Effect of Microbial Fermentation on the Fishy-Odor Compounds in Kelp (Laminaria japonica)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microbial Fermentation
2.3. Headspace-Solid Phase Microextraction (HS-SPME) Conditions
2.4. GC-MS Analysis
2.5. OAV Calculation
2.6. Headspace-Gas Chromatography-Ion Mobility Spectrometry (HS-GC-IMS) Analysis
2.7. Data Analysis
3. Results and Discussion
3.1. HS-SPME-GC-MS Analysis
3.1.1. Identification of Volatile Compounds in Kelp
3.1.2. Identification of Volatile Compounds in Fermented Kelp
3.2. OAV Analysis of Key Fishy-Odor Compounds in Kelp
3.3. HS-GC-IMS Analysis
3.4. Analysis Based on PCA Results and Heat Map Clustering
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yu, K.X.; Jantan, I.; Ahmad, R.; Wong, C.L. The major bioactive components of seaweeds and their mosquitocidal potential. Parasitol. Res. 2014, 113, 3121–3141. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Rhatigan, P.; Pérez-Lloréns, J.L. World cuisine of seaweeds: Science meets gastronomy. Int. J. Gastron. Food Sci. 2018, 14, 55–65. [Google Scholar] [CrossRef]
- Palmieri, N.; Forleo, M.B. The potential of edible seaweed within the western diet. A segmentation of Italian consumers. Int. J. Gastron. Food Sci. 2020, 20, 100202. [Google Scholar] [CrossRef]
- Rioux, L.-E.; Beaulieu, L.; Turgeon, S.L. Seaweeds: A traditional ingredients for new gastronomic sensation. Food Hydrocoll. 2017, 68, 255–265. [Google Scholar] [CrossRef]
- Birch, D.; Skallerud, K.; Paul, N.A. Who are the future seaweed consumers in a Western society? Insights from Australia. Br. Food J. 2019, 121, 603–615. [Google Scholar] [CrossRef] [Green Version]
- Rebours, C.; Marinho-Soriano, E.; Zertuche-Gonzalez, J.A.; Hayashi, L.; Vasquez, J.A.; Kradolfer, P.; Soriano, G.; Ugarte, R.; Abreu, M.H.; Bay-Larsen, I.; et al. Seaweeds: An opportunity for wealth and sustainable livelihood for coastal communities. J. Appl. Phycol. 2014, 26, 1939–1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouritsen, O.G.; Williams, L.; Bjerregaard, R.; Duelund, L. Seaweeds for umami flavour in the New Nordic Cuisine. Flavour 2012, 1, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Hua, Y.; Li, X.; Kong, X.; Chen, Y. Key volatile off-flavor compounds in peas (Pisum sativum L.) and their relations with the endogenous precursors and enzymes using soybean (Glycine max) as a reference. Food Chem. 2020, 333, 127469. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, Y.; Wang, B.; Song, H.; Zou, T. Screening of the volatile compounds in fresh and thermally treated watermelon juice via headspace-gas chromatography-ion mobility spectrometry and comprehensive two-dimensional gas chromatography-olfactory-mass spectrometry analysis. LWT-Food Sci. Technol. 2021, 137, 110478. [Google Scholar] [CrossRef]
- Ma, R.; Tian, Y.; Chen, L.; Jin, Z. Impact of cooling rates on the flavor of cooked rice during storage. Food Biosci. 2020, 35, 100563. [Google Scholar] [CrossRef]
- Seo, Y.S.; Bae, H.N.; Eom, S.H.; Lim, K.S.; Yun, I.H.; Chung, Y.H.; Jeon, J.M.; Kim, H.W.; Lee, M.S.; Lee, Y.B.; et al. Removal of off-flavors from sea tangle (Laminaria japonica) extract by fermentation with Aspergillus oryzae. Bioresour. Technol. 2012, 121, 475–479. [Google Scholar] [CrossRef]
- Takahashi, H.; Sumitani, H.; Inada, Y.; Mori, D. Identification of Volatile Compounds of Kombu (Laminaria spp.) and Their Odor Description. Nippon. Shokuhin Kagaku Kogaku Kaishi 2002, 49, 228–237. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Fang, Z. Recent development in efficient processing technology for edible algae: A review. Trends Food Sci. Technol. 2019, 88, 251–259. [Google Scholar] [CrossRef]
- Yang, X.; Yang, F.; Liu, Y.; Li, J.; Song, H. Off-flavor removal from thermal-treated watermelon juice by adsorbent treatment with β-cyclodextrin, xanthan gum, carboxymethyl cellulose sodium, and sugar/acid. LWT-Food Sci. Technol. 2020, 131, 109775. [Google Scholar] [CrossRef]
- Khalafu, S.H.S.; Mustapha, W.A.W.; Lim, S.J.; Maskat, M.Y. The effect of deodorization on volatile compositions of fucoidan extracted from brown seaweed (Sargassum sp.). AIP Conf. Proc. 2016, 1784, 30043. [Google Scholar] [CrossRef]
- Nedele, A.K.; Gross, S.; Rigling, M.; Zhang, Y. Reduction of green off-flavor compounds: Comparison of key odorants during fermentation of soy drink with Lycoperdon pyriforme. Food Chem. 2021, 334, 127591. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Li, Y.; Zhu, H.; Liu, Y.; Quan, K. Effect of Lactobacillus plantarum fermentation on the volatile flavors of mung beans. LWT-Food Sci. Technol. 2021, 146, 111434. [Google Scholar] [CrossRef]
- Vermeulen, N.; Czerny, M.; Gänzle, M.G.; Schieberle, P.; Vogel, R.F. Reduction of (E)-2-nonenal and (E,E)-2,4-decadienal during sourdough fermentation. J. Cereal Sci. 2007, 45, 78–87. [Google Scholar] [CrossRef]
- Gustaw, K.; Niedzwiedz, I.; Rachwal, K.; Polak-Berecka, M. New Insight into Bacterial Interaction with the Matrix of Plant-Based Fermented Foods. Foods 2021, 10, 1603. [Google Scholar] [CrossRef]
- López-Pérez, O.; del Olmo, A.; Picon, A.; Nuñez, M. Volatile compounds and odour characteristics during long-term storage of kombu seaweed (Laminaria ochroleuca) preserved by high pressure processing, freezing and salting. LWT-Food Sci. Technol. 2020, 118, 108710. [Google Scholar] [CrossRef]
- López-Pérez, O.; Picon, A.; Nuñez, M. Volatile compounds and odour characteristics of seven species of dehydrated edible seaweeds. Food Res. Int. 2017, 99, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Ferraces-Casais, P.; Lage-Yusty, M.A.; Rodriguez-Bernaldo de Quiros, A.; Lopez-Hernandez, J. Rapid identification of volatile compounds in fresh seaweed. Talanta 2013, 115, 798–800. [Google Scholar] [CrossRef]
- Maldonado-Robledo, G.; Rodriguez-Bustamante, E.; Sanchez-Contreras, A.; Rodriguez-Sanoja, R.; Sanchez, S. Production of tobacco aroma from lutein. Specific role of the microorganisms involved in the process. Appl. Microbiol. Biot. 2003, 62, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascues, E.; Calderon, F.; Benito, A.; Rauhut, D.; Santos, A.; et al. Effects on varietal aromas during wine making: A review of the impact of varietal aromas on the flavor of wine. Appl. Microbiol. Biot. 2019, 103, 7425–7450. [Google Scholar] [CrossRef] [PubMed]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- van Gemert, L.J. Odour Thresholds: Compilations of Odour Threshold Values in Air, Water and Other Media (Second Enlarged and Revised Edition); Oliemans Punter & Partners BV: Utrecht, The Netherlands, 2003. [Google Scholar]
- Darriet, P.; Pons, M.; Henry, R.; Dumont, O.; Findeling, V.; Cartolaro, P.; Calonnec, A.; Dubourdieu, D. Impact odorants contributing to the fungus type aroma from grape berries contaminated by powdery mildew (Uncinula necator); incidence of enzymatic activities of the yeast Saccharomyces cerevisiae. J. Agric. Food Chem. 2002, 50, 3277–3282. [Google Scholar] [CrossRef]
- Wanner, P.; Tressl, R. Purification and characterization of two enone reductases from Saccharomyces cerevisiae. Eur. J. Biochem. 1998, 255, 271–278. [Google Scholar] [CrossRef] [Green Version]
- La Guerche, S.; Dauphin, B.; Pons, M.; Blancard, D.; Darriet, P. Characterization of some mushroom and earthy off-odors microbially induced by the development of rot on grapes. J. Agric. Food Chem. 2006, 54, 9193–9200. [Google Scholar] [CrossRef]
- Huang, M.; Hu, H.; Ma, L.; Zhou, Q.; Yu, L.; Zeng, S. Carbon-carbon double-bond reductases in nature. Drug Metab. Rev. 2014, 46, 362–378. [Google Scholar] [CrossRef]
- Assaf, S.; Hadar, Y.; Dosoretz, C.G. 1-Octen-3-ol and 13-hydroperoxylinoleate are products of distinct pathways in the oxidative breakdown of linoleic acid by Pleurotus pulmonarius. Enzym. Microb. Tech. 1997, 21, 484–490. [Google Scholar] [CrossRef]
- Liu, J.; Tang, X.; Zhang, Y.; Zhao, W. Determination of the volatile composition in brown millet, milled millet and millet bran by gas chromatography/mass spectrometry. Molecules 2012, 17, 2271–2282. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Li, W.; Chen, X.; Feng, M.; Rui, X.; Jiang, M.; Dong, M. Microbiological, physicochemical and rheological properties of fermented soymilk produced with exopolysaccharide (EPS) producing lactic acid bacteria strains. LWT-Food Sci. Technol. 2014, 57, 477–485. [Google Scholar] [CrossRef]
- Fan, X.; Jiao, X.; Liu, J.; Jia, M.; Blanchard, C.; Zhou, Z. Characterizing the volatile compounds of different sorghum cultivars by both GC-MS and HS-GC-IMS. Food Res. Int. 2021, 140, 109975. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Ye, J.; Li, L.; Wang, X.; Wang, P.; Han, M.; Xu, X. Exploration of flavor and taste of soft-boiled chicken at different post-mortem aging time: Based on GC-IMS and multivariate statistical analysis. Food Biosci. 2021, 43, 101326. [Google Scholar] [CrossRef]
- Borsdorf, H.; Eiceman, G.A. Ion Mobility Spectrometry: Principles and Applications. Appl. Spectrosc. Rev. 2006, 41, 323–375. [Google Scholar] [CrossRef]
- Ge, S.; Chen, Y.; Ding, S.; Zhou, H.; Jiang, L.; Yi, Y.; Deng, F.; Wang, R. Changes in volatile flavor compounds of peppers during hot air drying process based on headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS). J. Sci. Food Agric. 2020, 100, 3087–3098. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Xiao, J.; Liu, J.; Tang, N.; Zhou, A. Variations of volatile flavour compounds in finger citron (Citrus medica L. var. sarcodactylis) pickling process revealed by E-nose, HS-SPME-GC-MS and HS-GC-IMS. Food Res. Int. 2020, 138, 109717. [Google Scholar] [CrossRef]
- Coleman, W.M. Tobacco Volatiles: Gas Chromatography. In Encyclopedia of Separation Science; Academic Press: Salt Lake City, UT, USA, 2000. [Google Scholar]
- Rizzi, G.P. The biogenesis of food-related Pyrazines. Food Rev. Int. 1988, 4, 375–400. [Google Scholar] [CrossRef]
- Vespermann, K.A.; Paulino, B.N.; Barcelos, M.C.; Pessoa, M.G.; Pastore, G.M.; Molina, G. Biotransformation of alpha- and beta-pinene into flavor compounds. Appl. Microbiol. Biot. 2017, 101, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
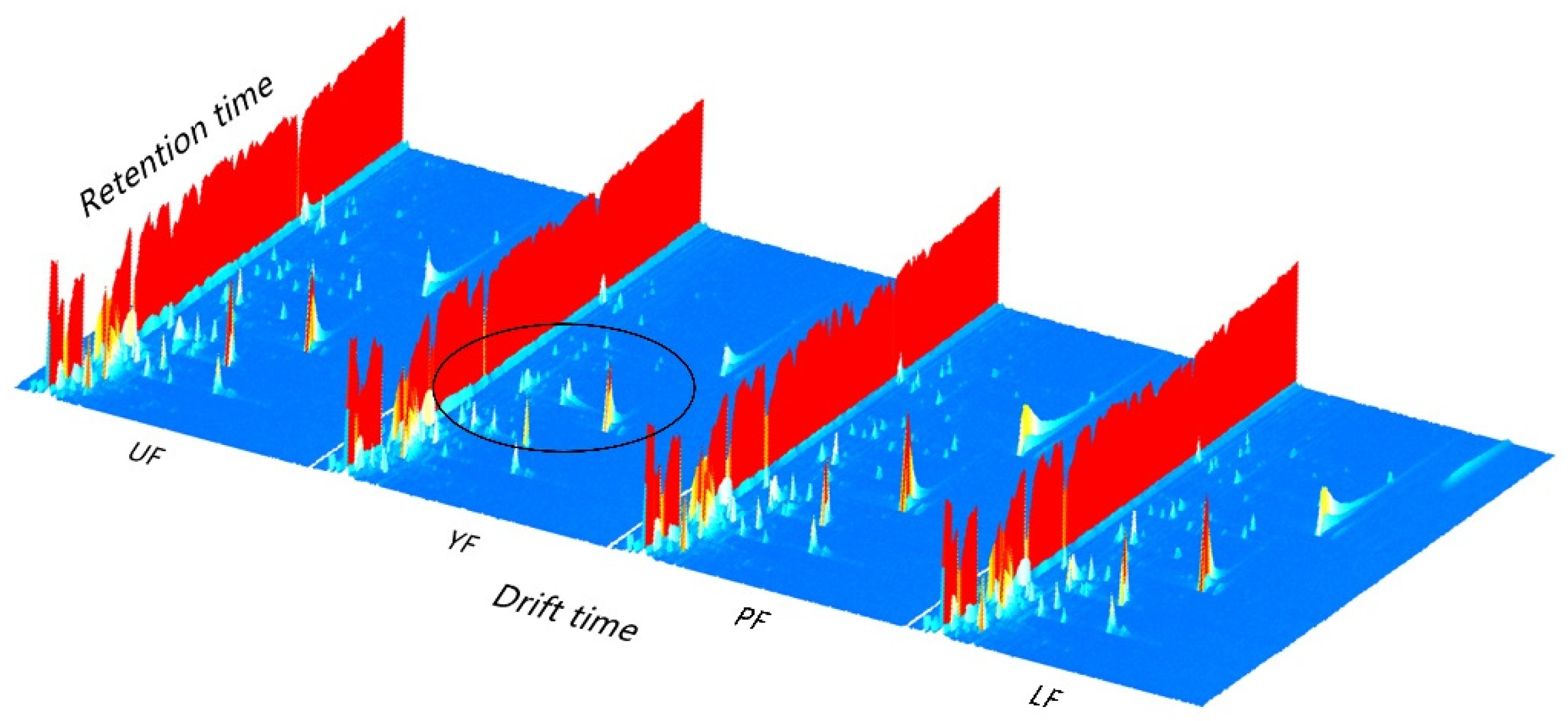
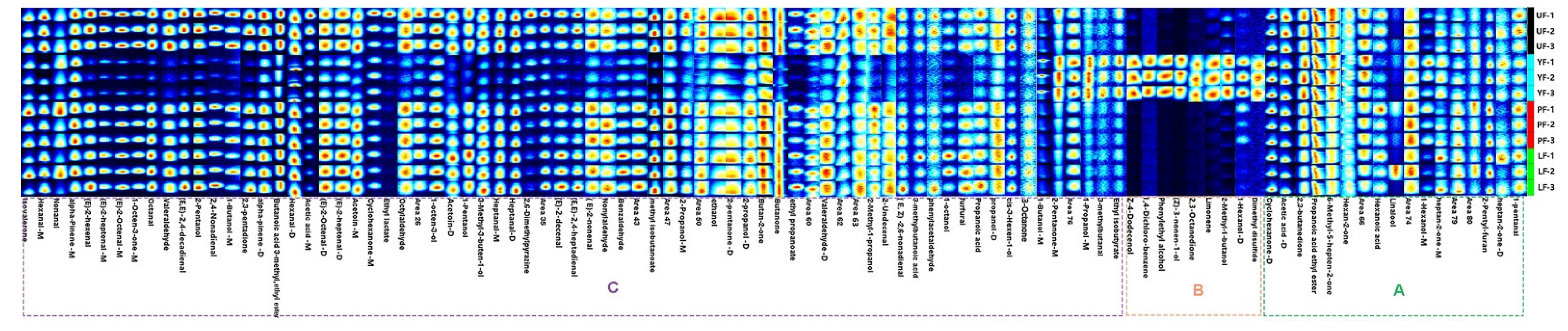

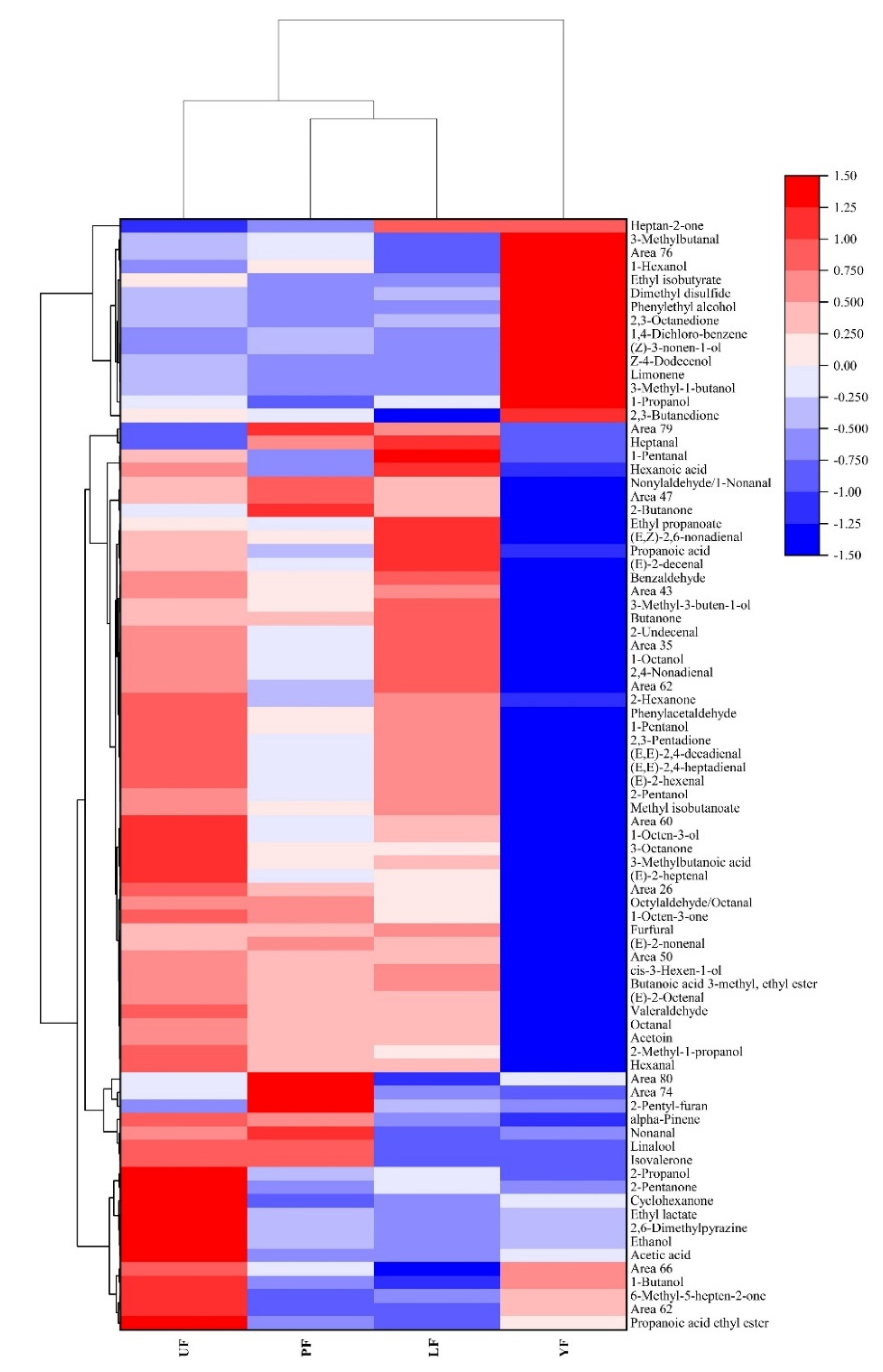
| No. | Compound | Rt a (min) | RI b | Concentration (μg/kg) | ||||
|---|---|---|---|---|---|---|---|---|
| Cal c | Ref d | UF | YF | PF | LF | |||
| Aldehydes | ||||||||
| 1 | Hexanal | 4.46 | 1087 | 1089 | 35.56 | 5.01 | 65.87 | 65.34 |
| 2 | (E)-2-pentenal | 5.46 | 1133 | 1130 | 2.19 | ND e | ND | 4.38 |
| 3 | Heptanal | 6.36 | 1182 | 1182 | 8.11 | ND | 8.32 | 12.68 |
| 4 | (E)-2-hexenal | 7.45 | 1212 | 1214 | ND | ND | 7.44 | 7.74 |
| 5 | (E)-4-heptenal | 7.63 | 1239 | 1243 | 1.87 | ND | 2.52 | ND |
| 6 | Octanal | 8.66 | 1283 | 1284 | 7.88 | 2.19 | 12.23 | 8.43 |
| 7 | (E)-2-heptenal | 9.54 | 1319 | 1321 | 20.21 | ND | ND | ND |
| 8 | Nonanal | 11.19 | 1385 | 1385 | 27.39 | 8.29 | 49.47 | 28.0 |
| 9 | (E)-2-octenal | 12.40 | 1417 | 1425 | 96.32 | 11.65 | 58.26 | 41.14 |
| 10 | (E,E)-2,4-heptadienal | 13.87 | 1478 | 1476 | 6.19 | ND | ND | ND |
| 11 | Benzaldehyde | 14.85 | 1512 | 1513 | 12.39 | ND | 7.66 | ND |
| 12 | (E)-2-nonenal | 15.12 | 1574 | 1573 | 126.76 | 10.38 | 55.79 | 48.60 |
| 13 | (E,Z)-2,6-nonadienal | 16.51 | 1574 | 1576 | 40.25 | 2.18 | 9.17 | 4.15 |
| 14 | (Z,Z)-3,6-nonadienal | 16.91 | 1589 | 1591 | 2.15 | ND | ND | ND |
| 15 | (E)-2-decenal | 17.86 | 1625 | 1625 | 15.16 | ND | ND | ND |
| 16 | 2,4-Nonadienal | 19.51 | 1687 | 1686 | 14.27 | ND | 0.198 | ND |
| 17 | 2-Undecenal | 20.97 | 1753 | 1755 | 10.86 | ND | ND | ND |
| 18 | (E,E)-2,4-decadienal | 21.37 | 1758 | 1758 | 94.75 | 5.07 | 23.04 | 14.41 |
| 19 | cis-4,5-Epoxy-(E)-2-decenal | 27.10 | 1997 | 2000 | 7.96 | ND | ND | ND |
| Alcohols | ||||||||
| 1 | 3-Methyl-1-butanol | 7.22 | 1201 | 1201 | ND | 90.11 | ND | ND |
| 2 | 1-Hexanol | 10.22 | 1346 | 1345 | 37.55 | 57.19 | ND | ND |
| 3 | (Z)-3-hexen-1-ol | 11.02 | 1370 | 1372 | ND | 0.69 | ND | ND |
| 4 | 1-Octen-3-ol | 13.18 | 1457 | 1455 | 67.87 | 34.80 | 10.28 | 11.43 |
| 5 | 1-Heptanol | 13.66 | 1451 | 1453 | ND | 23.42 | ND | ND |
| 6 | 2-Ethyl-1-hexanol | 14.02 | 1485 | 1486 | 78.63 | ND | ND | ND |
| 7 | 1-Phenyl-1-decanol | 15.44 | 1518 | - | ND | ND | ND | 3.52 |
| 8 | 1-Octanol | 15.69 | 1543 | 1540 | 29.54 | 35.80 | ND | ND |
| 9 | 1-Nonen-3-ol | 16.01 | 1554 | 1555 | ND | 0.34 | ND | ND |
| 10 | (Z)-2-octen-1-ol | 17.32 | 1604 | 1605 | 53.07 | 13.76 | 4.85 | ND |
| 11 | 1-Nonanol | 18.41 | 1646 | 1653 | 50.39 | 40.06 | ND | ND |
| 12 | Z-4-Dodecenol | 18.52 | 1630 | - | 1.14 | 10.69 | ND | ND |
| 13 | Z-2-Dodecenol | 19.15 | 1652 | - | 0.74 | ND | 6.80 | 4.71 |
| 14 | (Z)-3-nonen-1-ol | 19.60 | 1687 | 1688 | ND | 25.22 | ND | ND |
| 15 | (E)-2-nonen-1-ol | 19.96 | 1704 | 1703 | 84.99 | ND | ND | ND |
| 16 | (E)-6-nonen-1-ol | 19.97 | 1710 | 1714 | ND | 9.30 | ND | ND |
| 17 | 1-Decanol | 21.01 | 1760 | 1763 | ND | 3.04 | ND | ND |
| 18 | (Z)-5-decen-1-ol | 24.31 | 1886 | - | ND | 10.69 | ND | ND |
| 19 | Phenylethyl alcohol | 26.25 | 1928 | 1932 | ND | 20.25 | ND | ND |
| Ketones | ||||||||
| 1 | 3-Octanone | 8.05 | 1248 | 1248 | ND | 14.46 | ND | ND |
| 2 | 1-Octen-3-one | 8.98 | 1296 | 1295 | 39.57 | ND | 32.43 | 29.48 |
| 3 | 1-Hepten-3-one | 9.15 | 1303 | 1303 | 2.41 | ND | ND | 3.16 |
| 4 | 2,3-Octanedione | 9.68 | 1326 | 1325 | ND | 9.83 | ND | ND |
| 5 | 6-Methyl-5-hepten-2-one | 9.79 | 1329 | 1330 | 2.94 | 2.52 | 2.10 | ND |
| 6 | 4-Octen-3-one | 10.57 | 1360 | - | 1.19 | ND | ND | ND |
| 7 | (E,E)-3,5-octadien-2-one | 16.18 | 1562 | 1562 | 108.44 | ND | ND | ND |
| 8 | (E)-6,10-dimethyl-5,9-undecadien-2-one | 23.39 | 1848 | 1849 | 19.81 | ND | ND | ND |
| 9 | trans-á-Ionone | 25.5 | 1926 | 1926 | 204.63 | 35.06 | 28.38 | 17.37 |
| Halogens | ||||||||
| 1 | 1-Iodo-propane | 2.89 | 956 | 965 | 4.27 | ND | ND | ND |
| 2 | 1-Iodo-pentane | 5.42 | 1137 | 1164 | 14.64 | 13.26 | 7.03 | 9.43 |
| 3 | 3-Bromo-pentane | 9.50 | 1317 | - | 13.65 | ND | 39.0 | 30.88 |
| 4 | 1-Iodo-heptane | 10.33 | 1350 | 1384 | 4.11 | ND | 6.76 | 3.55 |
| 5 | 1,4-Dichloro-benzene | 13.1 | 1452 | 1450 | ND | 17.47 | ND | ND |
| Alkanes | ||||||||
| 1 | Tetradecane | 14.01 | 1408 | - | 108 | ND | ND | ND |
| 2 | Pentadecane | 14.12 | 1494 | 1500 | 334.44 | 27.42 | 30.10 | 20.16 |
| Furan | ||||||||
| 1 | 2-Pentyl-furan | 7.20 | 1220 | 1222 | 24.31 | 12.60 | 17.58 | 13.52 |
| Alkenes | ||||||||
| 1 | 1,4-Octadiene | 22.88 | 1799 | - | 7.81 | ND | ND | ND |
| 2 | 1-Tridecyne | 23.87 | 1873 | - | ND | 8.33 | ND | ND |
| 3 | 5-Ethyl-1-nonene | 24.58 | 1899 | - | ND | 15.47 | ND | ND |
| Esters | ||||||||
| 1 | 1-Octen-3-ol-acetate | 10.75 | 1367 | - | 3.10 | ND | ND | ND |
| 2 | Nonanoic acid, methyl ester | 14.92 | 1492 | 1491 | 13.71 | ND | ND | ND |
| 3 | Decanoic acid, methyl ester | 17.01 | 1597 | 1599 | ND | 9.68 | ND | ND |
| Acid | ||||||||
| 1 | Oxalic acid | 2.66 | 937 | - | ND | 31.31 | ND | ND |
| Benzene derivative | ||||||||
| 1 | p-Cymene | 7.98 | 1271 | 1268 | ND | ND | 6.81 | 5.84 |
| No. a | Compound | Odor Description b | Odor Threshold c (μg/kg) | OAV | |||
|---|---|---|---|---|---|---|---|
| UF | YF | PF | LF | ||||
| 1 | 1-Octen-3-one | metallic, mushroom, dirt | 0.01 | 3957.2 | 0 | 3242.8 | 2948 |
| 2 | (E,Z)-2,6-nonadienal | cucumber, cucumber peel, green, green leaves | 0.02 | 2012.7 | 109.1 | 458.6 | 207.7 |
| 3 | (E,E)-2,4-decadienal | fatty, green, wax, aldehyde, deep fried, fried fat, oily | 0.07 | 1353.6 | 72.5 | 329.1 | 205.8 |
| 4 | trans-á-Ionone | cedar, floral, artificial raspberry, cooked carrots, violet | 0.6 | 341.1 | 58.4 | 47.3 | 28.9 |
| 5 | (E)-2-nonenal | bast, cucumber, fatty, green, oxidized, stale, tallow | 0.4 | 316.9 | 26 | 139.5 | 121.5 |
| 6 | 2,4-Nonadienal | deep fried fat, fatty, fried potato, oily, soapy | 0.05 | 285.4 | 0 | 39.6 | 0 |
| 7 | cis-4,5-Epoxy-(E)-2-decenal | green, metallic | 0.13 | 61.2 | 0 | 0 | 0 |
| 8 | 1-Hepten-3-one | fatty, fruity, grass | 0.04 | 60.2 | 0 | 0 | 78.9 |
| 9 | (E)-2-decenal | fatty, green | 0.3 | 50.5 | 0 | 0 | 0 |
| 10 | 1-Octen-3-ol | fatty, fruity, grass, mushroom, raw mushrooms, sweet | 1.5 | 45.2 | 23.2 | 6.9 | 7.6 |
| 11 | (E)-2-octenal | almond, fatty, fruity, green, nutty, burdock, tallow | 3 | 32.1 | 3.9 | 19.4 | 13.7 |
| 12 | Nonanal | citrus, fatty, floral, green grass, pungent, soapy, tallow | 1.1 | 24.9 | 7.5 | 45 | 25.5 |
| 13 | 2-Undecenal | aldehyde, metallic, green | 0.78 | 13.9 | 0 | 0 | 0 |
| 14 | Octanal | aldehyde, fatty, fruity, orange peel, pungent, soapy | 0.9 | 8.8 | 2.4 | 13.6 | 9.4 |
| 15 | Hexanal | aldehyde, grass, green, leaves, vinous | 5 | 7.1 | 1 | 13.2 | 13.1 |
| 16 | 2-Pentyl-furan | fruity, green grass | 6 | 4.1 | 2 | 2.9 | 2.3 |
| 17 | Heptanal | fatty, green, heavy, oily, putty | 3 | 2.7 | 0 | 2.8 | 4.2 |
| 18 | (E)-2-heptenal | fatty, fruity, green, melting plastic, soapy, tallow | 13 | 1.6 | 0 | 0 | 0 |
| 19 | 1-Nonanol | green, sweet, oily | 45.5 | 1.1 | 0.9 | 0 | 0 |
| 20 | (Z)-2-octen-1-ol | fatty, rancid | 75 | 0.7 | 0.2 | 0.1 | 0 |
| 21 | (E)-2-nonen-1-ol | green, waxy melon | 130 | 0.7 | 0 | 0 | 0 |
| 22 | (E,E)-3,5-octadien-2-one | sweet, balsamic, vanilla, dill hay, oxidized | 150 | 0.7 | 0 | 0 | 0 |
| 23 | (E,E)-2,4-heptadienal | fatty, nutty | 15.4 | 0.4 | 0 | 0 | 0 |
| 24 | (E)-6,10-dimethyl-5,9-undecadien-2-one | floral, fruity, fatty, green, pear, apple, banana nuances | 60 | 0.3 | 0 | 0 | 0 |
| 25 | (E)-4-heptenal | dairy, biscuit, cream, fatty, fishy, sweet | 10 | 0.2 | 0 | 0.3 | 0 |
| 26 | 1-Octanol | green herbaceous | 130 | 0.2 | 0.3 | 0 | 0 |
| 27 | 1-Hexanol | fatty, floral, green | 250 | 0.2 | 0.2 | 0 | 0 |
| 28 | 2-Ethyl-1-hexanol | mild oily, sweet, slightly floral | 1280 | 0.1 | 0 | 0 | 0 |
| 29 | 6-Methyl-5-hepten-2-one | citrus, mushroom, pepper, rubber, strawberry | 50 | 0.1 | 0.1 | 0 | 0 |
| 30 | (E)-2-pentenal | almond, apple, green | 55 | 0 | 0 | 0 | 0.1 |
| 31 | Benzaldehyde | almond, bitter almond | 350 | 0 | 0 | 0 | 0 |
| 32 | (E)-2-hexenal | almond, bitter, green, heavy, stinkbug | 17 | 0 | 0 | 0.4 | 0.5 |
| 33 | Z-2-dodecenol | - | 41 | 0 | 0 | 0.2 | 0.1 |
| 34 | 3-Methyl-1-butanol | fruity, banana, sweet, fragrant, powerful | 250 | 0 | 0.4 | 0 | 0 |
| 35 | (Z)-3-halchexen-1-ol | green, grass | 200 | 0 | 0 | 0 | 0 |
| 36 | 1-Heptanol | herb | 330 | 0 | 0.1 | 0 | 0 |
| 37 | (E)-6-nonen-1-ol | powerful, melon, green | 1 | 0 | 9.3 | 0 | 0 |
| 38 | 1-Decanol | floral odor, orange flowers | 47 | 0 | 0.1 | 0 | 0 |
| 39 | Phenylethyl alcohol | rose-like, bitter, sweet, peach | 60 | 0 | 0.3 | 0 | 0 |
| 40 | 3-Octanone | earthy, ethereal, ketone, mushroom, resinous | 23 | 0 | 0.6 | 0 | 0 |
| 41 | 1-Octen-3-ol-acetate | lavender, metallic, mushroom-like | 90 | 0 | 0 | 0 | 0 |
| 42 | Decanoic acid, methyl ester | fruity odor, grape | 12 | 0 | 0.8 | 0 | 0 |
| 43 | p-Cymene | citrusy aroma, lemon | 0.01 | 0 | 0 | 681.5 | 584.2 |
| No. | Compound | DT a (ms) | RT b (s) | RI c | Comment | Signal Intensity | |||
|---|---|---|---|---|---|---|---|---|---|
| UF | YF | PF | LF | ||||||
| 1 | Propanoic acid ethyl ester | 1.1391 | 386.4 | 968.7 | 17,479.39 ± 1024 | 16,576.75 ± 167.44 | 16,524.81 ± 280.28 | 16,501.87 ± 518.37 | |
| 2 | Isovalerone | 1.8044 | 704.8 | 1169.4 | 9009.6 ± 227.5a | 5606.09 ± 32.26a | 8790.98 ± 368.81b | 5251.53 ± 141.78b | |
| 3 | Hexanal | 1.5578 | 563.6 | 1096.8 | monomer | 8524.21 ± 230.13a | 3730.53 ± 97.72c | 7197.59 ± 194.32b | 7510.7 ± 301.53b |
| Hexanal | 1.2572 | 573.4 | 1010.1 | dimer | |||||
| 4 | Nonanal | 1.9686 | 1042 | 1387.1 | 5197.54 ± 285.31b | 2884 ± 49.46c | 5830.12 ± 259.32a | 2462.1 ± 63.24d | |
| 5 | alpha-Pinene | 1.2863 | 434.2 | 1000 | monomer | 3617.51 ± 180.87a | 2491.84 ± 111.99b | 3376.24 ± 175.6a | 2741.81 ± 99.86b |
| alpha-Pinene | 1.6701 | 443.8 | 989.7 | dimer | |||||
| 6 | Acetic acid | 1.1521 | 1193.8 | 1447.7 | monomer | 3520.98 ± 141.86a | 2050.12 ± 61.5b | 1603.4 ± 24.57c | 1477.26 ± 44.32c |
| Acetic acid | 1.0503 | 1195 | 1443.2 | dimer | |||||
| 7 | Methyl isobutanoate | 1.1438 | 306.2 | 933.4 | 3057.95 ± 107.75a | 875.24 ± 25.49c | 2440.84 ± 87.13b | 3115.91 ± 48.24a | |
| 8 | Linalool | 2.2208 | 1363.2 | 1530.4 | 2279.88 ± 103.93a | 1526.01 ± 31.04c | 2135.22 ± 32.51b | 1395.21 ± 16.33d | |
| 9 | 1-Butanol | 1.3595 | 561.6 | 1106.9 | monomer | 1812.33 ± 61.59a | 1686.54 ± 50.19b | 1383.21 ± 13.83c | 1263.44 ± 70.66d |
| 1-Butanol | 1.9607 | 575 | 1107.2 | dimer | |||||
| 10 | 2,3-Pentadione | 1.3063 | 481.8 | 1062.3 | 1283.54 ± 26.19a | 301.81 ± 4.67d | 892.47 ± 13.59c | 1082.67 ± 45.74b | |
| 11 | 2-Pentanone | 1.1246 | 318.6 | 932.6 | monomer | 1259.46 ± 32.67a | 920.75 ± 5.33b | 940.09 ± 43.7b | 984.95 ± 46.74b |
| 2-Pentanone | 1.1279 | 459 | 924.3 | dimer | |||||
| 12 | Acetoin | 1.2489 | 824.6 | 1230.7 | monomer | 1042.84 ± 40.15a | 497.18 ± 7.47c | 964.55 ± 5.55b | 980.27 ± 40.56b |
| Acetoin | 1.2692 | 954 | dimer | ||||||
| 13 | 2-Butanone | 1.0591 | 306.8 | 911.6 | 1012.42 ± 60.74ab | 942.8 ± 33.22b | 1070.13 ± 39.01a | 1051.2 ± 22.03a | |
| 14 | 2-Pentyl-furan | 1.8771 | 845 | 1234.1 | 1017.81 ± 31.3b | 1014.2 ± 20.7b | 1321.08 ± 53.75a | 1073.31 ± 18.41b | |
| 15 | Ethanol | 1.1128 | 318.6 | 943.6 | 1023.83 ± 25.68a | 696.59 ± 17.59b | 679.46 ± 6.73b | 619.39 ± 10.84c | |
| 16 | 2,3-Butanedione | 1.1772 | 472.8 | 991 | 967.24 ± 14.53b | 1067.87 ± 26.78a | 899.04 ± 21.04c | 744.24 ± 22.23d | |
| 17 | Area 80 | 2.043 | 1040.2 | 918.01 ± 23.18b | 920.59 ± 9.11b | 992.1 ± 43.33a | 817.96 ± 24.82c | ||
| 18 | Valeraldehyde | 1.1838 | 438.4 | 997.5 | monomer | 872.69 ± 8.73a | 320.44 ± 4.91c | 759.89 ± 13.03b | 764.33 ± 27.02b |
| Valeraldehyde | 1.4196 | 435.2 | 999.8 | dimer | |||||
| 19 | (E)-2-Octenal | 1.3299 | 1156.6 | 1443.8 | monomer | 881.88 ± 25.04a | 402.72 ± 16.11c | 771.04 ± 15.42b | 785.5 ± 32.5b |
| (E)-2-Octenal | 1.8139 | 1153 | 1445.7 | dimer | |||||
| 20 | Heptanal | 1.3259 | 741.2 | 1197.3 | monomer | 757.72 ± 19.59c | 794.05 ± 15.57c | 1532.95 ± 40.16b | 1689.16 ± 61.52a |
| Heptanal | 1.6328 | 735.2 | 1198.6 | dimer | |||||
| 21 | Ethyl isovalerate | 1.2592 | 562.8 | 1088.1 | 829.7 ± 20.81a | 539.7 ± 13.95b | 807.02 ± 22.91a | 821.44 ± 36.92a | |
| 22 | Area 79 | 1.3336 | 562 | 822.54 ± 24.96c | 820.22 ± 21.21c | 1063.23 ± 28.41a | 1003.36 ± 38.63b | ||
| 23 | 2-Pentanol | 1.2869 | 629.8 | 1129.1 | 805.2 ± 24.16a | 282.82 ± 5.99c | 620.66 ± 12.41b | 832.07 ± 16.32a | |
| 24 | Butanone | 1.0596 | 325.8 | 957 | 787.6 ± 30.51a | 395.99 ± 15.84c | 733.39 ± 35.25b | 835.75 ± 17.51a | |
| 25 | (E)-2-hexenal | 1.5131 | 808.4 | 1233.8 | 781.79 ± 35.64a | 218.19 ± 9.81d | 529.04 ± 23.53c | 710.73 ± 7.18b | |
| 26 | (E)-2-heptenal | 1.6662 | 993 | 1338.8 | monomer | 677.47 ± 23.95a | 318 ± 11.84d | 490.28 ± 21.41c | 553.1 ± 11.25b |
| (E)-2-heptenal | 1.2518 | 996.2 | 1337.2 | dimer | |||||
| 27 | (E,E)-2,4-decadienal | 1.3576 | 1154.4 | 1815.7 | 678.43 ± 48.78 | 213.86 ± 3.78 | 486.83 ± 19.23 | 652.38 ± 32.62 | |
| 28 | 3-Methyl-3-buten-1-ol | 1.1785 | 812 | 1260.2 | 693.59 ± 26.87b | 322.38 ± 3.22d | 641.06 ± 10.99c | 721.52 ± 18.66a | |
| 29 | Area 62 | 1.0758 | 489.2 | 610.11 ± 24.82a | 545.76 ± 9.36b | 410.72 ± 8.69c | 408.34 ± 22.41c | ||
| 30 | Octanal | 1.4094 | 985.8 | 1294.1 | 562.22 ± 8.56a | 207.95 ± 6.24c | 501.38 ± 8.6b | 519.83 ± 23.52b | |
| 31 | Cyclohexanone | 1.4496 | 946.4 | 1312.5 | monomer | 516.94 ± 28.5a | 457.05 ± 14.06b | 439.72 ± 11.41b | 436.1 ± 11.65b |
| Cyclohexanone | 1.1517 | 947.2 | 1313.2 | dimer | |||||
| 32 | Area 47 | 1.139 | 484.6 | 500.01 ± 30.15a | 345.08 ± 3.45b | 524.37 ± 20.19a | 501.73 ± 25.09a | ||
| 33 | 6-Methyl-5-hepten-2-one | 1.0887 | 1002.2 | 1340.2 | 500.86 ± 9.82a | 454.41 ± 22.72b | 396.64 ± 11.26c | 414.56 ± 16.7c | |
| 34 | 2-Propanol | 1.0859 | 293.4 | 930.8 | monomer | 474.44 ± 23.96a | 328.65 ± 7.39c | 360.02 ± 23.35bc | 374.03 ± 12.31b |
| 2-Propanol | 1.084 | 295.2 | 933.8 | dimer | |||||
| 35 | 2-Hexanone | 1.1888 | 490.4 | 1054.2 | 485.91 ± 18.94a | 427.86 ± 11.06b | 439.5 ± 17.1b | 480.86 ± 16.83a | |
| 36 | 1-Octen-3-one | 1.6756 | 948.2 | 1315.7 | 447.93 ± 17.98a | 147.85 ± 2.54d | 417.78 ± 11.86b | 366.69 ± 12.83c | |
| 37 | Area 74 | 1.7416 | 448.4 | 369.09 ± 8.76b | 344.49 ± 5.28c | 446.71 ± 12.06a | 347.29 ± 14.37c | ||
| 38 | 1-Octen-3-ol | 1.1552 | 1171 | 1454.4 | 368.35 ± 16.55a | 303.28 ± 4.57c | 327.73 ± 8.28b | 359.54 ± 9.24a | |
| 39 | 1-Pentanol | 1.2483 | 819.6 | 1252.4 | 360.63 ± 22.33a | 132.63 ± 2.74d | 290.18 ± 5.69c | 323.29 ± 14.38b | |
| 40 | 1-Propanol | 1.2611 | 437.8 | 1011.7 | monomer | 457.04 ± 10.7b | 557.02 ± 20.74a | 423.87 ± 4.86c | 466.59 ± 14.16b |
| 1-Propanol | 1.1098 | 433 | 1031.7 | dimer | |||||
| 41 | 2,6-Dimethylpyrazine | 1.1352 | 1020.6 | 1351.7 | 293.16 ± 10.26a | 132.41 ± 3.61b | 121.36 ± 2.73c | 91.12 ± 2.68d | |
| 42 | 3-Methyl-1-butanol | 1.243 | 773 | 1204.1 | 265.2 ± 6.7b | 1022.17 ± 26.26a | 122.45 ± 1.88c | 108.4 ± 3.08d | |
| 43 | Area 62 | 1.1294 | 516.4 | 264.03 ± 4.05b | 184.45 ± 2.86d | 227.66 ± 3.87c | 274.86 ± 3.13a | ||
| 44 | (E,E)-2,4-heptadienal | 1.1434 | 1193.7 | 1482.4 | 259.04 ± 11.52a | 101.31 ± 2.37c | 198.92 ± 4.21b | 247.99 ± 11.14a | |
| 45 | Hexanoic acid | 1.3107 | 1768.2 | 1863.4 | 263.23 ± 5.26b | 221.44 ± 2.57c | 228.61 ± 9.65c | 279.05 ± 4.79a | |
| 46 | Area 50 | 1.117 | 351.6 | 237.68 ± 4.75a | 143.47 ± 5.84b | 231.95 ± 2.3a | 230.7 ± 5.83a | ||
| 47 | Area 43 | 1.1886 | 1261 | 209.37 ± 8.78b | 119.39 ± 1.18d | 195.49 ± 3.04c | 224.38 ± 8.17a | ||
| 48 | Z-4-Dodecenol | 1.4889 | 1258.2 | 1996.2 | 221.7 ± 10.69a | 1523.34 ± 39.9a | 126.52 ± 4.01c | 113.03 ± 4.77c | |
| 49 | 2,4-Nonadienal | 1.6144 | 1197.4 | 1668.5 | 219.26 ± 8.66a | 91.12 ± 2.29c | 161.72 ± 2.48b | 220.32 ± 5.95a | |
| 50 | Octylaldehyde | 1.3974 | 932 | 1299.3 | 188.96 ± 4.87a | 79.05 ± 0.92c | 186.55 ± 11.19a | 161.65 ± 7.31b | |
| 51 | Heptan-2-one | 1.6268 | 733.8 | 1189.6 | monomer | 190.74 ± 1.1d | 247.64 ± 2.5a | 203.41 ± 4.15c | 253.52 ± 3.82a |
| Heptan-2-one | 1.2591 | 731.2 | 1191.4 | dimer | |||||
| 52 | 1-Hexanol | 1.6343 | 1030 | 1405.1 | monomer | 186 ± 8.94d | 479.07 ± 5.5a | 293.7 ± 14.88b | 167.54 ± 6.53c |
| 1-Hexanol | 1.3215 | 1028.8 | 1405.7 | dimer | |||||
| 53 | Area 26 | 1.5704 | 951.4 | 179.22 ± 5.38a | 94.59 ± 3.85d | 163.4 ± 7.41b | 148.26 ± 3.88c | ||
| 54 | Ethyl lactate | 1.5308 | 1022.4 | 1358.3 | 178.42 ± 7.33a | 46.25 ± 1.51b | 45.9 ± 0.69b | 40.43 ± 0.86b | |
| 55 | (E)-2-nonenal | 1.404 | 1321.4 | 1562.3 | 169.76 ± 4.58b | 102.36 ± 1.54c | 178.34 ± 2.72a | 176.83 ± 5.02a | |
| 56 | (E)-2-decenal | 1.2193 | 1401.6 | 1647.8 | 176.05 ± 2.99b | 91.82 ± 2.82c | 143.27 ± 3.72c | 198.84 ± 5.37a | |
| 57 | Area 35 | 1.1726 | 1011.8 | 167.62 ± 4.32b | 115.66 ± 1.8d | 151.63 ± 6.74c | 181.77 ± 9.45a | ||
| 58 | Benzaldehyde | 1.1473 | 1316.4 | 1547 | 166.39 ± 8.32a | 123.56 ± 3.37c | 151.77 ± 5.53b | 173.95 ± 8.38a | |
| 59 | 3-Methylbutanoic acid | 1.2172 | 1548.8 | 1688.5 | 157.99 ± 4.64a | 69.03 ± 3.45d | 125.03 ± 5.62c | 134.01 ± 3.3b | |
| 60 | Nonylaldehyde | 1.4688 | 1099.8 | 1386.7 | 161.1 ± 3.33b | 64.07 ± 1.5d | 193.09 ± 9.65a | 158.94 ± 4.77c | |
| 61 | 2-Undecenal | 1.1014 | 1451.6 | 1755.8 | 160.57 ± 6.34b | 78.66 ± 2.04d | 121.01 ± 4.41c | 173.91 ± 6.21a | |
| 62 | (E,Z)-2,6-nonadienal | 1.3679 | 1405 | 1590.3 | 151.41 ± 3.72b | 84.88 ± 2.41d | 140.03 ± 1.41c | 178.14 ± 8.91a | |
| 63 | cis-3-Hexen-1-ol | 1.2591 | 1143 | 1433.4 | 148.1 ± 5.95a | 99.57 ± 1.54b | 143.21 ± 3.51a | 150.29 ± 5.86a | |
| 64 | Area 66 | 1.0893 | 380.4 | 140.43 ± 9.2a | 137.76 ± 2.7a | 130.92 ± 2.71ab | 125.55 ± 2.55b | ||
| 65 | Area 76 | 1.2492 | 853.4 | 127.03 ± 1.49b | 172.74 ± 7.84a | 133.15 ± 5.55b | 117.58 ± 1.83c | ||
| 66 | Ethyl isobutyrate | 1.3225 | 379.8 | 983.5 | 121.49 ± 2.08b | 159.34 ± 2.79a | 100.94 ± 1.52c | 100.49 ± 6.52c | |
| 67 | Phenylacetaldehyde | 1.2622 | 1435.4 | 1648.5 | 104.34 ± 4.76a | 51.12 ± 0.79d | 82.9 ± 0.84c | 96.85 ± 2.49b | |
| 68 | 2-Methyl-1-propanol | 1.1375 | 502 | 1101 | 93.02 ± 4.56a | 51.65 ± 0.91c | 85.89 ± 4.71b | 82.31 ± 2.22b | |
| 69 | Area 60 | 1.1018 | 347.6 | 94.71 ± 6.81a | 45.78 ± 1.61d | 68.68 ± 1.07c | 81.83 ± 2.21b | ||
| 70 | Propanoic acid | 1.1028 | 1232 | 974.5 | 91.15 ± 3.32b | 56.64 ± 1.92d | 75.83 ± 0.86c | 110.37 ± 4.57a | |
| 71 | 3-Methylbutanal | 1.4013 | 379 | 920.8 | 87.88 ± 1.32b | 124.45 ± 3.83a | 89.1 ± 1.78b | 71.72 ± 2.56c | |
| 72 | Furfural | 1.3234 | 1245.6 | 1472.6 | 65.17 ± 3.26a | 18.91 ± 0.49b | 67.18 ± 1.96a | 67.19 ± 1.04a | |
| 73 | 1-Octanol | 1.4643 | 1319 | 1566.6 | 61.05 ± 0.93a | 38.02 ± 0.96c | 53.09 ± 0.31b | 64.42 ± 3.88a | |
| 74 | Limonene | 1.2283 | 770.8 | 1212.1 | 45.15 ± 0.7b | 159.16 ± 4.12a | 21.51 ± 0.67c | 22.17 ± 0.25c | |
| 75 | 3-Octanone | 1.3285 | 925.4 | 1272.8 | 43.05 ± 1.62a | 26.46 ± 0.68c | 38.35 ± 1.92b | 37.23 ± 1.67b | |
| 76 | (Z)-3-nonen-1-ol | 1.1696 | 1399.6 | 1685.7 | 42.92 ± 0.43bc | 506.88 ± 8.69a | 47.93 ± 2.09b | 37.75 ± 0.22d | |
| 77 | Ethyl propanoate | 1.1427 | 348 | 911.3 | 41.12 ± 0.98b | 14.73 ± 0.17c | 38.26 ± 0.78b | 63.35 ± 3.05a | |
| 78 | 1-Pentanal | 1.2098 | 318 | 925.5 | 36.2 ± 1.47b | 33.92 ± 0.68c | 34.44 ± 0.52bc | 38.91 ± 0.8a | |
| 79 | 1,4-Dichloro-benzene | 1.4674 | 1199.8 | 1450 | 31.81 ± 0.83c | 193.32 ± 1.95a | 34.18 ± 0.86b | 28.83 ± 0.57d | |
| 80 | 2,3-Octanedione | 1.1697 | 1202.7 | 1325.6 | 24.49 ± 0.87bc | 144.76 ± 4.34a | 21.5 ± 1.5c | 26.84 ± 0.41b | |
| 81 | Phenylethyl alcohol | 1.4656 | 1801.5 | 1915.2 | 20.54 ± 0.42b | 211.13 ± 3.18a | 16.22 ± 0.48c | 17.55 ± 0.43bc | |
| 82 | Dimethyl disulfide | 1.1977 | 584 | 1111.1 | 12.38 ± 0.3b | 40.59 ± 0.47a | 11.41 ± 0.42c | 11.83 ± 0.54bc | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Jiang, B.; Zhong, F.; Chen, J.; Zhang, T. Effect of Microbial Fermentation on the Fishy-Odor Compounds in Kelp (Laminaria japonica). Foods 2021, 10, 2532. https://doi.org/10.3390/foods10112532
Zhu W, Jiang B, Zhong F, Chen J, Zhang T. Effect of Microbial Fermentation on the Fishy-Odor Compounds in Kelp (Laminaria japonica). Foods. 2021; 10(11):2532. https://doi.org/10.3390/foods10112532
Chicago/Turabian StyleZhu, Wenyang, Bo Jiang, Fang Zhong, Jingjing Chen, and Tao Zhang. 2021. "Effect of Microbial Fermentation on the Fishy-Odor Compounds in Kelp (Laminaria japonica)" Foods 10, no. 11: 2532. https://doi.org/10.3390/foods10112532
APA StyleZhu, W., Jiang, B., Zhong, F., Chen, J., & Zhang, T. (2021). Effect of Microbial Fermentation on the Fishy-Odor Compounds in Kelp (Laminaria japonica). Foods, 10(11), 2532. https://doi.org/10.3390/foods10112532






