High-Pressure Processing on Whole and Peeled Potatoes: Influence on Polyphenol Oxidase, Antioxidants, and Glycaemic Indices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Chemicals
2.3. HPP Treatment
2.4. Potato PPO Extraction and Activity Assay
2.5. Extraction of Phytochemicals
2.6. Total Phenolic Content
2.7. Antioxidant Activity (AOA)
2.7.1. Ferric-Reducing Antioxidant Power (FRAP)
2.7.2. DPPH Radical Scavenging Capacity
2.8. Liquid Chromatography-Mass Spectrometry Analysis
2.9. In Vitro Determination of Glycaemic Index (GI)
2.9.1. In Vitro Digestion
2.9.2. Glucose Analysis
2.10. Statistical Analysis
3. Results
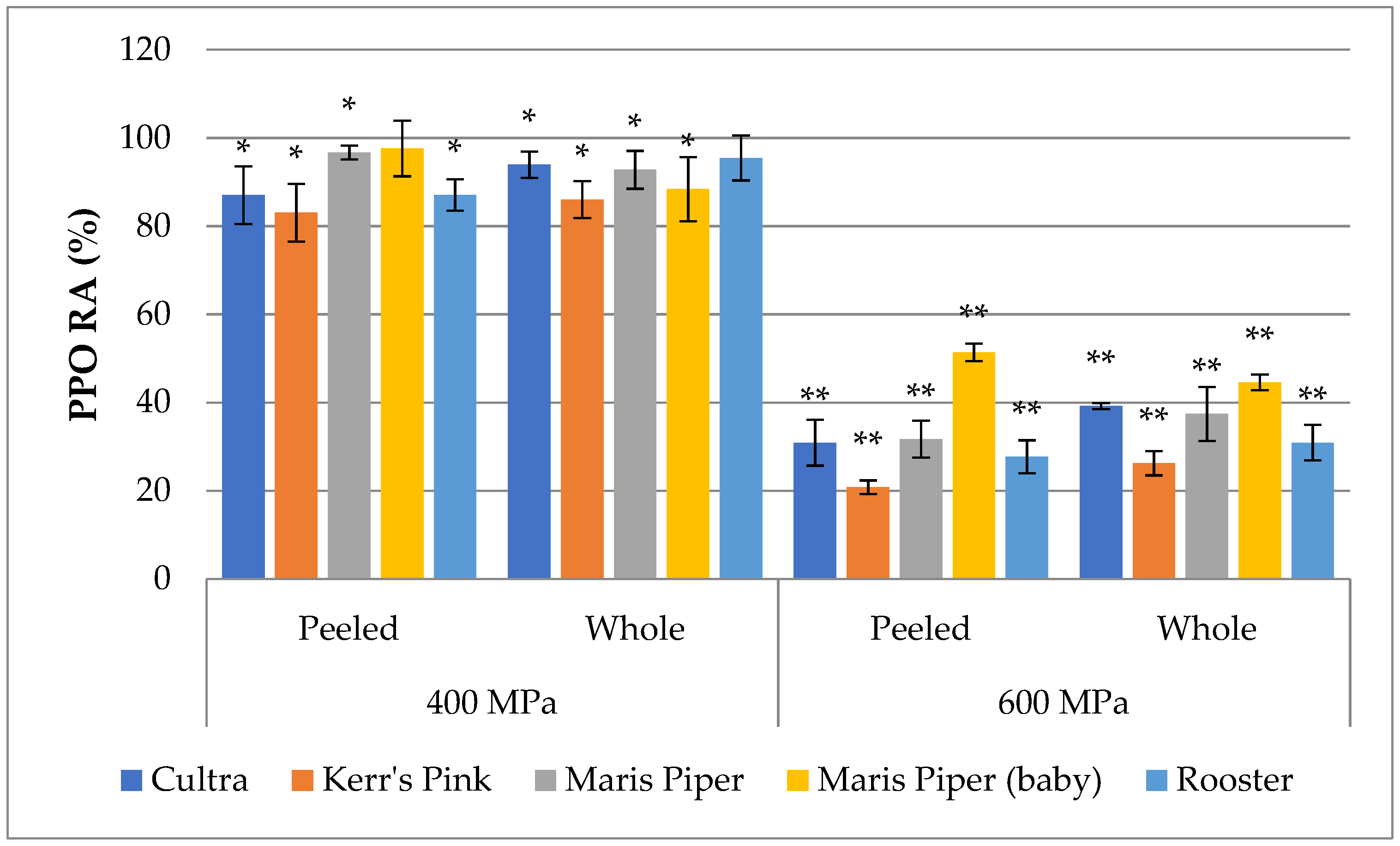
3.1. PPO Inactivation
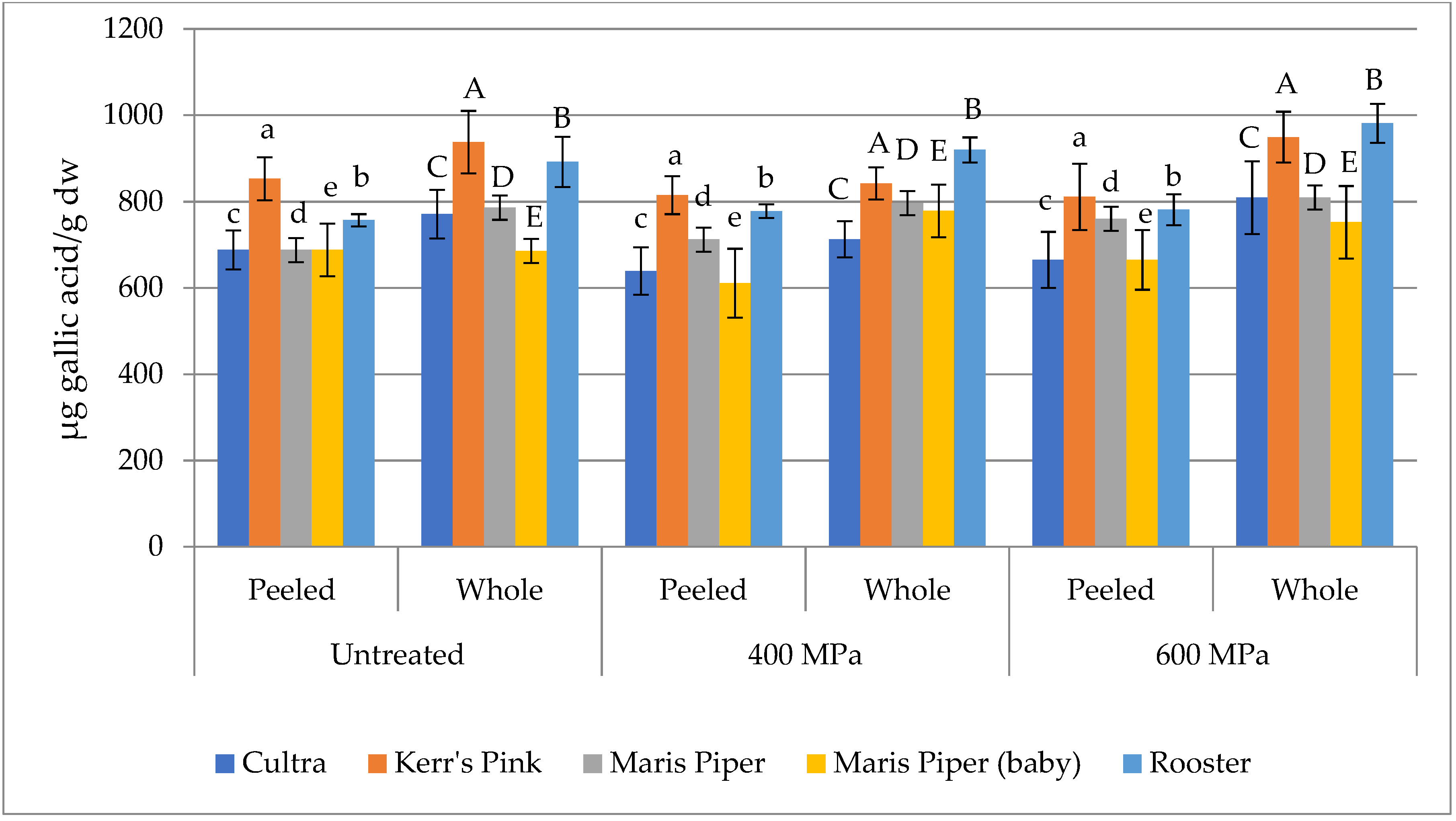
3.2. Total Phenolic Content
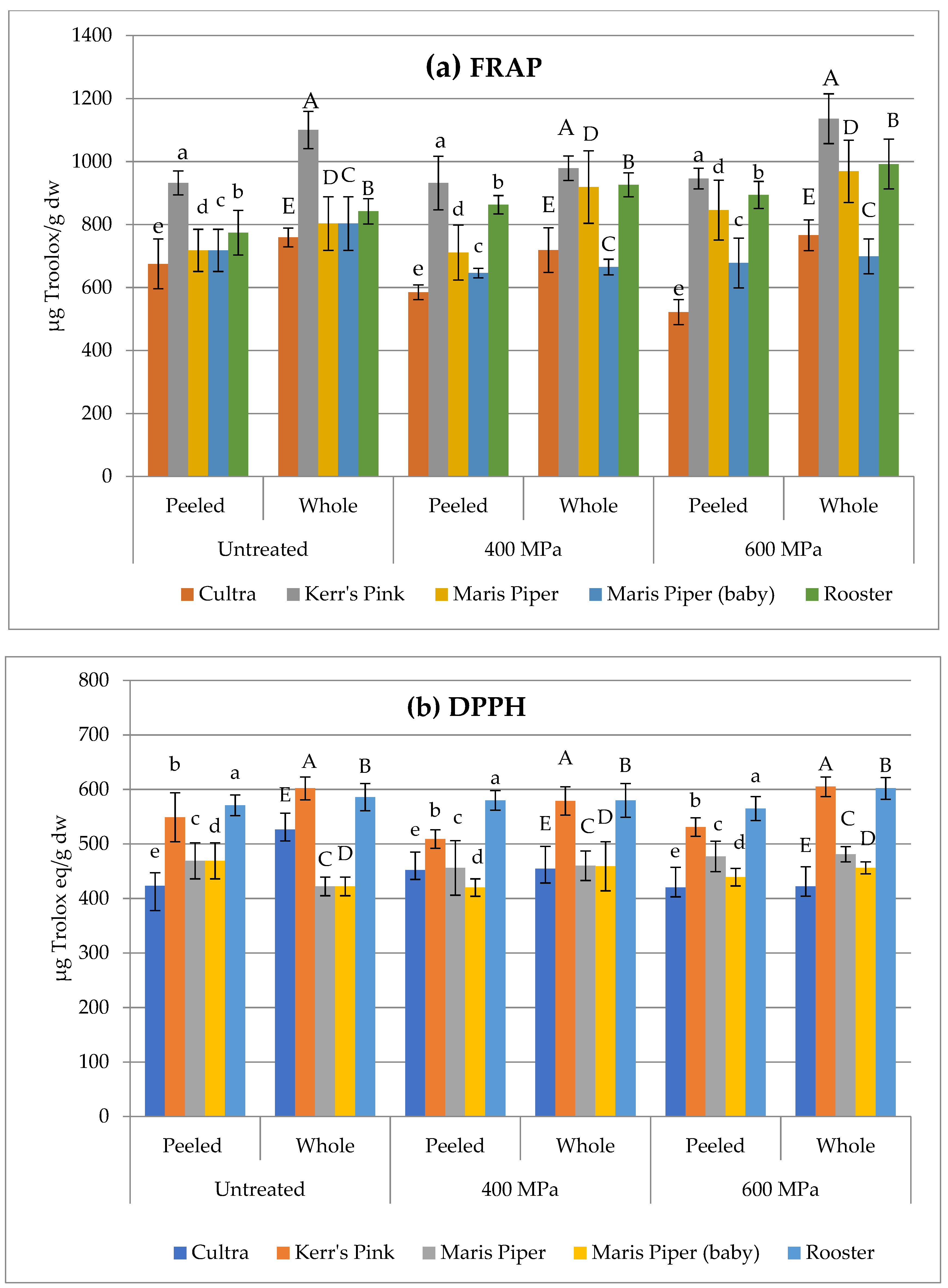
3.3. Antioxidant Activity
3.4. Effect of HPP on Polyphenols
3.5. Glycaemic Index (GI) of Potato Cultivars
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Burgos, G.; Zum Felde, T.; Andre, C.; Kubow, S. The Potato and Its Contribution to the Human Diet and Health. In The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind; Campos, H., Ortiz, O., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 37–74. ISBN 978-3-030-28683-5. [Google Scholar]
- McGill, C.R.; Kurilich, A.C.; Davignon, J. The role of potatoes and potato components in cardiometabolic health: A review. Ann. Med. 2013, 45, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Berrios, J.D.J.; Tang, J. Impact of food processing on the glycemic index (GI) of potato products. Food Res. Int. 2014, 56, 35–46. [Google Scholar] [CrossRef]
- Haase, N.U. The In Vitro Digestibility of Carbohydrates in Boiled and Processed Potatoes. Potato Res. 2015, 58, 91–102. [Google Scholar] [CrossRef]
- Henry, C.J.K.; Lightowler, H.J.; Strik, C.M.; Storey, M. Glycaemic index values for commercially available potatoes in Great Britain. Br. J. Nutr. 2005, 94, 917–921. [Google Scholar] [CrossRef]
- Lockyer, S.; Spiro, A.; Stanner, S. Dietary fibre and the prevention of chronic disease—Should health professionals be doing more to raise awareness? Nutr. Bull. 2016, 41, 214–231. [Google Scholar] [CrossRef]
- Dourado, C.; Pinto, C.; Barba, F.J.; Lorenzo, J.M.; Delgadillo, I.; Saraiva, J.A. Innovative non-thermal technologies affecting potato tuber and fried potato quality. Trends Food Sci. Technol. 2019, 88, 274–289. [Google Scholar] [CrossRef]
- Rocculi, P.; Romani, S.; Gómez Galindo, F.; Dalla Rosa, M. Effect of Minimal Processing on Physiology and Quality of Fresh-Cut Potatoes: A Review. Food 2009, 3, 18–30. [Google Scholar]
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.M.; Pavan, S.; Montemurro, C. Polyphenol oxidases in crops: Biochemical, physiological and genetic aspects. Int. J. Mol. Sci. 2017, 18, 377. [Google Scholar] [CrossRef]
- Yoruk, R.; Marshall, M.R. Physiochemical Properties and Function of Plant Polyphenol Oxidase. J. Food Biochem. 2003, 27, 361–422. [Google Scholar] [CrossRef]
- Marques Silva, F.V.; Sulaiman, A. Polyphenoloxidase in Fruit and Vegetables: Inactivation by Thermal and Non-Thermal Processes; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128140451. [Google Scholar]
- Alamar, M.C.; Tosetti, R.; Landahl, S.; Bermejo, A.; Terry, L.A. Assuring Potato Tuber Quality during Storage: A Future Perspective. Front. Plant Sci. 2017, 8, 2034. [Google Scholar] [CrossRef] [Green Version]
- San Martín, M.F.; Barbosa-Canovas, G.V.; Swanson, B.G. Food processing by high pressure. Crit. Rev. Food Sci. Nutr. 2002, 42, 627–645. [Google Scholar] [CrossRef]
- Andrés, V.; Villanueva, M.J.; Tenorio, M.D. The effect of high-pressure processing on colour, bioactive compounds, and antioxidant activity in smoothies during refrigerated storage. Food Chem. 2016, 192, 328–335. [Google Scholar] [CrossRef]
- Stratakos, A.C.; Inguglia, E.S.; Linton, M.; Tollerton, J.; Murphy, L.; Corcionivoschi, N.; Koidis, A.; Tiwari, B.K. Effect of high pressure processing on the safety, shelf life and quality of raw milk. Innov. Food Sci. Emerg. Technol. 2019, 52, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.-L.; Liu, W.; Zhao, J.; Yuan, C.; Song, Y.; Chen, D.; Ni, Y.-Y.; Li, Q.-H. The effect of high hydrostatic pressure on the microbiological quality and physical–chemical characteristics of Pumpkin (Cucurbita maxima Duch.) during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2014, 21, 24–34. [Google Scholar] [CrossRef]
- Tsikrika, K.; Walsh, D.; Joseph, A.; Burgess, C.M.; Rai, D.K. High-Pressure Processing and Ultrasonication of Minimally Processed Potatoes: Effect on the Colour, Microbial Counts, and Bioactive Compounds. Molecules 2021, 26, 2614. [Google Scholar] [CrossRef] [PubMed]
- Denoya, G.I.; Polenta, G.A.; Apóstolo, N.M.; Budde, C.O.; Sancho, A.M.; Vaudagna, S.R. Optimization of high hydrostatic pressure processing for the preservation of minimally processed peach pieces. Innov. Food Sci. Emerg. Technol. 2016, 33, 84–93. [Google Scholar] [CrossRef]
- Szczepańska, J.; Barba, F.J.; Skąpska, S.; Marszałek, K. High pressure processing of carrot juice: Effect of static and multi-pulsed pressure on the polyphenolic profile, oxidoreductases activity and colour. Food Chem. 2020, 307, 125549. [Google Scholar] [CrossRef]
- Denoya, G.I.; Nanni, M.S.; Apóstolo, N.M.; Vaudagna, S.R.; Polenta, G.A. Biochemical and microstructural assessment of minimally processed peaches subjected to high-pressure processing: Implications on the freshness condition. Innov. Food Sci. Emerg. Technol. 2016, 36, 212–220. [Google Scholar] [CrossRef]
- Woolf, A.B.; Wibisono, R.; Farr, J.; Hallett, I.; Richter, L.; Oey, I.; Wohlers, M.; Zhou, J.; Fletcher, G.C.; Requejo-Jackman, C. Effect of high pressure processing on avocado slices. Innov. Food Sci. Emerg. Technol. 2013, 18, 65–73. [Google Scholar] [CrossRef]
- Tsikrika, K.; Rai, D.K. The Effect of High Pressure Processing on Antioxidant Activity of Irish Potato Cultivars. Proceedings 2019, 11, 9. [Google Scholar] [CrossRef] [Green Version]
- Tsikrika, K.; O’Brien, N.; Rai, D.K. The Effect of High Pressure Processing on Polyphenol Oxidase Activity, Phytochemicals and Proximate Composition of Irish Potato Cultivars. Foods 2019, 8, 517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Benzie, I.; Strain, J. The ferric reducing ability of plasma as a measure of antioxodant. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables—Evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Goupy, P.; Hugues, M.; Boivin, P.; Amiot, M.J. Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts and of isolated phenolic compounds. J. Sci. Food Agric. 1999, 79, 1625–1634. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, A.C. Characterization of phenolic composition in lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, S.R.; Lee, Y.M.; Jang, H.H.; Kim, J.B. Analysis of Variation in Anthocyanin Composition in Korean Coloured Potato Cultivars by LC-DAD-ESI-MS and PLS-DA. Potato Res. 2018, 61, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.B.; Tiwari, B.K.; Gangopadhyay, N.; O’Donnell, C.P.; Brunton, N.P.; Rai, D.K. Ultrasonic extraction of steroidal alkaloids from potato peel waste. Ultrason. Sonochem. 2014, 21, 1470–1476. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P. Optimisation and validation of ultra-high performance liquid chromatographic-tandem mass spectrometry method for qualitative and quantitative analysis of potato steroidal alkaloids. J. Chromatogr. B 2015, 997, 110–115. [Google Scholar] [CrossRef]
- Englyst, O.N.; Hudson, G.J.; Englyst, H.N. Starch analysis in food. In Encyclopedia of Analytical Chemistry, 1st ed.; John Wiley & Sons: Chichester, UK, 2000; pp. 4246–4262. [Google Scholar]
- Thygesen, P.W.; Dry, I.B.; Robinson, S.P. Polyphenol oxidase in potato: A multigene family that exhibits differential expression patterns. Plant Physiol. 1995, 109, 525–531. [Google Scholar] [CrossRef] [Green Version]
- Aaby, K.; Grimsbo, I.H.; Hovda, M.B.; Rode, T.M. Effect of high pressure and thermal processing on shelf life and quality of strawberry purée and juice. Food Chem. 2018, 260, 115–123. [Google Scholar] [CrossRef]
- Marszałek, K.; Doesburg, P.; Starzonek, S.; Szczepańska, J.; Woźniak, Ł.; Lorenzo, J.M.; Skaopska, S.; Rzoska, S.; Barba, F.J. Comparative effect of supercritical carbon dioxide and high pressure processing on structural changes and activity loss of oxidoreductive enzymes. J. CO2 Util. 2019, 29, 46–56. [Google Scholar] [CrossRef]
- Ludikhuyze, L.; Van Loey, A.; Denys, I.S.; Hendrickx, M.E.G. Effects of High Pressure on Enzymes Related to Food Quality. In Ultra High Pressure Treatments of Foods; Springer: Boston, MA, USA, 2001; pp. 115–166. [Google Scholar]
- Chakraborty, S.; Kaushik, N.; Rao, P.S.; Mishra, H.N. High-pressure inactivation of enzymes: A review on its recent applications on fruit purees and juices. Compr. Rev. Food Sci. Food Saf. 2014, 13, 578–596. [Google Scholar] [CrossRef]
- Valcarcel, J.; Reilly, K.; Gaffney, M.; O’Brien, N.M. Antioxidant Activity, Total Phenolic and Total Flavonoid Content in Sixty Varieties of Potato (Solanum tuberosum L.) Grown in Ireland. Potato Res. 2015, 58, 221–244. [Google Scholar] [CrossRef]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic compounds in the potato and its byproducts: An overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef] [PubMed]
- Cilla, A.; Bosch, L.; Barberá, R.; Alegría, A. Effect of processing on the bioaccessibility of bioactive compounds—A review focusing on carotenoids, minerals, ascorbic acid, tocopherols and polyphenols. J. Food Compos. Anal. 2018, 68, 3–15. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Li, R.; Bi, X.; Liao, X. Effects of high hydrostatic pressure and high temperature short time on antioxidant activity, antioxidant compounds and color of mango nectars. Innov. Food Sci. Emerg. Technol. 2014, 21, 35–43. [Google Scholar] [CrossRef]
- Zheng, X.; Yu, Y.; Xiao, G.; Xu, Y.; Wu, J.; Tang, D.; Zhang, Y. Comparing product stability of probiotic beverages using litchi juice treated by high hydrostatic pressure and heat as substrates. Innov. Food Sci. Emerg. Technol. 2014, 23, 61–67. [Google Scholar] [CrossRef]
- Da Silveira, T.F.F.; Cristianini, M.; Kuhnle, G.G.; Ribeiro, A.B.; Filho, J.T.; Godoy, H.T. Anthocyanins, non-anthocyanin phenolics, tocopherols and antioxidant capacity of açaí juice (Euterpe oleracea) as affected by high pressure processing and thermal pasteurization. Innov. Food Sci. Emerg. Technol. 2019, 55, 88–96. [Google Scholar] [CrossRef]
- Yuan, B.; Danao, M.G.C.; Stratton, J.E.; Weier, S.A.; Weller, C.L.; Lu, M. High pressure processing (HPP) of aronia berry purée: Effects on physicochemical properties, microbial counts, bioactive compounds, and antioxidant capacities. Innov. Food Sci. Emerg. Technol. 2018, 47, 249–255. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Cao, X.; Chen, F.; Hu, X.; Liao, X. Comparison of high hydrostatic pressure and high temperature short time processing on quality of purple sweet potato nectar. Innov. Food Sci. Emerg. Technol. 2012, 16, 326–334. [Google Scholar] [CrossRef]
- Di Scala, K.; Vega-Gálvez, A.; Ah-Hen, K.; Nuñez-Mancilla, Y.; Tabilo-Munizaga, G.; Pérez-Won, M.; Giovagnoli, C. Chemical and physical properties of aloe vera (Aloe barbadensis Miller) gel stored after high hydrostatic pressure processing | Propriedades químicas e físicas do gel de aloe vera (Aloe barbadensis Miller) armazenado após processamento sob alta pressão hi. Cienc. Tecnol. Aliment. 2013, 33, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Friedman, M.; Levin, C.E. Analysis and Biological Activities of Potato Glycoalkaloids, Calystegine Alkaloids, Phenolic Compounds, and Anthocyanins. Adv. Potato Chem. Technol. 2009, 127–161. [Google Scholar] [CrossRef]
- Jeż, M.; Wiczkowski, W.; Zielińska, D.; Białobrzewski, I.; Błaszczak, W. The impact of high pressure processing on the phenolic profile, hydrophilic antioxidant and reducing capacity of purée obtained from commercial tomato varieties. Food Chem. 2018, 261, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, S.; De Jong, D.; Griffiths, H.; Halitschke, R.; De Jong, W. The potato R locus codes for dihydroflavonol 4-reductase. Theor. Appl. Genet. 2009, 119, 931–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Palazon, A.; Suthanthangjai, W.; Kajda, P.; Zabetakis, I. The effects of high hydrostatic pressure on β-glucosidase, peroxidase and polyphenoloxidase in red raspberry (Rubus idaeus) and strawberry (Fragaria x ananassa). Food Chem. 2004, 88, 7–10. [Google Scholar] [CrossRef]
- Ek, K.L.; Wang, S.; Copeland, L.; Brand-Miller, J.C. Discovery of a low-glycaemic index potato and relationship with starch digestion in vitro. Br. J. Nutr. 2014, 111, 699–705. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Sahoo, U.; Baisakha, B.; Okpani, O.A.; Ngangkham, U.; Parameswaran, C.; Ngangkham, U.; Basak, N.; Kumar, G.; Sharma, S.G. Resistant starch could be decisive in determining the glycemic index of rice cultivars. J. Cereal Sci. 2018, 79, 348–353. [Google Scholar] [CrossRef]
- Panlasigui, L.N.; Thompson, L.U.; Juliano, B.O.; Perez, C.M.; Yiu, S.H.; Greenberg, G.R. Rice varieties with similar amylose content differ in starch digestibility and glycemic response in humans. Am. J. Clin. Nutr. 1991, 54, 871–877. [Google Scholar] [CrossRef]
- Elizondo-Montemayor, L.; Hernández-Brenes, C.; Ramos-Parra, P.A.; Moreno-Sánchez, D.; Nieblas, B.; Rosas-Pérez, A.M.; Lamadrid-Zertuche, A.C. High hydrostatic pressure processing reduces the glycemic index of fresh mango puree in healthy subjects. Food Funct. 2015, 6, 1352–1360. [Google Scholar] [CrossRef]
- Huang, H.-W.; Hsu, C.-P.; Wang, C.-Y. Healthy expectations of high hydrostatic pressure treatment in food processing industry. J. Food Drug Anal. 2019, 28. [Google Scholar] [CrossRef]
- Nasehi, B.; Javaheri, S. Application of high hydrostatic pressure in modifying functional properties of starches: A Review. Middle-East J. Sci. Res. 2012, 11, 856–861. [Google Scholar]
- Colussi, R.; Kaur, L.; da Rosa Zavareze, E.; Dias, A.R.G.; Stewart, R.B.; Singh, J. High pressure processing and retrogradation of potato starch: Influence on functional properties and gastro-small intestinal digestion in vitro. Food Hydrocoll. 2018, 75, 131–137. [Google Scholar] [CrossRef]
| Cultivar | Treatment | Polyphenols | Anthocyanins (Rutin Equivalent) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ferulic Acid | Chlorogenic Acid | Caffeic Acid | Quinic Acid | 4-Coumaric Acid | Rutin | Pelargonidin-O-Feruloylrutinoside-O-Hexoside | Pelargonidin-O-Rutinoside-O-Hexoside | ||
| Cultra | Untreated | 0.4 ± 0.02 b | 35.9 ± 1.1 ab | 18.0 ± 1.6 b | 71.3 ± 2.9 ab | 3.6 ± 0.5 b | 0.66 ± 0.1 b | ND | ND |
| 400 MPa | 3.1 ± 0.7 a | 37.5 ± 3.1 ab | 23.5 ± 4.5 a | 99.0 ± 1.8 a | 10.1 ± 1.5 a | 0.85 ± 0.2 b | ND | ND | |
| 600 MPa | 2.7 ± 0.3 a | 34.8 ± 2.1 ab | 19.7 ± 4.2 a | 86.2 ± 2.7 b | 9.0 ± 0.5 a | 1.85 ± 0.2 a | ND | ND | |
| Kerr’s Pink | Untreated | 0.5 ± 0.2 b | 54.0 ± 1.3 a | 18.3 ± 2.5 ab | 32.5 ± 3.7 b | 2.4 ± 0.8 ab | 0.5 ± 0.1 a | 1.8 ± 0.3 b | 2.0 ± 0.2 a |
| 400 MPa | 0.6 ± 0.2 b | 50.4 ± 1.8 b | 48.3 ± 1.3 b | 75.2 ± 3.5 a | 23.6 ± 1.1 a | 0.5 ± 0.1 a | 3.5 ± 1.0 a | 2.7 ± 0.6 a | |
| 600 MPa | 0.9 ± 0.3 b | 47.5 ± 3.3 b | 60.4 ± 1.9 a | 72.9 ± 3.2 a | 20.5 ± 0.1 b | 0.6 ± 0.1 a | 3.0 ± 0.3 a | 2.5 ± 0.5 a | |
| Maris Piper | Untreated | 0.4 ± 0.1 b | 55.3 ± 4.1 a | 28.3 ± 3.6 ab | 48.8 ± 1.7 ab | 10.4 ± 0.8 b | 5.1 ± 0.3 a | ND | ND |
| 400 MPa | 3.4 ± 0.2 a | 38.3 ± 9.80 ab | 56.8 ± 7.9 b | 57.0 ± 5.4 b | 15.2 ± 0.6 a | 5.6 ± 0.2 a | ND | ND | |
| 600 MPa | 3.2 ± 0.1 a | 38.9 ± 4.8 ab | 68.2 ± 8.9 a | 88.8 ± 4.6 a | 15.5 ± 0.6 a | 5.4 ± 0.2 a | ND | ND | |
| Maris Piper (baby) | Untreated | 0.4 ± 0.1 b | 55.3 ± 4.1 a | 28.3 ± 3.6 ab | 48.8 ± 1.7 ab | 10.4 ± 0.8 ab | 5.1 ± 0.3 a | ND | ND |
| 400 MPa | 3.3 ± 0.1 a | 43.9 ± 3.8 b | 80.1 ± 3.8 a | 77.9 ± 2.2 b | 17.4 ± 0.8 b | 4.9 ± 0.4 a | ND | ND | |
| 600 MPa | 3.6 ± 0.3 a | 44.6 ± 3.6 b | 77.3 ± 4.0 b | 107 ± 12 a | 19.6 ± 1.2 a | 4.4 ± 0.7 a | ND | ND | |
| Rooster | Untreated | 0.5 ± 0.01 b | 60.8 ± 2.0 a | 16.3 ± 2.0 ab | 50.1 ± 2.9 ab | 9.4 ± 1.1 ab | 1.8 ± 0.4 a | 9.9 ± 0.5 b | 6.5 ± 1.7 b |
| 400 MPa | 0.4 ± 0.03 b | 36.6 ± 5.1 ab | 21.3 ± 4.3 b | 86.8 ± 7.0 a | 24.5 ± 3.6 a | 2.3 ± 0.4 a | 14.7 ± 3.2 a | 11.1 ± 5.4 a | |
| 600 MPa | 0.4 ± 0.03 b | 30.7 ± 2.6 ab | 24.5 ± 0.7 a | 76.5 ± 8.2 b | 16.1 ± 2.3 b | 2.1 ± 0.2 a | 18.2 ± 3.5 a | 11.8 ± 3.0 a | |
| Cultivar | Treatment | Polyphenols | |||||
|---|---|---|---|---|---|---|---|
| Ferulic Acid | Chlorogenic Acid | Caffeic Acid | Quinic Acid | 4-Coumaric Acid | Rutin | ||
| Cultra | Untreated | ND | 35.2 ± 2.5 a | 5.7 ± 1.1 a | 32.9 ± 2.6 ab | 2.3 ± 0.4 a | 0.5 ± 0.1 a |
| 400 MPa | ND | 19.5 ± 1.0 b | 5.9 ± 1.1 a | 58.3 ± 4.5 b | 1.9 ± 0.4 a | 0.3 ± 0.1 b | |
| 600 MPa | ND | 2.5 ± 0.5 ab | 0.4 ± 0.1 b | 111.2 ± 6.9 a | ND | 0.3 ± 0.1 b | |
| Kerr’s Pink | Untreated | 0.6 ± 0.1 a | 47.5 ± 8.3 a | 6.3 ± 1.0 a | 36.1 ± 3.1 ab | 1.9 ± 0.3 a | 0.6 ± 0.1 a |
| 400 MPa | 0.5 ± 0.05 a | 26.4 ± 4.4 b | 6.9 ± 1.7 a | 48.7 ± 2.3 b | 1.3 ± 0.2 b | 0.2 ± 0.1 b | |
| 600 MPa | 0.5 ± 0.05 a | 1.1 ± 0.2 ab | 0.2 ± 0.05 b | 57.9 ± 2.5 a | 1.5 ± 0.1 b | 0.2 ± 0.1 b | |
| Maris Piper | Untreated | 0.6 ± 0.1 b | 38.1 ± 4.9 a | 34.1 ± 2.7 ab | 27.1 ± 3.2 ab | 1.4 ± 0.1 a | 0.7 ± 0.1 a |
| 400 MPa | 1.6 ± 0.5 a | 4.1 ± 0.5 b | 39.5 ± 1.3 b | 85.2 ± 1.5 b | 1.2 ± 0.1 b | 0.3 ± 0.2 b | |
| 600 MPa | 2.3 ± 0.4 a | 4.2 ± 0.2 b | 43.5 ± 1.5 a | 138 ± 7.9 a | 1.0 ± 0.2 b | 0.3 ± 0.1 b | |
| Maris Piper (baby) | Untreated | 0.5 ± 0.1 b | 38.5 ± 3.7 a | 31.4 ± 1.6 a | 21.5 ± 6.3 ab | 1.4 ± 0.3 a | 0.8 ± 0.2 a |
| 400 MPa | 2.3 ± 1.0 a | 3.2 ± 0.6 b | 26.7 ± 0.7 b | 57.2 ± 1.8 b | 1.1 ± 0.2 b | 0.6 ± 0.1 a | |
| 600 MPa | 2.4 ± 0.8 a | 2.5 ± 0.2 b | 28.9 ± 1.4 a | 116 ± 11 a | 1.2 ± 0.2 b | 0.7 ± 0.1 a | |
| Rooster | Untreated | 0.3 ± 0.05 b | 50.9 ± 4.7 a | 14.1 ± 0.8 a | 36.1 ± 2.9 ab | 2.5 ± 0.5 a | 0.8 ± 0.2 a |
| 400 MPa | 2.0 ± 0.1 a | 10.6 ± 0.5 b | 5.6 ± 0.4 b | 63.2 ± 4.3 b | 2.2 ± 0.3 a | 0.4 ± 0.1 b | |
| 600 MPa | 2.5 ± 0.1 a | 5.5 ± 0.7 ab | 3.0 ± 0.2 ab | 77.1 ± 1.7 a | 2.1 ± 0.4 a | 0.4 ± 0.2 b | |
| Cultivars | Treatment | Rapidly Available Glucose (g/100 g) | Total Glucose (g/100 g) | Glycaemic Index | Glycaemic Load |
|---|---|---|---|---|---|
| Cultra | Untreated | 14.38 ± 1.02 a | 19.31 ± 0.77 a | 79.10 ± 6.79 a | 14.09 ± 1.07 a |
| 400 MPa | 11.73 ± 0.39 b | 18.92 ± 2.27 a | 68.36 ± 10.52 a | 11.73 ± 0.72 b | |
| 600 MPa | 14.93 ± 0.75 a | 20.18 ± 3.43 a | 80.97 ± 7.72 a | 14.92 ± 1.07 a | |
| Kerr’s Pink | Untreated | 10.77 ± 0.18 a | 11.39 ± 0.51 a | 97.79 ± 3.42 a | 10.24 ± 0.17 a |
| 400 MPa | 9.23 ± 1.29 a | 12.06 ± 4.15 a | 85.00 ± 12.05 a | 9.16 ± 1.69 a | |
| 600 MPa | 9.37 ± 1.95 a | 17.33 ± 2.10 b | 62.14 ± 10.65 b | 9.86 ± 1.77 a | |
| Maris Piper | Untreated | 10.97 ± 0.66 a | 17.87 ± 2.99 a | 66.71 ± 13.05 a | 11.06 ± 1.24 a |
| 400 MPa | 11.39 ± 0.03 a | 17.69 ± 3.04 a | 73.20 ± 9.51 a | 11.76 ± 0.45 a | |
| 600 MPa | 12.65 ± 3.36 a | 18.21 ± 2.56 a | 67.74 ± 3.02 a | 11.61 ± 1.25 a | |
| Maris Piper (baby) | 400 MPa | 12.18 ± 5.73 a | 19.41 ± 2.98 a | 117.70 ± 5.80 a | 10.43 ± 3.91 a |
| 600 MPa | 11.40 ± 1.18 a | 17.78 ± 1.73 a | 65.52 ± 0.02 b | 10.77 ± 1.09 a | |
| Rooster | Untreated | 12.38 ± 2.76 a | 16.38 ± 3.17 a | 81.60 ± 5.36 a | 12.31 ± 2.65 a |
| 400 MPa | 12.23 ± 1.60 a | 17.30 ± 1.77 a | 78.74 ± 11.50 a | 12.32 ± 1.18 a | |
| 600 MPa | 12.04 ± 0.84 a | 20.97 ± 3.36 a | 62.03 ± 8.96 b | 11.75 ± 0.75 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsikrika, K.; Muldoon, A.; O’Brien, N.M.; Rai, D.K. High-Pressure Processing on Whole and Peeled Potatoes: Influence on Polyphenol Oxidase, Antioxidants, and Glycaemic Indices. Foods 2021, 10, 2425. https://doi.org/10.3390/foods10102425
Tsikrika K, Muldoon A, O’Brien NM, Rai DK. High-Pressure Processing on Whole and Peeled Potatoes: Influence on Polyphenol Oxidase, Antioxidants, and Glycaemic Indices. Foods. 2021; 10(10):2425. https://doi.org/10.3390/foods10102425
Chicago/Turabian StyleTsikrika, Konstantina, Aine Muldoon, Nora M. O’Brien, and Dilip K. Rai. 2021. "High-Pressure Processing on Whole and Peeled Potatoes: Influence on Polyphenol Oxidase, Antioxidants, and Glycaemic Indices" Foods 10, no. 10: 2425. https://doi.org/10.3390/foods10102425
APA StyleTsikrika, K., Muldoon, A., O’Brien, N. M., & Rai, D. K. (2021). High-Pressure Processing on Whole and Peeled Potatoes: Influence on Polyphenol Oxidase, Antioxidants, and Glycaemic Indices. Foods, 10(10), 2425. https://doi.org/10.3390/foods10102425








