Phytochemical Fortification in Fruit and Vegetable Beverages with Green Technologies
Abstract
1. Introduction
2. Nutraceutical Food Supplements
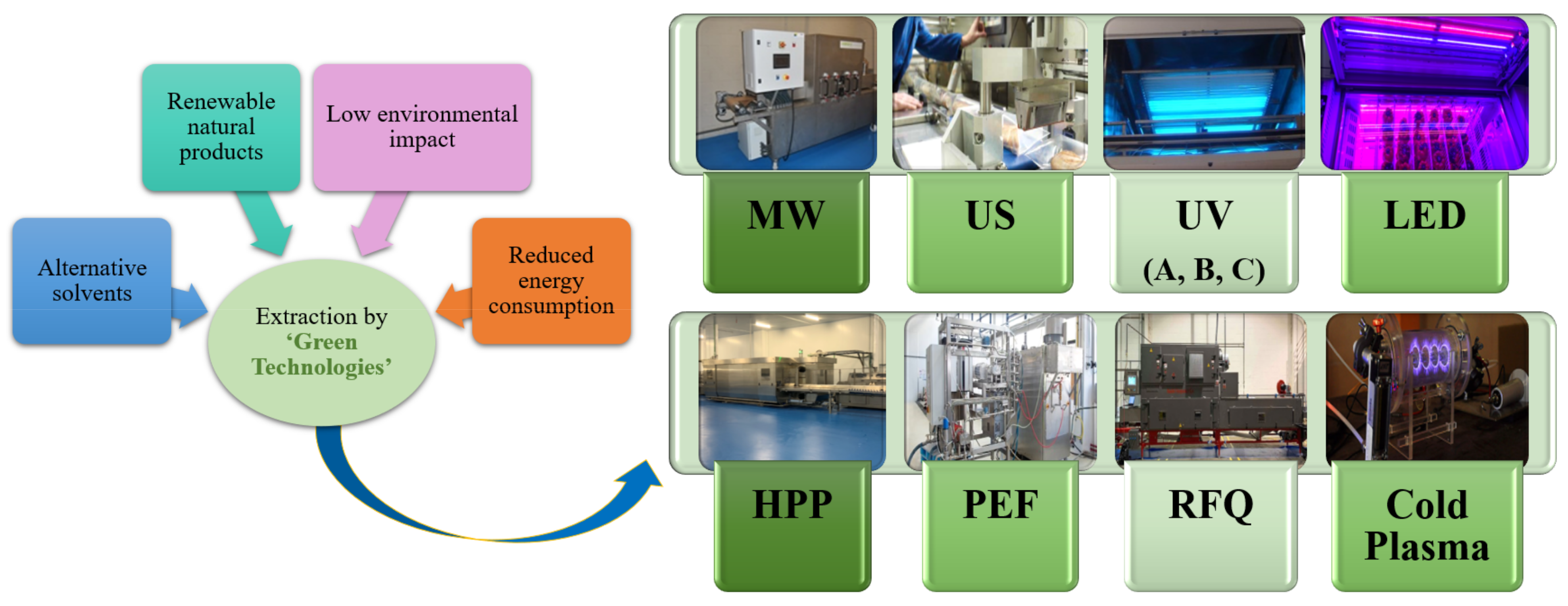
3. Preservation of Fruit and Vegetable Beverages Using Green Technologies
3.1. Microwaves
3.2. Ultrasounds
3.3. Ultraviolet
3.4. Light Emitting Diodes (LEDs)
3.5. High Hydrostatic Pressure Treatment
3.6. Pulsed Electric Fields
3.7. Radiofrequency
3.8. Cold Plasma Treatment
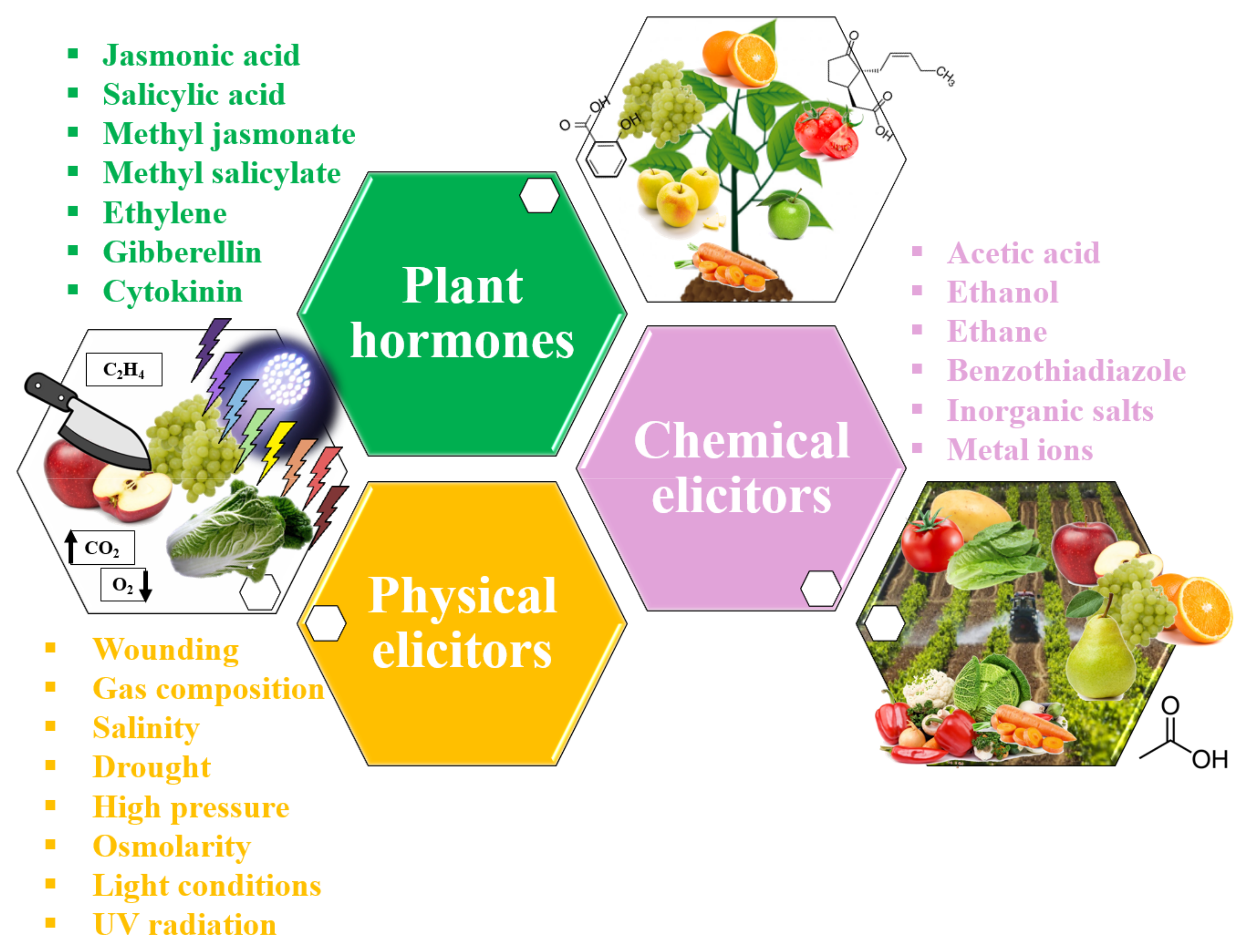
4. Phytochemical Elicitors in Fruit and Vegetables Used in Beverages
5. Green Technologies as Elicitors of the Fortification of Fruit and Vegetable Beverages
5.1. Ultraviolet
5.2. High-Pressure Processing
5.3. Pulsed Electric Fields
5.4. Ultrasound
5.5. Cold Plasma
5.6. Combined Technologies
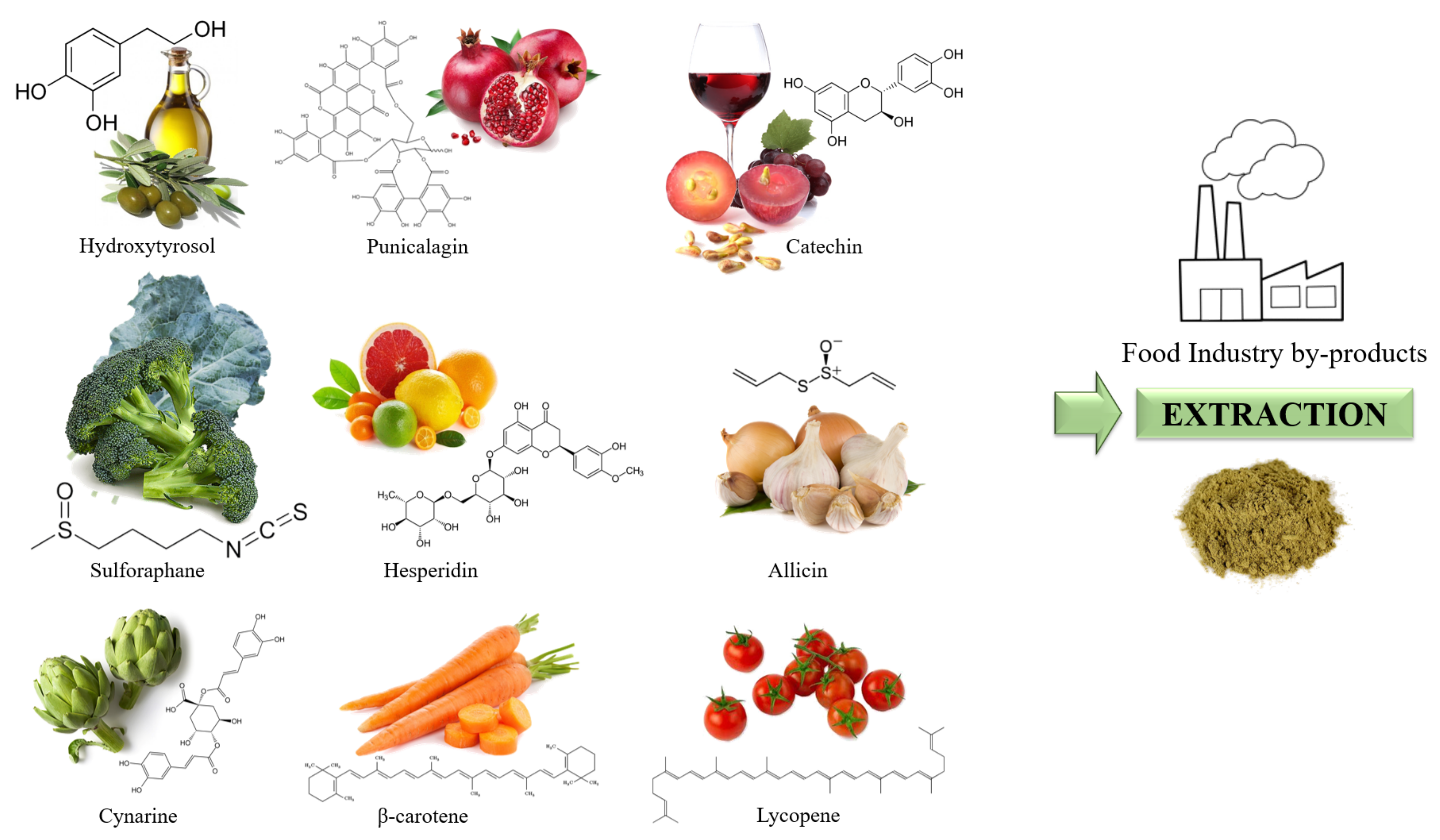
6. Fortification of Beverages Using Natural Products
6.1. Fortification of Beverages by Adding Plant Extracts and Other Health-Promoting Compounds
6.2. Fortification of Beverages by Adding Algae
6.3. Phytochemical Fortification of Beverages during Fermentation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeFelice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar] [CrossRef]
- Contor, L. Functional Food Science in Europe. Nutr. Metab. Cardiovasc. Dis. 2001, 11, 20–23. [Google Scholar] [CrossRef][Green Version]
- Guhr, G.; Lachance, P.A. Chapter 32: Role of Phytochemicals in Chronic Disease Prevention. 311-364. In Nutraceuticals: Designer Foods III: Garlic, Soy and Licorice; Lachance, P.A., Ed.; Wiley: Hoboken, NJ, USA, 1997. [Google Scholar] [CrossRef]
- Télessy, I.G. Nutraceuticals. In The Role of Functional Food Security in Global Health; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 409–421. ISBN 978-0-12-813148-0. [Google Scholar] [CrossRef]
- Infusino, F.; Marazzato, M.; Mancone, M.; Fedele, F.; Mastroianni, C.M.; Severino, P.; Ceccarelli, G.; Santinelli, L.; Cavarretta, E.; Marullo, A.G.M.; et al. in SARS-CoV-2 Infection: A Scoping Review. Nutrients 2020, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Verástegui, L.L.; Martínez-Hernández, G.B.; Castillejo, N.; Gómez, P.A.; Artés, F.; Artés-Hernández, F. Bioactive Compounds and Enzymatic Activity of Red Vegetable Smoothies during Storage. Food Bioprocess Technol. 2016, 9. [Google Scholar] [CrossRef]
- Morales-de la Peña, M.; Welti-Chanes, J.; Martín-Belloso, O. Application of Novel Processing Methods for Greater Retention of Functional Compounds in Fruit-Based Beverages. Beverages 2016, 2, 14. [Google Scholar] [CrossRef]
- Shahbaz, H.M.; Kim, J.U.; Kim, S.H.; Park, J. Advances in Nonthermal Processing Technologies for Enhanced Microbiological Safety and Quality of Fresh Fruit and Juice Products; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128114476. [Google Scholar]
- Buzrul, S.; Alpas, H.; Largeteau, A.; Demazeau, G. Inactivation of Escherichia coli and Listeria innocua in kiwifruit and pineapple juices by high hydrostatic pressure. Int. J. Food Microbiol. 2008, 124, 275–278. [Google Scholar] [CrossRef]
- Di Cagno, R.; Minervini, G.; Rizzello, C.G.; De Angelis, M.; Gobbetti, M. Effect of lactic acid fermentation on antioxidant, texture, color and sensory properties of red and green smoothies. Food Microbiol. 2011, 28, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi, M.; Otón, M.; Artés, F.; Artés-Hernández, F.; Gómez, P.A.; Aguayo, E. Semi-industrial microwave treatments positively affect the quality of orange-colored smoothies. J. Food Sci. Technol. 2016, 53, 3695–3703. [Google Scholar] [CrossRef]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Artés, F.; Artés-Hernández, F. Use of postharvest UV-B and UV-C radiation treatments to revalorize broccoli byproducts and edible florets. Innov. Food Sci. Emerg. Technol. 2017, 43, 77–83. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.N.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and potential health benefits of the Mediterranean diet: Views from experts around the world. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Medicines 2018, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.; Klöpping-Ketelaars, I.W.A.A.; Van Den Berg, R.; Vaes, W.H.J. Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. J. Agric. Food Chem. 2008, 56, 10505–10509. [Google Scholar] [CrossRef] [PubMed]
- Lozanovski, V.J.; Houben, P.; Hinz, U.; Hackert, T.; Herr, I.; Schemmer, P. Pilot study evaluating broccoli sprouts in advanced pancreatic cancer (POUDER trial)—study protocol for a randomized controlled trial. Trials 2014, 15, 204. [Google Scholar] [CrossRef]
- Parker, R.S.; Swanson, J.E.; You, C.-S.; Edwards, A.J.; Huang, T. Bioavailability of carotenoids in human subjects. Proc. Nutr. Soc. 1999, 58, 155–162. [Google Scholar] [CrossRef]
- Desmarchelier, C.; Borel, P. Overview of carotenoid bioavailability determinants: From dietary factors to host genetic variations. Trends Food Sci. Technol. 2017, 69, 270–280. [Google Scholar] [CrossRef]
- Martínez, L.; Ros, G.; Nieto, G. Fe, Zn and Se bioavailability in chicken meat emulsions enriched with minerals, hydroxytyrosol and extra virgin olive oil as measured by Caco-2 cell model. Nutrients 2018, 10, 969. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Cooperstone, J.L.; Ralston, R.A.; Riedl, K.M.; Haufe, T.C.; Schweiggert, R.M.; King, S.A.; Timmers, C.D.; Francis, D.M.; Lesinski, G.B.; Clinton, S.K.; et al. Enhanced bioavailability of lycopene when consumed as cis-isomers from tangerine compared to red tomato juice, a randomized, cross-over clinical trial. Mol. Nutr. Food Res. 2015, 59, 658–669. [Google Scholar] [CrossRef]
- Gallego-Jara, J.; de Diego, T.; del Real, Á.; Écija-Conesa, A.; Manjón, A.; Cánovas, M. Lycopene overproduction and in situ extraction in organic-aqueous culture systems using a metabolically engineered Escherichia coli. AMB Express 2015, 5, 1–11. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Gómez, P.A.; Talavera, N.V.G.; Hernández, F.A.; Saiz, T.M.; Álvarez, C.S.; Artés, F. Vitamin C, folates and antioxidant plasma status after consumption of kailan—hybrid broccoli. Consumption of kalian—hybrid broccoli cooking effects. ACS Food Sci. Technol. 2021, 62, 327–337. [Google Scholar] [CrossRef]
- Jayachandran, L.E.; Chakraborty, S.; Rao, P.S. Effect of high pressure processing on physicochemical properties and bioactive compounds in litchi based mixed fruit beverage. Innov. Food Sci. Emerg. Technol. 2015, 28, 1–9. [Google Scholar] [CrossRef]
- Fernandez, M.V.; Denoya, G.I.; Agüero, M.V.; Jagus, R.J.; Vaudagna, S.R. Optimization of high pressure processing parameters to preserve quality attributes of a mixed fruit and vegetable smoothie. Innov. Food Sci. Emerg. Technol. 2018, 47, 170–179. [Google Scholar] [CrossRef]
- Arjmandi, M.; Otón, M.; Artés, F.; Artés-Hernández, F.; Gómez, P.A.; Aguayo, E. Continuous microwave pasteurization of a vegetable smoothie improves its physical quality and hinders detrimental enzyme activity. Food Sci. Technol. Int. 2017, 23, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Markowski, J.; Celejewska, K.; Rosłonek, A.; Kosmala, M. Impact of different thermal preservation technologies on the quality of apple-based smoothies. LWT—Food Sci. Technol. 2017, 85, 470–473. [Google Scholar] [CrossRef]
- Amador-Espejo, G.G.; Chávez-Ocegueda, J.; Cruz-Cansino, N.; Suárez-Jacobo, A.; Gutiérrez-Martínez, P.; Valencia-Flores, D.; Velázquez Estrada, R. Thermosonication parameter effects on physicochemical changes, microbial and enzymatic inactivation of fruit smoothie. J. Food Sci. Technol. 2020, 57, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.O.; Brígida, A.I.S.; Sá, D.D.G.C.F.; Carvalho, C.W.P.; Silva, J.P.L.; Matta, V.M.; Freitas, S.P. Effect of sonication on the quality attributes of juçara, banana and strawberry smoothie. J. Food Sci. Technol. 2019, 56, 5531–5537. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Grimi, N.; Vorobiev, E.; Esteve, M.J.; Frígola, A. Changes of Antioxidant Compounds in a Fruit Juice-Stevia rebaudiana Blend Processed by Pulsed Electric Technologies and Ultrasound. Food Bioprocess Technol. 2016, 9, 1159–1168. [Google Scholar] [CrossRef]
- Rabelo, M.C.; Bang, W.Y.; Nair, V.; Alves, R.E.; Jacobo-Velázquez, D.A.; Sreedharan, S.; de Miranda, M.R.A.; Cisneros-Zevallos, L. UVC light modulates vitamin C and phenolic biosynthesis in acerola fruit: Role of increased mitochondria activity and ROS production. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB light doses and harvesting time differentially tailor glucosinolate and phenolic profiles in broccoli sprouts. Molecules 2017, 22, 1065. [Google Scholar] [CrossRef]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB Light, and Methyl Jasmonate, Alone or Combined, Redirect the Biosynthesis of Glucosinolates, Phenolics, Carotenoids, and Chlorophylls in Broccoli Sprouts. Int. J. Mol. Sci. 2017, 18, 2330. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Zamora, L.; Artés–Hernández, F. Periodical Uv-B Radiation Hormesis in Biosynthesis of Kale Sprouts Nutraceuticals. Plant Physiol. Biochem. 2021, 165, 274–285. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Castillejo, N.; Artés, F. Postharvest UV-B and Photoperiod with Blue + Red LEDs as Strategies to Stimulate Carotenogenesis in Bell Peppers. Appl. Sci. 2021, 11, 3736. [Google Scholar] [CrossRef]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Artés, F.; Artés-Hernández, F. Effects of UV-B and UV-C combination on phenolic compounds biosynthesis in fresh-cut carrots. Postharvest Biol. Technol. 2017, 127, 99–104. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the role of red:Blue LED lights on resource use efficiency and nutritional properties of indoor grown sweet basil. Front. Plant Sci. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.L.C.; Ramos, A.L.S.; Ramos, E.M.; Piccoli, R.H.; Cristianini, M. Natural antimicrobials as additional hurdles to preservation of foods by high pressure processing. Trends Food Sci. Technol. 2015, 45, 60–85. [Google Scholar] [CrossRef]
- Wang, C.Y.; Huang, H.W.; Hsu, C.P.; Yang, B.B. Recent Advances in Food Processing Using High Hydrostatic Pressure Technology. Crit. Rev. Food Sci. Nutr. 2016, 56, 527–540. [Google Scholar] [CrossRef]
- Raso, J.; Condón, S.; Álvarez, I. Non-Thermal Processing: Pulsed Electric Field. Encycl. Food Microbiol. Second Ed. 2014, 2, 966–973. [Google Scholar] [CrossRef]
- Swamy, G.J.; Muthukumarappan, K. Microwave and Radiofrequency Processing of Plant-Related Food Products; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128157817. [Google Scholar]
- Datta, A.K.; Davidson, P.M. Microwave and radio frequency processing. J. Food Sci. 2000, 65, 32–41. [Google Scholar] [CrossRef]
- López, M.; Calvo, T.; Prieto, M.; Múgica-Vidal, R.; Muro-Fraguas, I.; Alba-Elías, F.; Alvarez-Ordóñez, A. A review on non-thermal atmospheric plasma for food preservation: Mode of action, determinants of effectiveness, and applications. Front. Microbiol. 2019, 10, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Tappi, S.; Berardinelli, A.; Ragni, L.; Dalla Rosa, M.; Guarnieri, A.; Rocculi, P. Atmospheric gas plasma treatment of fresh-cut apples. Innov. Food Sci. Emerg. Technol. 2014, 21, 114–122. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Keener, K.M. Cold Plasma Processing of Fruit Juices; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128024911. [Google Scholar]
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.J.; Gilmore, B.F. Microbiological interactions with cold plasma. J. Appl. Microbiol. 2017, 123, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Angelova, Z.; Georgiev, S.; Roos, W. Elicitation of plants. Biotechnol. Biotechnol. Equip. 2006, 20, 72–83. [Google Scholar] [CrossRef]
- Keen, N.T. Specific Elicitors of Plant Phytoalexin Production: Detenninants of Race Specificity in Pathogens? Science 1975, 187, 74–75. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L. The use of controlled postharvest abiotic stresses as a tool for enhancing the nutraceutical content and adding-value of fresh fruits and vegetables. J. Food Sci. 2003, 68, 1560–1565. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. An alternative use of horticultural crops: Stressed plants as biofactories of bioactive phenolic compounds. Agriculture 2012, 2, 259–271. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. Correction: An alternative use of horticultural crops: Stressed plants as biofactories of bioactive phenolic compounds. Agriculture 2013, 3, 596–598. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Cisneros-Zevallos, L. Biosynthesis of phenolic antioxidants in carrot tissue increases with wounding intensity. Food Chem. 2012, 134, 615–624. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. UVA, UVB and UVC light enhances the biosynthesis of phenolic antioxidants in fresh-cut carrot through a synergistic effect with wounding. Molecules 2017, 22, 668. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Hernández, E.; Nair, V.; Welti-Chanes, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Wounding and UVB light synergistically induce the biosynthesis of phenolic compounds and ascorbic acid in red prickly pears (Opuntia ficusindica cv. Rojo Vigor). Int. J. Mol. Sci. 2019, 20, 5327. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. Postharvest UV-B and UV-C radiation enhanced the biosynthesis of glucosinolates and isothiocyanates in brassicaceae sprouts. Postharvest Biol. Technol. 2021, 181, 1–11. [Google Scholar] [CrossRef]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Aguayo, E.; Gómez, P.A.; Artés, F.; Artés-Hernández, F. UV-C and hyperoxia abiotic stresses to improve healthiness of carrots: Study of combined effects. J. Food Sci. Technol. 2016, 53, 3465–3476. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Callejas, A.; Otón, M.; Artés, F.; Artés-Hernández, F. Combined effect of UV-C pretreatment and high oxygen packaging for keeping the quality of fresh-cut Tatsoi baby leaves. Innov. Food Sci. Emerg. Technol. 2012, 14, 115–121. [Google Scholar] [CrossRef]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Improving the phytochemical composition of broccoli sprouts by elicitation. Food Chem. 2011, 129, 35–44. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Huertas, J.-P.; Navarro-Rico, J.; Gómez, P.A.; Artés, F.; Palop, A.; Artés-Hernández, F. Inactivation kinetics of foodborne pathogens by UV-C radiation and its subsequent growth in fresh-cut kailan-hybrid broccoli. Food Microbiol. 2015, 46, 263–271. [Google Scholar] [CrossRef]
- Pierscianowski, J.; Popović, V.; Biancaniello, M.; Bissonnette, S.; Zhu, Y.; Koutchma, T. Continuous-flow UV-C processing of kale juice for the inactivation of E. coli and assessment of quality parameters. Food Res. Int. 2021, 140, 110085. [Google Scholar] [CrossRef] [PubMed]
- García Carrillo, M.; Ferrario, M.; Guerrero, S.; García Carrillo, M. Study of the inactivation of some microorganisms in turbid carrot-orange juice blend processed by ultraviolet light assisted by mild heat treatment. J. Food Eng. 2017, 212, 213–225. [Google Scholar] [CrossRef]
- Fundo, J.F.; Miller, F.A.; Mandro, G.F.; Tremarin, A.; Brandão, T.R.S.; Silva, C.L.M. UV-C light processing of Cantaloupe melon juice: Evaluation of the impact on microbiological, and some quality characteristics, during refrigerated storage. LWT 2019, 103, 247–252. [Google Scholar] [CrossRef]
- Amanina, A.K.Z.; Rosnah, S.; Noranizan, M.A.; Alifdalino, S. Comparison of UV-C and thermal pasteurisation for the quality preservation of pineapple-mango juice blend. Food Res. 2019, 3, 362–372. [Google Scholar] [CrossRef]
- Türkmen, F.U.; Takci, H.A.M. Ultraviolet-C and ultraviolet-B lights effect on black carrot (Daucus carota ssp. sativus) juice. J. Food Meas. Charact. 2018, 12, 1038–1046. [Google Scholar] [CrossRef]
- Baykuş, G.; Akgün, M.P.; Unluturk, S. Effects of ultraviolet-light emitting diodes (UV-LEDs) on microbial inactivation and quality attributes of mixed beverage made from blend of carrot, carob, ginger, grape and lemon juice. Innov. Food Sci. Emerg. Technol. 2021, 67, 102572. [Google Scholar] [CrossRef]
- Riganakos, K.A.; Karabagias, I.K.; Gertzou, I.; Stahl, M. Comparison of UV-C and thermal treatments for the preservation of carrot juice. Innov. Food Sci. Emerg. Technol. 2017, 42, 165–172. [Google Scholar] [CrossRef]
- Hurtado, A.; Guàrdia, M.D.; Picouet, P.; Jofré, A.; Bañón, S.; Ros, J.M. Shelf-life extension of multi-vegetables smoothies by high-pressure processing compared with thermal treatment. Part I: Microbial and enzyme inhibition, antioxidant status, and physical stability. J. Food Process. Preserv. 2019, 43, e14139. [Google Scholar] [CrossRef]
- Hurtado, A.; Guàrdia, M.D.; Picouet, P.; Jofré, A.; Bañón, S.; Ros, J.M. Shelf-life extension of multi-vegetables smoothies by high pressure processing compared with thermal treatment. Part II: Retention of selected nutrients and sensory quality. J. Food Process. Preserv. 2019, 43, e14210. [Google Scholar] [CrossRef]
- Hurtado, A.; Picouet, P.; Jofré, A.; Guàrdia, M.D.; Ros, J.M.; Bañón, S. Application of High Pressure Processing for Obtaining “Fresh-Like” Fruit Smoothies. Food Bioprocess Technol. 2015, 8, 2470–2482. [Google Scholar] [CrossRef]
- Keenan, D.F.; Rößle, C.; Gormley, R.; Butler, F.; Brunton, N.P. Effect of high hydrostatic pressure and thermal processing on the nutritional quality and enzyme activity of fruit smoothies. LWT—Food Sci. Technol. 2012, 45, 50–57. [Google Scholar] [CrossRef]
- Zacconi, C.; Giosuè, S.; Marudelli, M.; Scolari, G. Microbiological quality and safety of smoothies treated in different pressure–temperature domains: Effects on indigenous fruit microbiota and Listeria monocytogenes and their survival during storage. Eur. Food Res. Technol. 2015, 241, 317–328. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.C.; Wang, Y.; Zhao, F.; Sun, Z.; Liao, X. Quality comparison of carrot juices processed by high-pressure processing and high-temperature short-time processing. Innov. Food Sci. Emerg. Technol. 2016, 33, 135–144. [Google Scholar] [CrossRef]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Otón, M.; Artés, F.; Artés-Hernández, F. High Hydrostatic Pressure Treatments for Keeping Quality of Orange Vegetables Smoothies. Acta Hortic. 2018, 1194, 575–580. [Google Scholar] [CrossRef]
- Pallarés, N.; Berrada, H.; Tolosa, J.; Ferrer, E. Effect of high hydrostatic pressure (HPP) and pulsed electric field (PEF) technologies on reduction of aflatoxins in fruit juices. LWT 2021, 142, 111000. [Google Scholar] [CrossRef]
- Raj, A.S.; Chakraborty, S.; Rao, P.S. Thermal assisted high-pressure processing of Indian gooseberry (Embilica officinalis L.) juice Impact on colour and nutritional attributes. LWT—Food Sci. Technol. 2019, 99, 119–127. [Google Scholar] [CrossRef]
- Moreira, R.M.; Martins, M.L.; de Castro Leite Júnior, B.R.; Martins, E.M.F.; Ramos, A.M.; Cristianini, M.; da Rocha Campos, A.N.; Stringheta, P.C.; Silva, V.R.O.; Canuto, J.W.; et al. Development of a juçara and Ubá mango juice mixture with added Lactobacillus rhamnosus GG processed by high pressure. LWT—Food Sci. Technol. 2017, 77, 259–268. [Google Scholar] [CrossRef]
- De Ancos, B.; Rodrigo, M.J.; Sánchez-Moreno, C.; Pilar Cano, M.; Zacarías, L. Effect of high-pressure processing applied as pretreatment on carotenoids, flavonoids and vitamin C in juice of the sweet oranges “Navel” and the red-fleshed “Cara Cara”. Food Res. Int. 2020, 132, 109105. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Esteve, M.J.; Frigola, A. Ascorbic acid is the only bioactive that is better preserved by high hydrostatic pressure than by thermal treatment of a vegetable beverage. J. Agric. Food Chem. 2010, 58, 10070–10075. [Google Scholar] [CrossRef] [PubMed]
- Saldaña, G.; Puértolas, E.; Monfort, S.; Raso, J.; Álvarez, I. Defining treatment conditions for pulsed electric field pasteurization of apple juice. Int. J. Food Microbiol. 2011, 151, 29–35. [Google Scholar] [CrossRef]
- Timmermans, R.A.H.; Nederhoff, A.L.; Nierop Groot, M.N.; van Boekel, M.A.J.S.; Mastwijk, H.C. Effect of electrical field strength applied by PEF processing and storage temperature on the outgrowth of yeasts and moulds naturally present in a fresh fruit smoothie. Int. J. Food Microbiol. 2016, 230, 21–30. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.A.; Sun, D.W.; Wang, M.S.; Liu, Z.W.; Zhang, Z.H. Combined effects of sonication and pulsed electric field on selected quality parameters of grapefruit juice. LWT—Food Sci. Technol. 2015, 62, 890–893. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Cortes, C.; Zulueta, A.; Frigola, A.; Esteve, M.J. Influence of pulsed electric field processing on the quality of fruit juice beverages sweetened with Stevia rebaudiana. Food Bioprod. Process. 2017, 101, 214–222. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Hu, B.; Lei, S.; Zeng, X. Sonication enhances polyphenolic compounds, sugars, carotenoids and mineral elements of apple juice. Ultrason. Sonochem. 2014, 21, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.; Asif, M.N.; Ahmad, M.H.; Imran, M.; Arshad, M.S.; Hassan, S.; Khan, M.I.; Nisa, M.U.; Iqbal, M.M.; Muhammad, N. Ultrasound-Assisted Optimal Development and Characterization of Stevia-Sweetened Functional Beverage. J. Food Qual. 2019, 2019. [Google Scholar] [CrossRef]
- Keenan, D.F.; Tiwari, B.K.; Patras, A.; Gormley, R.; Butler, F.; Brunton, N.P. Effect of sonication on the bioactive, quality and rheological characteristics of fruit smoothies. Int. J. Food Sci. Technol. 2012, 47, 827–836. [Google Scholar] [CrossRef]
- Jabbar, S.; Abid, M.; Hu, B.; Wu, T.; Hashim, M.M.; Lei, S.; Zhu, X.; Zeng, X. Quality of carrot juice as influenced by blanching and sonication treatments. LWT—Food Sci. Technol. 2014, 55, 16–21. [Google Scholar] [CrossRef]
- Ahmad, K.; Imran, M.; Ahmad, T.; Ahmad, M.H.; Khan, M.K. Impact of ultrasound processing on physicochemical and bioactive attributes of grape based optimized fruit beverage. Pakistan J. Agric. Sci. 2020, 57, 1117–1124. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.A.; Wang, M.S.; Liu, Z.W.; Han, Z.; Zhang, Z.H.; Hong, J.; Jabbar, S. A potential of ultrasound on minerals, micro-organisms, phenolic compounds and colouring pigments of grapefruit juice. Int. J. Food Sci. Technol. 2015, 50, 1144–1150. [Google Scholar] [CrossRef]
- Guerrouj, K.; Sánchez-Rubio, M.; Taboada-Rodríguez, A.; Cava-Roda, R.M.; Marín-Iniesta, F. Sonication at mild temperatures enhances bioactive compounds and microbiological quality of orange juice. Food Bioprod. Process. 2016, 99, 20–28. [Google Scholar] [CrossRef]
- De Albuquerque, J.G.; Escalona-Buendía, H.B.; de Magalhães Cordeiro, A.M.T.; dos Santos Lima, M.; de Souza Aquino, J.; da Silva Vasconcelos, M.A. Ultrasound treatment for improving the bioactive compounds and quality properties of a Brazilian nopal (Opuntia ficus-indica) beverage during shelf-life. LWT—Food Sci. Technol. 2021, 149, 111814. [Google Scholar] [CrossRef]
- Mehta, D.; Sharma, N.; Bansal, V.; Sangwan, R.S.; Yadav, S.K. Impact of ultrasonication, ultraviolet and atmospheric cold plasma processing on quality parameters of tomato-based beverage in comparison with thermal processing. Innov. Food Sci. Emerg. Technol. 2019, 52, 343–349. [Google Scholar] [CrossRef]
- Surowsky, B.; Fröhling, A.; Gottschalk, N.; Schlüter, O.; Knorr, D. Impact of cold plasma on Citrobacter freundii in apple juice: Inactivation kinetics and mechanisms. Int. J. Food Microbiol. 2014, 174, 63–71. [Google Scholar] [CrossRef]
- Rodríguez, Ó.; Gomes, W.F.; Rodrigues, S.; Fernandes, F.A.N. Effect of indirect cold plasma treatment on cashew apple juice (Anacardium occidentale L.). LWT—Food Sci. Technol. 2017, 84, 457–463. [Google Scholar] [CrossRef]
- Illera, A.E.; Chaple, S.; Sanz, M.T.; Ng, S.; Lu, P.; Jones, J.; Carey, E.; Bourke, P. Effect of cold plasma on polyphenol oxidase inactivation in cloudy apple juice and on the quality parameters of the juice during storage. Food Chem. X 2019, 3, 100049. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, R.; Gan, Z.; Shao, T.; Zhang, X.; He, M.; Sun, A. Effect of cold plasma on blueberry juice quality. Food Chem. 2019, 290, 79–86. [Google Scholar] [CrossRef]
- Bursać Kovačević, D.; Gajdoš Kljusurić, J.; Putnik, P.; Vukušić, T.; Herceg, Z.; Dragović-Uzelac, V. Stability of polyphenols in chokeberry juice treated with gas phase plasma. Food Chem. 2016, 212, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, A.A.; Aba, R.P.M.; Tayamora, D.J.L.; Colambo, J.C.R.; Siringan, M.A.T.; Rosario, L.M.D.; Tumlos, R.B.; Ramos, H.J. Reference organism selection for microwave atmospheric pressure plasma jet treatment of young coconut liquid endosperm. Food Control 2016, 69, 74–82. [Google Scholar] [CrossRef]
- Shi, X.M.; Zhang, G.J.; Wu, X.L.; Li, Y.X.; Ma, Y.; Shao, X.J. Effect of low-temperature plasma on microorganism inactivation and quality of freshly squeezed orange juice. IEEE Trans. Plasma Sci. 2011, 39, 1591–1597. [Google Scholar] [CrossRef]
- Xu, L.; Garner, A.L.; Tao, B.; Keener, K.M. Microbial Inactivation and Quality Changes in Orange Juice Treated by High Voltage Atmospheric Cold Plasma. Food Bioprocess Technol. 2017, 10, 1778–1791. [Google Scholar] [CrossRef]
- Almeida, F.D.L.; Cavalcante, R.S.; Cullen, P.J.; Frias, J.M.; Bourke, P.; Fernandes, F.A.N.; Rodrigues, S. Effects of atmospheric cold plasma and ozone on prebiotic orange juice. Innov. Food Sci. Emerg. Technol. 2015, 32, 127–135. [Google Scholar] [CrossRef]
- Herceg, Z.; Kovačević, D.B.; Kljusurić, J.G.; Jambrak, A.R.; Zorić, Z.; Dragović-Uzelac, V. Gas phase plasma impact on phenolic compounds in pomegranate juice. Food Chem. 2016, 190, 665–672. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Režek Jambrak, A.; Milošević, S.; Dragović-Uzelac, V.; Zorić, Z.; Herceg, Z. The effect of gas phase plasma treatment on the anthocyanin and phenolic acid content of sour cherry Marasca (Prunus cerasus var. Marasca) juice. LWT—Food Sci. Technol. 2015, 62, 894–900. [Google Scholar] [CrossRef]
- Ma, T.J.; Lan, W.S. Effects of non-thermal plasma sterilization on volatile components of tomato juice. Int. J. Environ. Sci. Technol. 2015, 12, 3767–3772. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Wan, Z.; Colonna, W.; Keener, K.M. Effect of high voltage atmospheric cold plasma on white grape juice quality. J. Sci. Food Agric. 2017, 97, 4016–4021. [Google Scholar] [CrossRef]
- Ferrario, M.; Alzamora, S.M.; Guerrero, S. Study of the inactivation of spoilage microorganisms in apple juice by pulsed light and ultrasound. Food Microbiol. 2015, 46, 635–642. [Google Scholar] [CrossRef]
- Tremarin, A.; Brandão, T.R.S.; Silva, C.L.M. Application of ultraviolet radiation and ultrasound treatments for Alicyclobacillus acidoterrestris spores inactivation in apple juice. LWT—Food Sci. Technol. 2017, 78, 138–142. [Google Scholar] [CrossRef]
- Sauceda-Gálvez, J.N.; Codina-Torrella, I.; Martinez-Garcia, M.; Hernández-Herrero, M.M.; Gervilla, R.; Roig-Sagués, A.X. Combined effects of ultra-high pressure homogenization and short-wave ultraviolet radiation on the properties of cloudy apple juice. LWT 2021, 136, 110286. [Google Scholar] [CrossRef]
- Umair, M.; Jabbar, S.; Senan, A.M.; Sultana, T.; Nasiru, M.M.; Shah, A.A.; Zhuang, H.; Jianhao, Z. Influence of Combined E ff ect of Ultra-Sonication and High-Voltage Cold Plasma Treatment on Quality. Foods 2019, 8, 593. [Google Scholar] [CrossRef]
- Gomes, W.F.; Tiwari, B.K.; Rodriguez, Ó.; de Brito, E.S.; Fernandes, F.A.N.; Rodrigues, S. Effect of ultrasound followed by high pressure processing on prebiotic cranberry juice. Food Chem. 2017, 218, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, B.; Sun, Z. Quality parameters and bioactive compound bioaccessibility changes in probiotics fermented mango juice using ultraviolet-assisted ultrasonic pre-treatment during cold storage. LWT 2021, 137, 110438. [Google Scholar] [CrossRef]
- Walkling-Ribeiro, M.; Noci, F.; Cronin, D.A.; Lyng, J.G.; Morgan, D.J. Shelf life and sensory evaluation of orange juice after exposure to thermosonication and pulsed electric fields. Food Bioprod. Process. 2009, 87, 102–107. [Google Scholar] [CrossRef]
- Kouniaki, S.; Kajda, P.; Zabetakis, I. The effect of high hydrostatic pressure on anthocyanins and ascorbic acid in blackcurrants (Ribes nigrum). Flavour Fragr. J. 2004, 19, 281–286. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Barba, F.J.; Esteve, M.J.; Frígola, A. High pressure processing of fruit juice mixture sweetened with Stevia rebaudiana Bertoni: Optimal retention of physical and nutritional quality. Innov. Food Sci. Emerg. Technol. 2013, 18, 48–56. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Martínez-Hernández, G.B.; Aguayo, E.; Gómez, P.A.; Artés, F. Fresh-cut fruit and vegetables: Emerging eco-friendly techniques for sanitation and preserving safety. In Postharvest Handling; Kahramanoglu, I., Ed.; InTech: London, UK, 2017; pp. 7–45. [Google Scholar]
- Ruiz-Rico, M.; Pérez-Esteve, É.; Lerma-García, M.J.; Marcos, M.D.; Martínez-Máñez, R.; Barat, J.M. Protection of folic acid through encapsulation in mesoporous silica particles included in fruit juices. Food Chem. 2017, 218, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, T.M.; Pinto, V.S.; Soares, V.R.F.; Marotz, D.; Cichoski, A.J.; Zepka, L.Q.; Jacob Lopes, E.; de Bona da Silva, C.; de Menezes, C.R. Viability of microencapsulated Lactobacillus acidophilus by complex coacervation associated with enzymatic crosslinking under application in different fruit juices. Food Res. Int. 2021, 141, 110190. [Google Scholar] [CrossRef]
- Stebbins, N.B.; Howard, L.R.; Prior, R.L.; Brownmiller, C.; Mauromoustakos, A. Stabilization of anthocyanins in blackberry juice by glutathione fortification. Food Funct. 2017, 8, 3459–3468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.; Wei, Z.; Yin, B.; Man, C.; Jiang, Y. Enhancement of functional characteristics of blueberry juice fermented by Lactobacillus plantarum. LWT 2021, 139, 110590. [Google Scholar] [CrossRef]
- Trigo, J.P.; Alexandre, E.M.C.; Oliveira, A.; Saraiva, J.A.; Pintado, M. Fortification of carrot juice with a high-pressure-obtained pomegranate peel extract: Chemical, safety and sensorial aspects. Int. J. Food Sci. Technol. 2020, 55, 1599–1605. [Google Scholar] [CrossRef]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Aguayo, E.; Gómez, P.A.; Artés, F.; Artés-Hernández, F. A Functional Smoothie from Carrots with Induced Enhanced Phenolic Content. Food Bioprocess Technol. 2017, 10, 491–502. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Santacruz, A.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Postharvest Wounding Stress in Horticultural Crops as a Tool for Designing Novel Functional Foods and Beverages with Enhanced Nutraceutical Content: Carrot Juice as a Case Study. J. Food Sci. 2019, 84, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Bontsidis, C.; Mallouchos, A.; Terpou, A.; Nikolaou, A.; Batra, G.; Mantzourani, I.; Alexopoulos, A.; Plessas, S. Microbiological and chemical properties of chokeberry juice fermented by novel lactic acid bacteria with potential probiotic properties during fermentation at 4◦c for 4 weeks. Foods 2021, 10, 768. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.L.; O’Callaghan, Y.C.; Neugart, S.; Piggott, C.O.; Connolly, A.; Jansen, M.A.K.; Krumbein, A.; Schreiner, M.; FitzGerald, R.J.; O’Brien, N.M. The hydroxycinnamic acid content of barley and brewers’ spent grain (BSG) and the potential to incorporate phenolic extracts of BSG as antioxidants into fruit beverages. Food Chem. 2013, 141, 2567–2574. [Google Scholar] [CrossRef] [PubMed]
- Aljobair, M.O.; Albaridi, N.A.; Alkuraieef, A.N.; AlKehayez, N.M. Physicochemical properties, nutritional value, and sensory attributes of a nectar developed using date palm puree and spirulina. Int. J. Food Prop. 2021, 24, 845–858. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Kokkinomagoulos, E.; Hatzikamari, M.; Bekatorou, A. Emmer-based beverage fortified with fruit juices. Appl. Sci. 2021, 11, 3116. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Hernández, G.B.; Goffi, V.; Gómez, P.A.; Aguayo, E.; Artés, F.; Artés-Hernández, F. Natural vitamin B12 and fucose supplementation of green smoothies with edible algae and related quality changes during their shelf life. J. Sci. Food Agric. 2018, 98, 2411–2421. [Google Scholar] [CrossRef]
- Rúa Aller, J.; González González, S.; Sanz Gómez, J.; del Valle Fernandez, M.P.; García-Armesto, M.R. Assessment of (-) epicatechin as natural additive for improving safety and functionality in fresh “Piel de Sapo” melon juice. Food Sci. Nutr. 2021, 9, 2925–2935. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.; de Oliveira Torres, L.R.; Silva, A.S.; Barbosa, C.H.; Vilarinho, F.; Ramos, F.; de Quirós, A.R.B.; Khwaldia, K.; Sendón, R. Industrial multi-fruits juices by-products: Total antioxidant capacity and phenolics profile by LC–MS/MS to ascertain their reuse potential. Eur. Food Res. Technol. 2020, 246, 2271–2282. [Google Scholar] [CrossRef]
- Fernandez, M.V.; Bengardino, M.; Jagus, R.J.; Agüero, M.V. Enrichment and preservation of a vegetable smoothie with an antioxidant and antimicrobial extract obtained from beet by-products. LWT 2020, 117, 108622. [Google Scholar] [CrossRef]
- Dogan, K.; Akman, P.K.; Tornuk, F. Role of non-thermal treatments and fermentation with probiotic Lactobacillus plantarum on in vitro bioaccessibility of bioactives from vegetable juice. J. Sci. Food Agric. 2021, 101, 4779–4788. [Google Scholar] [CrossRef]
- Xu, X.; Bi, S.; Lao, F.; Chen, F.; Liao, X.; Wu, J. Induced changes in bioactive compounds of broccoli juices after fermented by animal- and plant-derived Pediococcus pentosaceus. Food Chem. 2021, 357, 129767. [Google Scholar] [CrossRef]
- Saad, A.M.; Mohamed, A.S.; El-Saadony, M.T.; Sitohy, M.Z. Palatable functional cucumber juices supplemented with polyphenols-rich herbal extracts. LWT 2021, 148, 111668. [Google Scholar] [CrossRef]
- Mohammad Bagher Hashemi, S.; Jafarpour, D.; Jouki, M. Improving bioactive properties of peach juice using Lactobacillus strains fermentation: Antagonistic and anti-adhesion effects, anti-inflammatory and antioxidant properties, and Maillard reaction inhibition. Food Chem. 2021, 365, 130501. [Google Scholar] [CrossRef] [PubMed]
- Aderinola, T. Nutritional, Antioxidant and Quality Acceptability of Smoothies Supplemented with Moringa oleifera Leaves. Beverages 2018, 4, 104. [Google Scholar] [CrossRef]
- Habibi, A.; Keramat, J.; Hojjatoleslamy, M.; Tamjidi, F. Preparation of Fish Oil Microcapsules by Complex Coacervation of Gelatin–Gum Arabic and their Utilization for Fortification of Pomegranate Juice. J. Food Process Eng. 2017, 40, 1–11. [Google Scholar] [CrossRef]
- Sahin, O.I.; Ozturk, B. Microalgal biomass—a bio-based additive: Evaluation of green smoothies during storage. Int. Food Res. J. 2021, 28, 309–316. [Google Scholar]
- Kranz, P.; Braun, N.; Schulze, N.; Kunz, B. Sensory quality of functional beverages: Bitterness perception and bitter masking of olive leaf extract fortified fruit smoothies. J. Food Sci. 2010, 75, S308–S311. [Google Scholar] [CrossRef]
- Michaličková, D.; Belović, M.; Ilić, N.; Kotur-Stevuljević, J.; Slanař, O.; Šobajić, S. Comparison of Polyphenol-Enriched Tomato Juice and Standard Tomato Juice for Cardiovascular Benefits in Subjects with Stage 1 Hypertension: A Randomized Controlled Study. Plant Foods Hum. Nutr. 2019, 74, 122–127. [Google Scholar] [CrossRef]
- Aduloju, A.T.V.-; Nwanja, N.M.; Ezegbe, C.C.; Okocha, K.S.; Aduloju, T.A. Phytochemicals and Vitamin Properties of Smoothie Flavoured with Mint Leaves Extract. Int. J. Biochem. Res. Rev. 2020, 29, 24–30. [Google Scholar] [CrossRef]
- Tarazona-Díaz, M.P.; Aguayo, E. Influence of acidification, pasteurization, centrifugation and storage time and temperature on watermelon juice quality. J. Sci. Food Agric. 2013, 93, 3863–3869. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, A.; Ramos-Campo, D.J.; Fernández-Lobato, B.; Rubio-Arias, J.A.; Alacid, F.; Aguayo, E. Biochemical, physiological, and performance response of a functional watermelon juice enriched in L-citrulline during a half-marathon race. Food Nutr. Res. 2017, 61, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tarazona-Díaz, M.P.; Alacid, F.; Carrasco, M.; Martínez, I.; Aguayo, E. Watermelon juice: Potential functional drink for sore muscle relief in Athletes. J. Agric. Food Chem. 2013, 61, 7522–7528. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Alacid, F.; Rubio-Arias, J.A.; Fernández-Lobato, B.; Ramos-Campo, D.J.; Aguayo, E. Consumption of Watermelon Juice Enriched in l -Citrulline and Pomegranate Ellagitannins Enhanced Metabolism during Physical Exercise. J. Agric. Food Chem. 2017, 65, 4395–4404. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Rodrigues, C.; Garcia-Oliveira, P.; Lourenço-Lopes, C.; Silva, S.A.; Garcia-Perez, P.; Carvalho, A.P.; Domingues, V.F.; Barroso, M.F.; Delerue-Matos, C.; et al. Screening of Bioactive Properties in Brown Algae from the Northwest Iberian Peninsula. Foods 2021, 10, 1915. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Castillejo, N.; Carrión-Monteagudo, M.M.; Artés, F.; Artés-Hernández, F. Nutritional and bioactive compounds of commercialized algae powders used as food supplements. Food Sci. Technol. Int. 2018, 24, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Klug, T.V.; Collado, E.; Martínez-Hernández, G.B.; Artés, F.; Artés-Hernández, F. Effect of stevia supplementation of kale juice spheres on their quality changes during refrigerated shelf life. J. Sci. Food Agric. 2019, 99, 2384–2392. [Google Scholar] [CrossRef] [PubMed]
| Technology | Beverage Type and Components | Treatment Conditions | Optimum Conditions | Shelf Life | Main Results Obtained | Reference |
|---|---|---|---|---|---|---|
| ULTRAVIOLET | Black carrot juice | 254 and 365 nm; 15 W LMP lamps: 2.16·10−3 and 1.50·10−3 kJ/m2 respectively; 0–60 min; 25 °C | 365 nm 1.5·10−3 kJ/m2 | - | Increased TPC No effect on colour Approx. 1 log reduction in microbial spoilage | [65] |
| Carrot juice | 30 W LMP lamps; 11.4 kJ/m2 | 253.7 nm 11.4 kJ/m2 | 12 d at 5 °C | Good sensory parameters during storage | [67] | |
| Carrot-carob-ginger-lemon-grape juice | 280 and 365 nm; 0.6–0.4 mW LEDs; single and combined wavelength for 10–100 min; 25 °C | Combined 280/365 nm 0.77/2.2 kJ/m2 | - | Increased TPC and TAC 4.05 log reductions of inoculated Escherichia coli Minimum effect on physical properties | [66] | |
| Carrot-orange juice | 30 W LMP lamps; 0–10.6 kJ/m2; 0–15 min; 1.6 L/min; 20 °C | 253.7 nm 10.6 kJ/m2 | - | Up to 2.5–5.9 log reductions in inoculated E. coli, Pseudomonas fluorescens, and saccharomyces cerevisiae | [62] | |
| Kale juice | 25 W LMP lamps: 74 and 108.3 mJ/cm2; 0.14 L/min; RT | 253.7 nm 1.08 kJ/m2 | 4 d at 4 °C | Reduction by 20% in TPC Up to 5-log reduction of inoculated E. coli No effect on viscosity, chlorophyll content, colour, TAC, PPO, and POD Increased sedimentation rate Higher PME activity | [61] | |
| Melon juice | 15 W LMP lamps; 4 and 16 kJ/m2; 5 and 20 min; 25 °C | 254 nm 16 kJ/m2 | 13 d at 5 °C | Better retention of TAC and colour No effect on TPC Moulds and yeasts did not grow | [63] | |
| Pineapple-mango juice | 55 W LPM lamps; 0.08 kJ/m2; 8.65 s | 254 nm; 8 kJ/m2 | 9 weeks at 4 °C | Minimal degradation of ascorbic acid TPC and TAC retention | [64] | |
| HPP | Apple-carrot-zucchini-pumpkin-leek smoothie | 350 MPa; 10 °C; 5 min | 350 MPa 10 °C 5 min | 28 d at 4 °C | High retention of vitamin C during storage Retention of antioxidants (TPC and flavonoids) Lower microbiological load Higher oxidation due to earlier clarification | [68,69] |
| Apple-orange-strawberry-banana smoothie | 350–600 MPa; 10 °C; 3–5 min | 350 MPa 10 °C 5 min | 48 h at 4 °C | Does not affect phenolics and flavonoids Preserves vitamin C and flavours Ensures microbial quality | [70] | |
| Apple-strawberry-banana-orange smoothie | 450–600 MPa; 20 °C; 5–10 min | 600 MPa 20 °C 5 min | 10 h at 4 °C | Retains ascorbic acid High PPO inactivation rate (83%) | [71] | |
| Berries-grape-orange-strawberry-apple smoothie | 100–300 MPa; −5–45 °C; 5 min | 300 MPa 45 °C 5 min | 15 d at 4 and 20 °C | Reduction of up to 6-log of mesophilic lactobacilli | [72] | |
| Carrot juice | 550 MPa; <38 °C; 6 min | 550 MPa <38 °C 6 min | 20 d at 4 °C | Better carotenoid (α- and β-carotene), phenolic, polyacetylene and TAC retention Better preservation of nutritional compounds Higher rheological properties Better sensory attributes | [73] | |
| Carrot-pumpkin smoothie | 300–600 MPa; 23 °C; 5 min | 400 MPa 23 °C 5 min | 7 d at 5 °C | Mild TPC reduction (<15%) Better TPC preservation during storage High microbial control (≈6 log lower counts) No high physicochemical changes (SSC, pH and colour) | [74] | |
| Grape juice | 500 MPa; 45 °C; 5 min | 500 MPa 45 °C 5 min | - | Reduction of 17–29% in aflatoxins | [75] | |
| Indian gooseberry juice | 200–500 MPa; 30–60 °C; 5 min | 500 MPa 30 °C 5 min | - | Increase in TPC and TAC up to 50 °C Less vitamin C degradation | [76] | |
| Juçara-mango juice | 600 MPa; 25 °C; 5 min | 600 MPa 25 °C 5 min | - | Does not affect anthocyanin content Good sensory properties | [77] | |
| Orange juice | 0–200 MPa; 25 °C; 1 min (whole peeled orange) + 400 MPa;40 °C; 1 min (juice) | 200 MPa 25°C 1 min + 400 MPa 40 °C 1 min | - | Non-additive effect in flavonoids and vitamin C 12-fold increase in content of colourless carotenoids | [78] | |
| Tomato-pepper-celery-cucumber-onion-carrot-lemon beverage | 100–400 MPa; <30 °C; 2–9 min | 400 MPa <30 °C 2–5 min | - | Good preservation of vitamin C Slight colour change | [79] | |
| PEF | Apple juice | 20–30 kV/cm; 5–125 µs; ≤55 °C | 25 kV/cm 63 µs ≤55 °C | - | >5 log reduction cycles of E. coli, L. monocytogenes, Staphylococcus aureus, and Salmonella typhimurium | [80] |
| Apple-strawberry-banana smoothie | 13.5–24 kV/cm; 100–290 Hz; 8.7–24.1 pulses; 3 µs; 130 L/h; <58 °C | 24 kV/cm; 100 Hz 8.7 pulses <58 °C | 27 d at 7 °C & 7 d at 4 °C | Highest inactivation of moulds and yeasts | [81] | |
| Grape juice | 3 kV/cm; 238 pulses up to 500 kJ/kg; sample: 215 mL; <75 °C | 3 kV/cm 238 pulses <75 °C | - | Reductions by 24–84% in aflatoxins | [75] | |
| Grapefruit juice | 20 kV/cm; 1 kHz; 80 mL/min; <45 °C | 20 kV/cm; 1 kHz <45 °C | - | Lower non-enzymatic browning and viscosity than the untreated sample | [82] | |
| Mango-papaya-stevia juice | 20–40 kV/cm; 2.5 µs; 30 mL/min; 100–360 µs; <50 °C | 21 kV/cm 360 µs <50 °C | - | Greatest content of bioactive compounds Minimal colour changes | [83] | |
| ULTRASOUND | Apple juice | 2 W/cm2; 25 kHz; 70%; 30–60 min; 20 °C; sample: 60 mL | 2 W/cm2; 25 kHz 30 min 20 °C | - | The highest polyphenolic and sugars content Higher minerals and total carotenoids (60 min) | [84] |
| Apple-carrot-stevia juice | 750 W; 20 kHz; 20–80%; 15 min; sample: 100 mL | 750 W; 20 kHz; 60% 15 min | - | Better phenolic profile Better radical scavenging activity | [85] | |
| Apple-strawberry-banana-orange smoothie | 1.5 kW; 20 kHz; 40–100%; 25 °C; sample: 200 mL | 1.5 kW; 20 kHz; 70% (42.7 μm) 3 min 25 °C | - | Better TPC preservation Higher flavonoid content | [86] | |
| Carrot juice | 750 W; 20 kHz; pulses of 5 s on and 5 s off; 70%; 15 °C; sample: 250 mL | 750 W; 20 kHz; 70% 15 °C | 48 h at 4 °C | Enhancement of colouring pigments, sugar, chlorogenic acid and some mineral contents Decreased microbial population | [87] | |
| Grape-apple juice | 750 W; 20 kHz; 100%; 20–40 min; sample: 100 mL | 750 W; 20 kHz; 100% 20 min | - | Increased phenolic profile and TAC Higher organic acids | [88] | |
| Grapefruit juice | 720 W; 28 kHz; 70%; 30–90 min; 20 °C | 720 W; 28 kHz; 70% 90 min 20 °C | - | Improvement in sugar, carotenoid, mineral and phenolic content Decreased spoilage microbe population | [89] | |
| Strawberry-banana-juçara smoothie | 73.5–250 W; 20 kHz; 7–19 min; <60 °C; sample: 200 mL | 147 W; 20 kHz 2 min <60 °C | - | The highest anthocyanin retention | [30] | |
| Orange juice | 33.31 W/mL; 24 kHz; 105 µm; 1–30 min; <46 °C; sample: 30 mL | 33.31 W/mL; 24 kHz; 105 µm 30 min <46 °C | 28 d at 5 °C | Increased phenolic and flavonoid content Higher vitamin C retention Better sensorial properties | [90] | |
| Nopal beverage | 240 W; 42 kHz; 10–40 min; <34 °C; sample: 300 mL | 240 W; 42 kHz 40 min <34 °C | 28 d at 4 °C | Higher stability for bioactive compounds Ascorbic acid reduction Best acceptability Low changes in colour | [91] | |
| Tomato-coconut water-beetroot juice-based beverage | 240 V; 37 kHz; 10 and 15 min | 240 V; 37 kHz 10 min | - | High sinapic and gallic acid contents Ascorbic acid reduction 1 log reduction of yeast and mould | [92] | |
| COLD PLASMA | Apple juice | Atmospheric jet; 65 V; 1.1 MHz; 0–0.1% oxygen-argon gas flow: 5 slm; 0–8 min | Atmospheric jet; 65 V 0.1% O2 in Ar gas 8 min | 24 h | Reduction of C. freundii by ~5 log cycles | [93] |
| Apple juice (cashew) | Indirect plasma field under 30 kPa; 80 kHz; nitrogen gas flow: 10–50 mL/min; 5–15 min; sample: 10 mL | Indirect plasma; 30 kPa 10 mL N2/min 5 min | - | Increased TPC and TAC Higher vitamin C retention | [94] | |
| Apple juice (cloudy) | Spark and glow discharge; 7.9–10.9 kV; 20–65 kHz; 1–5 min | Spark discharge 10.5 kV 5 min | 28 d at 4 °C | Increased TPC and TAC PPO inactivation Lighter juice colour | [95] | |
| Blueberry juice | Single-electrode atmospheric jet; 11 kV; 1 kHz; 0–1% oxygen-argon gas flow: 1 L/min; 2–6 min | Single-electrode 11 kV 1% O2 in Ar gas 6 min | - | Increased TPC and TAC Higher content of anthocyanin and vitamin C (at 0% O2 and 2–4 min) than heat treatment 7.2 log reduction of Bacillus | [96] | |
| Chokeberry juice | Single-electrode atmospheric jet; 25 kHz; argon gas flow: 0.75 dm3/min; time: 3–5 min; 24 °C | Single-electrode 5–7 cm3 3 min | - | Polyphenolic content stability | [97] | |
| Coconut liquid endosperm | Atmospheric jet powered by a microwave generator; 450–650 W; air gas flow: 5 L/min; time: 0–25 min | Atmospheric jet 450 W 22–24 min | - | Reduction of initial counts of S. enterica and E. coli by 4 log cycles | [98] | |
| Orange juice | DBD-low-temperature plasma; 30 kV; 60 kHz; time: 3–12 s and 5–20 s; sample: 50 µL and 4 mL, respectively | DBD; 30 kV 10 s | 16 d at 4 °C | Absence of E. coli in juice inoculated with 4.20 × 107 CFU/mL Preservation of vitamin C content | [99] | |
| Orange juice | DBD-atmosphere CP; 90 kV; 60 Hz; time: 30–120 s; sample: 25–50 mL; atmosphere gas: air or 65% O2 | DBD; 90 kV 2 min direct plasma 50 mL 65% O2 | 24 h at 4 °C | Reduction of 4.7-log of S. enterica Reduced PME enzyme activity Higher vitamin C retention in air-packaging compared to the high-oxygen atmosphere. | [100] | |
| Orange juice with oligosaccharides | DBD-atmosphere CP (direct and indirect plasma field); 70 kV; 50 Hz; time: 15–60 s; sample: 20 mL | DBD 70 kV Direct plasma field | 24 h at RT | 12% oligosaccharide loss preservation of TPC, TAC and colour | [101] | |
| Pomegranate juice | Single-electrode atmospheric jet; 2.5 kV; 25 kHz; argon gas flow: 0.75–1.25 dm3/min; time: 3–5 min; sample: 3–5 cm3 | Single-electrode; 2.5 kV 0.75 dm3/min 3 min 5 cm3 | - | Greater anthocyanin stability Less colour changing with higher gas flow | [97] | |
| Pomegranate juice | Single-electrode atmospheric jet; 2.5 kV; 25 kHz; argon gas flow: 0.75–1.25 dm3/min; time: 3–5 min; sample: 3–5 cm3 | Single-electrode; 2.5 kV 1 dm3/min 5 min 3 cm3 | - | Better phenolic compound stability | [102] | |
| COLD PLASMA | Sour cherry Marasca juice | Single-electrode atmospheric plasma jet; 2.5 kV; 25 kHz; argon gas flow: 0.75–1.25 L/min; time: 3–5 min; sample: 2–4 mL | Single-electrode; 2.5 kV 3 min 3 mL | - | Highest anthocyanin and phenol content | [103] |
| Tomato juice | DBD; 10 kV; 5 min; 30 °C | DBD; 10 kV 5 min 30 °C | - | No effects on flavour and aroma Lower volatile compound release | [104] | |
| Tomato-coconut water-beetroot juice-based beverage | DBD; 60 kV; 50 Hz; time: 10 and 15 min; sample: 100 mL | DBD; 60 kV 10 min | - | Improvement of TPC | [92] | |
| White grape juice | DBD; 80 kV; 60 Hz; time: 1–4 min; 24 °C | DBD; 80 kV 1 min 24 °C | - | Higher bioactive compound levels | [105] | |
| COMBINED TECHNOLOGIES | Apple juice | US + PEF US: 600 W; 20 kHz; 80%; 20–44 °C; 10–30 min; sample: 95 mL PEF: 23.9–71.6 J/cm2; 360 µs; 2–60 s; sample: 5 mL; <56 °C | US30 + PEF60 | 15 d at 5 °C | 5.8-log reduction of S. cerevisiae | [106] |
| Apple juice | UV + US; US + UV UV: 254 nm; 15 W lamps: 13.44 W/m2; 5–25 min US: 120–480 W; 35 kHz; 5–25 min | US: 120 W; 5 min + UV: 254 nm; 20.2 kJ/m2 | - | 5-log reduction of A. acidoterrestris | [107] | |
| Apple juice (cloudy) | HPP + UV HPP: 0–300 MPa; 32 °C; sample: 13 L UV: 254 nm; 55 W lamp; 14.3–28.7 J/mL; 20 °C; sample: 70 mL | HPP: 300 MPa; 32 °C + UV: 254 nm; 28.7 J/mL | - | Increased TPC by 277.6% Reduced PME activity | [108] | |
| Carrot juice | CP + US CP: DBD; 70 kV; time: 3 × 4 min, US: 750 W; 20 kHz; pulses of 5 s on and 5 s off; 80%; <20 °C; 3 min; sample: 100 mL | CP: DBD; 70 kV + US: 750 W; 3 min; <20 °C | - | Better stability Higher TPC, carotenoid, lycopene, and lutein Up to 2 log reductions of mesophilic and yeast and moulds | [109] | |
| Cranberry juice | US + HPP US: 600–1200 W/L; 18 kHz; <25 °C; 5 min HPP: 450 MPa; 11.5 °C; 5 min | US: 1.2 kW/L; 5 min; 25 °C + HPP: 450 MPa; 5 min; 11.5 °C | - | Higher anthocyanin content Good preservation of FOS | [110] | |
| Mango juice | US-UV: 600 W; 20 kHz; pulses of 5 s on and 5 s off; 10 min; 3600 J/mL; sample: 100 mL UV: 254 nm; 8 W lamp; | US-UV: 600 W; 254 nm; 3.6 kJ/mL; 10 min | 30 d at 4 °C | Increased the bioaccessibility of ascorbic acid, TPC, and carotenoids by 102%, 114%, and 32%, respectively Good retention up to 30 d | [111] | |
| Orange juice | US + PEF US: 500 W; 30 kHz; 55 °C; 10 min; sample: 800 mL PEF: 40 kV/cm; 15 Hz; 100 µs | US: 500 W; 10 min; 55 °C + PEF: 40 kV/cm | 168 d at 25 °C | Lower colour differences Similar attributes to heat treatment | [112] |
| Beverage Type and Components | Fortification Conditions | Optimum Conditions | Shelf Life | Results | Reference |
|---|---|---|---|---|---|
| Apple and orange juices | Encapsulation of folic acid (synthesised) in mesoporous silica particles | Encapsulated | - | Improved stability Controlled release after consumption by modifying vitamin bioaccessibility | [116] |
| Apple and orange juices | Free and microencapsulated L. acidophilus (10 and 30%) | Microencapsulated | 63 d at 4 °C | Extended survival | [117] |
| Blackberry juice | Glutathione, galacturonic acid, diethylenetriaminepentaacetic acid, and tannic acid (500 mg/L) | Glutathione | 5 weeks at 30 °C | Great retention of the anthocyanin content | [118] |
| Blueberry juice | Lactobacillus plantarum strain J26 | L. plantarum | - | Increased TPC by 43% Increased anthocyanin content by 15% | [119] |
| Carrot juice | Pomegranate peel extract obtained by HPP (0–2.5 mg/mL) | 2.5 mg/mL | 42 d at 4 °C | Higher TAC Improved microbial safety | [120] |
| Carrot juice | Carrot shreds under combination of UV-C (4 kJ/m2) and 72 h at 15 °C in air or hyperoxia (80 kPa O2) conditions | Non-UV-C + 80 kPa O2 (72 h at 15 °C) | 14 d at 5 °C | Increased TPC by 2060% Good microbial quality | [121] |
| Carrot juice | Carrot slices, peeled and unpeeled (48 h at 15 °C) + blanching (80 °C; 6 min) | Unpeeled carrot slices + blanching | - | Increased by 3600 and 195% in chlorogenic and TPC, respectively Increased minerals by 7–40% | [122] |
| Chokeberry juice | Lacticaseibacillus paracasei strain SP5 (10 g/mL) | L. paracasei | 4 weeks at 4 °C | Increased TPC by 49% | [123] |
| Cranberry juice | 0–10% (v/v) of pale brewers’ spent grains (BSG) 0–10% (v/v) of black BSG | 10% of pale or black BSG | - | Increased TAC by 120% | [124] |
| Date-puree nectar | Spirulina (0–20%) | 10% of spirulina | - | Higher total sugar and carotenoid contents Better sensory attributes | [125] |
| Emmer-based beverage | Lactiplantibacillus plantarum 2035 + blueberry, aronia or grape juice | L. plantarum + aronia juice | 4 weeks at 4 °C | Higher TPC and TAC High viable counts during storage | [126] |
| Grape-broccoli-cucumber smoothie | 2.2% of alga (sea lettuce, kombu, wakame, thongweed, dulse, Irish moss, nori, chlorella, or spirulina) | Chlorella and spirulina | 24 d at 5 °C | The highest vitamin C content High fucose content for thongweed, kombu, and wakame-based smoothies | [127] |
| Melon juice | (−) Epicatequin (1.25–5 g/mL) | 2.5 g/mL | 10 d at 4 °C | Increased TPC by 724% | [128] |
| Multi-fruit juice byproducts | Ginger and apple (50:50, w/w) and apple, carrot, beet, and ginger (50:29:20:1, w/w) juice obtained by fresh and freeze-dried byproducts | Ginger and apple juice obtained by freeze-dried by-products | - | Higher TPC, TAC, and flavonoids | [129] |
| Orange- apple-carrot-beet smoothie | Beet leaf extract (30% v/v) | Beet leaf extract (30%) | 21 d at 5 °C | Increased TPC content by 50% High TAC | [130] |
| Orange-celery-carrot-lemon juice | Fermentation by L. plantarum strain HFC8 | L. plantarum | - | Low mesophilic aerobic bacteria and yeast and mould counts Increased TPC, flavonoid, and anthocyanin content | [131] |
| Pasteurised broccoli juice | Fermentation by Pediococcus pentosaceus (isolated from fermented cherry juice and pickled pig’s ear) | P. pentosaceus of animal origin | - | Increase riboflavin and β-carotene content Decrease free amino acids Both obtained high sulforaphane content | [132] |
| Pasteurised cucumber juice | Cinnamon, clove, mint, and ginger extracts (200–800 µg/mL) | 200 µg/mL of clove extract | 6 months RT | Better sensory attributes Higher TPC and flavonoid content Good retention for 6 months | [133] |
| Pasteurised peach juice | Lactobacillus acidophilus PTCC 1643 and Lactobacillus fermentum PTCC 1744 | L. acidophilus | - | Inhibited Maillard reaction by 36.7%; 38.5% of anti-inflammatory activity Increased TAC by 74% | [134] |
| Pineapple- banana-apple smoothie | Moringa leaves (0–4.5%) | 4.5% of moringa leaves | - | Increased vitamin C and E by 227% and 102%, respectively Highest TPC and TAC Lower sensorial quality | [135] |
| Pomegranate juice | 0–0.1% of fish oil microcapsules by complex coacervation | 0.1% fish oil microcapsules | 42 d at 4 °C | 16% of eicosapentaenoic acid and 11% of docosahexaenoic acid were released after 42 d Good sensory quality up to 0.07% microcapsules | [136] |
| Spinach-green apple-cucumber smoothie | 2.5% of alga (Chlorella vulgaris and Dunaliella salina) | D. salina | 28 d at 5 °C | Higher TPC and TAC Great sensory attributes | [137] |
| Strawberry- banana smoothie | Olive leaf extract (-OLE- 0–25 mg/100 g) + Sucrose (0–4 g/100 g)/sodium cyclamate (0–114.4 mg/100 g)/sodium chloride (0–40 mg/100 g) | OLE (20 mg/100 g) + sodium chloride (40 mg/100 g) | - | High TPC 40% less bitter taste perception | [138] |
| Tomato juice | Polyphenols from 0.5% of tomato extracts | Tomato extract (0.5%) | - | High TPC, lycopene, and β-carotene contents | [139] |
| Watermelon- apple-banana smoothie | Mint leaf extract (0–8%) | Mint leaf extract (8%) | - | High vitamin A, C, flavonoid, and TPC | [140] |
| Watermelon juice | Citric acid, malic acid, or lemon juice (pH = 3.8) | Non-centrifuged and addition of citric acid or lemon juice (pH = 3.8) | 20 d at 4° C | Retention of sensory and functional qualities | [141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artés-Hernández, F.; Castillejo, N.; Martínez-Zamora, L.; Martínez-Hernández, G.B. Phytochemical Fortification in Fruit and Vegetable Beverages with Green Technologies. Foods 2021, 10, 2534. https://doi.org/10.3390/foods10112534
Artés-Hernández F, Castillejo N, Martínez-Zamora L, Martínez-Hernández GB. Phytochemical Fortification in Fruit and Vegetable Beverages with Green Technologies. Foods. 2021; 10(11):2534. https://doi.org/10.3390/foods10112534
Chicago/Turabian StyleArtés-Hernández, Francisco, Noelia Castillejo, Lorena Martínez-Zamora, and Ginés Benito Martínez-Hernández. 2021. "Phytochemical Fortification in Fruit and Vegetable Beverages with Green Technologies" Foods 10, no. 11: 2534. https://doi.org/10.3390/foods10112534
APA StyleArtés-Hernández, F., Castillejo, N., Martínez-Zamora, L., & Martínez-Hernández, G. B. (2021). Phytochemical Fortification in Fruit and Vegetable Beverages with Green Technologies. Foods, 10(11), 2534. https://doi.org/10.3390/foods10112534










