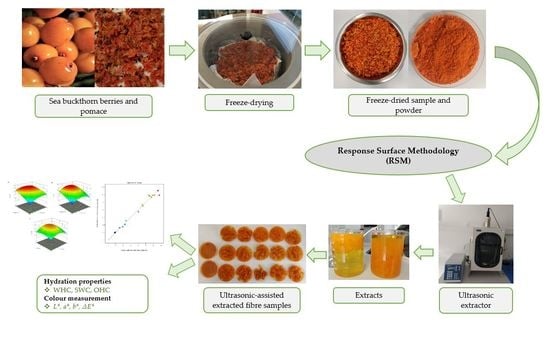
Valorisation of Sea Buckthorn Pomace by Optimization of Ultrasonic-Assisted Extraction of Soluble Dietary Fibre Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Extraction of Dietary Fibre from Sea Buckthorn Pomace
2.3. Yield Determination
2.4. RSM Design for Optimization of Extraction Yield
2.5. Water Holding Capacity (WHC)
2.6. Swelling Capacity (SWC)
2.7. Oil Holding Capacity (OHC)
2.8. Colour Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Ultrasound-Assisted Extraction of SDF
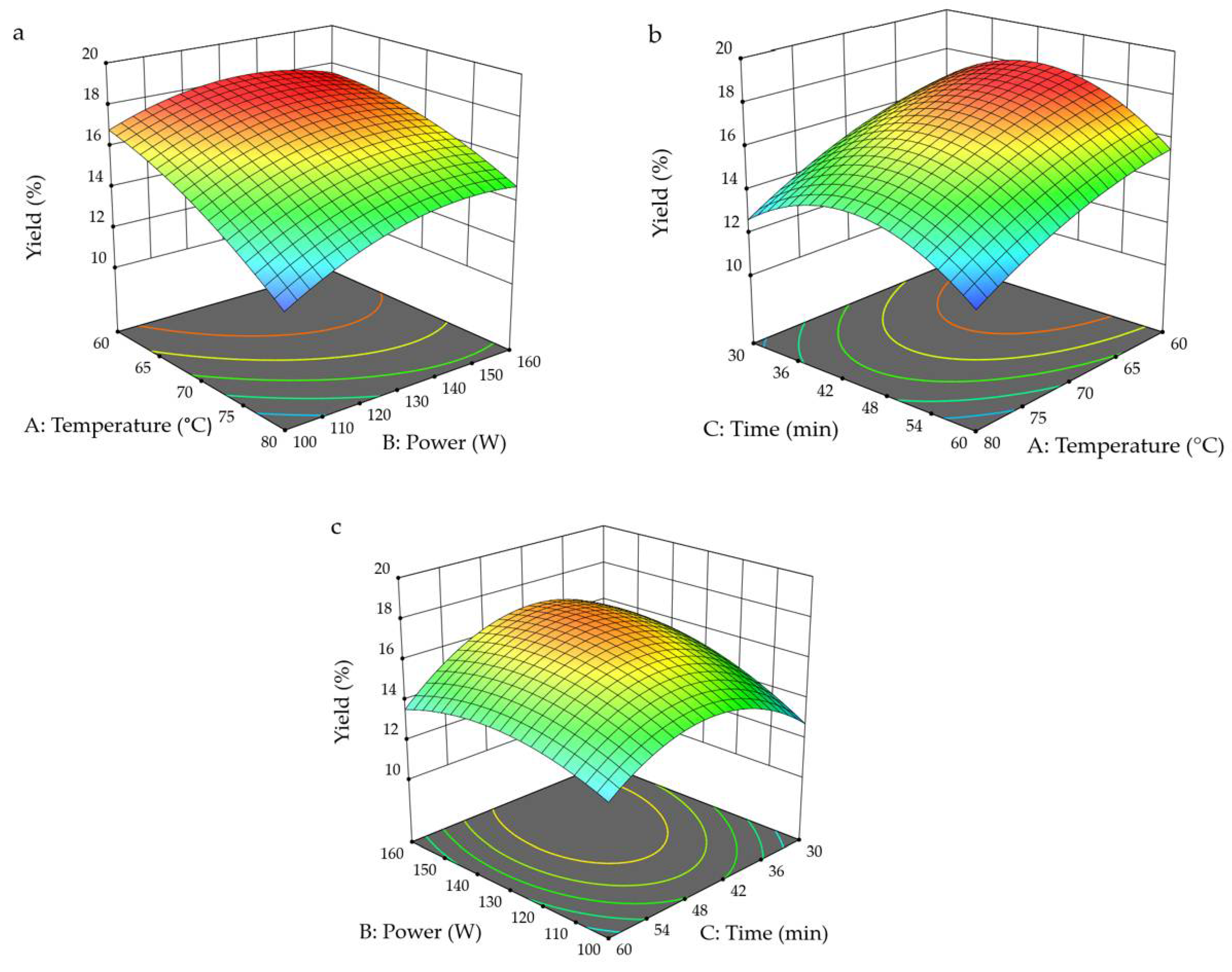
3.2. Response Surface Analysis
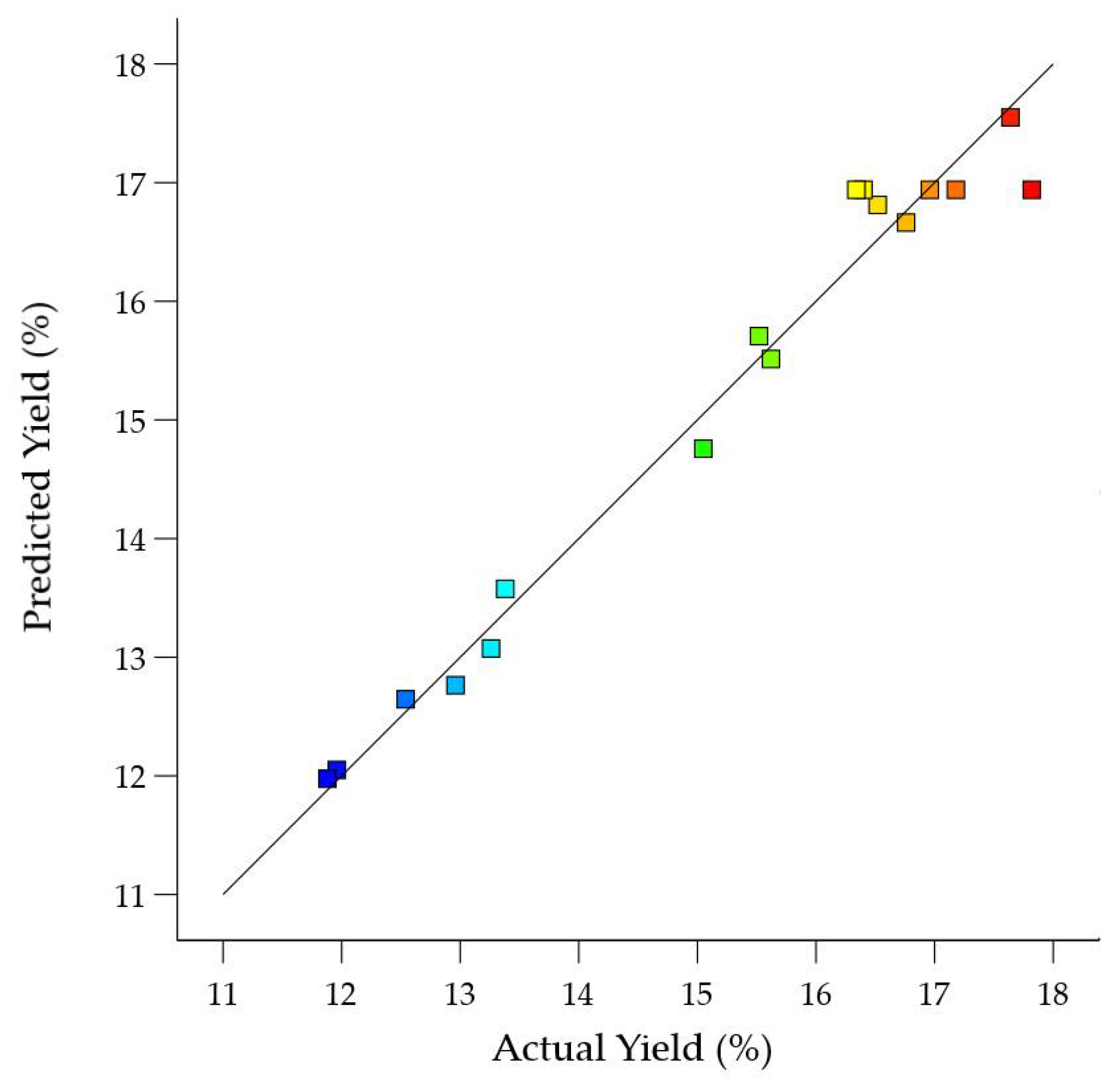
3.3. Optimization of Parameters and Verification of the Model
3.4. Hydration Properties and Colour Evaluation of Sea Buckthorn Pomace Dietary Fibres
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, X.F.; Yang, B.; Cai, W.; Li, D. Effect of sea buckthorn (Hippophae rhamnoides L.) on blood lipid profiles: A systematic review and meta-analysis from 11 independent randomized controlled trials. Trends Food Sci. Technol. 2017, 61, 1–10. [Google Scholar] [CrossRef]
- Kitrytė, V.; Povilaitis, D.; Kraujalienė, V.; Šulniūtė, V.; Pukalskas, A.; Venskutonis, P.R. Fractionation of sea buckthorn pomace and seeds into valuable components by using high pressure and enzyme-assisted extraction methods. LWT Food Sci. Technol. 2017, 85, 534–538. [Google Scholar] [CrossRef]
- Chen, C.; Xu, X.M.; Chen, Y.; Yu, M.Y.; Wen, F.Y.; Zhang, H. Identification, quantification and antioxidant activity of acylated flavonol glycosides from sea buckthorn (Hippophae rhamnoides ssp. sinensis). Food Chem. 2013, 141, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Destandau, E.; Le Floch, G.; Lucchesi, M.E.; Elfakir, C. Antimicrobial, antioxidant and phytochemical investigations of sea buckthorn (Hippophaë rhamnoides L.) leaf, stem, root and seed. Food Chem. 2012, 131, 754–760. [Google Scholar] [CrossRef]
- Bal, L.M.; Meda, V.; Naik, S.N.; Satya, S. Sea buckthorn berries: A potential source of valuable nutrients for nutraceuticals and cosmoceuticals. Food Res. Int. 2011, 44, 1718–1727. [Google Scholar] [CrossRef]
- Madawala, S.R.; Brunius, C.; Adholeya, A.; Tripathi, S.B.; Hanhineva, K.; Hajazimi, E.; Shi, L.; Dimberg, L.; Landberg, R. Impact of location on composition of selected phytochemicals in wild sea buckthorn (Hippophae rhamnoides). J. Food Compos. Anal. 2018, 72, 115–121. [Google Scholar] [CrossRef]
- Marciniak, B.; Kontek, R.; Żuchowski, J.; Stochmal, A. Novel bioactive properties of low-polarity fractions from sea-buckthorn extracts (Elaeagnus rhamnoides (L.) A. Nelson)–(in vitro). Biomed. Pharmacother. 2021, 135, 111141. [Google Scholar] [CrossRef]
- Xu, Y.J.; Kaur, M.; Dhillon, R.S.; Tappia, P.S.; Dhalla, N.S. Health benefits of sea buckthorn for the prevention of cardiovascular diseases. J. Funct. Foods 2011, 3, 2–12. [Google Scholar] [CrossRef]
- Gani, A.; Jan, R.; Ashwar, B.A.; Ashraf-ul, Z.; Shah, A.; Gani, A. Encapsulation of saffron and sea buckthorn bioactives: Its utilization for development of low glycemic baked product for growing diabetic population of the world. LWT Food Sci. Technol. 2021, 142, 111035. [Google Scholar] [CrossRef]
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary Fibre from Underutilized Plant Resources—A Positive Approach for Valorisation of Fruit and Vegetable Wastes. Sustainability 2020, 12, 5401. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Górnaś, P.; Pugajeva, I.; Segliņa, D. Seeds recovered from by-products of selected fruit processing as a rich source of tocochromanols: RP-HPLC/FLD and RP-UPLC-ESI/MSn study. Eur. Food Res. Technol. 2014, 239, 519–524. [Google Scholar] [CrossRef]
- Górnaś, P.; Soliven, A.; Segliņa, D. Seed oils recovered from industrial fruit by-products are a rich source of tocopherols and tocotrienols: Rapid separation of α/β/γ/δ homologues by RP-HPLC/FLD. Euro. J. Lipid Sci. Technol. 2015, 117, 773–777. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Viskelis, P.; Bobinas, Č.; Mieželienė, A.; Alenčikienė, G.; Venskutonis, P.R. Raspberry marc extracts increase antioxidative potential, ellagic acid, ellagitannin and anthocyanin concentrations in fruit purees. LWT Food Sci. Technol. 2016, 66, 460–467. [Google Scholar] [CrossRef]
- Kryževičiūtė, N.; Kraujalis, P.; Venskutonis, P.R. Optimization of high pressure extraction processes for the separation of raspberry pomace into lipophilic and hydrophilic fractions. J. Supercrit. Fluids 2016, 108, 61–68. [Google Scholar] [CrossRef]
- Grunovaitė, L.; Pukalskienė, M.; Pukalskas, A.; Venskutonis, P.R. Fractionation of black chokeberry pomace into functional ingredients using high pressure extraction methods and evaluation of their antioxidant capacity and chemical composition. J. Funct. Foods 2016, 24, 85–96. [Google Scholar] [CrossRef]
- Kapasakalidis, P.G.; Rastall, R.A.; Gordon, M.H. Effect of a cellulase treatment on extraction of antioxidant phenols from black currant (Ribes nigrum L.) pomace. J. Agric. Food Chem. 2009, 57, 4342–4351. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saft. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Streppel, M.T.; Ocké, M.C.; Boshuizen, H.C.; Kok, F.J.; Kromhout, D. Dietary fibre intake in relation to coronary heart disease and all-cause mortality over 40 y: The Zutphen Study. Am. J. Clin. Nutr. 2008, 88, 1119–1125. [Google Scholar] [CrossRef]
- Park, Y.; Brinton, L.A.; Subar, A.F.; Hollenbeck, A.; Schatzkin, A. Dietary fibre intake and risk of breast cancer in postmenopausal women: The National Institutes of Health– AARP Diet and Health Study. Am. J. Clin. Nutr. 2009, 90, 664–671. [Google Scholar] [CrossRef]
- Han, S.; Jiao, J.; Zhang, W.; Xu, J.; Wan, Z.; Zhang, W.; Gao, X.; Qin, L. Dietary fibre prevents obesity-related liver lipotoxicity by modulating sterol-regulatory element binding protein pathway in C57BL/6J mice fed a high-fat/cholesterol diet. Sci. Rep. 2015, 5, 15256. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.A.; Kushi, L.H.; Jacobs Jr, D.R.; Slavin, J.; Sellers, T.A.; Folsom, A.R. Carbohydrates, dietary fibre, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 2000, 71, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Maran, J.P.; Priya, B. Ultrasound-assisted extraction of polysaccharide from Nephelium lappaceum L. fruit peel. Int. J. Biol. Macromol. 2014, 70, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.Q.; Chin, N.L.; Yusof, Y.A.; Sowndhararajan, K. Comparison of acidic and enzymatic pectin extraction from passion fruit peels and its gel properties. J. Food Process Eng. 2016, 39, 501–511. [Google Scholar] [CrossRef]
- Wang, A.; Wu, L.; Li, X. Optimization of ultrasonic-assisted preparation of dietary fibre from corn pericarp using response surface methodology. J. Sci. Food Agric. 2013, 93, 2922–2926. [Google Scholar] [CrossRef]
- Raghavendra, S.N.; Rastogi, N.K.; Raghavarao, K.S.M.S.; Tharanathan, R.N. Dietary fibre from coconut residue: Effects of different treatments and particle size on the hydration properties. Euro. Food Res. Technol. 2004, 218, 563–567. [Google Scholar] [CrossRef]
- Sangnark, A.; Noomhorm, A. Effect of particle sizes on functional properties of dietary fibre prepared from sugarcane bagasse. Food Chem. 2003, 80, 221–229. [Google Scholar] [CrossRef]
- Sharma, M.; Bhat, R. Extraction of Carotenoids from Pumpkin Peel and Pulp: Comparison between Innovative Green Extraction Technologies (Ultrasonic and Microwave-Assisted Extractions Using Corn Oil). Foods 2021, 10, 787. [Google Scholar] [CrossRef]
- Zhang, X.; Cavender, G.A.; Lewandowski, K.R.; Cox, G.O.; Paton, C.M. Sensory Analysis of a Processed Food Intended for Vitamin A Supplementation. Foods 2020, 9, 232. [Google Scholar] [CrossRef]
- Segura, L.I.; Salvadori, V.O.; Goñi, S.M. Characterisation of liquid food colour from digital images. Int. J. Food Prop. 2017, 20, S467–S477. [Google Scholar] [CrossRef]
- Pauli, H. Proposed extension of the CIE recommendation on Uniform colour spaces, colour difference equations, and metric colour terms. J. Opt. Soc. Am. 1976, 66, 866–867. [Google Scholar] [CrossRef]
- Pingret, D.; Fabiano-Tixier, A.S.; Chemat, F. Ultrasound-assisted extraction ‘in’ Natural product extraction: Principles and applications. R. Soc. Chem. Camb. 2013, 21, 89–112. [Google Scholar]
- Zaibunnisa, A.H.; Norasikin, S.; Mamot, S.; Osman, H. An experimental design approach for the extraction of volatile compounds from turmeric leaves (Curcuma domestica) using pressurized liquid extraction (PLE). J. Food Sci. Technol. 2009, 42, 233–238. [Google Scholar]
- Zzaman, W.; Bhat, R.; Yang, T.A. Application of response surface methodology to optimize roasting conditions in Cocoa Beans subjected to superheated steam treatments in relevance to antioxidant compounds and activities. Dry. Technol. 2014, 32, 1104–1111. [Google Scholar] [CrossRef]
- Chen, K.; Yan, W.; Zhang, X.; Tang, X.; Han, X.; Li, J. Ultrasound-assisted extraction of dietary fibre from Citrus Changshan-huyou peels. J. Chem. Pharmaceut. Res. 2014, 6, 312–318. [Google Scholar]
- Hussain, S.; Li, J.; Jin, W.; Yan, S.; Wang, Q. Effect of micronisation on dietary fibre content and hydration properties of lotus node powder fractions. Int. J. Food Sci. Technol. 2018, 53, 590–598. [Google Scholar] [CrossRef]
- Kurek, M.A.; Karp, S.; Wyrwisz, J.; Niu, Y. Physicochemical properties of dietary fibres extracted from gluten-free sources: Quinoa (Chenopodium quinoa), amaranth (Amaranthus caudatus) and millet (Panicum miliaceum). Food Hydrocoll. 2018, 85, 321–330. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Li, R.; Li, J. Effect of micronization technology on physicochemical and antioxidant properties of dietary fibre from buckwheat hulls. Biocatal. Agric. Biotechnol. 2014, 3, 30–34. [Google Scholar] [CrossRef]
- Huang, Y.L.; Ma, Y.S. The effect of extrusion processing on the physiochemical properties of extruded orange pomace. Food Chem. 2016, 192, 363–369. [Google Scholar] [CrossRef]
- Tejada-Ortigoza, V.; Garcia-Amezquita, L.E.; Serna-Saldívar, S.O.; Martín-Belloso, O.; Welti-Chanes, J. High hydrostatic pressure and mild heat treatments for the modification of orange peel dietary fibre: Effects on hygroscopic properties and functionality. Food Bioproc. Techol. 2018, 11, 110–121. [Google Scholar] [CrossRef]
- Hutchings, J.B. Food Colour Mechanisms. In Food Colour and Appearance, 2nd ed.; Hutchings, J.B., Ed.; Aspen Publishers, Inc.: Gaithersburg, MD, USA, 1999; pp. 453–592. [Google Scholar]
- Aaby, K.; Martinsen, B.K.; Borge, G.I.; Røen, D. Bioactive compounds and colour of sea buckthorn (Hippophae rhamnoides L.) purees as affected by heat treatment and high-pressure homogenization. Int. J. Food Prop. 2020, 23, 651–664. [Google Scholar] [CrossRef]
- Yan, H.; Kerr, W.L. Total phenolics content, anthocyanins, and dietary fibre content of apple pomace powders produced by vacuum-belt drying. J. Sci. Food Agric. 2013, 93, 1499–1504. [Google Scholar] [CrossRef]
- Bender, A.B.; Speroni, C.S.; Salvador, P.R.; Loureiro, B.B.; Lovatto, N.M.; Goulart, F.R.; Lovatto, M.T.; Miranda, M.Z.; Silva, L.P.; Penna, N.G. Grape pomace skins and the effects of its inclusion in the technological properties of muffins. J. Culin. Sci. Technol. 2017, 15, 143–157. [Google Scholar] [CrossRef]
| Independent Variables | Symbol | Levels | |||
|---|---|---|---|---|---|
| Un-Coded | Coded | −1 | 0 | 1 | |
| Ultrasonic temperature (°C) | X1 | X1 | 60 | 70 | 80 |
| Ultrasonic power (W) | X2 | X2 | 100 | 130 | 160 |
| Ultrasonic time (min) | X3 | X3 | 30 | 45 | 60 |
| Coded Variable Levels | Yield of Soluble Dietary Fibre (%) | ||||
|---|---|---|---|---|---|
| Run | X1 | X2 | X3 | Actual Yield | Predicted Yield |
| 1 | −1 (60) | 0 (130) | 1 (60) | 15.62 | 15.51 |
| 2 | 0 (70) | −1 (100) | −1 (30) | 12.96 | 12.76 |
| 3 | 0 (70) | 1 (160) | −1 (30) | 15.52 | 15.71 |
| 4 | −1 (60) | 0 (130) | −1 (30) | 16.76 | 16.66 |
| 5 | 0 (70) | 0 (130) | 0 (45) | 16.40 | 16.94 |
| 6 | −1 (60) | −1 (100) | 0 (45) | 16.52 | 16.81 |
| 7 | 0 (70) | 0 (130) | 0 (45) | 16.96 | 16.94 |
| 8 | 1 (80) | 0 (130) | −1 (30) | 12.54 | 12.65 |
| 9 | 0 (70) | 0 (130) | 0 (45) | 17.82 | 16.94 |
| 10 | 0 (70) | 0 (130) | 0 (45) | 16.34 | 16.94 |
| 11 | 0 (70) | 1 (160) | 1 (60) | 13.38 | 13.58 |
| 12 | 0 (70) | −1 (100) | 1 (60) | 13.26 | 13.07 |
| 13 | 1 (80) | −1 (100) | 0 (45) | 11.96 | 12.05 |
| 14 | 0 (70) | 0 (130) | 0 (45) | 17.18 | 16.94 |
| 15 | −1 (60) | 1 (160) | 0 (45) | 17.64 | 17.55 |
| 16 | 1 (80) | 0 (130) | 1 (60) | 11.88 | 11.98 |
| 17 | 1 (80) | 1 (160) | 0 (45) | 15.05 | 14.76 |
| Response | Source | Sum of Squares | df | Mean Square | F-Value | Model p-Value | Lack of Fit | R2 |
|---|---|---|---|---|---|---|---|---|
| Mean | 3909.16 | 1 | 3909.16 | |||||
| Yield (%) | Linear | 36.13 | 3 | 12.04 | 4.96 | 0.0165 * | 0.0244 * | 0.4259 |
| 2FI | 2.52 | 3 | 0.8387 | 0.2885 | 0.8328 | 0.0146 * | 0.3131 | |
| Quadratic | 27.22 | 3 | 9.07 | 34.16 | 0.0001 ** | 0.80 | 0.9373 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 65.86 | 9 | 7.32 | 27.56 | 0.0001 ** |
| A | 28.54 | 1 | 28.54 | 107.47 | <0.0001 ** |
| B | 5.93 | 1 | 5.93 | 22.35 | 0.0021 ** |
| C | 1.66 | 1 | 1.66 | 6.24 | 0.0411 * |
| AB | 0.9702 | 1 | 0.9702 | 3.65 | 0.0975 |
| AC | 0.0576 | 1 | 0.0576 | 0.2169 | 0.6555 |
| BC | 1.49 | 1 | 1.49 | 5.61 | 0.0498 * |
| A2 | 1.59 | 1 | 1.59 | 5.97 | 0.0445 * |
| B2 | 4.5 | 1 | 4.5 | 16.94 | 0.0045 ** |
| C2 | 19.04 | 1 | 19.04 | 71.68 | <0.0001 ** |
| Residual | 1.86 | 7 | 0.2655 | ||
| Lack of Fit | 0.3748 | 3 | 0.1249 | 0.3368 | 0.801 |
| Pure Error | 1.48 | 4 | 0.371 | ||
| Cor Total | 67.72 | 16 | |||
| C.V% | 3.4 | ||||
| R2 | 0.9726 | ||||
| Adjusted R2 | 0.9373 |
| Sample | Hydration Properties | Colour Measurement | |||||
|---|---|---|---|---|---|---|---|
| WHC (g g−1) | SWC (mL g−1) | OHC (g g−1) | L* | a* | b* | ∆E* | |
| STP | 4.17 ± 0.04 c | 3.48 ± 0.06 c | 0.89 ± 0.03 c | 54.71 ± 0.72 b | 52.35 ± 1.04 a | 79.28 ± 0.62 b | 79.93 ± 0.50 a |
| IDF | 6.30 ± 0.02 b | 5.75 ± 0.07 b | 1.25 ± 0.03 b | 72.64 ± 0.21 a | 32.85 ± 0.79 c | 82.47 ± 0.19 a | 74.18 ± 0.30 b |
| SDF | 7.25 ± 0.10 a | 7.24 ± 0.05 a | 1.49 ± 0.02 a | 54.53 ± 0.31 b | 43.54 ± 0.03 b | 71.33 ± 0.50 c | 68.40 ± 0.39 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Sharma, M.; Bhat, R. Valorisation of Sea Buckthorn Pomace by Optimization of Ultrasonic-Assisted Extraction of Soluble Dietary Fibre Using Response Surface Methodology. Foods 2021, 10, 1330. https://doi.org/10.3390/foods10061330
Hussain S, Sharma M, Bhat R. Valorisation of Sea Buckthorn Pomace by Optimization of Ultrasonic-Assisted Extraction of Soluble Dietary Fibre Using Response Surface Methodology. Foods. 2021; 10(6):1330. https://doi.org/10.3390/foods10061330
Chicago/Turabian StyleHussain, Shehzad, Minaxi Sharma, and Rajeev Bhat. 2021. "Valorisation of Sea Buckthorn Pomace by Optimization of Ultrasonic-Assisted Extraction of Soluble Dietary Fibre Using Response Surface Methodology" Foods 10, no. 6: 1330. https://doi.org/10.3390/foods10061330
APA StyleHussain, S., Sharma, M., & Bhat, R. (2021). Valorisation of Sea Buckthorn Pomace by Optimization of Ultrasonic-Assisted Extraction of Soluble Dietary Fibre Using Response Surface Methodology. Foods, 10(6), 1330. https://doi.org/10.3390/foods10061330