Protein Fractionation of Green Leaves as an Underutilized Food Source—Protein Yield and the Effect of Process Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Biomass
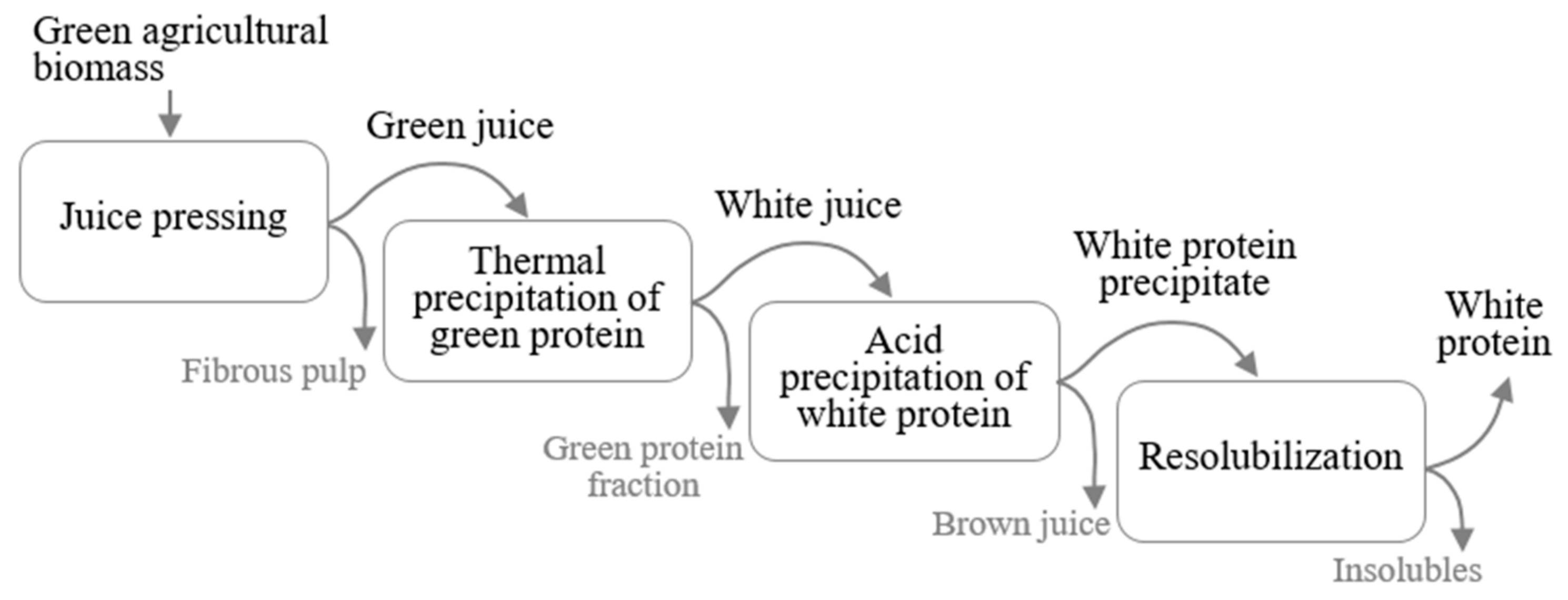
2.2. Protein Fractionation from Green Leaves
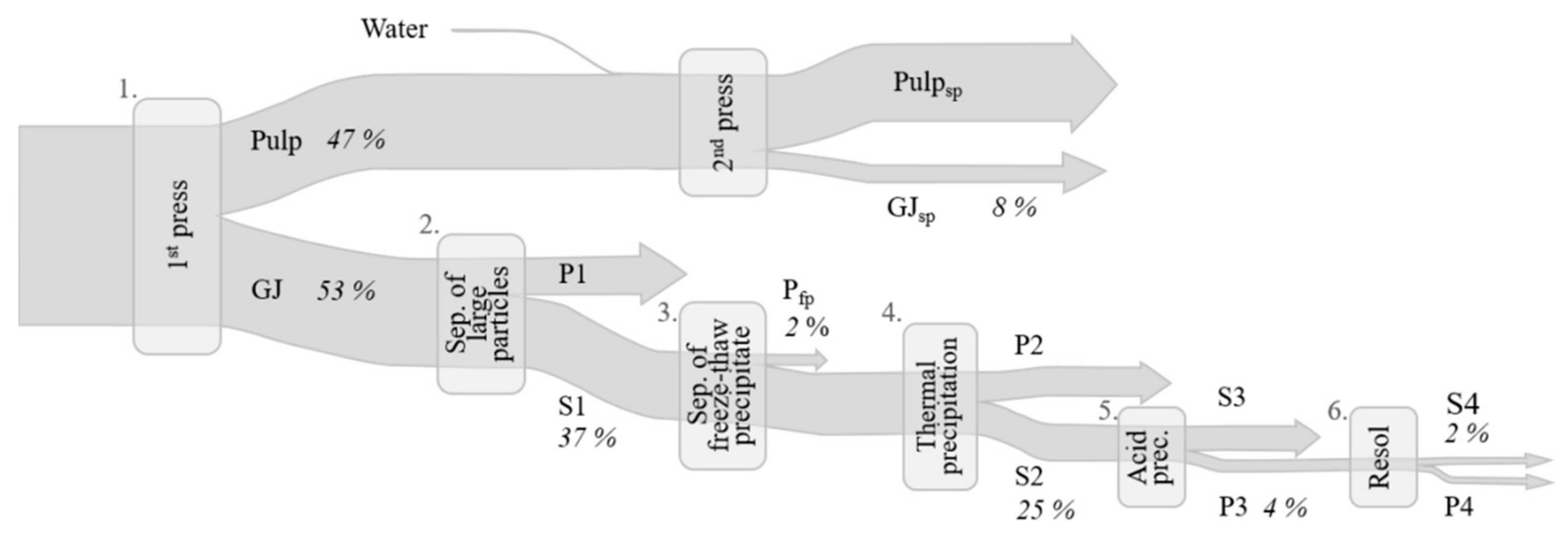
2.2.1. Juice Pressing
2.2.2. Thermal Precipitation
2.2.3. Acid Precipitation and Resolubilization of the White Protein Fraction
2.3. Thermal and Acid Precipitation Tests to Determine Differences between Biomass Sources
2.3.1. Thermal Precipitation
2.3.2. Acid Precipitation
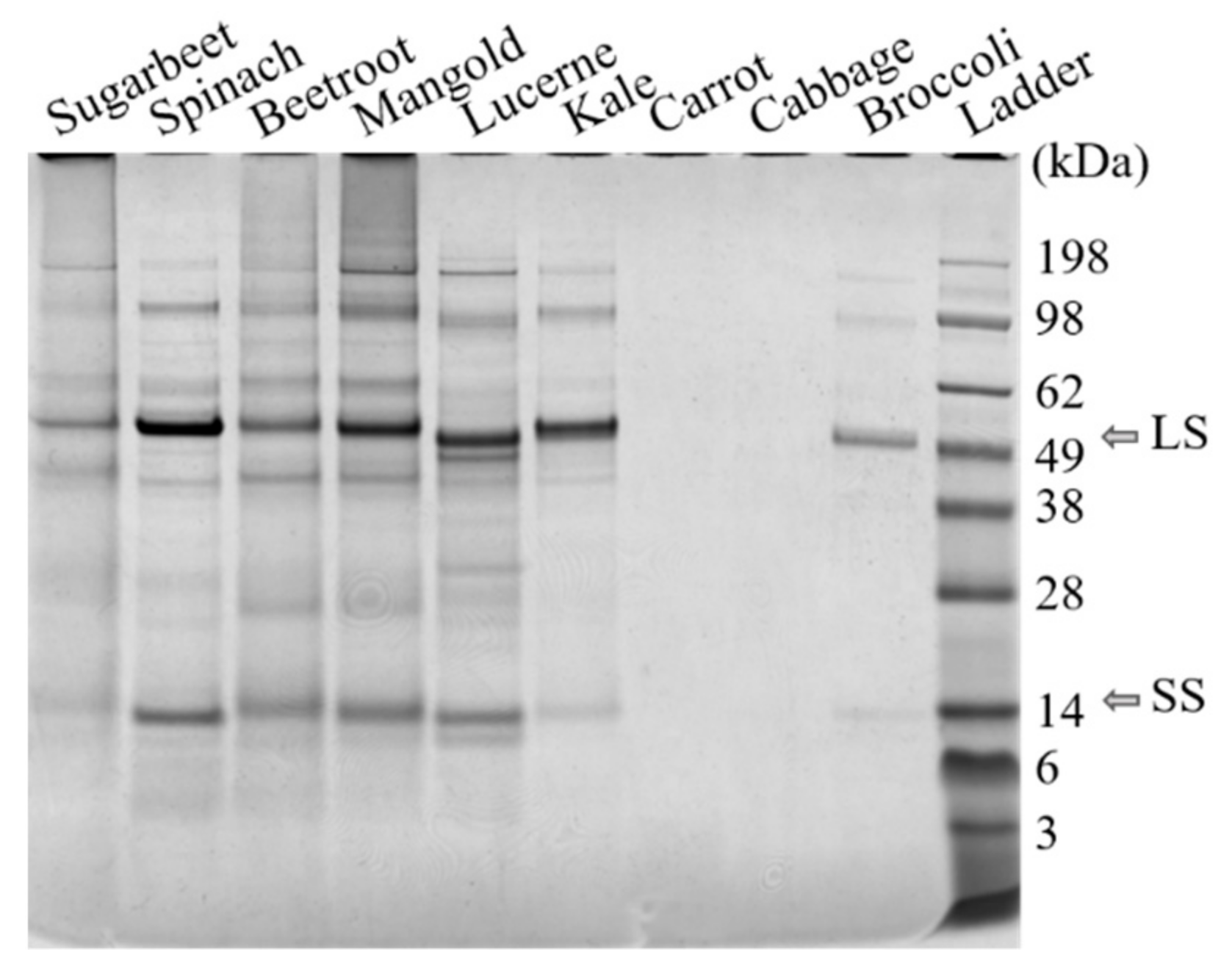
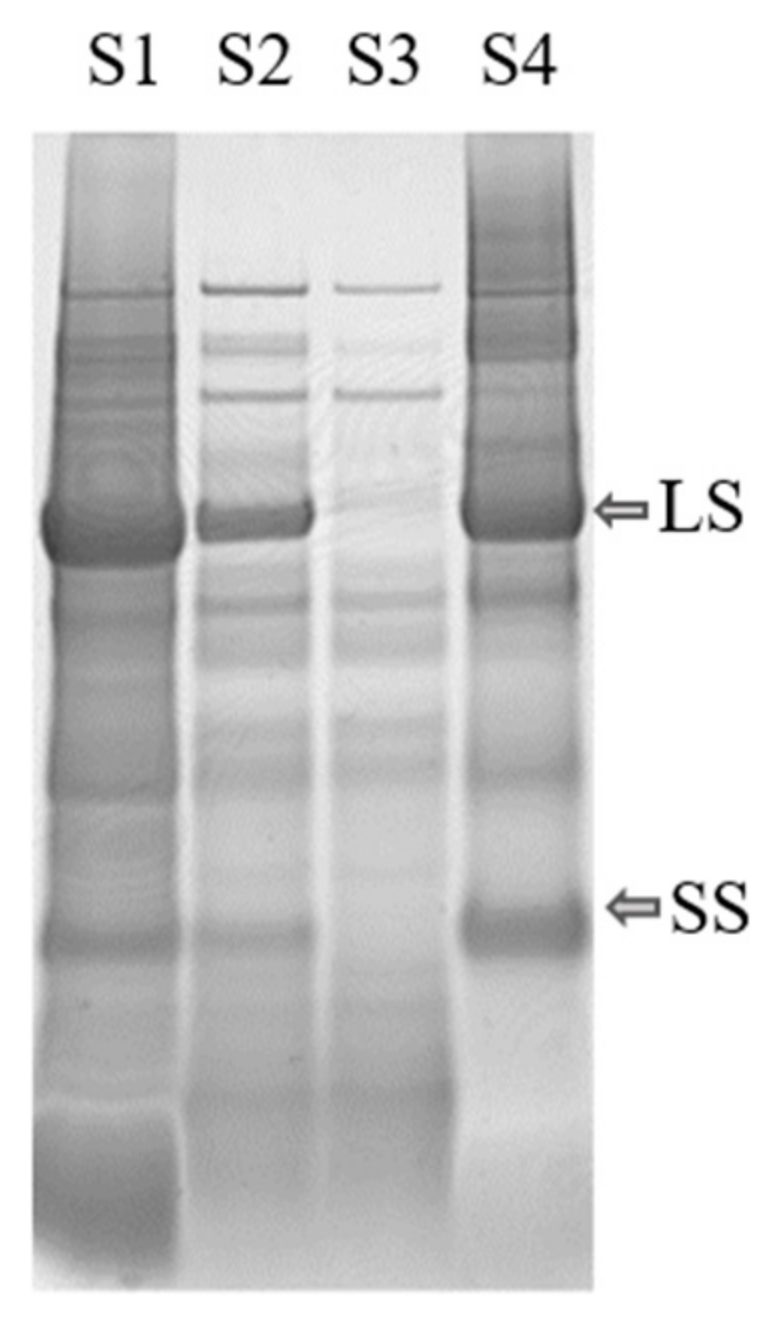
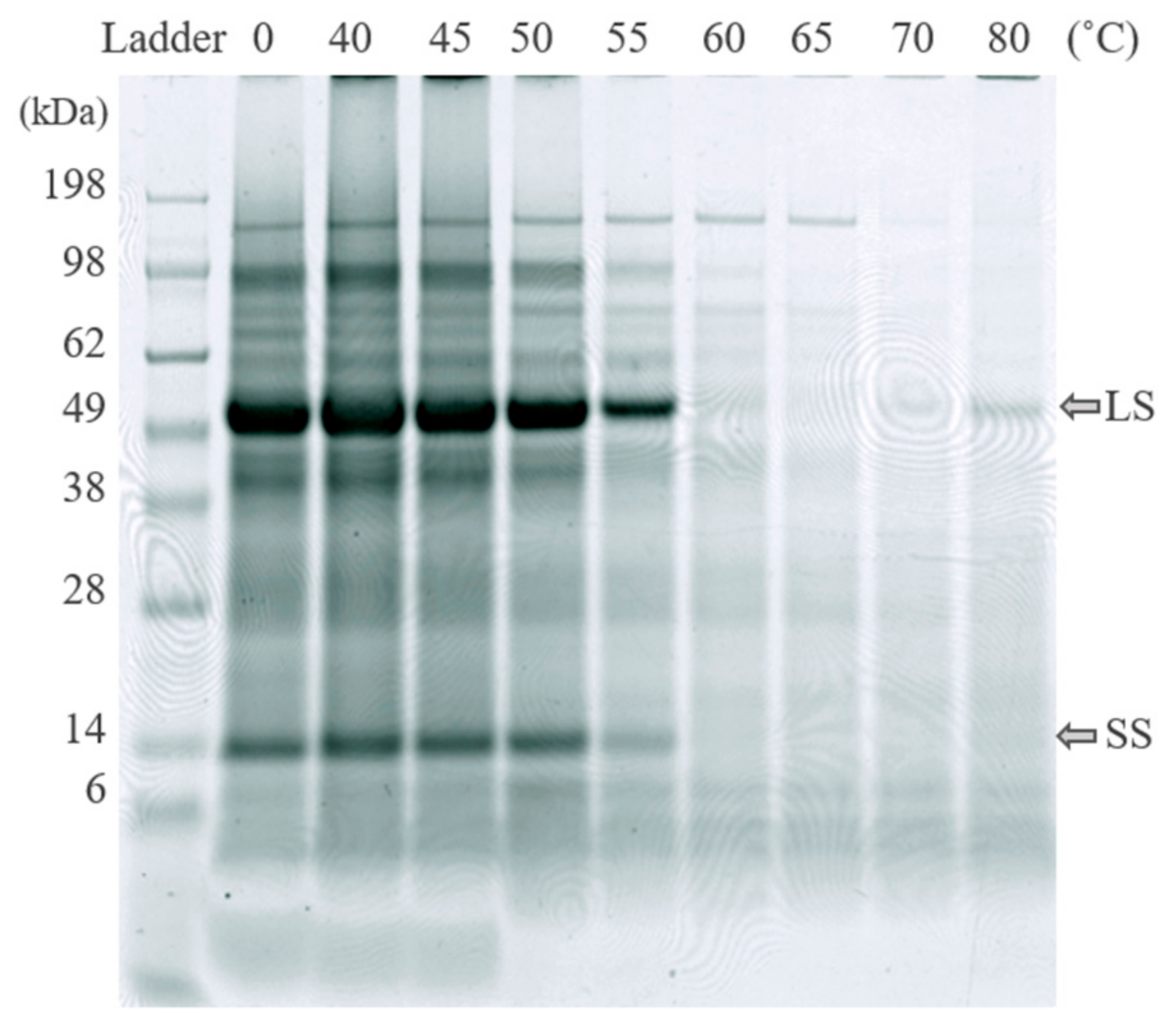
2.3.3. SDS-PAGE Analysis
2.4. Dry Matter and Nitrogen Determinations
2.5. Yield Calculations
2.6. Calculation of Theoretical pI
2.7. Statistical Evaluation
3. Results and Discussion
3.1. Effect of Biomass Source on Protein Fractionation
3.2. Differences in Protein Fractionation between Biomass Types
3.2.1. Juice Pressing
3.2.2. Green Protein Fractionation and White Juice Production
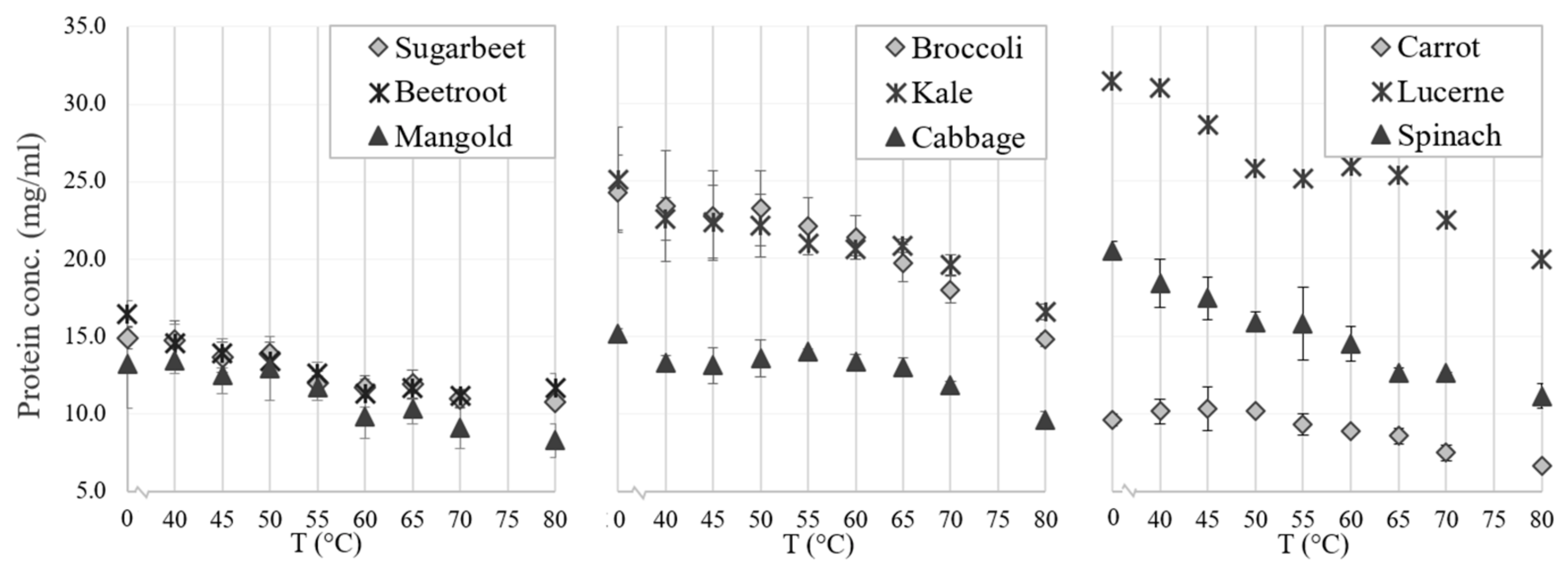
3.2.3. Acid Precipitation of the White Protein Fraction
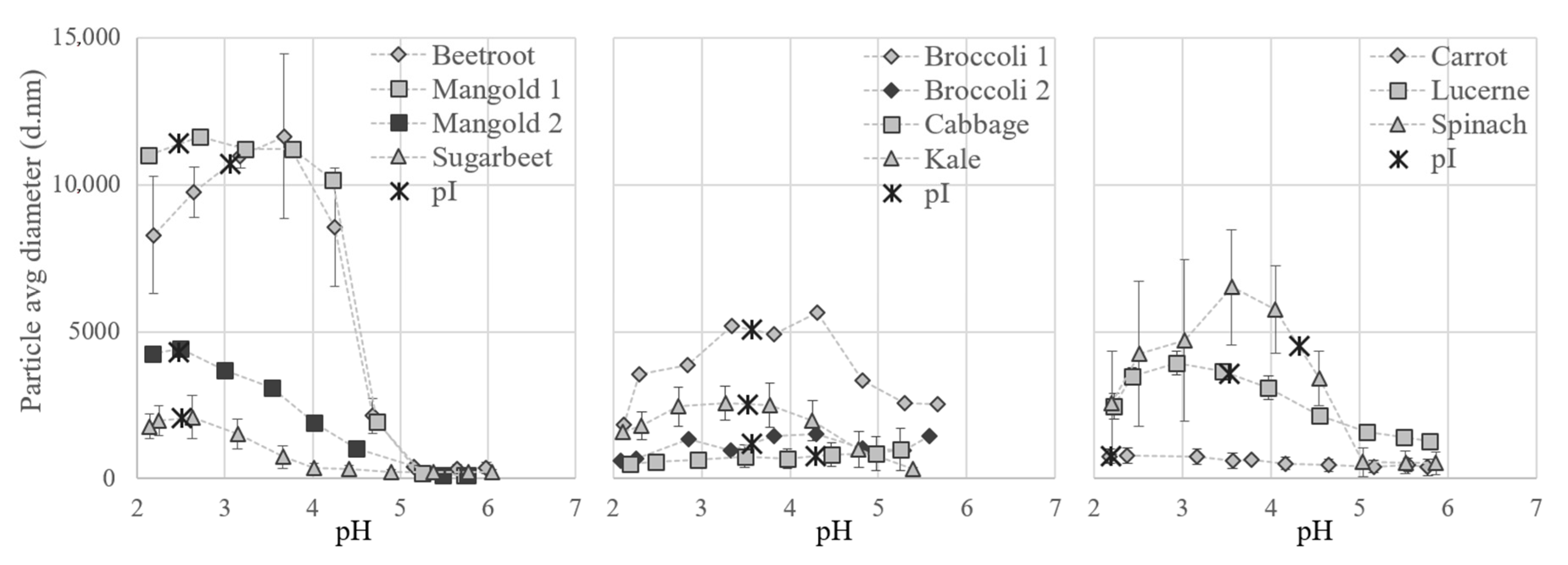
3.2.4. Resolubilization of the White Protein Fraction
3.3. Use of Green Biomass in Industrial Protein Fractionation for Food Ingredients, Additives, and Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barclays. Carving Up the Alternative Meat Market. Available online: https://www.investmentbank.barclays.com/our-insights/carving-up-the-alternative-meat-market.html (accessed on 6 July 2020).
- Smart Protein Project. Plant-Based Foods in Europe: How Big Is the Market? Smart Protein Plant-Based Food Sector Report by Smart Protein Project, European Union’s Horizon 2020 Research and Innovation Programme (No 862957). 2020. Available online: https://knowledge4policy.ec.europa.eu/publication/plant-based-foods-europe-how-big-market_en (accessed on 12 October 2021).
- Furu, M. Marknadsanalys och Potential för Växtbaserade Proteiner. 2020. Available online: https://www.lrf.se/globalassets/dokument/mitt-lrf/nyheter/2020/marknadsanalys-och-potential-for-vaxtbaserade-proteiner.pdf (accessed on 27 March 2020).
- USDA. Oilseeds: World Markets and Trade; Monthly Report July 2021; United States Department of Agriculture: Economics, Statistics and Market Information System: Washington, DC, USA, 2021; Available online: https://usda.library.cornell.edu/concern/publications/tx31qh68h?locale=en (accessed on 16 August 2021).
- Sozer, N.; Poutanen, K. Plant protein ingredients for future foods. Agro FOOD Ind. Hi-Tech 2015, 26, 56–59. [Google Scholar]
- Pirie, N.W. The direct use of leaf protein in human nutrition. Chem. Ind. 1942, 61, 45–48. [Google Scholar]
- Di Stefano, E.; Agyei, D.; Njoku, E.N.; Udenigwe, C.C. Plant RuBisCo: An underutilized protein for food applications. J. Am. Oil Chem. Soc. 2018, 95, 1063–1074. [Google Scholar] [CrossRef]
- Van de Velde, F.; Alting, A.; Pouvreau, L. From waste product to food ingredient: The extraction of abundant plant protein RuBisCo. New Food 2011, 14, 10–13. [Google Scholar]
- Berndtsson, E.; Nynäs, A.-L.; Newson, W.R.; Langton, M.; Andersson, R.; Johansson, E.; Olsson, M.E. The underutilised side streams of broccoli and kale–valorisation via proteins and phenols. In Sustainable Governance and Management of Food Systems: Ethical Perspectives; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019; pp. 74–81. [Google Scholar]
- Andersson, I.; Backlund, A. Structure and function of Rubisco. Plant Physiol. Biochem. 2008, 46, 275–291. [Google Scholar] [CrossRef]
- Martin, A.H.; Castellani, O.; de Jong, G.A.H.; Bovetto, L.; Schmitt, C. Comparison of the functional properties of RuBisCO protein isolate extracted from sugar beet leaves with commercial whey protein and soy protein isolates. J. Sci. Food Agric. 2019, 99, 1568–1576. [Google Scholar] [CrossRef]
- Lamsal, B.P.; Koegel, R.G.; Gunasekaran, S. Some physicochemical and functional properties of alfalfa soluble leaf proteins. LWT—Food Sci. Technol. 2007, 40, 1520–1526. [Google Scholar] [CrossRef]
- Hojilla-Evangelista, M.P.; Selling, G.W.; Hatfield, R.; Digman, M. Extraction, composition, and functional properties of dried alfalfa (Medicago sativa L.) leaf protein. J. Sci. Food Agric. 2016, 97, 882–888. [Google Scholar] [CrossRef]
- Patel, M.; Berry, J.O. Rubisco gene expression in C4 plants. J. Exp. Bot. 2008, 59, 1625–1634. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Kinsella, J.E. Composition of alfalfa leaf protein isolates. J. Food Sci. 1975, 40, 1156–1161. [Google Scholar] [CrossRef]
- Merodio, C.; Sabater, B. Preparation and properties of a white protein fraction in high yield from sugar beet (Beta vulgaris L.) leaves. J. Sci. Food Agric. 1987, 44, 237–243. [Google Scholar] [CrossRef]
- Knuckles, B.E.; Kohler, G.O. Functional properties of edible protein concentrates from alfalfa. J. Agric. Food Chem. 1982, 30, 748–752. [Google Scholar] [CrossRef]
- Tamayo Tenorio, A.; Gieteling, J.; de Jong, G.A.; Boom, R.M.; van der Goot, A.J. Recovery of protein from green leaves: Overview of crucial steps for utilisation. Food Chem. 2016, 203, 402–408. [Google Scholar] [CrossRef]
- Edwards, R.H.; Miller, R.E.; De Fremery, D.; Knuckles, B.E.; Bickoff, E.M.; Kohler, G.O. Pilot plant production of an edible white fraction leaf protein concentrate from alfalfa. J. Agric. Food Chem. 1975, 23, 620–626. [Google Scholar] [CrossRef]
- Martin, A.H.; Nieuwland, M.; de Jong, G.A.H. Characterization of heat-set gels from RuBisCO in comparison to those from other proteins. J. Agric. Food Chem. 2014, 62, 10783–10791. [Google Scholar] [CrossRef]
- Sheen, S.J. Comparison of chemical and functional properties of soluble leaf proteins from four plant species. J. Agric. Food Chem. 1991, 39, 681–685. [Google Scholar] [CrossRef]
- Amer, B.; Juul, L.; Møller, A.; Møller, H.; Dalsgaard, T. Improved solubility of proteins from white and red clover–Inhibition of redox enzymes. Int. J. Food Sci. Technol. 2020, in press. [Google Scholar] [CrossRef]
- Bray, W.J.; Humphries, C. Preparation of white leaf protein concentrate using a polyanionic flocculant. J. Sci. Food Agric. 1979, 30, 171–176. [Google Scholar] [CrossRef]
- Fiorentini, R.; Galoppini, C. Pilot plant production of an edible alfalfa protein concentrate. J. Food Sci. 1981, 46, 1514–1517. [Google Scholar] [CrossRef]
- Colas, D.; Doumeng, C.; Pontalier, P.; Rigal, L. Green crop fractionation by twin-screw extrusion: Influence of the screw profile on alfalfa (Medicago sativa) dehydration and protein extraction. Chem. Eng. Process. Process Intensif. 2013, 72, 1–9. [Google Scholar] [CrossRef]
- De Fremery, D.; Miller, R.E.; Edwards, R.H.; Knuckles, B.E.; Bickoff, E.M.; Kohler, G.O. Centrifugal separation of white and green protein fractions from alfalfa juice following controlled heating. J. Agric. Food Chem. 1973, 21, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; Hernández, T.; Martinez, C. Production and chemical composition of alfalfa protein concentrate obtained by freezing. Anim. Feed Sci. Technol. 1998, 72, 169–174. [Google Scholar] [CrossRef]
- Jwanny, E.W.; Montanari, L.; Fantozzi, P. Protein production for human use from Sugarbeet: Byproducts. Bioresour. Technol. 1993, 43, 67–70. [Google Scholar] [CrossRef]
- Kiskini, A.; Vissers, A.; Vincken, J.-P.; Gruppen, H.; Wierenga, P.A. Effect of plant age on the quantity and quality of proteins extracted from sugar beet (Beta vulgaris L.) leaves. J. Agric. Food Chem. 2016, 64, 8305–8314. [Google Scholar] [CrossRef]
- Lamsal, B.P.; Koegel, R.G.; Boettcher, M.E. Separation of protein fractions in alfalfa juice: Effects of some pre-treatment methods. Trans. ASAE 2003, 46, 715–720. [Google Scholar] [CrossRef]
- Merodio, C.; Martin, M.; Sabater, B. Improved separation of green and soluble leaf proteins by pH shift. J. Agric. Food Chem. 1983, 31, 957–959. [Google Scholar] [CrossRef]
- Zhang, W.; Grimi, N.; Jaffrin, M.Y.; Ding, L. Leaf protein concentration of alfalfa juice by membrane technology. J. Membr. Sci. 2015, 489, 183–193. [Google Scholar] [CrossRef]
- Sedlar, T.; Čakarević, J.; Tomić, J.; Popović, L. Vegetable by-products as new sources of functional proteins. Plant Foods Hum. Nutr. 2021, 76, 31–36. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R. RStudio; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.E.; de Fremery, D.; Bickoff, E.M.; Kohler, G.O. Soluble protein concentrate from alfalfa by low-temperature acid precipitation. J. Agric. Food Chem. 1975, 23, 1177–1179. [Google Scholar] [CrossRef]
- Santamaría-Fernández, M.; Ytting, N.K.; Lübeck, M. Influence of the development stage of perennial forage crops for the recovery yields of extractable proteins using lactic acid fermentation. J. Clean. Prod. 2019, 218, 1055–1064. [Google Scholar] [CrossRef]
- Béghin, V.; Bizot, H.; Audebrand, M.; Lefebvre, J.; Libouga, D.G.; Douillard, R. Differential scanning calorimetric studies of the effects of ions and pH on ribulose 1, 5-bisphosphate carboxylase/oxygenase. Int. J. Biol. Macromol. 1993, 15, 195–200. [Google Scholar] [CrossRef]
- Betschart, A.; Kinsella, J.E. Extractability and solubility of leaf protein. J. Agric. Food Chem. 1973, 21, 60–65. [Google Scholar] [CrossRef]
- Bhatia, S.; Dahiya, R. Concepts and Techniques of Plant Tissue Culture Science. In Modern Applications of Plant Biotechnology in Pharmaceutical Sciences; Bhatia, S., Sharma, K., Dahiya, R., Bera, T., Eds.; Academic Press: Boston, MA, USA, 2015; Chapter 4; pp. 121–156. [Google Scholar]
- Kobbi, S.; Bougatef, A.; Balti, R.; Mickael, C.; Fertin, B.; Chaabouni, S.; Dhulster, P.; Nedjar, N. Purification and Recovery of RuBisCO Protein from Alfalfa Green Juice: Antioxidative Properties of Generated Protein Hydrolysate. Waste Biomass Valorization 2017, 8, 493–504. [Google Scholar] [CrossRef]
- Firdaous, L.; Fertin, B.; Khelissa, O.; Dhainaut, M.; Nedjar, N.; Chataigné, G.; Ouhoud, L.; Lutin, F.; Dhulster, P. Adsorptive removal of polyphenols from an alfalfa white proteins concentrate: Adsorbent screening, adsorption kinetics and equilibrium study. Sep. Purif. Technol. 2017, 178, 29–39. [Google Scholar] [CrossRef]
- Tenorio, A.T.; Schreuders, F.; Zisopoulos, F.; Boom, R.; Van der Goot, A. Processing concepts for the use of green leaves as raw materials for the food industry. J. Clean. Prod. 2017, 164, 736–748. [Google Scholar] [CrossRef]
- Muneer, F.; Hovmalm, H.P.; Svensson, S.-E.; Newson, W.R.; Johansson, E.; Prade, T. Economic viability of protein concentrate production from green biomass of intermediate crops: A pre-feasibility study. J. Clean. Prod. 2021, 294, 126304. [Google Scholar] [CrossRef]
- Prade, T.; Muneer, F.; Berndtsson, E.; Nynäs, A.-L.; Svensson, S.-E.; Newson, W.R.; Johansson, E. Protein fractionation of broccoli (Brassica oleracea, var Italica) and kale (Brassica oleracea, var. Sabellica) leaves—A pre-feasibility assessment and evaluation of fraction phenol and fibre content. Food Bioprod. Process. 2021, in press. [Google Scholar]
| Biomass Source | Collection Date | %N | %DM | |
|---|---|---|---|---|
| Broccoli * | Brassica oleracea, var. italica | 2 October 2017 | 3.2 | 13.3 |
| Cabbage * | Brassica oleracea, var. capitata | 30 August 2017 | 2.1 | 11.0 |
| Kale * | Brassica oleracea, var. sabellica | 23 October 2017 | 3.0 | 13.3 |
| Mangold | Beta vulgaris, subsp. vulgaris, var. cicla | 30 August 2017 | 2.1 | 8.4 |
| Beetroot * | Beta vulgaris, subsp. vulgaris, var. Red hawk | 13 September 2017 | 3.2 | 9.9 |
| Sugarbeet * | Beta vulgaris, subsp. vulgaris, var. Lombok | 12 October 2018 | 3.0 | 13.0 |
| Carrot * | Daucus carota subsp. sativus | 28 June 2018 | 2.1 | 17.7 |
| Lucerne | Medicago sativa | 25 May 2018 | 2.8 | 20.9 |
| Spinach | Spinacia oleracea | Retail ** | 4.8 | 10.6 |
| (a) | Process Step | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield of N (%) | BM Pressing | Second BM Pressing | Separation of Particles | Thermal Precipitation | Acid Precipitation | Full Process | |||||
| Biomass | BM to GJ | BM to GJsp | BM to S1 | BM to S2 | BM to P3 | BM to S4 | |||||
| Broccoli | 28.2 ± 2.0 BC | 7.2± 1.5 BC | 14.8 ± 1.4 BC | 15.5 ± 2.2 B | 2.9 ± 0.7 CD | 0.4 ± 0.0 A | |||||
| Cabbage | 37.4 ± 5.4 C | 7.9 ± 2.0 BC | 9.3 ± 2.4 AB | 14.4 ± 3.1 AB | 0.2 ± 0.1 AB | 0.2 ± 0.1 A | |||||
| Kale | 30.3 ± 3.3 BC | 5.0 ± 0.2 AB | 19.3 ± 0.7 CD | 18.7 ± 0.7 BC | 2.1 ± 0.2 ABCD | 0.5 ± 0.1 AB | |||||
| Mangold | 53.1 ± 1.0 D | 8.4 ± 0.6 C | 36.9 ± 1.3 E | 24.6 ± 4.6 C | 3.7 ± 1.6 D | 1.9 ± 1.1 B | |||||
| Beetroot | 35.5 ± 2.1 C | 6.3 ± 0.4 ABC | 21.9 ± 2.1 D | 17.5 ± 0.3 BC | 3.8 ± 0.1 D | 1.0 ± 0.5 AB | |||||
| Sugarbeet | 14.6 ± 4.0 A | 3.7 ± 0.6 A | 12.1 ± 1.5 AB | 11.72 ± 2.4 AB | 1.22 ± 0.3 ABC | 0.92 ± 0 AB | |||||
| Carrot | 20.9 ± 2.7 AB | 5.3 ± 0.4 AB | 6.0 ± 0.5 A | 7.2 ± 0.4 A | 0.2 ± 0.2 A | 0.1 ± 0.1 A | |||||
| Lucerne | 52.1 ± 5.4 D | 15.1 ± 0.9 D | 42.9 ± 4.4 E | 20.0 ± 0.9 BC | 3.31 BCD | 1.51 AB | |||||
| Spinach | 27.6 ± 5.0 BC | 8.9 ± 1.3 C | 25.1 ± 0.4 D | 18.0 ± 3.8 BC | 3.02 ± 0.1 CD | 1.02 ± 0.4 AB | |||||
| (b) | Process Step | ||||||||||
| Yield of N (%) | Separation of Particles | Separation of Freeze-Thaw Precipitate | Thermal Precipitation | Acid Precipitation | |||||||
| Biomass | GJ to S1 | S1 to Pfp | S1 to S2 | S2 to P3 | |||||||
| Broccoli | 52.6 ± 4.2 AB | 12.6 ± 1.9 A | 84.2 ± 0.5 CD | 18.8 ± 1.7 BC | |||||||
| Cabbage | 24.9 ± 5.9 A | 42.2 ± 14.1 B | 89.9 ± 1.8 DE | 1.2 ± 1.1 A | |||||||
| Kale | 64.1 ± 6.4 BC | 6.5 ± 1.4 A | 84.3 ± 2.5 CD | 11.4 ± 1.2 B | |||||||
| Mangold | 69.6 ± 3.6 BC | 4.6 ± 3.9 A | 52.0 ± 6.0 A | 15.2 ± 5.4 BC | |||||||
| Beetroot | 61.6 ± 3.5 BC | 6.0 ± 6.0 A | 84.9 ± 16.1 CD | 21.6 ± 0.3 C | |||||||
| Sugarbeet | 79.7 ± 31.4 BC | 1.2 ± 0.2 A | 61.42 ± 13.2 ABC | 10.52 ± 0.5 AB | |||||||
| Carrot | 29.2 ± 5.1 A | 1.8 ± 0.1 A | 110.6 ± 8.0 E | 2.8 ± 2.4 A | |||||||
| Lucerne | 82.5 ± 8.5 BC | 7.7 ± 1.5 A | 52.2 ± 4.4 AB | 16.11 BC | |||||||
| Spinach | 93.3 ± 17.9 C | 7.1 ± 0.7 A | 79.5 ± 10.1 BCD | 16.92 ± 4.0 BC | |||||||
| (c) | |||||||||||
| % N | Flow in the Process | ||||||||||
| Biomass | BM * | GJ | Pulp | GJsp | Pulpsp | S1 | Pfp | S2 | P3 | S4 | P4 |
| Broccoli | 3.2 | 2.1 ± 0.1 | 1.9 ± 0.2 | 2.4 ± 0.1 | 1.9 ± 0.2 | 1.7 ± 0.1 | 5.8 ± 0.2 | 2.0 ± 0.2 | 7.9 ± 0.2 | 1.8 ± 0.4 | 13.6 ± 0.2 |
| Cabbage | 2.1 | 1.7 ± 0.2 | 1.2 ± 0.1 | 1.5 ± 0.3 | 1.3 ± 0.2 | 0.5 ± 0.1 | 8.2 ± 0.3 | 0.8 ± 0.2 | 1.2 ± 0.0 | 0.9 ± 0.1 | 0.7 ± 0.5 |
| Kale | 3 | 2.7 ± 0.2 | 1.5 ± 0.2 | 2.9 ± 0.1 | 2.2 ± 0.1 | 2.2 ± 0.0 | 1.7 ± 0.1 | 2.3 ± 0.1 | 7.9 ± 0.4 | 2.6 ± 0.4 | 12.3 ± 1.2 |
| Mangold | 2.1 | 2.3 ± 0.1 | 2.4 ± 0.4 | 2.6 ± 0.2 | 1.4 ± 0.1 | 1.9 ± 0.2 | 4.3 ± 0.3 | 1.6 ± 0.3 | 7.2 ± 0.6 | 3.6 ± 0.9 | 13.7 ± 0.4 |
| Beetroot | 3.2 | 3.0 ± 0.0 | 3.3 ± 0.2 | 3.6 ± 0.1 | 1.6 ± 0.2 | 2.6 ± 0.1 | 5.5 ± 0.3 | 2.2 ± 0.1 | 9.2 ± 0.7 | 3.3 ± 0.7 | 14.0 ± 0.3 |
| Sugarbeet | 3 | 1.1 ± 0.2 | 1.3 ± 0.3 | 1.6 ± 0.2 | 2.6 ± 0.5 | 1.0 ± 0.1 | 5.7 ± 0.4 | 1.1 ± 0.1 | 2.62 ± 1.5 | 2.32 ± 0.9 | 0.52 ± 0.1 |
| Carrot | 2.5 | 1.5 ± 0.1 | 2.1 ± 0.0 | 2.3 ± 0.1 | 3.2 ± 0.3 | 0.6 ± 0.1 | 8.0 ± 0.2 | 0.6 ± 0.0 | 1.1 ± 0.4 | 0.6 ± 0.1 | |
| Lucerne | 2.8 | 3.8 ± 0.3 | 2.0 ± 0.1 | 4.1 ± 0.1 | 5.1 ± 0.2 | 4.3 ± 0.1 | 8.0 ± 0.0 | 3.2 ± 0.1 | 7.21 | 4.01 | 12.91 |
| Spinach | 5.1 | 4.4 ± 0.3 | 4.9 ± 0.2 | 4.1 ± 0.1 | 1.5 ± 0.3 | 4.2 ± 0.3 | 2.9 ± 0.5 | 4.0 ± 0.2 | 8.1 ± 1.8 | 3.3 ± 0.5 | 14.1 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nynäs, A.-L.; Newson, W.R.; Johansson, E. Protein Fractionation of Green Leaves as an Underutilized Food Source—Protein Yield and the Effect of Process Parameters. Foods 2021, 10, 2533. https://doi.org/10.3390/foods10112533
Nynäs A-L, Newson WR, Johansson E. Protein Fractionation of Green Leaves as an Underutilized Food Source—Protein Yield and the Effect of Process Parameters. Foods. 2021; 10(11):2533. https://doi.org/10.3390/foods10112533
Chicago/Turabian StyleNynäs, Anna-Lovisa, William R. Newson, and Eva Johansson. 2021. "Protein Fractionation of Green Leaves as an Underutilized Food Source—Protein Yield and the Effect of Process Parameters" Foods 10, no. 11: 2533. https://doi.org/10.3390/foods10112533
APA StyleNynäs, A.-L., Newson, W. R., & Johansson, E. (2021). Protein Fractionation of Green Leaves as an Underutilized Food Source—Protein Yield and the Effect of Process Parameters. Foods, 10(11), 2533. https://doi.org/10.3390/foods10112533







