In Vitro Genotoxicity Assessment of Functional Ingredients: Betaine, Choline, and Taurine
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
2.3. Miniaturized Ames Test
2.4. In Vitro MN Test
2.5. Enzyme-Modified Comet Assay
2.6. Proliferation Assay
2.7. Statistical Analysis
3. Results
3.1. Miniaturized Ames Test
3.2. In Vitro MN Test
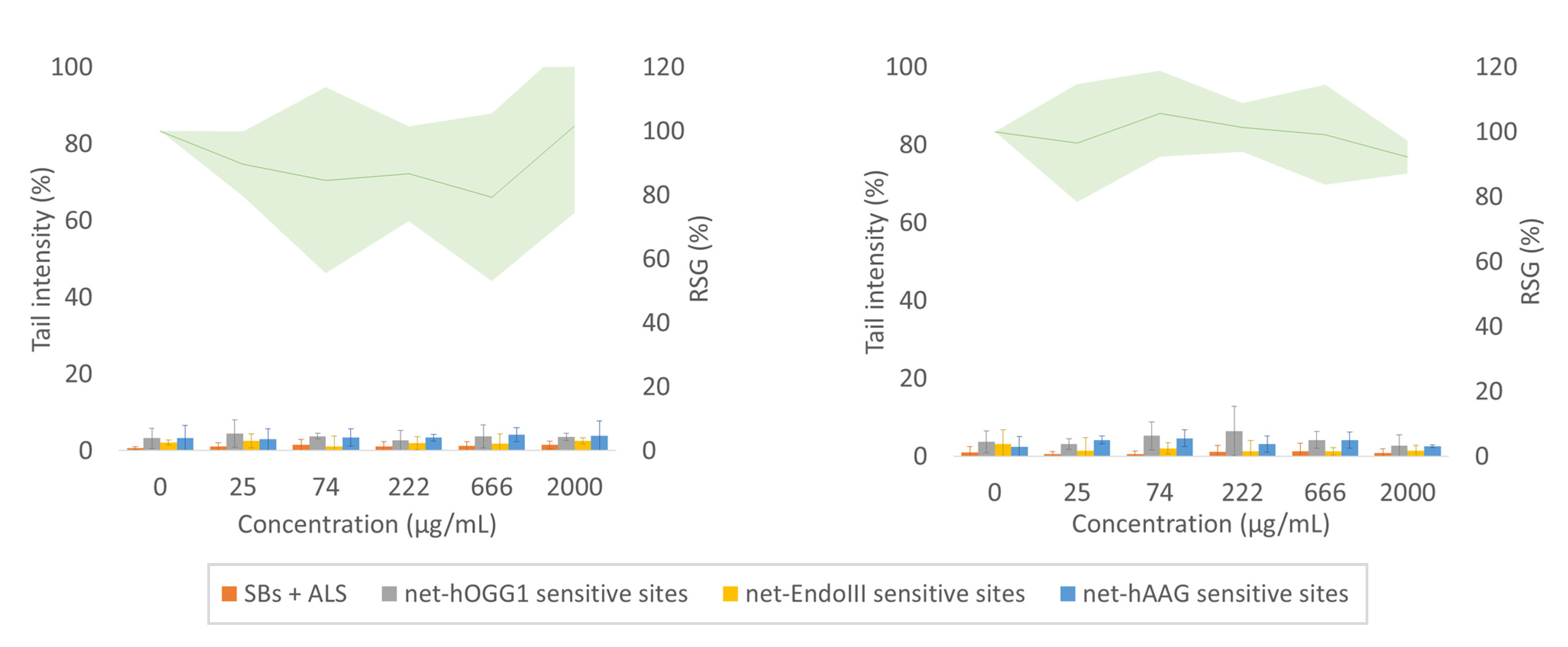
3.3. Enzyme-Modified Comet Assay and Proliferation Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vettorazzi, A.; de Cerain, A.L.; Sanz-Serrano, J.; Gil, A.G.; Azqueta, A. European regulatory framework and safety assessment of food-related bioactive compounds. Nutrients 2020, 12, 613. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (EFSA NDA Panel); Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of betaine as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control. Off. J. Eur. Union 2013, 181, 35–56. [Google Scholar]
- Schaffer, S.; Kim, H.W. Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomol. Ther. 2018, 26, 225–241. [Google Scholar] [CrossRef]
- European Commission. EU Register of Nutrition and Health Claims Made on Foods (v.3.5). Available online: https://ec.europa.eu/food/safety/labelling_nutrition/claims/register/public/ (accessed on 12 January 2021).
- Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to betaine. EFSA J. 2011, 9, 1–14. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, 136, 1–40. [Google Scholar]
- Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to choline. EFSA J. 2011, 9, 1–23. [Google Scholar] [CrossRef]
- Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to choline. EFSA J. 2014, 12, 1–9. [Google Scholar] [CrossRef][Green Version]
- European Commission. Commission Regulation (EU) 2015/391 of 9 March 2015 refusing to authorise certain health claims made on foods and referring to children’s development and health. Off. J. Eur. Union 2015, 65, 15–17. [Google Scholar]
- Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to taurine. EFSA J. 2009, 7, 1–17. [Google Scholar] [CrossRef]
- Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to taurine. EFSA J. 2011, 9, 1–19. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2015/2283 on novel foods. Off. J. Eur. Union 2015, 327, 1–22. [Google Scholar]
- European Commission. Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. Off. J. Eur. Union 2006, 404, 26–38. [Google Scholar]
- European Commission. Regulation (EC) No 178/2002 of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Off. J. Eur. Communities 2002, 31, 1–24. [Google Scholar]
- Nutrition and Allergies (NDA). Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA J. 2016, 14. [Google Scholar] [CrossRef]
- FAO/WHO. Chapter 4: Hazard Identification and Characterization: Toxicological and Human Studies. In Principles and Methods for the Risk Assessment of Chemicals in Food; World Health Organization: Geneva Switzerland, 2009; pp. 1–190. ISBN 9789241572408. [Google Scholar]
- European Food Safety Authority. Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA J. 2011, 9, 2379. [Google Scholar] [CrossRef]
- Nutrition and Allergies (NDA). Opinion of the Scientific Panel on Dietetic products, nutrition and allergies related to an application concerning the use of betaine as a novel food in the EU. EFSA J. 2005, 3, 1–17. [Google Scholar] [CrossRef]
- Nutrition and Allergies (NDA). Safety of betaine as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017 establishing the Union list of novel foods in accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. Off. J. Eur. Union 2017, 351, 72–201. [Google Scholar]
- Caine, J.J.; Geracioti, T.D. Taurine, energy drinks, and neuroendocrine effects. Cleve. Clin. J. Med. 2016, 83, 895–904. [Google Scholar] [CrossRef]
- Nutrition and Allergies (NDA). The use of taurine and D-glucurono-γ-lactone as constituents of the so-called “energy” drinks. EFSA J. 2009, 935, 1–31. [Google Scholar]
- Nutrition and Allergies (NDA). Scientific Opinion on safety and efficacy of choline chloride as a feed additive for all animal species. EFSA J. 2011, 9, 1–15. [Google Scholar] [CrossRef]
- International Organization for Migration. Section 12: Choline. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998; pp. 390–422. ISBN 978-0-309-06554-2. [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD). Choline Chloride; OECD: Berlin, Germany, 2004; pp. 1–135. [Google Scholar]
- Scientific Committee on Food (SCF). Opinion on Caffeine, Taurine and D-Glucurono-γ-Lactone as Constituents of So-Called “Energy” Drinks; SCF: Brussels, Belgium, 1999; pp. 1–12. [Google Scholar]
- Scientific Committee on Food (SCF). Opinion of the Scientific Committee on Food on Additional Information on "Energy” Drinks; SCF: Brussels, Belgium, 2003; pp. 1–26. [Google Scholar]
- Burke, D.A.; Wedd, D.J.; Burlinson, B. Use of the Miniscreen assay to screen novel compounds for bacterial mutagenicity in the pharmacentical industry. Mutagenesis 1996, 11, 201–205. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Test No. 471: Bacterial Reverse Mutation Test. OECD Guidel. Test. Chem. 2020, 1–12. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Test No. 487: In Vitro Mammalian Cell Micronucleus Test. OECD Guidel. Test. Chem. 2014, 1–29. [Google Scholar] [CrossRef]
- Muruzabal, D.; Langie, S.A.S.; Pourrut, B.; Azqueta, A. The enzyme-modified comet assay: Enzyme incubation step in 2 vs. 12-gels/slide systems. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 845, 402981. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C.; O’Donovan, M.R.; Martin, E.A. hOGG1 recognizes oxidative damage using the comet assay with greater specificity than FPG or ENDOIII. Mutagenesis 2006, 21, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Duthie, S.J.; Dobson, V.L. Direct enzymic detection of endogenous oxidative base damage in human lymphocyte DNA. Carcinogenesis 1993, 14, 1733–1735. [Google Scholar] [CrossRef]
- Muruzabal, D.; Sanz-Serrano, J.; Sauvaigo, S.; Gützkow, K.B.; López de Cerain, A.; Vettorazzi, A.; Azqueta, A. Novel approach for the detection of alkylated bases using the enzyme-modified comet assay. Toxicol. Lett. 2020, 330, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Hoorn, A.J.W. Dimethylglycine and chemically related amines tested for mutagenicity under potential nitrosation conditions. Mutat. Res. Toxicol. 1989, 222, 343–350. [Google Scholar] [CrossRef]
- Litton Bionetics. Mutagenicity Evaluation of Choline Chloride, 000067-48-1; Report No. PB-266 891; Litton Bionetics: Frederick, MD, USA, 1977; pp. 1–19. [Google Scholar]
- Haworth, S.; Lawlor, T.; Mortelmans, K.; Speck, W.; Zeiger, E. Salmonella mutagenicity test results for 250 chemicals. Environ. Mutagen. 1983, 5, 3–49. [Google Scholar] [CrossRef]
- Sussmuth, R.; Lingens, F. Mutagenic Actions of Chlorocholine Chloride. Mutat. Res. 1976, 40, 229–236. [Google Scholar] [CrossRef]
- Galloway, S.; Bloom, A.; Resnick, M.; Margolin, B.; Nakamura, F.; Archer, P.; Zeiger, E. Development of a standard protocol for in vitro cytogenetic testing with Chinese hamster ovary cells: Comparison of results for 22 compounds in two laboratories. Environ. Mutagen. 1985, 7, 1–51. [Google Scholar] [CrossRef]
- Cozzi, R.; Ricordy, R.; Bartolini, F.; Perticone, P.; De Salvia, R.; Sapienza, L. Taurine and Ellagic Acid: Two Differently-Acting Natural Antioxidants. Environ. Mol. Mutagen. 1995, 26, 248–254. [Google Scholar] [CrossRef]
- Yamada, K.; Lim, B.O.; Nonaka, M.; Sugano, M. Measurements of Mutagenic and Antimutagenic Activities of Bile Acids by Rec-assay. Biosci. Biotechnol. Biochem. 1993, 57, 599–602. [Google Scholar] [CrossRef][Green Version]
- Turkez, H.; Aydin, E. The Effects of Taurine on Permethrininduced Cytogenetic and Oxidative Damage in Cultured Human Lymphocytes. Arch. Ind. Hyg. Toxicol. 2012, 63, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Upston, J.M.; Terentis, A.C.; Stocker, R. Tocopherol-mediated peroxidation of lipoproteins: Implications for vitamin E as a potential antiatherogenic supplement. FASEB J. 1999, 13, 977–994. [Google Scholar] [CrossRef]
- Lever, M.; Sizeland, P.C.M.; Frampton, C.M.; Chambers, S.T. Short and long-term variation of plasma glycine betaine concentrations in humans. Clin. Biochem. 2004, 37, 184–190. [Google Scholar] [CrossRef]
- Tiihonen, K.; Saarinen, M.T. Effect of Dietary Betaine on Metabolic Syndrome Risk Factors in Asian. J. Diabetes Metab. 2016, 7. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and Its Panel on Folate Other B Vitamins and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998; Volume 78, ISBN 978-0-309-06554-2. [Google Scholar]
- Trautwein, E.A.; Hayes, K.C. Plasma and whole blood taurine concentrations respond differently to taurine supplementation (humans) and depletion (cats). Z. Ernahrungswiss. 1995, 34, 137–142. [Google Scholar] [CrossRef] [PubMed]

| Nutrient | Status | Health Relationship | EFSA Opinion Reference | Commission Regulation (EU) | |
|---|---|---|---|---|---|
| Betaine | Authorized | Contribution to normal homocysteine metabolism | >500 mg/portion or 1.5 g/day >4 g/day increase cholesterolemia | [6] | [7] |
| Choline | Authorized | Contribution to normal homocysteine metabolism | Food which contains at least 82.5 mg/100 g or 100 mL or per single portion of food | [8] | [7] |
| Contribution to normal lipid metabolism | |||||
| Maintenance of normal liver function | |||||
| Non-authorized | Contribution to normal cognitive function | [8] | |||
| Maintenance of normal neurological function | |||||
| Development of brain of infants and young children (0–3 years) | [9] | [10] | |||
| Taurine | Non-authorized | Delay in the onset of fatigue and enhancement of physical performance | [11] | ||
| Protection of DNA, proteins and lipids from oxidative damage | |||||
| Energy-yielding metabolism | |||||
| Contribution to normal cognitive function | [12] | ||||
| Immune system protection | |||||
| Delay in the onset of physical fatigue during exercise | |||||
| Maintenance of normal cardiac function | |||||
| Supports “metabolism processes” | |||||
| Maintenance of normal muscle function | |||||
| Reference | Regulatory Context | Aim | Conclusion | In Vitro Genotoxicity Assessment | |
|---|---|---|---|---|---|
| Betaine | EFSA Journal 2005; 191, 1–17 [19]. | Use as a novel food ingredient in the context of Regulation (EC) No 258/97 *. | In beverages, cereal product, confectionary and dairy product. Proposed intake of 4 g/day. | Some safety concerns remained unresolved; safety for the intended use not established. | (−) AT, (−) CA. |
| EFSA Journal 2017; 15(11): 5057 [20]. | Safety as a novel food pursuant to Regulation (EC) No 258/97 *. | Foods for intense muscular effort. Proposed intake of 2.5 g/day or 1.25 g/serving. | Safe at maximum intake of 400 mg/day (6 mg/kg bw/day for sportspeople > 10 years) in addition to the background exposure. | No genotoxicity concerns based on EFSA, 2005. | |
| EFSA Journal 2019; 17(4): 5658 [2]. | Safety as a novel food pursuant to Regulation (EU) 2015/2283. | Foods for sportspeople (>10 years), weight control, and special medical purposes. Max use level ranged from 20–500 mg/100 g. | New conditions are safe (if <400 mg/day including supplements), except for medical purposes for <18 years | - | |
| Choline | EFSA Journal 2011; 9(9): 2353 [24]. | Authorization of additives for use in animal nutrition in the context of Regulation (EC) No 1831/2003. | Safety of choline for the target species, consumers, users and environment, and its efficacy. | No UL has been established in Europe to date. Refers to IOM report of a UL of 3500 mg/person/day for adults [25]. | No genotoxicity concerns [26]: (−) AT (3x), (+) CA, (−) CA (2x), (+) SCE, (−) SCE (2x), (−) GCA. |
| Taurine | SCF, 1999 [27] | Inclusion as constituents of “energy” drinks. | Safety of taurine as constituent of “energy drinks. | Insufficient available data. | No genotoxic potential **: (−) SCE, (−) CA, (−) RA. |
| SCF, 2003 [28]. | Inclusion as constituents of “energy” drinks. | Safety of taurine as constituent of “energy” drinks. | Concerns related with behavioral effects and impaired motor performance at the lowest dose tested (300 mg/kg/day bw in rats); more neurological studies are needed. | No genotoxicity concerns based on SCF, 1999. | |
| EFSA Journal 2009; 935, 1–31 [23]. | Use as constituents of “energy” drinks in the context of Regulation (EC) 178/2002. | Safety of taurine as individual ingredient of “energy” drinks. | The exposure to taurine at the levels presently used in “energy” drinks is not of safety concern, but more exposure data are needed. | No new studies available. No genotoxicity concerns based on SCF, 1999. |
| Concentration (µg/well) | Revertants/Well | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TA1535 | TA97a | TA98 | TA100 | TA102 | ||||||
| S9− | S9+ | S9− | S9+ | S9− | S9+ | S9− | S9+ | S9− | S9+ | |
| Betaine | ||||||||||
| 0 | 17 ± 12 | 3 ± 1 | 36 ± 7 | 56 ± 2 | 5 ± 3 | 9 ± 0 | 42 ± 8 | 44 ± 3 | 78 ± 13 | 66 ± 9 |
| 21 | 2 ± 0 | 4 ± 2 | 45 ± 3 | 68 ± 1 | 6 ± 4 | 7 ± 0 | 34 ± 4 | 44 ± 8 | 92 ± 19 | 86 ± 13 |
| 62 | 2 ± 1 | 5 ± 1 | 37 ± 5 | 63 ± 7 | 4 ± 2 | tox | 38 ± 12 | 42 ± 7 | 94 ± 13 | 72 ± 15 |
| 185 | 1 ± 1 | 2 ± 2 | 37 ± 6 | 56 ± 4 | 4 ± 1 | tox | 33 ± 8 | 46 ± 4 | 86 ± 19 | 77 ± 18 |
| 556 | 3 ± 2 | 3 ± 2 | 40 ± 5 | 57 ± 5 | 4 ± 1 | tox | 45 ± 5 | 37 ± 4 | 89 ± 23 | 67 ± 6 |
| 1667 | 3 ± 1 | 4 ± 1 | 35 ± 3 | 54 ± 7 | 5 ± 3 | tox | 43 ± 6 | 41 ± 5 | 106 ± 20 | 85 ± 14 |
| 5000 | 3 ± 0 | 3 ± 1 | 39 ± 3 | 60 ± 7 | 3 ± 1 | tox | 45 ± 3 | 44 ± 1 | 82 ± 12 | 83 ± 36 |
| Choline | ||||||||||
| 0 | 10 ± 2 | 10 ± 3 | 47 ± 1 | 64 ± 15 | 8 ± 2 | 9 ± 3 | 51 ± 4 | 50 ± 7 | 111 ± 20 | 117 ± 8 |
| 21 | 10 ± 2 | 10 ± 3 | 49 ± 6 | 72 ± 9 | 6 ± 1 | 9 ± 4 | 42 ± 3 | 47 ± 10 | 88 ± 26 | 77 ± 2 |
| 62 | 10 ± 2 | 8 ± 2 | 43 ± 5 | 65 ± 8 | 7 ± 1 | 9 ± 4 | 36 ± 8 | 40 ± 6 | 101 ± 16 | 86 ± 12 |
| 185 | 6 ± 5 | 8 ± 4 | 42 ± 7 | 68 ± 6 | 7 ± 3 | 9 ± 1 | 39 ± 7 | 51 ± 5 | 96 ± 30 | 108 ± 22 |
| 556 | 8 ± 3 | 7 ± 2 | 48 ± 3 | 71 ± 14 | 6 ± 1 | 6 ± 1 | 44 ± 5 | 50 ± 6 | 106 ± 13 | 107 ± 14 |
| 1667 | 6 ± 1 | 7 ± 3 | 43 ± 12 | 66 ± 6 | 7 ± 3 | 5 ± 3 | 36 ± 10 | 52 ± 6 | 99 ± 12 | 95 ± 24 |
| 5000 | 10 ± 4 | 8 ± 2 | 45 ± 4 | 66 ± 6 | 5 ± 1 | 8 ± 2 | 37 ± 9 | 48 ± 5 | 98 ± 13 | 89 ± 24 |
| Taurine | ||||||||||
| 0 | 13 ± 1 | 9 ± 2 | 34 ± 5 | 59 ± 5 | 6 ± 4 | 9 ± 5 | 37 ± 1 | 42 ± 5 | 119 ± 13 | 152 ± 14 |
| 7 | 11 ± 4 | 9 ± 0 | 34 ± 5 | 45 ± 8 | 7 ± 3 | 5 ± 1 | 32 ± 2 | 33 ± 12 | 111 ± 7 | 135 ± 4 |
| 21 | 8 ± 2 | 6 ± 1 | 30 ± 5 | 42 ± 7 | 8 ± 1 | 10 ± 2 | 31 ± 1 | 41 ± 5 | 110 ± 7 | 130 ± 6 |
| 62 | 13 ± 2 | 13 ± 2 | 43 ± 6 | 52 ± 7 | 6 ± 2 | 8 ± 1 | 35 ± 7 | 40 ± 6 | 134 ± 15 | 139 ± 4 |
| 185 | 9 ± 1 | 11 ± 2 | 40 ± 10 | 50 ± 13 | 4 ± 2 | 11 ± 1 | 33 ± 3 | 44 ± 2 | 114 ± 17 | 114 ± 4 |
| 556 | 6 ± 3 | 7 ± 2 | 34 ± 2 | 48 ± 7 | 7 ± 1 | 9 ± 3 | 34 ± 9 | 45 ± 2 | 115 ± 8 | 119 ± 7 |
| 1667 | 5 ± 1 | 9 ± 4 | 33 ± 6 | 43 ± 5 | 7 ± 1 | 8 ± 2 | 33 ± 4 | 35 ± 2 | 126 ± 37 | 126 ± 9 |
| C+ | 145 ± 107 | 92 ± 11 | 248 ± 44 | 460 ± 40 | 375 ± 60 | 213 ± 149 | 225 ± 19 | 358 ± 34 | 262 ± 58 | 153 ± 17 |
| Treatment (µg/mL) | Experiment 1 | Experiment 2 | Mean ± min/max | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MN/103 c. | RS | MN/103 c. | RS | MN/103 c. | RS | |||||
| Betaine | 3 h (S9−) | 0 | 2.8 | 100 | 3.4 | 100 | 3.1 | ±0.3 | 100 | ±0 |
| 74 | 3.5 | 96 | 3.9 | 95 | 3.7 | ±0.2 | 96 | ±0 | ||
| 222 | 6.9 | 107 | 5.8 | 93 | 6.4 | ±0.6 | 100 | ±7 | ||
| 667 | 4.5 | 103 | 5.7 | 100 | 5.1 | ±0.6 | 102 | ±2 | ||
| 2000 | 4.9 | 103 | 3.8 | 96 | 4.3 | ±0.5 | 99 | ±4 | ||
| 3 h (S9+) | 0 | 3.0 | 86 | 4.7 | 130 | 3.9 | ± 0.9 | 108 | ± 22 | |
| 74 | 2.4 | 67 | 3.3 | 117 | 2.9 | ±0.5 | 92 | ±25 | ||
| 222 | 4.0 | 86 | 7.6 | 142 | 5.8 | ±1.8 | 114 | ±28 | ||
| 667 | 6.9 | 90 | 5.5 | 130 | 6.2 | ±0.7 | 110 | ±20 | ||
| 2000 | 5.7 | 100 | 8.3 | 91 | 7.0 | ±1.3 | 96 | ±5 | ||
| 24 h (S9−) | 0 | 3.3 | 100 | 5.5 | 100 | 4.4 | ±1.1 | 100 | ±0 | |
| 74 | 5.0 | 58 | 4.5 | 99 | 4.8 | ±0.3 | 79 | ±21 | ||
| 222 | 3.6 | 47 | 3.6 | 87 | 3.6 | ±0.0 | 67 | ±20 | ||
| 667 | 5.6 | 52 | 2.7 | 81 | 4.2 | ±1.5 | 67 | ±15 | ||
| 2000 | 7.3 | 59 | 8.3 | 81 | 7.8 | ±0.5 | 70 | ±11 | ||
| Choline | 3 h (S9−) | 0 | 2.8 | 100 | 3.5 | 100 | 3.2 | ±0.4 | 100 | ±0 |
| 74 | 3.7 | 100 | 3.1 | 109 | 3.4 | ±0.3 | 104 | ±4 | ||
| 222 | 9.1 | 95 | 2.4 | 150 | 5.8 | ±3.3 | 122 | ±27 | ||
| 667 | 6.7 | 119 | 2.8 | 142 | 4.7 | ±1.9 | 130 | ±11 | ||
| 2000 | 5.3 | 97 | 6.6 | 110 | 6.0 | ±0.7 | 103 | ±6 | ||
| 3 h (S9+) | 0 | 4.7 | 160 | 5.8 | 132 | 5.3 | ±0.6 | 146 | ±14 | |
| 74 | 6.8 | 102 | 4.5 | 105 | 5.7 | ±1.2 | 104 | ±2 | ||
| 222 | 5.2 | 93 | 7.2 | 110 | 6.2 | ±1.0 | 102 | ±9 | ||
| 667 | 4.5 | 106 | 8.8 | 140 | 6.7 | ±2.2 | 123 | ±17 | ||
| 2000 | 4.8 | 104 | 4.6 | 103 | 4.7 | ±0.1 | 104 | ±1 | ||
| 24 h (S9−) | 0 | 2.5 | 100 | 3.2 | 100 | 2.9 | ±0.4 | 100 | ±0 | |
| 74 | 2.7 | 85 | 3.6 | 107 | 3.2 | ±0.5 | 96 | ±11 | ||
| 222 | 2.8 | 84 | 5.1 | 98 | 4.0 | ±1.2 | 91 | ±7 | ||
| 667 | 3.9 | 68 | 4.6 | 95 | 4.3 | ±0.4 | 82 | ±14 | ||
| 2000 | 3.5 | 80 | 6.0 | 92 | 4.8 | ±1.3 | 86 | ±6 | ||
| Taurine | 3 h (S9−) | 0 | 7.2 | 100 | 7.4 | 100 | 7.3 | ±0.1 | 100 | ±0 |
| 74 | 1.9 | 117 | 9.4 | 81 | 5.7 | ±3.8 | 99 | ±18 | ||
| 222 | 3.1 | 104 | 5.9 | 104 | 4.5 | ±1.4 | 104 | ±0 | ||
| 667 | 2.1 | 102 | 9.2 | 107 | 5.7 | ±3.6 | 105 | ±3 | ||
| 2000 | 3.3 | 114 | 6.8 | 92 | 5.1 | ±1.8 | 103 | ±11 | ||
| 3 h (S9+) | 0 | 5.6 | 81 | 14.3 | 125 | 10.0 | ±4.4 | 103 | ±22 | |
| 74 | 5.0 | 119 | 11.9 | 110 | 8.5 | ±3.5 | 115 | ±5 | ||
| 222 | 4.2 | 99 | 6.5 | 106 | 5.4 | ±1.2 | 103 | ±4 | ||
| 667 | 3.4 | 98 | 10.4 | 116 | 6.9 | ±3.5 | 107 | ±9 | ||
| 2000 | 6.1 | 76 | 11.2 | 96 | 8.7 | ±2.6 | 86 | ±10 | ||
| 24 h (S9−) | 0 | 5.2 | 100 | 2.7 | 100 | 4.0 | ±1.3 | 100 | ±0 | |
| 74 | 6.4 | 116 | 4.1 | 81 | 5.3 | ±1.2 | 99 | ±17 | ||
| 222 | 6.3 | 83 | 4.0 | 75 | 5.2 | ±1.1 | 79 | ±4 | ||
| 667 | 10.1 | 76 | 6.1 | 64 | 8.1 | ±2.0 | 70 | ±6 | ||
| 2000 | 6.1 | 65 | 3.6 | 74 | 4.9 | ±1.3 | 70 | ±5 | ||
| CP (S9−) | 6.1 | ±2.5 | ||||||||
| CP (S9+) | 38.1 | ±22.3 | ||||||||
| COL | 89.1 | ±42.4 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanz-Serrano, J.; Vettorazzi, A.; Muruzabal, D.; Azqueta, A.; López de Cerain, A. In Vitro Genotoxicity Assessment of Functional Ingredients: Betaine, Choline, and Taurine. Foods 2021, 10, 339. https://doi.org/10.3390/foods10020339
Sanz-Serrano J, Vettorazzi A, Muruzabal D, Azqueta A, López de Cerain A. In Vitro Genotoxicity Assessment of Functional Ingredients: Betaine, Choline, and Taurine. Foods. 2021; 10(2):339. https://doi.org/10.3390/foods10020339
Chicago/Turabian StyleSanz-Serrano, Julen, Ariane Vettorazzi, Damian Muruzabal, Amaya Azqueta, and Adela López de Cerain. 2021. "In Vitro Genotoxicity Assessment of Functional Ingredients: Betaine, Choline, and Taurine" Foods 10, no. 2: 339. https://doi.org/10.3390/foods10020339
APA StyleSanz-Serrano, J., Vettorazzi, A., Muruzabal, D., Azqueta, A., & López de Cerain, A. (2021). In Vitro Genotoxicity Assessment of Functional Ingredients: Betaine, Choline, and Taurine. Foods, 10(2), 339. https://doi.org/10.3390/foods10020339







