ed.; Hamid Said; Academic Press: Irvine, CA, USA, 2018; Volumes 1–2. [Google Scholar]
- Salter-Venzon, D.; Kazlova, V.; Ford, S.I.; Intra, J.; Klosner, A.E.; Gellenbeck, K.W. Evidence for decreased interaction and improved carotenoid bioavailability by sequential delivery of a supplement. Food Sci. Nutr. 2016, 5, 424–433. [Google Scholar] [CrossRef]
- Cardoso, L.A.C.; Karp, S.G.; Vendruscolo, F.; Kanno, K.Y.F.; Zoz, L.I.C.; Carvalho, J.C. Biotechnological Production of Carotenoids and Their Applications in Food and Pharmaceutical Products. In Carotenoids; Cvetkovic, D.J., Nikolic, G.S., Eds.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Dickinson, A.J.; Lehner, K.; Mi, J.; Jia, K.-P.; Mijar, M.; Dinneny, J.; Al-Babili, S.; Benfey, P.N. β-Cyclocitral is a conserved root growth regulator. Proc. Natl. Acad. Sci. USA 2019, 116, 10563–10567. [Google Scholar] [CrossRef] [PubMed]
- Ilg, A.; Bruno, M.; Beyer, P.; Al-Babili, S. Tomato carotenoid cleavage dioxygenases 1A and 1B: Relaxed double bond specificity leads to a plenitude of dialdehydes, mono-apocarotenoids and isoprenoid volatiles. FEBS Open Bio 2014, 4, 584–593. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Desmarchelier, C.; Margier, M.; Reboul, E.; Martínez, A.J.M.; Borel, P. Phytoene and Phytofluene Isolated from a Tomato Extract are Readily Incorporated in Mixed Micelles and Absorbed by Caco-2 Cells, as Compared to Lycopene, and SR-BI is Involved in their Cellular Uptake. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E. Mechanisms of Carotenoid Intestinal Absorption: Where Do We Stand? Nutrients 2019, 11, 838. [Google Scholar] [CrossRef]
- Sharif, A.; Akhtar, M.F.; Akhtar, B.; Saleem, A.; Manan, M.; Shabbir, M.; Ashraf, M.; Peerzada, S.; Ahmed, S.; Raza, M. Genotoxic and cytotoxic potential of whole plant extracts of Kalanchoe laciniata by Ames and MTT assay. EXCLI J. 2017, 16, 593–601. [Google Scholar]
- Guil-Guerrero, J.L.; Ramos-Bueno, R.; Rodríguez-García, I.; López-Sánchez, C. Cytotoxicity Screening of Several Tomato Extracts. J. Med. Food 2011, 14, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Calviello, G.; Serini, S.; Maggiano, N.; Lanza, P.; O Ranelletti, F.; Bartoli, G.M. β-carotene at high concentrations induces apoptosis by enhancing oxy-radical production in human adenocarcinoma cells. Free. Radic. Biol. Med. 2001, 30, 1000–1007. [Google Scholar] [CrossRef]
- Palozza, P.; Bellovino, D.; Simone, R.; Boninsegna, A.; Cellini, F.; Monastra, G.; Gaetani, S. Effect of β-carotene-rich tomato lycopene β-cyclase (tlcy-b) on cell growth inhibition in HT-29 colon adenocarcinoma cells. Br. J. Nutr. 2008, 102, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, C.; Kamiloglu, S.; Capanoglu, E.; Van Camp, J. Cell Systems to Investigate the Impact of Polyphenols on Cardiovascular Health. Nutrients 2015, 7, 9229–9255. [Google Scholar] [CrossRef] [PubMed]
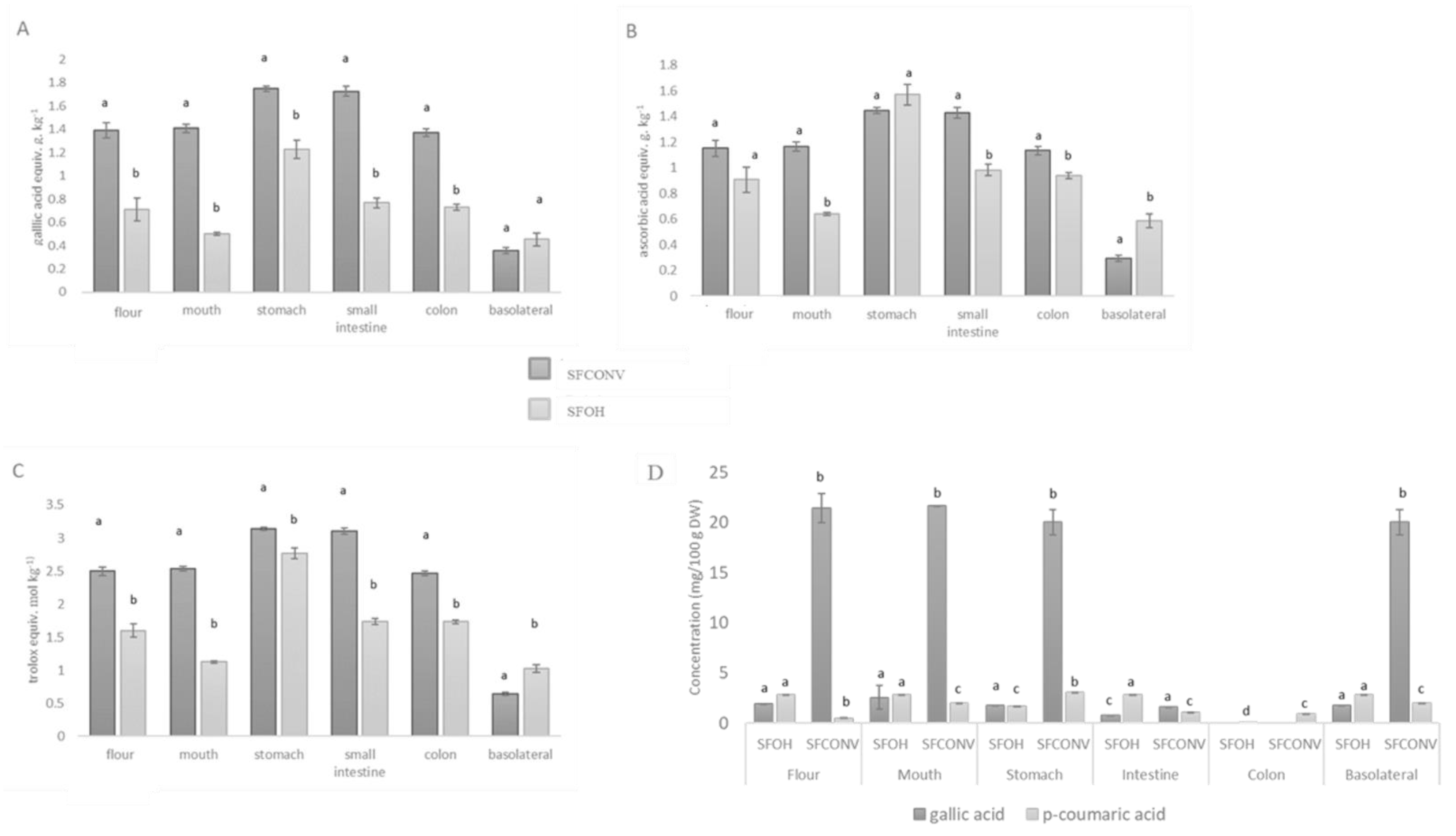
 represent without metabolization and
represent without metabolization and  with metabolization. Results are the means of three determinations ± standard deviation.
with metabolization. Results are the means of three determinations ± standard deviation.
 represent without metabolization and
represent without metabolization and  with metabolization. Results are the means of three determinations ± standard deviation.
with metabolization. Results are the means of three determinations ± standard deviation.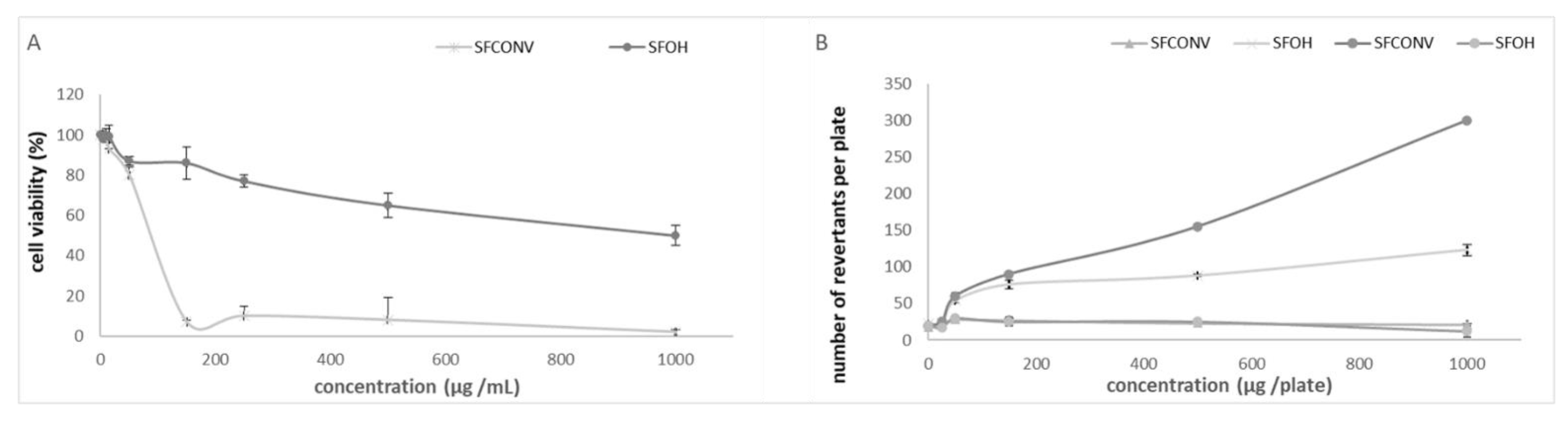
| Chemical Composition (g/100 g DW) | Sample (g/100 g) | |||
|---|---|---|---|---|
| SFOH | SFCONV | |||
| Proximate composition | Ash | 3.32 ± 0.21 a | 2.98 ± 0.16 a | |
| Protein | 18.72 ± 0.47 a | 16.29 ± 0.59 b | ||
| Total Fatty acids | 21.12 ± 0.51 a | 17.82 ± 0.3 b | ||
| Dietary Fiber | ||||
| TDF | 62.47 ±1.24 a | 59.06 ± 0.67 b | ||
| IDF | 50.99 ± 0.16 a | 46.01 ± 0.13 b | ||
| SDF | 10.86 ± 0.85 a | 12.98 ± 0.64 b | ||
| Phenolic composition | Free phenolic extract (FPC) | TPC | 1.46 ± 0.05 a | 0.80 ± 0.01 b |
| ABTS | 0.28 ± 0.01 b | 0.99 ± 0.15 a | ||
| ORAC | 2.44 ± 0.34 a | 1.60 ± 0.09 b | ||
| Bound phenolic extract (BPC) | TPC | 0.42 ± 0.03 b | 0.73 ± 0.12 a | |
| ABTS | 2.23 ± 0.21 a | 2.63 ± 0.11 a | ||
| ORAC | 2.41 ± 0.22 a | 2.71 ± 0.43 a | ||
| SFOH | SFCONV | |||
|---|---|---|---|---|
| SDF | IDF | SDF | IDF | |
| Klason Lignin | n.d. | 13.06 ± 0.52 b | n.d. | 14.09 ± 0.27 a |
| Glucose (as cellulose) | 24.42 ± 0.43 a | 55.32 ± 1.21 b | 32.1 ± 0.56 c | 50.23 ± 1.76 d |
| Hemicellulose | 15.12 ± 0.54 a | 25.2 ± 0.10 b | 12.71 ± 1.21 a | 24.72 ± 0.30 b |
| Xylose | * | 13.2 ± 0.10 a | * | 15.1 ± 0.17 b |
| Galactose | 1.87 ± 0.21 | * | * | 0.31 ± 0.021 |
| Mannose | * | * | 4.10 ± 0.12 a | * |
| Arabinose | 13.25 ± 0.86 | 12.81 ± 0.28 | 12.71 ± 1.21 | 9.31 ± 0.51 |
| Uronic acids** | 67.41 ± 2.15 a | 81.92 ± 1.98 b | 58.93 ± 2.56 c | 78.49 ± 2.32 b |
| Resistant Protein | n.d. | 16.03 ± 0.05 a | n.d. | 11.69 ± 0.03 b |
| Bond Phenolic compounds *** | 7.80 ± 0.43 a | 31.18 ± 2.31 b | 4.99 ± 0.35 c | 34.10 ± 1.21 b |
| Recovery Index (%) | Bioaccessibility (%) | ||||||
|---|---|---|---|---|---|---|---|
| Bioactivities | Samples | Mouth | Stomach | Small Intestine | Colon | Basolateral | |
| Total Phenol | SFCONV | 101.42 ± 2.34 a | 124.11 ± 1.28 b | 121.99 ± 2.03 b | 90.78 ± 1.12 d | 9.22 ± 1.07 e | 7.03 ± 0.42 α |
| SFOH | 70.44 ± 1.56 a | 172.86 ± 2.34 b | 122.18 ± 1.24 c | 103.04 ± 1.28 d | 15.32 ± 1.25 e | 11.14 ± 0.76 β | |
| ABTS | SFCONV | 103.08 ± 2.23 a | 134.35 ± 1.78 b | 124.14 ± 2.02 c | 98.49 ± 2.45 a | 18.1 ± 1.96 d | 14.58 ± 0.13 α |
| SFOH | 81.45 ± 1.87 a | 175.71 ± 2.56 b | 108.26 ± 1.95 c | 92.51 ± 1.96 | 7.49 ± 0.06 d | 6.47 ± 0.15 β | |
| Orac | SFCONV | 109.34 ± 2.25 a | 121.65 ± 1.25 b | 119.60 ± 1.21 b | 90.49 ± 2.54 | 9.51 ± 0.07 d | 7.37 ± 0.31 α |
| SFOH | 89.19 ± 2.54 a | 160.36 ± 2.46 b | 108.26 ± 2.38 c | 87.50 ± 1.26 | 12.50 ± 1.01 d | 10.35 ± 0.12 β | |
| Phenolic compounds | |||||||
| gallic acid | SFCONV | 101.19 ± 1.87 a | 93.56 ± 1.17 b | 7.62 ± 0.97 c | n.d. | 93.56 ± 1.87 b | 92.46 ± 1.03 α |
| SFOH | 134.31 ± 1.12 | 8.45 ± 0.2 3 b | 29.76 ± 0.79 c | n.d. | 100.00 ± 2.05 d | 77.07 ± 1.44 β | |
| 4-hydroxybenzoic acid | SFCONV | 100.38 ± 1.56 a | 87.64 ± 1.68 b | 12.74 ± 0.26 c | n.d. | 87.64 ± 1.67 b | 87.31 ± 1.38 α |
| SFOH | 102.54 ± 1.69 a | 84.92 ± 1.78 b | 70.98 ± 1.45 c | 70.98 ± 0.13 c | 13.94 ± 1.36 | 16.42 ± 1.22 β | |
| p-coumaric acid | SFCONV | 79.35 ± 2.56 a | 121.14 ± 1.27 b | 42.49 ± 0.08 c | 36.15 ± 0.89 d | 78.65 ± 1.23 a | 64.92 ± 1.12 α |
| SFOH | 89.07 ± 1.98 a | 59.51 ± 1.25 b | 100.42 ± 2.76 c | 0.42 ± 0.03 d | 99.37 ± 3.01 c | 49.74 ± 1.12β | |
| rutin | SFCONV | 17.01 ± 0.54 a | n.d. | 68.83 ± 1.05 b | n.d. | 58.83 ± 2.16 c | 46.08 ± 1.56α |
| SFOH | 7.84 ± 0.05 a | n.d | n.d. | n.d. | 7.56 ± 0.34 a | 99.99 ± 0.66 β | |
| Mouth | Stomach | Intestine | Colon | Basolateral | Bioaccessibility | |||
|---|---|---|---|---|---|---|---|---|
| Compounds | Mz | Samples | (%) | |||||
| n.i. | 525 | SFOH | 84.85 ± 2.31 a | 57.97 ± 1.3 b | 45.52 ± 1.56 c | 40.33 ± 1.87 c | 27.17 ± 1.84 d | 59.67 ± 1.83 β |
| SFCONV | 86.32 ± 2.05 a | 60.21 ± 1.31 b | 49.23 ± 1.55 c | 41.32 ± 1.98 d | 31.24 ± 2.13 e | 63.46 ± 1.76 α | ||
| n.i. | 527 | SFOH | 91.55 ± 1.95 a | 16.86 ± 0.25 b | 15.68 ± 0.99 b | 92.48 ± 2.06 a | 1.18 ± 1.23 c | 7.52 ± 0.74 α |
| SFCONV | 93.41 ± 1.28 a | 89.31 ± 2.45 b | 44.66 ± 2.87 c | 90.23 ± 2.77 a,b | 3.06 ± 0.22 d | 6.85 ± 0.32 β | ||
| phytofluene | 542 | SFOH | 72.25 ± 1.04 a | 36.46 ± 0.78 b | 29.14 ± 1.23 c | 69.82 ± 1.76 a | 8.79 ± 0.97 d | 30.18 ± 0.98 α |
| SFCONV | 26.40 ± 0.27 b | 11.53 ± 0.69 c | 37.08 ± 1.39 a | 35.54 ± 1.43 a | 3.21 ± 0.06 d | 8.66 ± 0.45 β | ||
| lycopene | 536 | SFOH | n.q | n.q | n.q | n.q | n.q | n.q |
| SF | n.q | n.q | n.q | n.q | n.q. | n.q | ||
| lutein | 569 | SFOH | 113.9 ± 2.76 a | 62.52 ± 1.43 b | 57.65 ± 1.43 c | 19.26 ± 1.25 e | 46.55 ± 1.54 d | 80.74 ± 1.32 α |
| SFCONV | 25.53 ± 0.34 c | 11.15 ± 0.98 d | 35.85 ± 1.41 a | 30.53 ± 1.23 b | 5.21 ± 0.09 e | 14.53 ± 0.57 β | ||
| n.i. | 633 | SFOH | 103.8 ± 2.54 a | 58.61 ± 1.77 b | 33.06 ± 1.04 c | 12.98 ± 0.12 d | 35.82 ± 2.31 c | 108.32 ± 2.25 α |
| SFCONV | n.d | n.d | n.d | n.d | n.d | n.q | ||
| Compounds (Name) | 1,2-dimethylindole | 2,6-dimethylbenzaldehyde | Benzoic Acid | β-cyclocitral | 3,4-diethenyl-1,6-dimethyl- | Camphenol | Linalyl Acetate and Linalool | |
|---|---|---|---|---|---|---|---|---|
| Samples | m/z quantifiers | 133 | 105 | 118 | 119 | 93 | 93 | 93 |
| Molecular formula | C10H11N | C9H10O | C7H6O2 | C10H16O | C12H18 | C10H16O | C12H20O2/C10H18O | |
| MW | 145 | 134 | 122 | 152 | 162 | 152 | 196/154 | |
| Conventional method | CBF | 226.6 ± 13.4 | 0.4 ± 0.1 | 14.4 ± 4.5 | 3.3 ± 0.5 | 0.4 ± 0.1 | 14.1 ± 0.3 | 0.0 ± 0.0 |
| Mouth | 190.9 ± 5.4 | 2.7 ± 0.1 | 14.0 ± 0.9 | 2.0 ± 0.1 | 0.4 ± 0.0 | 9.2 ± 2.5 | 0.8 ± 0.3 | |
| Stomach | 189.0 ± 3.9 | 2.2 ± 0.4 | 4.1 ± 0.8 | 3.2 ± 0.4 | 0.9 ±0.1 | 10.4 ± 1.1 | 0.3 ± 0.1 | |
| Small Intestine | 113.1 ± 4.5 | 0.0 ± 0.0 | 1.6 ± 0.6 | 1.3 ±0.1 | 0.0 ± 0.0 | 0.4 ± 0.1 | 0.0 ± 0.0 | |
| Colon | 1.3 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 1.10 ± 0.3 | 0.0 ± 0.0 | |
| Basolateral | 4.6 ±0.6 | 0.0 ± 0.0 | 20.8 ± 3.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Ohmic conventional | CBF | 232.1 ± 21.7 | 0.0 ±0.0 | 8.6 ± 1.4 | 2.8 ± 0.2 | 0.1 ± 0.0 | 2.8 ± 0.3 | 0.0 ± 0.0 |
| Mouth | 155.5 ± 12.43 | 1.5 ± 0.3 | 6.6 ± 1.8 | 2.6 ± 0.3 | 0.1 ± 0.0 | 3.1 ± 0.4 | 0.3 ± 0.1 | |
| Stomach | 208.5 ± 15.84 | 1.3 ± 0.2 | 4.6 ± 0.7 | 4.8 ± 0.5 | 0.1 ± 0.0 | 3.7 ± 0.7 | 0.2 ± 0.0 | |
| Small Intestine | 79.5 ± 7.49 | 0.0 ± 0.0 | 5.5 ± 0.8 | 1.3 ± 0.1 | 0.0 ± 0.0 | 0.5 ± 0.1 | 0.0 ± 0.0 | |
| Colon | 19.0 ± 4.61 | 0.0 ± 0.0 | 2.0 ±0.3 | 0.0 ± 0.0 | 0.3 ± 0.1 | 3.5 ± 0.7 | 0.0 ± 0.0 | |
| Basolateral | 17.3 ± 3.95 | 0.0 ± 0.0 | 39.4 ± 4.9 | 0.6 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, M.C.; Ribeiro, T.B.; Oliveira, C.; Batista, P.; Castro, P.; Monforte, A.R.; Rodrigues, A.S.; Teixeira, J.; Pintado, M. In Vitro Gastrointestinal Digestion Impact on the Bioaccessibility and Antioxidant Capacity of Bioactive Compounds from Tomato Flours Obtained after Conventional and Ohmic Heating Extraction. Foods 2021, 10, 554. https://doi.org/10.3390/foods10030554
Coelho MC, Ribeiro TB, Oliveira C, Batista P, Castro P, Monforte AR, Rodrigues AS, Teixeira J, Pintado M. In Vitro Gastrointestinal Digestion Impact on the Bioaccessibility and Antioxidant Capacity of Bioactive Compounds from Tomato Flours Obtained after Conventional and Ohmic Heating Extraction. Foods. 2021; 10(3):554. https://doi.org/10.3390/foods10030554
Chicago/Turabian StyleCoelho, Marta C., Tânia B. Ribeiro, Carla Oliveira, Patricia Batista, Pedro Castro, Ana Rita Monforte, António Sebastião Rodrigues, José Teixeira, and Manuela Pintado. 2021. "In Vitro Gastrointestinal Digestion Impact on the Bioaccessibility and Antioxidant Capacity of Bioactive Compounds from Tomato Flours Obtained after Conventional and Ohmic Heating Extraction" Foods 10, no. 3: 554. https://doi.org/10.3390/foods10030554
APA StyleCoelho, M. C., Ribeiro, T. B., Oliveira, C., Batista, P., Castro, P., Monforte, A. R., Rodrigues, A. S., Teixeira, J., & Pintado, M. (2021). In Vitro Gastrointestinal Digestion Impact on the Bioaccessibility and Antioxidant Capacity of Bioactive Compounds from Tomato Flours Obtained after Conventional and Ohmic Heating Extraction. Foods, 10(3), 554. https://doi.org/10.3390/foods10030554