Fatty Acid Composition from Olive Oils of Portuguese Centenarian Trees Is Highly Dependent on Olive Cultivar and Crop Year
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Fatty Acid Composition
2.3. Statistical Analysis
3. Results and Discussions
3.1. Effect of Olive Cultivar on Fatty Acid Composition
3.2. Effect of Crop Year on the Fatty Acid Composition
3.3. Cultivar and Crop Year Discrimination According to Fatty Acid Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paolini, M.; Bontempo, L.; Camin, F. Compound-specific δ13C and δ2H analysis of olive oil fatty acids. Talanta 2017, 174, 38–43. [Google Scholar] [CrossRef]
- Boskou, D.; Blekas, G.; Tsimidou, M. Olive oil composition. In Olive Oil, Chemistry and Technology; Boskou, D., Ed.; AOCS Press: Champaign, IL, USA, 2006; pp. 41–72. ISBN 9780128043547. [Google Scholar]
- EFSA European Commission Regulation EC, No. 432/2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, L136, 1–40.
- Ros, E.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Fitó, M.; Martínez, J.A.; Corella, D. Mediterranean Diet and Cardiovascular Health: Teachings of the PREDIMED Study. Adv. Nutr. 2014, 5, 330S–336S. [Google Scholar] [CrossRef] [PubMed]
- IOC (International Olive Council). Revising the Trade Standard Applying to Olive Oils and Olive-Pomance Oils. COI/T.15/NC No 3/Rev. 15. 2019. Available online: https://www.internationaloliveoil.org/what-we-do/chemistry-standardisation-unit/standards-and-methods/ (accessed on 3 October 2020).
- European Commission Regulation EEC/1924/2006 from 20th December: On nutrition and health claims made on foods. Off. J. Eur. Union. 1991, 404, 9–30.
- Dewapriya, P.; Himaya, S.W.A.; Li, Y.X.; Kim, S.K. Tyrosol exerts a protective effect against dopaminergic neuronal cell death in in vitro model of Parkinson’s disease. Food Chem. 2013, 141, 1147–1157. [Google Scholar] [CrossRef]
- Medina, J.M.; Tabernero, A. Chapter 156—The neurotrophic effect of oleic acid: Implications for olive oil in health and disease A2—Preedy, victor R. In Olives and Olive Oil in Health and Disease Prevention; Watson, R.R., Ed.; Academic Press: San Diego, CA, USA, 2010; pp. 1405–1412. [Google Scholar]
- García-Inza, G.P.; Hall, A.J.; Rousseaux, M.C. Proportion of oleic acid in olive oil as influenced by the dimensions of the daily temperature oscillation. Sci. Hortic. 2018, 227, 305–312. [Google Scholar] [CrossRef]
- Orlandi, F.; Bonofiglio, T.; Romano, B.; Fornaciari, M. Qualitative and quantitative aspects of olive production in relation to climate in southern Italy. Sci. Hortic. 2012, 138, 151–158. [Google Scholar] [CrossRef]
- Rondanini, D.P.; Castro, D.N.; Searles, P.S.; Rousseaux, M.C. Contrasting patterns of fatty acid composition and oil accumulation during fruit growth in several olive varieties and locations in a non-Mediterranean region. Eur. J. Agron. 2014, 52, 237–246. [Google Scholar] [CrossRef]
- Hernández, M.L.; Padilla, M.N.; Sicardo, M.D.; Mancha, M.; Martínez-Rivas, J.M. Effect of different environmental stresses on the expression of oleate desaturase genes and fatty acid composition in olive fruit. Phytochemistry 2011, 72, 178–187. [Google Scholar]
- Rondanini, D.P.; Castro, D.N.; Searles, P.S.; Rousseaux, M.C. Fatty acid profiles of varietal virgin olive oils (Olea europaea L.) from mature orchards in warm arid valleys of Northwestern Argentina (La Rioja). Grasas y Aceites 2011, 62, 399–409. [Google Scholar] [CrossRef]
- García-Inza, G.P.; Castro, D.N.; Hall, A.J.; Rousseaux, M.C. Responses to temperature of fruit dry weight, oil concentration, and oil fatty acid composition in olive (Olea europaea L. var. Arauco). Eur. J. Agron. 2014, 54, 107–115. [Google Scholar]
- Bakhouche, A.; Lozano-Sánchez, J.; Beltrán-Debón, R.; Joven, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic characterization and geographical classification of commercial Arbequina extra-virgin olive oils produced in southern Catalonia. Food Res. Int. 2013, 50, 401–408. [Google Scholar] [CrossRef]
- Borges, T.H.; Pereira, J.A.; Cabrera-Vique, C.; Lara, L.; Oliveira, A.F.; Seiquer, I. Characterization of Arbequina virgin olive oils produced in different regions of Brazil and Spain: Physicochemical properties, oxidative stability and fatty acid profile. Food Chem. 2017, 215, 454–462. [Google Scholar] [CrossRef]
- Dabbou, S.; Dabbou, S.; Chehab, H.; Brahmi, F.; Taticchi, A.; Servili, M.; Hammami, M. Chemical composition of virgin olive oils from Koroneiki cultivar grown in Tunisia with regard to fruit ripening and irrigation regimes. Int. J. Food Sci. Technol. 2011, 46, 577–585. [Google Scholar] [CrossRef]
- Portarena, S.; Farinelli, D.; Lauteri, M.; Famiani, F.; Estic, M.; Brugnoli, E. Stable isotope and fatty acid compositions of monovarietal olive oils: Implications of ripening stage and climate effects as determinants in traceability studies. Food Control 2015, 57, 129–135. [Google Scholar] [CrossRef]
- Pereira, J.A.; Casal, S.; Bento, A.; Oliveira, M.B.P.P. Influence of olive storage period on oil quality of three Portuguese cultivars of Olea europea, Cobrançosa, Madural and Verdeal Transmontana. J. Agric. Food Chem. 2002, 50, 6335–6340. [Google Scholar] [CrossRef] [PubMed]
- European Community Regulation EEC/2568/91 from 11th July: On the characteristics of olive oil and olive-pomace oil and on the relevant methods of analysis. Off. J. Eur. Union 1991, 248, 1–83.
- Cadima, J.; Cerdeira, J.O.; Minhoto, M. Computational aspects of algorithms for variable selection in the context of principal components. Comput. Stat. Data Anal. 2004, 47, 225–236. [Google Scholar] [CrossRef]
- Cadima, J.; Cerdeira, J.O.; Silva, P.D.; Minhoto, M. The Subselect R Package. 2012. Available online: http://cran.rproject.org/web/packages/subselect/vignettes/subselect.pdf (accessed on 15 February 2016).
- Kuhn, M.; Johnson, K. Applied Predictive Modeling; Springer Science Business Media: New York, NY, USA, 2013. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S (Statistics and Computing), 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Kritioti, A.; Menexes, G.; Drouza, C. Chemometric characterization of virgin olive oils of the two major Cypriot cultivars based on their fatty acid composition. Food Res. Int. 2018, 103, 426–437. [Google Scholar] [CrossRef]
- Xiang, C.; Xu, Z.; Liu, J.; Li, T.; Yang, Z.; Ding, C. Quality, composition, and antioxidant activity of virgin olive oil from introduced varieties at Liangshan. LWT 2017, 78, 226–234. [Google Scholar] [CrossRef]
- Wanga, J.; Ma, L.; Gómez-del-Campo, M.; Zhang, D.; Deng, Y.; Jia, Z. Youth tree behavior of olive (Olea europaea L.) cultivars in Wudu, China: Cold and drought resistance, growth, fruit production, and oil quality. Sci. Hortic. 2018, 236, 106–122. [Google Scholar] [CrossRef]
- Saad, N.; Delattre, C.; Urdaci, M.; Schmitter, J.M.; Bressollier, P. An overview of the last advances in probiotic and prebiotic field. LWT 2012, 50, 11–16. [Google Scholar] [CrossRef]
- García-Mesa, J.A.; Pereira-Caro, G.; Fernández-Hernández, A.; García-Ortíz Civantos, C.; Mateos, R. Influence of lipid matrix in the bitterness perception of virgin olive oil. Food Qual. Prefer 2008, 19, 421–430. [Google Scholar] [CrossRef]
- Youssef, O.; Flamini, G.; Mokhar, G.; Nabil, B.Y.; Daoud, D.; Mokhtar, Z. The compositional quality and volatile compounds of samples from the blend of monovarietal olive oils cultivated in Tunisia. Int. J. Food Sci. Technol. 2011, 46, 678–686. [Google Scholar] [CrossRef]
- Ceci, L.N.; Mattar, S.B.; Carelli, A.A. Chemical quality and oxidative stability of extra virgin olive oils from San Juan province (Argentina). Food Res. Int. 2017, 100, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Tena, N.; Aparicio, R.; García-González, D.L. Virgin olive oil stability study by mesh cell-FTIR spectroscopy. Talanta 2017, 167, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Dabbou, S.; Brahmi, F.; Taamali, A.; Issaoui, M.; Ouni, Y.; Braham, M.; Hammami, M. Extra virgin olive oil components and oxidative stability from olives grown in Tunisia. J. Am. Oil. Chem. Soc. 2010, 87, 1199–1209. [Google Scholar] [CrossRef]
- Mailer, R.J.; Ayton, J.; Graham, K. The influence of growing region, cultivar and harvest timing on the diversity of Australian olive oil. J. Am. Oil. Chem. Soc. 2010, 87, 877–884. [Google Scholar] [CrossRef]
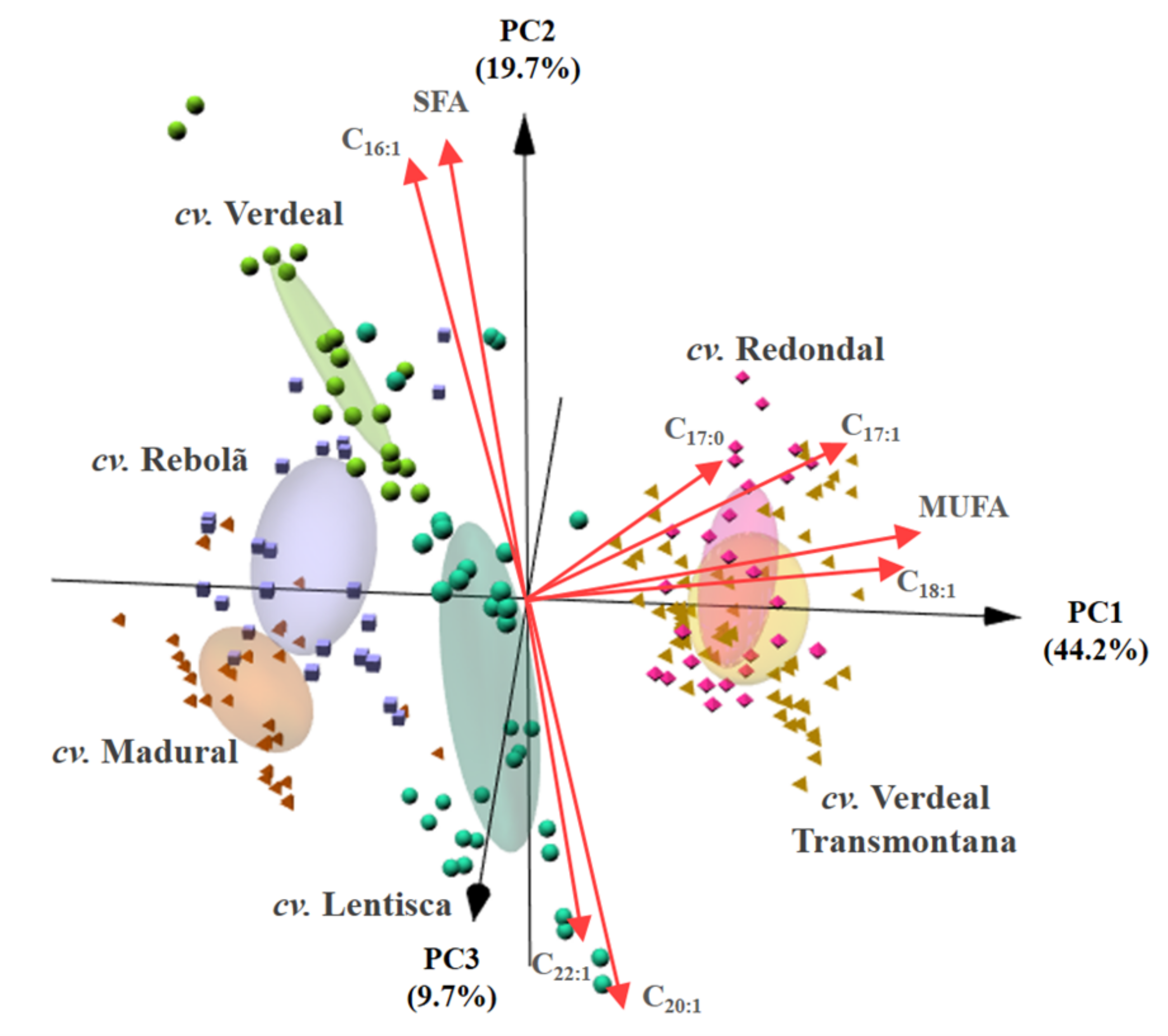
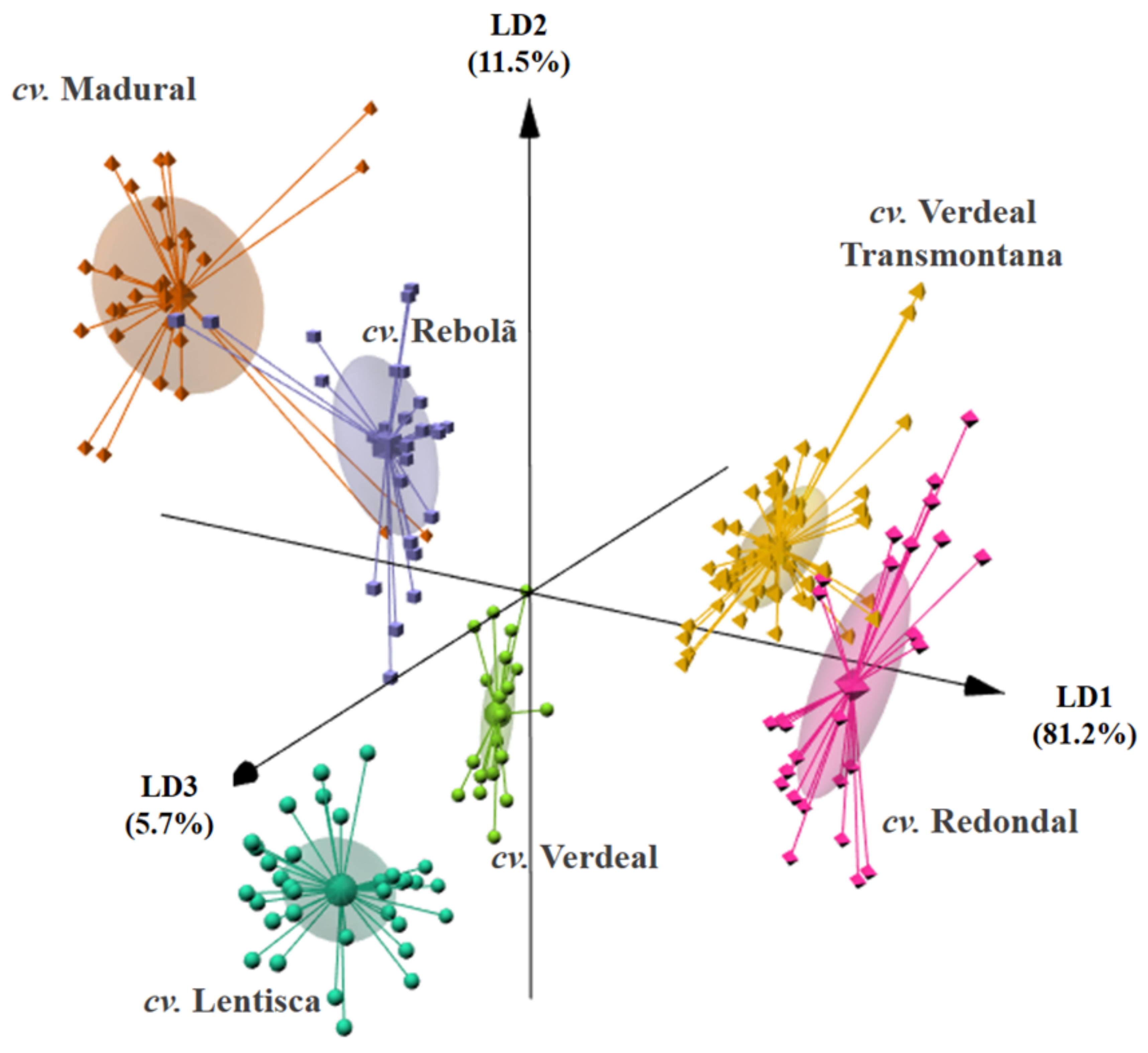
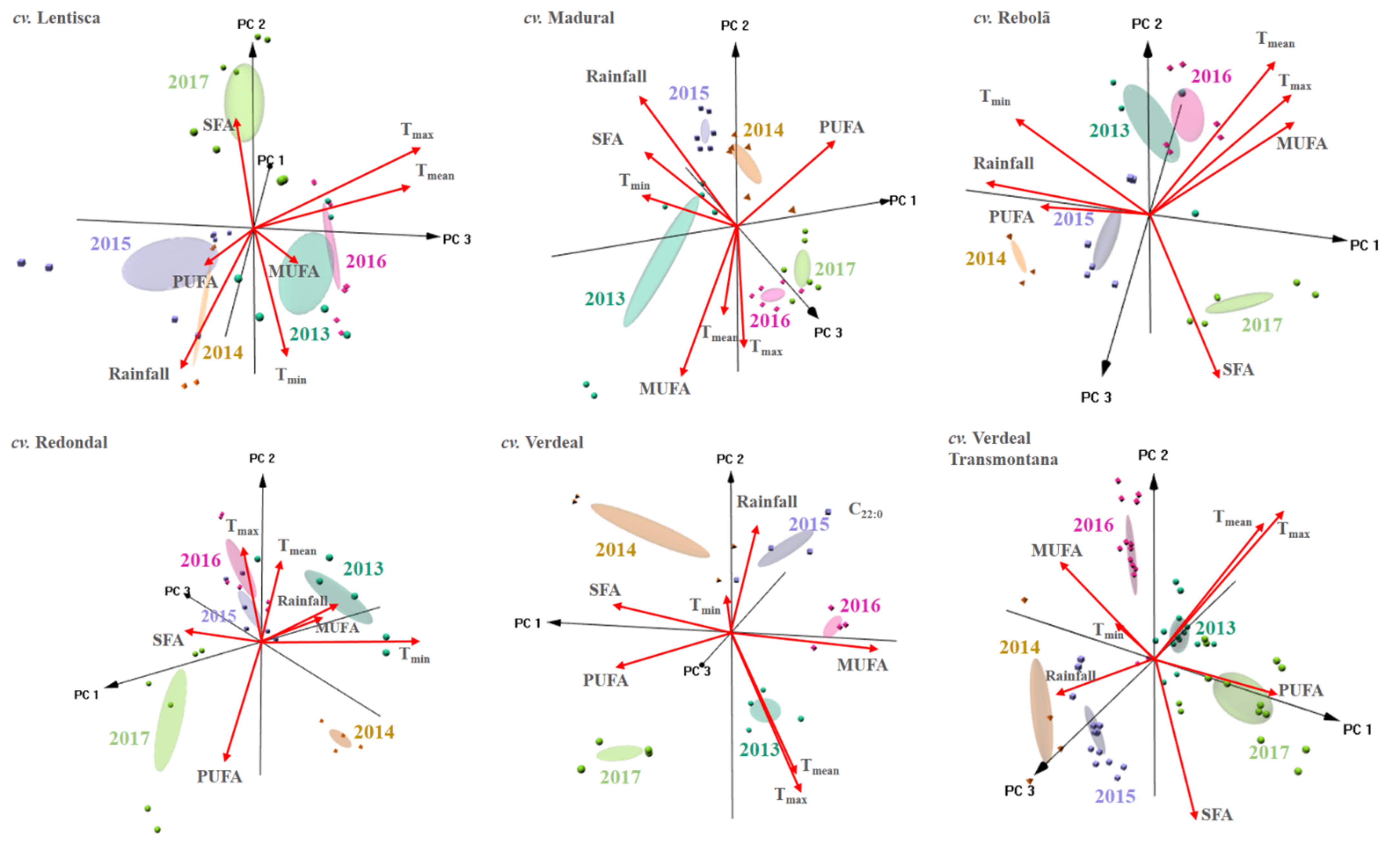
| Figure | Year | cv. Lentisca | cv. Madural | cv. Rebolã | cv. Redondal | cv. Verdeal | cv. Verdeal Transmontana | p-Value * |
|---|---|---|---|---|---|---|---|---|
| Palmitic acid (C16:0) | 2013 | 9.9 ± 0.4 a;C | 12.4 ± 0.6 a;A | 12.3 ± 0.7 a,b;A | 10.6 ± 0.4 a,b;B,C | 13.0 ± 0.1 a;A | 11.0 ± 0.3 a,b;B | <0.0001 |
| 2014 | 9.9 ± 0.4 a;C | 11.7 ± 0.3 b;B,C | 12.8 ± 0.5 a,b;B | 10.2 ± 0.3 b;C | 14.8 ± 2.1 a;A | 11.1 ± 0.7 a,b;B,C | <0.0001 | |
| 2015 | 10.6 ± 0.4 a;C | 12.6 ± 0.1 a;A | 12.9 ± 0.4 a;A | 10.9 ± 0.3 a;B,C | 12.8 ± 0.2 a;A | 11.3 ± 0.4 a;B | <0.0001 | |
| 2016 | 10.2 ± 1.0 a;D | 11.3 ± 0.3 b;B,C | 12.0 ± 0.4 b;A,B; | 10.7 ± 0.4 a,b;C,D | 13.0 ± 0.1 a;A | 10.3 ± 0.5 c;D | <0.0001 | |
| 2017 | 11.0 ± 1.4 a;B | 11.1 ± 0.1 b;B | 13.0 ± 0.2 a;A | 11.2 ± 0.2 a;B | 14.1 ± 0.2 a;A | 10.7 ± 0.6 b,c;B | <0.0001 | |
| p-value * | 0.1650 | <0.0001 | 0.0018 | 0.0022 | 0.0453 | <0.0001 | ||
| Mean (Min.–Max.) | 10.4 (9.1–12.5) | 11.8 (10.8–13.1) | 12.6 (11.4–13.3) | 10.8 (9.9–11.5) | 13.5 (12.7–16.7) | 10.8 (9.8–11.9) | ||
| Palmitoleic acid (C16:1) | 2013 | 0.68 ± 0.13 a;B | 0.76 ± 0.28 a;B | 0.71 ± 0.17 c;B | 0.80 ± 0.07 b;B | 1.23 ± 0.03 b;A | 0.71 ± 0.05 a;B | <0.0001 |
| 2014 | 0.61 ± 0.16 a;C | 0.48 ± 0.01 b;C | 1.30 ± 0.03 a;A | 1.03 ± 0.04 a;B | 1.39 ± 0.05 a;A | 0.58 ± 0.07 b,c;C | <0.0001 | |
| 2015 | 0.79 ± 0.17 a;C | 0.57 ± 0.01 ª,b;D | 0.99 ± 0.11 b;B | 0.70 ± 0.05 c;C,D | 1.25 ± 0.03 b;A | 0.68 ± 0.06 a,b;C,D | <0.0001 | |
| 2016 | 0.69 ± 0.24 a;B,C | 0.48 ± 0.01 b,D | 0.83 ± 0.05 b,c;B | 0.76 ± 0.07 b,c;B,C | 1.18 ± 0.02 b;A | 0.59 ± 0.10 c;C,D | <0.0001 | |
| 2017 | 0.79 ± 0.28 a;B,C | 0.53 ± 0.01 b;D | 0.83 ± 0.08 b,c;B | 0.79 ± 0.03 b,c;B,C | 1.41 ± 0.02 a;A | 0.61 ± 0.09 b,c;C,D | <0.0001 | |
| p-value * | 0.5670 | 0.0033 | <0.0001 | <0.0001 | <0.0001 | 0.0002 | ||
| Mean (Min.–Max.) | 0.72 (0.43–1.15) | 0.56 (0.46–1.13) | 0.92 (0.55–1.34) | 0.80 (0.66–1.08) | 1.29 (1.16–1.44) | 0.64 (0.49–0.84) | ||
| Stearic acid (C18:0) | 2013 | 2.87 ± 0.40 a;B | 2.18 ± 0.23 c;C | 2.41 ± 0.25 a,b;B,C | 3.37 ± 0.33 a;A | 2.59 ± 0.02 a;B,C | 2.65 ± 0.11 b;B | <0.0001 |
| 2014 | 2.69 ± 0.23 a;A | 2.44 ± 0.04 b;A,B,C | 1.84 ± 0.04 c,D | 2.27 ± 0.15 c,C | 2.35 ± 0.09 b;B,C | 2.60 ± 0.14 b;A,B | <0.0001 | |
| 2015 | 2.54 ± 0.29 a;A,C | 2.25 ± 0.04 b,c;C,D | 2.07 ± 0.09 b,c;D | 2.84 ± 0.27 b;A | 2.45 ± 0.03 b;B,C | 2.68 ± 0.14 b;A,B | <0.0001 | |
| 2016 | 2.70 ± 0.18 a;A,B | 2.31 ± 0.10 b,c,C | 1.97 ± 0.10 b,c,C | 2.88 ± 0.42 ª,b;A | 2.38 ± 0.03 b,B,C | 2.70 ± 0.25 b;A,B | <0.0001 | |
| 2017 | 2.92 ± 0.35 a;A,B,C | 2.81 ± 0.23 ª;B,C | 2.74 ± 0.45 a;B,C | 3.16 ± 0.09 ª,b;A,B | 2.45 ± 0.02 b,C | 3.35 ± 0.45 a;A | 0.0004 | |
| p-value * | 0.1360 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Mean (Min.-Max.) | 2.75 (2.11–3.42) | 2.40 (1.89–3.10) | 2.22 (1.80–3.37) | 2.92 (2.13–3.77) | 2.45 (2.26–2.63) | 2.83 (2.33–3.89) | ||
| Oleic acid (C18:1) | 2013 | 77.0 ± 2.5 a;B | 70.5 ± 1.8 a,b;C | 72.6 ± 2.5 a,b;C | 80.4 ± 0.3 b;A | 76.8 ± 0.2 a;B | 80.4 ± 0.2 b;A | <0.0001 |
| 2014 | 79.2 ± 1.1 a;B | 70.9 ± 0.4 a;D | 70.0 ± 0.5 b;D | 81.6 ± 0.1 a;A | 74.2 ± 2.1 b;C | 80.8 ± 0.9 a,b;A,B | <0.0001 | |
| 2015 | 77.6 ± 2.4 a;B | 69.0 ± 0.5 b;D | 73.3 ± 1.8 a,b;C | 80.8 ± 0.3 a,b;A | 76.8 ± 0.3 a;B | 80.2 ± 0.5 b;A | <0.0001 | |
| 2016 | 79.2 ± 1.2 a;B | 71.2 ± 0.3 a,E | 75.3 ± 0.6 a,D | 80.9 ± 0.7 a,b;A | 76.9 ± 0.1 a,C | 81.3 ± 0.4 a;A | <0.0001 | |
| 2017 | 77.1 ± 1.2 a;B | 69.7 ± 0.6 a,b;D | 72.4 ± 2.0 b;C | 79.3 ± 1.0 c;A | 74.1 ± 0.4 b;C | 79.3 ± 0.6 c;A | <0.0001 | |
| p-value * | 0.0856 | 0.0022 | 0.0019 | <0.0001 | 0.0005 | <0.0001 | ||
| Mean (Min.–Max.) | 78.0 (74.2–80.6) | 70.3 (68.3–72.9) | 72.9 (69.6–75.8) | 80.6 (78.1–81.8) | 75.8 (72.4–77.1) | 80.4 (78.3–82.1) | ||
| Linoleic acid (C18:2) | 2013 | 7.27 ± 2.77 a;B,C | 11.77 ± 2.18 b;A | 9.68 ± 2.81 a,b:A,B | 2.10 ± 0.11 b;C | 4.32 ± 0.05 c;C | 2.76 ± 0.15 b;C | <0.0001 |
| 2014 | 5.56 ± 1.63 a;B | 12.08 ± 0.31 ª,b;A | 11.74 ± 0.92 a;A | 2.12 ± 0.01 b;C | 5.25 ± 0.04 b;B | 2.31 ± 0.12 c;C | <0.0001 | |
| 2015 | 6.40 ± 2.70 a;C | 13.17 ± 0.42 a,b;A | 8.65 ± 1.32 ª,b;B | 1.90 ± 0.12 b;D | 4.76 ± 0.24 b,c;C | 2.55 ± 0.19 b,c;D | <0.0001 | |
| 2016 | 5.33 ± 2.03 a;C | 12.39 ± 0.49 ª,b;A | 7.82 ± 0.45 b;B | 2.06 ± 0.16 b;D | 4.56 ± 0.12 c,C | 2.55 ± 0.06 b,c;D | <0.0001 | |
| 2017 | 6.13 ± 2.46 a;C | 13.63 ± 0.28 ª;A | 8.98 ± 2.16 ª,b;B | 2.75 ± 0.48 a;D | 6.08 ± 0.48 a;C | 3.22 ± 0.34 a;D | <0.0001 | |
| p-value * | 0.6370 | 0.0253 | 0.0227 | <0.0001 | <0.0001 | <0.0001 | ||
| Mean (Min.–Max.) | 6.15 (3.33–9.73) | 12.61 (8.90–14.0) | 9.17 (6.06–12.55) | 2.19 (1.77–3.34) | 4.99 (4.27–6.50) | 2.74 (2.16–3.76) | ||
| Linolenic acid (C18:3) | 2013 | 0.93 ± 0.20 a;B,C | 1.27 ± 0.19 a;A | 1.17 ± 0.23 a;A,B | 0.90 ± 0.07 a;C | 0.96 ± 0.06 ª;B,C | 0.81 ± 0.06 a;C | <0.0001 |
| 2014 | 0.82 ± 0.13 a;C | 1.20 ± 0.16 a;A | 1.07 ± 0.02 a,b;A,B | 0.90 ± 0.01 a;B,C | 0.89 ± 0.05 ª,b;B,C | 0.74 ± 0.04 a,b;C | <0.0001 | |
| 2015 | 0.89 ± 0.23 a;B | 1.23 ± 0.06 a;A | 0.92 ± 0.04 b,c;B | 0.88 ± 0.05 a,b;B,C | 0.87 ± 0.01 b;B,C | 0.76 ± 0.04 a,b,C | <0.0001 | |
| 2016 | 0.74 ± 0.09 a;C | 1.15 ± 0.02 a;A | 0.92 ± 0.12 b,c;B | 0.80 ± 0.02 b,c;B,C | 0.88 ± 0.02 b;B | 0.74 ± 0.05 b;C | <0.0001 | |
| 2017 | 0.83 ± 0.14 a;B,C | 1.11 ± 0.03 a;A | 0.86 ± 0.04 c;B,C | 0.77 ± 0.04 c;C,D | 0.94 ± 0.03 a,b;B | 0.74 ± 0.07 b;D | <0.0001 | |
| p-value * | 0.2930 | 0.1610 | 0.0014 | <0.0001 | 0.0134 | 0.0051 | ||
| Mean (Min.–Max.) | 0.84 (0.65–1.19) | 1.19 (1.00–1.41) | 0.97 (0.80–1.37) | 0.85 (0.73–0.97) | 0.91 (0.84–1.01) | 0.76 (0.65–0.89) | ||
| Arachidic acid (C20:0) | 2013 | 0.41 ± 0.04 a;B | 0.35 ± 0.03 b;C | 0.37 ± 0.03 b;B,C | 0.51 ± 0.03 a,A | 0.38 ± 0.02 a;B,C | 0.48 ± 0.02 b,A | <0.0001 |
| 2014 | 0.37 ± 0.06 a;A | 0.48 ± 0.15 a;A | 0.35 ± 0.01 b;A | 0.41 ± 0.03 b;A | 0.33 ± 0.03 b;A | 0.49 ± 0.03 a,b;A | 0.0279 | |
| 2015 | 0.35 ± 0.05 a;B | 0.34 ± 0.01 b;B | 0.38 ± 0.02 b;B | 0.48 ± 0.04 a;A | 0.37 ± 0.02 a,b;B | 0.49 ± 0.02 b;A | <0.0001 | |
| 2016 | 0.39 ± 0.06 a;B | 0.36 ± 0.01 b;B | 0.36 ± 0.01 b;B | 0.48 ± 0.05 a;A | 0.39 ± 0.01 a;B | 0.50 ± 0.03 a,b;A | <0.0001 | |
| 2017 | 0.40 ± 0.07 a;C | 0.37 ± 0.02 a,b,C | 0.41 ± 0.03 a;B,C | 0.49 ± 0.02 a;A,B | 0.36 ± 0.02 a,b;C | 0.53 ± 0.05 a;A | <0.0001 | |
| p-value * | 0.3670 | 0.0088 | 0.0005 | 0.0044 | 0.0092 | 0.0030 | ||
| Mean (Min.–Max.) | 0.39 (0.29–0.48) | 0.38 (0.30–0.68) | 0.38 (0.34–0.44) | 0.48 (0.39–0.54) | 0.37 (0.30–0.41) | 0.50 (0.44–0.61) | ||
| Eicosenoic acid (C20:1) | 2013 | 0.36 ± 0.10 a;A | 0.34 ± 0.03 a;A | 0.30 ± 0.03 ª,b;A,B | 0.33 ± 0.03 b,c;A | 0.24 ± 0.01 b,c;B | 0.32 ± 0.02 b,c;A | 0.0063 |
| 2014 | 0.34 ± 0.09 a;A | 0.34 ± 0.01 a;A | 0.32 ± 0.01 ª;A,B | 0.39 ± 0.01 a;A | 0.25 ± 0.02 a;B | 0.35 ± 0.02 a;A | 0.0014 | |
| 2015 | 0.33 ± 0.10 a;A,B | 0.32 ± 0.01 a,b;A,B | 0.30 ± 0.01 ª;A,B | 0.36 ± 0.02 a,b;A | 0.26 ± 0.01 a,b;B | 0.34 ± 0.01 a,b;A | 0.0230 | |
| 2016 | 0.30 ± 0.08 a;A,B | 0.32 ± 0.00 a,b;A | 0.31 ± 0.04 ª;A,B | 0.33 ± 0.01 b,c;A | 0.24 ± 0.01 a,b;B | 0.32 ± 0.01 b,c;A | 0.0215 | |
| 2017 | 0.30 ± 0.08 a;A | 0.30 ± 0.01 b;A | 0.26 ± 0.01 b;A,B | 0.32 ± 0.00 c;A | 0.22 ± 0.01 c;B | 0.31 ± 0.02 c;A | 0.0004 | |
| p-value * | 0.7560 | 0.0038 | 0.0040 | <0.0001 | 0.0006 | <0.0001 | ||
| Mean (Min.-Max.) | 0.32 (0.22–0.45) | 0.32 (0.28–0.39) | 0.30 (0.24–0.37) | 0.34 (0.29–0.40) | 0.24 (0.21–0.28) | 0.33 (0.28–0.36) |
| Year–Month | Temperature Records (°C) | Rainfall (mm) | ||
|---|---|---|---|---|
| Minimum | Mean | Maximum | ||
| 2013 | ||||
| August | 16.8 ± 2.2 | 25.6 ± 9.3 | 34.4 ± 3.4 | 0.0 |
| September | 14.2 ± 2.2 | 22.4 ± 8.8 | 30.5 ± 3.8 | 30.0 |
| October | 11.0 ± 3.8 | 16.4 ± 6.4 | 21.9 ± 2.9 | 111.4 |
| 2014 | ||||
| August | 14.3 ± 2.6 | 22.7 ± 8.9 | 31.2 ± 2.6 | 0.4 |
| September | 14.5 ± 4.5 | 21.0 ± 7.6 | 27.6 ± 4.5 | 84.8 |
| October | 10.9 ± 3.0 | 17.1 ± 6.8 | 23.2 ± 3.0 | 77.2 |
| 2015 | ||||
| August | 14.5 ± 2.6 | 23.2 ± 9.4 | 31.9 ± 4.4 | 20.2 |
| September | 11.9 ± 3.0 | 19.5 ± 8.3 | 27.1 ± 3.5 | 50.4 |
| October | 9.8 ± 3.3 | 15.2 ± 6.2 | 20.6 ± 2.7 | 109.4 |
| 2016 | ||||
| August | 15.8 ± 2.4 | 25.1 ± 9.9 | 34.5 ± 3.2 | 32.2 |
| September | 12.9 ± 2.8 | 21.4 ± 9.6 | 30.0 ± 5.5 | 24.2 |
| October | 9.9 ± 2.7 | 16.3 ± 7.2 | 22.6 ± 3.8 | 41.6 |
| 2017 | ||||
| August | 15.0 ± 2.8 | 24.4 ± 10.0 | 33.8 ± 3.4 | 1.2 |
| September | 10.8 ± 3.1 | 19.9 ± 9.6 | 28.9 ± 3.1 | 0.2 |
| October | 8.9 ± 3.7 | 17.7 ± 10.0 | 26.5 ± 5.3 | 28.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, N.; Casal, S.; Pinho, T.; Cruz, R.; Peres, A.M.; Baptista, P.; Pereira, J.A. Fatty Acid Composition from Olive Oils of Portuguese Centenarian Trees Is Highly Dependent on Olive Cultivar and Crop Year. Foods 2021, 10, 496. https://doi.org/10.3390/foods10030496
Rodrigues N, Casal S, Pinho T, Cruz R, Peres AM, Baptista P, Pereira JA. Fatty Acid Composition from Olive Oils of Portuguese Centenarian Trees Is Highly Dependent on Olive Cultivar and Crop Year. Foods. 2021; 10(3):496. https://doi.org/10.3390/foods10030496
Chicago/Turabian StyleRodrigues, Nuno, Susana Casal, Teresa Pinho, Rebeca Cruz, António M. Peres, Paula Baptista, and José Alberto Pereira. 2021. "Fatty Acid Composition from Olive Oils of Portuguese Centenarian Trees Is Highly Dependent on Olive Cultivar and Crop Year" Foods 10, no. 3: 496. https://doi.org/10.3390/foods10030496
APA StyleRodrigues, N., Casal, S., Pinho, T., Cruz, R., Peres, A. M., Baptista, P., & Pereira, J. A. (2021). Fatty Acid Composition from Olive Oils of Portuguese Centenarian Trees Is Highly Dependent on Olive Cultivar and Crop Year. Foods, 10(3), 496. https://doi.org/10.3390/foods10030496













