Alternative Proteins as a Source of Bioactive Peptides: The Edible Snail and Generation of Hydrolysates Containing Peptides with Bioactive Potential for Use as Functional Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Substrate Materials
2.2. Generation of Snail Protein Hydrolysates
2.3. Freeze-Drying
2.4. Proximate Analysis
2.4.1. Protein
2.4.2. Ash
2.4.3. Lipid Content
2.4.4. Water Activity
2.5. ACE-I Inhibition Assay for Potential to Reduce Blood Pressure
2.6. LC-MS/MS Analysis
2.7. Data Analysis of MS/MS Results
2.8. In Silico Identification of Novel Peptides
3. Results
3.1. Yields and Proximate Analysis of Snail Protein Hydrolysates
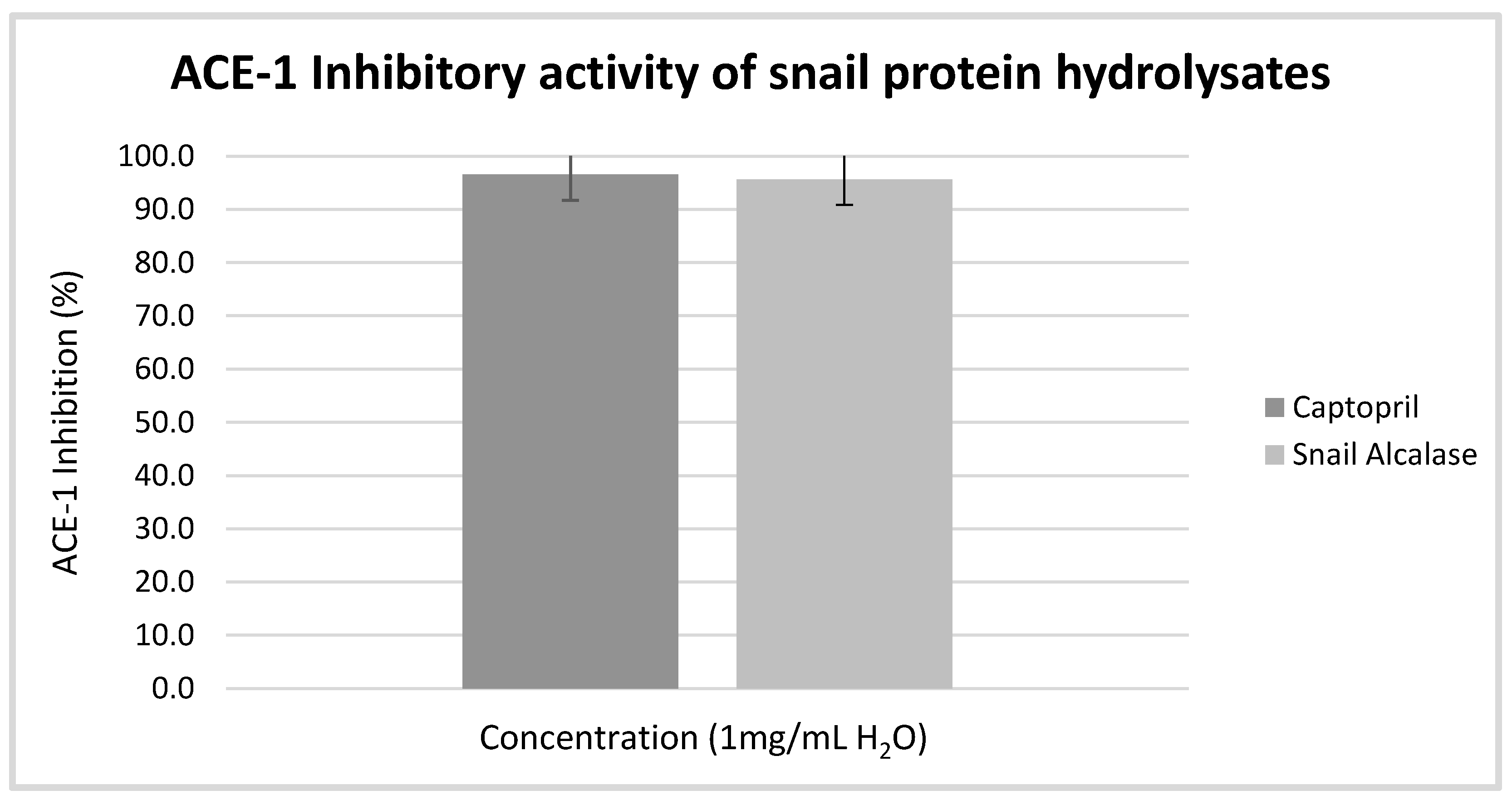
3.2. Angiotensin Converting Enzyme I Inhibition Assay
3.3. Identification of Proteins and Peptides in Snail Alcalase® Hydrolysates
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cagiltay, F.; Erkan, N.; Tosun, D.; Selcuk, A. Amino acid, fatty acid, vitamin and mineral contents of the edible garden snail (Helix aspersa). J. Fish. Sci. 2011, 5, 354–363. [Google Scholar]
- Bazán, M. Caracol terrestre, un Nuevo product de exportación. Tattersall 2005, 194, 6–7. [Google Scholar]
- López, N.L.; Recabal, G.M.; Carrasco, C.A. Preparation and evaluation of appertized from snail Helix aspersa M. Acta Agronómica 2015, 64, 1–10. [Google Scholar] [CrossRef]
- Ghosh, S.; Jung, C.; Meyer-Rochow, V.B. Snail as mini-livestock: Nutritional potential of farmed Pomacea canaliculata (Ampullariidae). Agric. Nat. Resour. 2017, 51, 504–511. [Google Scholar] [CrossRef]
- Engmann, F.N.; Afoakwah, N.A.; Darko, P.O.; Sefah, W. Proximate and mineral composition of snail (Achatina achatina) meat; Any nutritional justification for acclaimed health benefits? J. Basic Appl. Sci. Res. 2013, 3, 8–15. [Google Scholar]
- Adeyeye, S.A.O.; Bolaji, O.T.; Abegude, T.A.; Adesina, T.O. Processing and utilization of snail meat in alleviating protein malnutrition in Africa: A review. Nutr. Food Sci. 2020, 50, 1085–1097. [Google Scholar] [CrossRef]
- Górka, A.; Oklejewicz, B.; Duda, M. Nutrient content and antioxidant properties of eggs of the land snail Helix aspersa maxima. J. Nutr. Food Sci. 2017, 7, 1–4. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Dziuba, J.; Iwaniak, A.; Dziuba, M.; Darewicz, M. BIOPEP database and other programs for processing bioactive peptide sequences. J. AOAC Int. 2008, 91, 965–980. [Google Scholar] [CrossRef]
- Pearman, N.A.; Ronander, E.; Smith, A.M.; Morris, G.A. The identification and characterisation of novel bioactive peptides derived from porcine liver. Curr. Res. Food Sci. 2020. [Google Scholar] [CrossRef]
- Petsantad, P.; Sangtanoo, P.; Srimongkol, P.; Sarsavoey, T.; Reamtong, O.; Chaitanawisnti, N.; Karnchanatat, A. The antioxidant potential of peptides obtained from the spotted Babylon snail (Babylonia areolata) in treating human colon adenocarcinoma (Caco-2) cells. RSC Adv. 2020, 10, 25746–25757. [Google Scholar] [CrossRef]
- Hamid, S.A.; Ruhaya, A.H.N.; Sarbon, N.M. Optimization of enzymatic hydrolysis conditions of golden apple snail (Pomacea canaliculata) protein by Alcalase. Int. Food Res. J. 2015, 22, 2041–2049. [Google Scholar]
- Adler-Nissen, J. Enzymatic Hydrolysis of Food Protein; Elsevier Applied Science Publishers Ltd.: London, UK, 1986. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists Inc.: Gaithersburg, MD, USA, 1990. [Google Scholar]
- Kolar, K. Gravimetric determination of moisture and ash in meat and meat products. NMKL Interlaboratory study. J. AOAC Int. 1992, 75, 1016–1022. [Google Scholar] [CrossRef]
- Naik, A.; Mora, L.; Hayes, M. Characterisation of seasonal Mytilus edulis by-products and generation of bioactive hydrolysates. Appl. Sci. 2020, 10, 6892. [Google Scholar] [CrossRef]
- Shilov, I.V.; Seymour, S.L. The Paragon Algorithm, a Next Generation Search Engine That Uses Sequence Temperature Values and Feature Probabilities to Identify Peptides from Tandem Mass Spectra. Mol. Cell Proteom. 2007, 6, 1638–1655. [Google Scholar] [CrossRef] [PubMed]
- Drevet, P. Preuves de l’effet antihypertenseur d’un hydrolysat de coproduit d’escargot terrestre (Helix aspersa)—Identification des peptides impliqués. Ann. Cardiol. Angéiol. 2017, 66, 140–148. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Drevet, P. Hydrolysat peptidique d’escargots, fractions peptidiques, proceed de fabrication, utilization et compositions pour inhiber l’enzyme de conversion de l’angiotensine. France Patent application FR3023717A1 and FR3023717B1, 8 December 2017. [Google Scholar]
- Zamyatnin, A.A. Fragmentomics of natural peptide structures. Biochemistry 2009, 74, 1575–1585. [Google Scholar] [CrossRef]
- Liu, Z.P.; Wu, L.Y.; Wang, Y.; Zhan, X.S.; Chen, L. Bridging protein local structures and protein functions. Amino Acids 2008, 35, 627–650. [Google Scholar] [CrossRef]
- Iwaniak, A.; Hrynkiewicz, M.; Minkiewicz, P.; Bucholska, J.; Darewicz, M. Soybean (Glycine max) protein hydrolysates as sources of peptide bitter-tasting indicators: An analysis based on hybrid and fragmentomic approaches. Appl. Sci. 2020, 10, 2514. [Google Scholar] [CrossRef]
- Udenigwe, C. Bioinformatic approaches, prospects and challenges of food bioactive peptide research. Trends Food Sci. Technol. 2014, 36, 137–143. [Google Scholar] [CrossRef]
- Tahir, R.A.; Bashir, A.; Yousaf, M.N.; Ahmed, A.; Dali, Y.; Khan, S. In Silico identification of angiotensin-converting enzyme inhibitory peptides from MRJP1. PLoS ONE 2020, 15, e0228265. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakesh, R.; Perera, C.O. Partial purification and characterisation of bioactive peptides from cooked New Zealand Green-lipped Mussel (Perna canaliculus) protein hydrolysates. Foods 2020, 9, 879. [Google Scholar] [CrossRef] [PubMed]
| Protein Accession Number | Peptide Sequences Identified a | Theoretical Molecular Weight (MW) b |
|---|---|---|
| sp|P00780|SUBC_BACLI | AAGNSGSSGNTNTIGYPAKY | 1928.89 |
| sp|P00780|SUBC_BACLI | AQTVPYGIPLIK | 1298.76 |
| sp|P00780|SUBC_BACLI | AQTVPYGIPLIKADKVQAQ | 2039.14 |
| sp|P00780|SUBC_BACLI | ASHPDLNVVGGA | 1135.56 |
| sp|P00780|SUBC_BACLI | ASHPDLNVVGGAS | 1222.59 |
| sp|P00780|SUBC_BACLI | AVDSNSNRASFS | 1253.56 |
| sp|P00780|SUBC_BACLI | AYNTDGNGHGTHVA | 1413.59 |
| sp|P00780|SUBC_BACLI | DNTTGVLGVAPSVSLY | 1591.81 |
| sp|P00780|SUBC_BACLI | DTGIQASHPDLNVVGGA | 1649.80 |
| sp|P00780|SUBC_BACLI | GAVDSNSNRASFS | 1310.59 |
| sp|P00780|SUBC_BACLI | GIPLIKADKVQAQ | 1379.81 |
| sp|P00780|SUBC_BACLI | ILSKHPNLSAS | 1165.65 |
| sp|P00780|SUBC_BACLI | NSSGSGSYSGIVSGIE | 1499.67 |
| sp|P00780|SUBC_BACLI | NSSGSGSYSGIVSGIEWATTN | 2072.93 |
| sp|P00780|SUBC_BACLI | NTDGNGHGTHVA | 1179.49 |
| sp|P00780|SUBC_BACLI | SLGGASGSTAMKQ | 1209.57 |
| sp|P00780|SUBC_BACLI | STYPTNTYATL | 1230.58 |
| sp|P00780|SUBC_BACLI | STYPTNTYATLN | 1344.62 |
| sp|P00780|SUBC_BACLI | VDSNSNRASFS | 1182.53 |
| sp|O16808|ACT_MAYDE | AGDDAPRAVFPS | 1201.57 |
| sp|O16808|ACT_MAYDE | GFAGDDAPRAVFPS | 1405.66 |
| sp|O16808|ACT_MAYDE | GQKDSYVGDEAQSKRGILTL | 2164.11 |
| sp|O16808|ACT_MAYDE | KSYELPDGQVITIG | 1518.79 |
| sp|O16808|ACT_MAYDE | KSYELPDGQVITIGNE | 1761.88 |
| sp|P80057|GSEP_BACLD | SEPIGNTVGYF | 1182.56 |
| sp|P80057|GSEP_BACLD | WQHSGPIAISE | 1223.59 |
| sp|P02461|CO3A1_HUMAN | GEPGQAGPSGPPGPPGAIGPSGP | 1937.91 |
| sp|Q8RB67|DNAJ_CALS4 | GEGEPGLRGGPN | 1139.52 |
| sp|Q9BXJ0|C1QT5_HUMAN | GEAGPAGPTGPAG | 1037.48 |
| sp|B4UGJ6|Y4073_ANASK | EGAVALGAGLALGGSRE | 1526.81 |
| sp|O45218|ADAS_CAEEL | LDPANIFASANLIDI | 1585.84 |
| sp|Q03A18|ATPB_LACP3 | GDPIDGGEAFGP | 1130.49 |
| sp|A5WGE2|LPXA_PSYWF | IGNNVILGGNAG | 1097.58 |
| sp|O60784|TOM1_HUMAN | SAEGPPGPPSGPA | 1119.52 |
| sp|P45586|CU79A_LOCMI | LGGGLGGIGL | 812.48 |
| sp|O24385|CPI7_SOLTU | VDDDKDFIPF | 1209.56 |
| sp|Q38YA9|GPDA_LACSS | MPITNAIYNVL | 1247.66 |
| sp|Q5RF96|SPCS1_PONAB | SGAVAIAFPGLEGPPA | 1452.76 |
| sp|P54334|XKDO_BACSU | GGGLVGGI | 628.35 |
| sp|Q75JF3|CLCC_DICDI | LIGGLLG | 641.41 |
| sp|Q3UHE1|PITM3_MOUSE | AGPSGDSPGSSSR | 1142.50 |
| sp|Q8IQG1|MOB2_DROME | LLGGILG | 641.41 |
| sp|Q38XN0|MRAY_LACSS | IIGGLIG | 641.41 |
| sp|A4R2R1|NST1_MAGO7 | NQHYPPGIGPLNAP | 1490.72 |
| sp|L0E307|PHQO_PENFE | YLKPVPIVPGLP | 1319.79 |
| sp|Q80XI7|VOME_MOUSE | GGLGIGGLL | 755.45 |
| sp|Q8BM72|HSP13_MOUSE | VTGVAIQAGIDGGSWP | 1526.77 |
| sp|C1A8U3|DAPF_GEMAT | FVKMTGSGNDF | 1233.53 |
| sp|P80057|GSEP_BACLD | GYPGDKTAGTQWQHSGPIAISE | 2299.09 |
| sp|O42350|CO1A2_LITCT | AGLNGGLGPSGPA | 1067.52 |
| sp|P00780|SUBC_BACLI | SHPDLNVVGGA | 1064.53 |
| sp|P00780|SUBC_BACLI | AAGNSGSSGNTNTIGYPA | 1637.73 |
| sp|Q5FRT2|PROA_GLUOX | LIDAAIAPAL | 966.58 |
| sp|P00780|SUBC_BACLI | SKHPNLSAS | 939.48 |
| sp|P00780|SUBC_BACLI | APGAGVY | 633.31 |
| sp|Q9M3B0|PME34_ARATH | MPVSQIQADIIV | 1314.67 |
| sp|Q9Z470|AROA_CORGL | ATAGAIIGLAVDG | 1127.62 |
| sp|A1XGT3|TI214_RANMC | LIVLPSLI | 866.58 |
| sp|D4GP41|KGSDH_HALVD | GATLVAGGGVPE | 1026.53 |
| sp|Q8X226|CAS1_CRYNH | FGLWVLNWI | 1147.61 |
| sp|Q9C6V3|AGL86_ARATH | AGAGAGAAPL | 754.40 |
| sp|P27483|GRP1_ARATH | GAGGGLGGGHGGGIGGGAGGGSGGGLGGGIGGGAGG | 2447.13 |
| sp|P27393|CO4A2_ASCSU | GDDGLPGAPGRPG | 1146.54 |
| sp|P00780|SUBC_BACLI | NSSGSGSYSGIVS | 1200.53 |
| sp|B2A865|ACCDA_NATTJ | EGGSGGALALTV | 1030.53 |
| sp|Q8WXI7|MUC16_HUMAN | PSLLSLPATTSP | 1182.65 |
| sp|Q9H7P9|PKHG2_HUMAN | RGGGGGGPR | 769.39 |
| sp|Q1HVF7|EBNA1_EBVA8 | GAGGGAGAGGGAGAGGGAGAGGGAG | 1555.67 |
| sp|Q80XI7|VOME_MOUSE | GGEGGGLGIGGLL | 1055.56 |
| sp|Q7W0A6|MUTL_BORPE | AGVPDGAAPDTAYAGEPA | 1628.73 |
| sp|Q8IA41|GLT11_DROME | EPILLNNQ | 939.50 |
| sp|Q8K4I4|BPIA1_RAT | NGLVGGLLG | 798.46 |
| sp|B8ITX3|MCH_METNO | VAEAAGVPL | 825.46 |
| sp|Q9LEJ0|ENO1_HEVBR | SIEDPFDQD | 1064.43 |
| sp|P86950|SLP2_PINMA | GIGGGGIIGGGPI | 1023.57 |
| sp|Q5U9X3|GPC6A_DANRE | IIGGLFPI | 828.51 |
| sp|Q9NG98|TOP3A_DROME | GGGGGPGPGPGGG | 879.38 |
| sp|O16808|ACT_MAYDE | RVAPEEHPVLL | 1258.70 |
| sp|P34804|COL40_CAEEL | SEPGPAGPAGDAGPDGAPGNAGAPGA | 2114.91 |
| sp|Q2KIN5|HEM3_BOVIN | WSLNGAETMQ | 1136.48 |
| sp|Q9FZC4|FOX1_ARATH | PAGTPKTVLLGRP | 1305.78 |
| sp|P12575|FUS_SENDF | IVVMVVIL | 884.58 |
| sp|B0TMM7|ADEC_SHEHH | LDALAPLI | 824.50 |
| sp|P00780|SUBC_BACLI | SHPDLNVVGGAS | 1151.56 |
| sp|P49597|P2C56_ARATH | AGPFRPF | 790.41 |
| sp|Q7TZN1|PKNF_MYCBO | TEAPLPIE | 868.45 |
| sp|Q6AZY7|SCAR3_HUMAN | GDPGSLGPLGPQ | 1094.52 |
| sp|P17140|CO4A2_CAEEL | QDGLPGLPGNKG | 1152.58 |
| sp|A5G0G6|LEUC_ACICJ | LGMNPDKLKPGE | 1297.67 |
| sp|Q7SIB2|CO4A1_BOVIN | GPAGVPGLPGAKGDHGFPGSSGPRGD | 2343.14 |
| sp|Q96MP8|KCTD7_HUMAN | FGDVLNF | 810.39 |
| sp|Q8TZZ2|MPTA_PYRFU | EDIALEDMI | 1047.48 |
| sp|Q7Z5A4|PRS42_HUMAN | APGPEAGPPL | 904.47 |
| sp|O48534|DEXHD_ARATH | VQVGVAINGE | 984.52 |
| sp|Q9QY06|MYO9B_MOUSE | DAGLSPGSQGDSK | 1217.55 |
| sp|Q4K758|SYM_PSEF5 | ITQYFDPE | 1011.45 |
| sp|Q9Y4K4|M4K5_HUMAN | SSDPNFMLQ | 1038.43 |
| sp|Q8SWH6|Y206_ENCCU | DVPVEEMAVG | 1044.48 |
| sp|Q82EX7|DNAJ1_STRAW | GAGGGFGGGI | 748.35 |
| sp|B2GDS9|DNLJ_LACF3 | AGDIIPE | 713.36 |
| sp|Q9Z8N1|PHSG_CHLPN | AIEDIALI | 856.49 |
| sp|Q9FCC1|BIOD_STRCO | GAPLLGAVPAGAGS | 1136.62 |
| sp|Q6CZR3|GPH_PECAS | IGGDDVIVK | 914.51 |
| sp|P0C062|GRSA_BREBE | GGEGLARGYWK | 1192.60 |
| sp|P21840|VSM5_TRYBR | GEDQETFHSRFWDQ | 1781.73 |
| sp|Q97CT6|METK_THEVO | DTSFGVGFAP | 996.46 |
| sp|Q2KJ58|R3GEF_BOVIN | SVGPCKSHRESLGGLPE | 1751.86 |
| sp|Q10Y85|RIMO_TRIEI | GTPAYNLPN | 945.46 |
| sp|Q4K5F9|PROA_PSEF5 | NEVDSSSVMVNASTRF | 1741.79 |
| sp|D5AV94|TKT_RHOCB | FVGMEGFGASAPA | 1239.56 |
| sp|Q9KPI4|Y2383_VIBCH | AAAQLALGGML | 1014.55 |
| sp|Q8SY41|BCAS3_DROME | GLGVQVWAIPANGEAVE | 1708.88 |
| sp|Q9DD48|MKRN2_SERQU | GGGGAGGGGAGIGGAGGGP | 1239.56 |
| sp|O48928|C77A3_SOYBN | TALAFFISGLIF | 1298.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayes, M.; Mora, L. Alternative Proteins as a Source of Bioactive Peptides: The Edible Snail and Generation of Hydrolysates Containing Peptides with Bioactive Potential for Use as Functional Foods. Foods 2021, 10, 276. https://doi.org/10.3390/foods10020276
Hayes M, Mora L. Alternative Proteins as a Source of Bioactive Peptides: The Edible Snail and Generation of Hydrolysates Containing Peptides with Bioactive Potential for Use as Functional Foods. Foods. 2021; 10(2):276. https://doi.org/10.3390/foods10020276
Chicago/Turabian StyleHayes, Maria, and Leticia Mora. 2021. "Alternative Proteins as a Source of Bioactive Peptides: The Edible Snail and Generation of Hydrolysates Containing Peptides with Bioactive Potential for Use as Functional Foods" Foods 10, no. 2: 276. https://doi.org/10.3390/foods10020276
APA StyleHayes, M., & Mora, L. (2021). Alternative Proteins as a Source of Bioactive Peptides: The Edible Snail and Generation of Hydrolysates Containing Peptides with Bioactive Potential for Use as Functional Foods. Foods, 10(2), 276. https://doi.org/10.3390/foods10020276







