Commercial Bio-Packaging to Preserve the Quality and Extend the Shelf-Life of Vegetables: The Case-Study of Pumpkin Samples Studied by a Multimethodological Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biodegradable Commercial Films
2.2. Pumpkin and Packaged Food Preparation
2.3. Biofilms Analyses
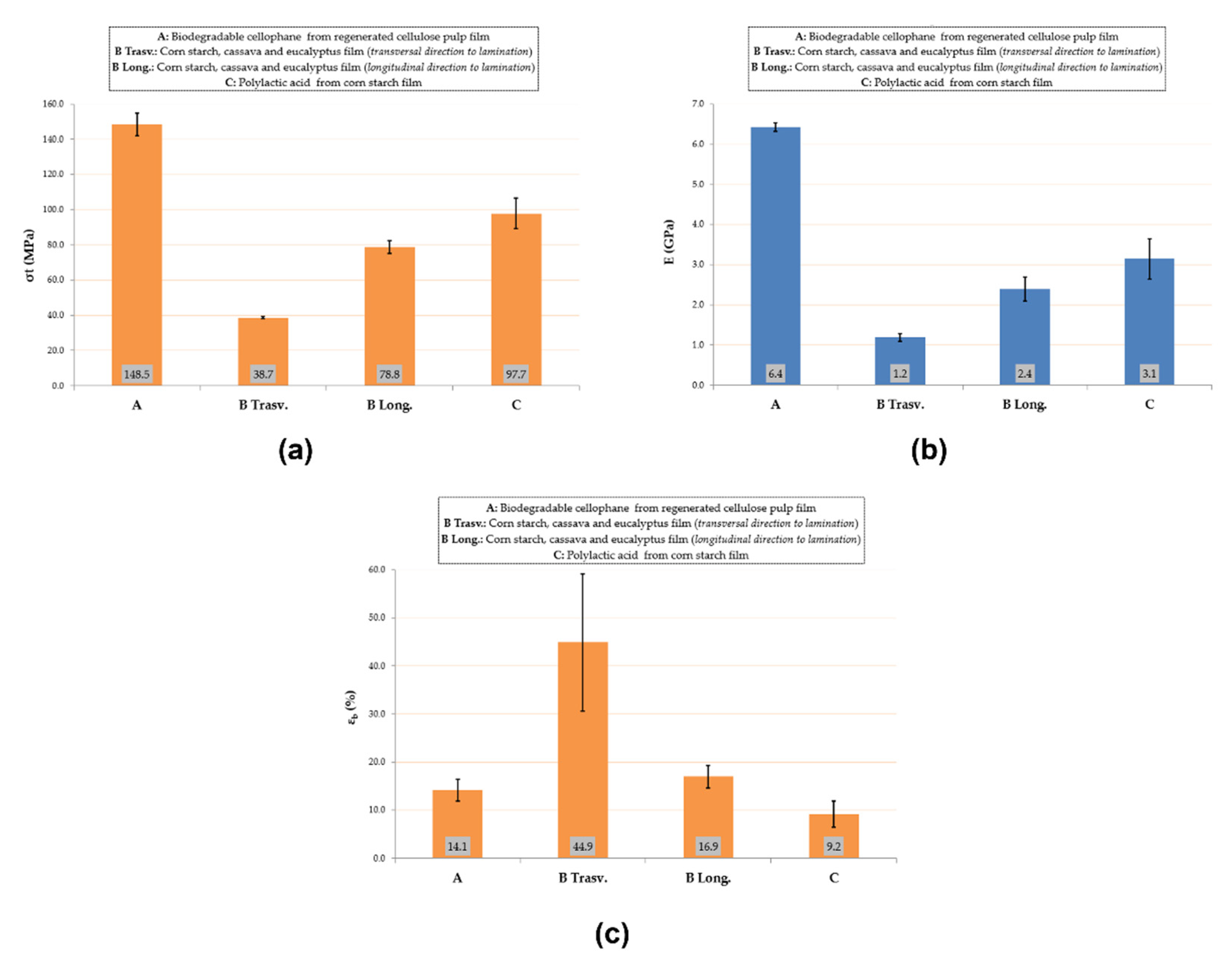
2.3.1. Mechanical Characterization: Tensile Test
2.3.2. Gas Permeability Testing: Oxygen Transmission Rate (OTR) and Carbon Dioxide Transmission Rate (CO2TR)
2.3.3. NMR Analysis
2.3.4. Spectrophotometric Analysis of Chlorophylls, Total Carotenoids, Total Polyphenols and Antioxidant Activity
2.3.5. Biogenic Amines (BAs) Determination
2.3.6. Microbiological Analysis
2.3.7. Mass Loss Measurement
2.3.8. Water Activity Measurement
2.3.9. pH Evaluation
2.3.10. Carbon Dioxide Measurement
2.3.11. Aroma Compounds by SPME-GC-MS Analysis
2.3.12. Portable Time Domain (TD)-NMR Relaxometry
3. Results and Discussion
3.1. Mechanical and Chemical/Physical Characterization of the Commercial Biofilm
3.1.1. Mechanical Characterization: Tensile Test
3.1.2. Gas Permeability Testing: Oxygen Transmission Rate (OTR) and Carbon Dioxide Transmission Rate (CO2TR)
3.2. Chemical Profile of Fresh and Packaged Samples
3.2.1. NMR Based Metabolite Profile
3.2.2. Total Carotenoids, Chlorophylls a and b, Total Polyphenols and Antioxidant Activity
3.2.3. Biogenic Amines Content
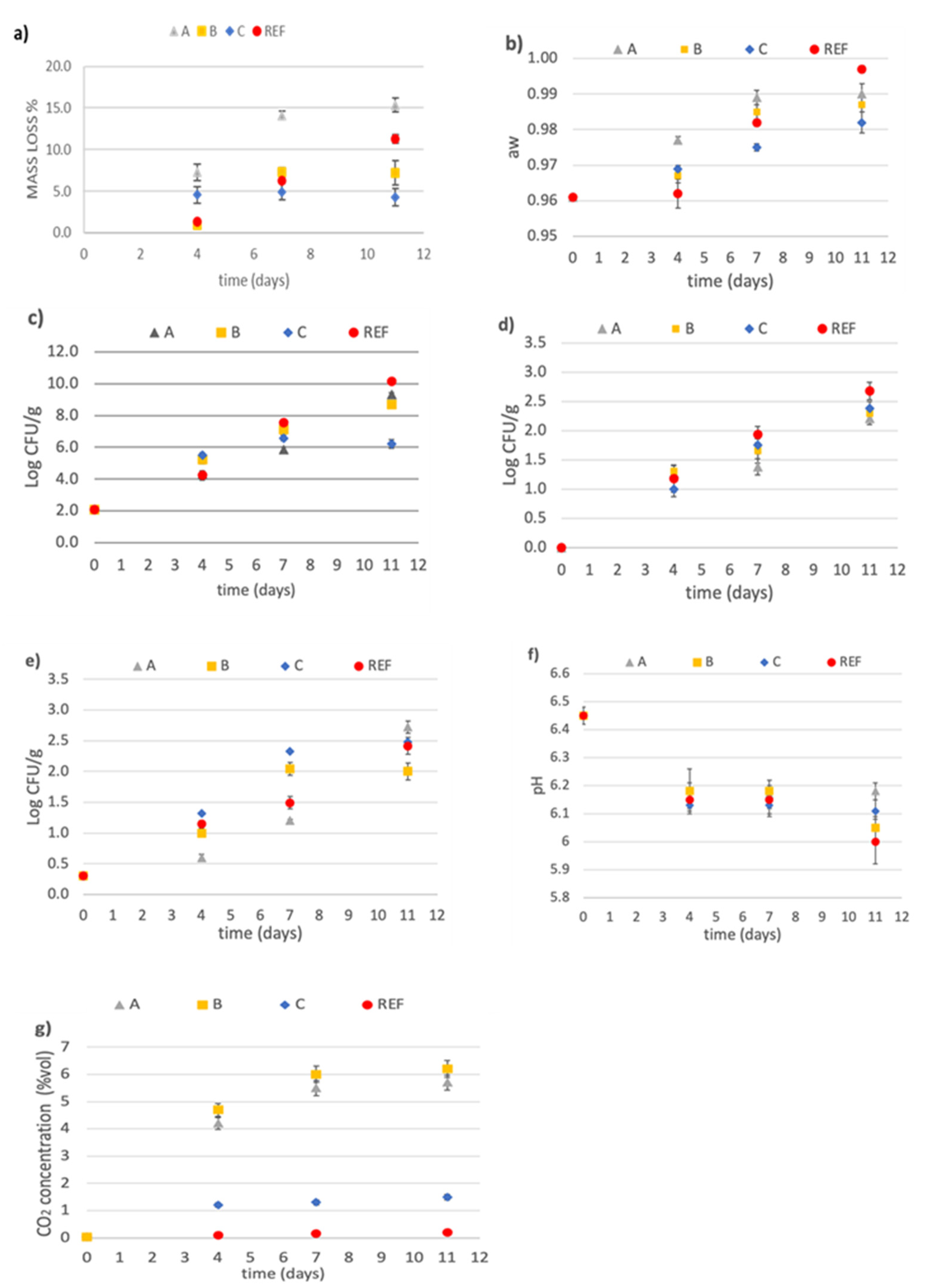
3.3. Microbiological Analysis
3.3.1. Mass Loss
3.3.2. Water Activity Evaluation
3.3.3. Microbiological Analysis
3.3.4. pH Value
3.3.5. Carbon Dioxide Headspace Concentration
3.3.6. Aroma Compounds
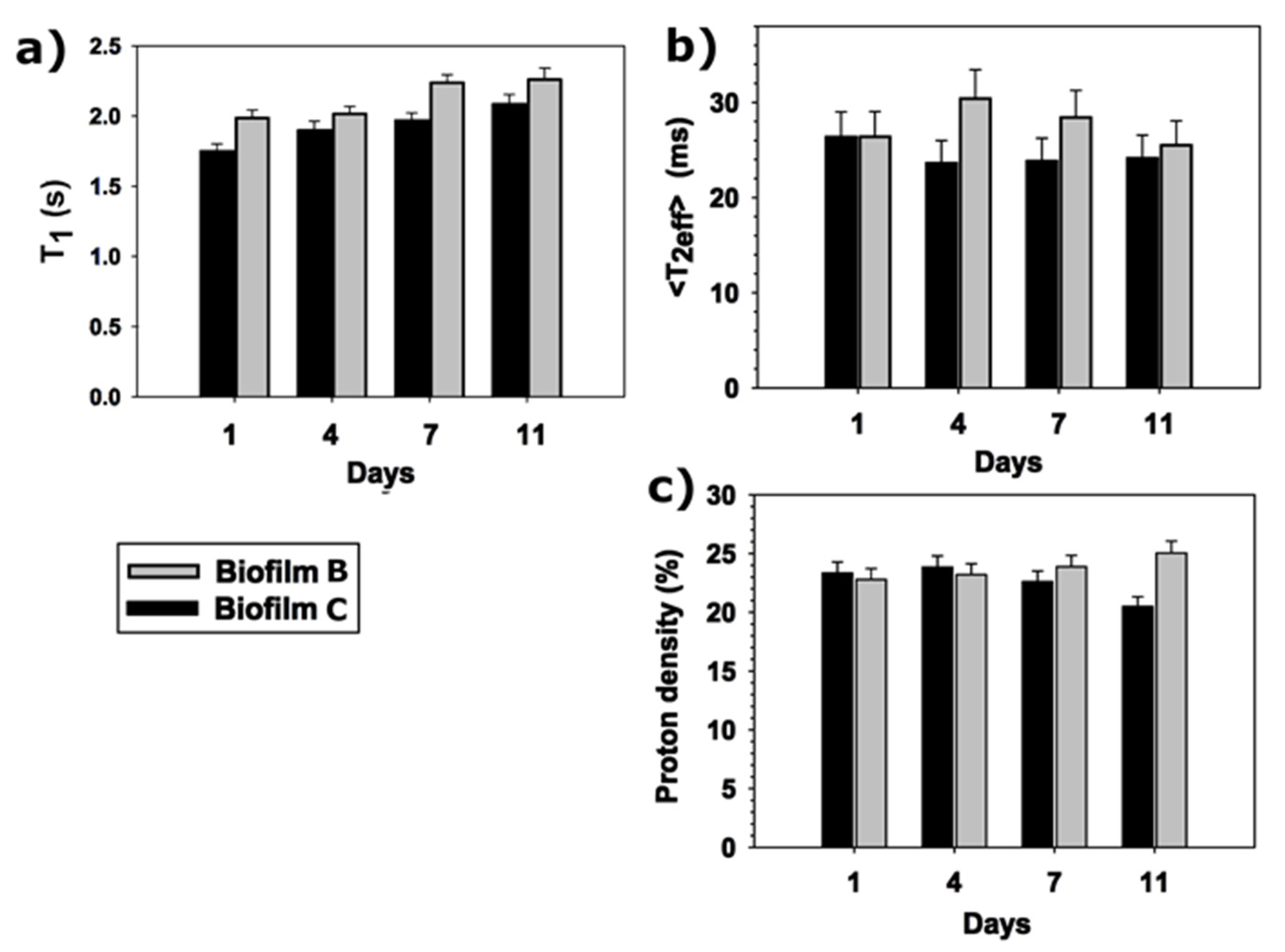
3.4. T1 and T2 Relaxation Times of Fresh Pumpkin Packaged in Biofilm B and C by Portable TD-NMR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lima, K.S.; Sanches, A.G.; Cordeiro, C.A.M. Evalution of packaging in the conservation of minimally processed squash. Appl. Res. Agrotechnol. Guarapuava-PR 2019, 12, 75–84. [Google Scholar] [CrossRef]
- Habibunnisa, R.; Baskaran, R.; Prasad, K.; Mysore, S. Storage behaviour of minimally processed pumpkin (Cucurbita maxima) under modified atmosphere packaging conditions. Eur. Food Res. Technol. 2001, 212, 165–169. [Google Scholar]
- Lucera, A.; Simsek, F.; Conte, A.; Del Nobile, M.A. Minimally processed butternut squash shelf life. J. Food Eng. 2012, 113, 322–328. [Google Scholar] [CrossRef]
- Ingallina, C.; Maccelli, A.; Spano, M.; Di Matteo, G.; Di Sotto, A.; Giusti, A.M.; Vinci, G.; Di Giacomo, S.; Rapa, M.; Ciano, S.; et al. Chemico-Biological Characterization of Torpedino Di Fondi® Tomato Fruits: A Comparison with San Marzano Cultivar at Two Ripeness Stages. Antioxidants 2020, 9, 1027. [Google Scholar] [CrossRef]
- Mannina, L.; Sobolev, A.P.; Viel, S. Liquid state 1H high field NMR in food analysis. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 66, 1–39. [Google Scholar] [CrossRef] [PubMed]
- ISO 527-3:2008 Plastics—Determination of Tensile Properties Test Conditions for Films and Sheets. Available online: https://www.iso.org/standard/70307.html (accessed on 3 October 2021).
- ISO 15105-2:2003 Plastics—Film and Sheeting—Determination of Gas-Transmission Rate—Part 2: Equal-Pressure Method. Available online: https://www.iso.org/standard/37514.html (accessed on 17 July 2021).
- Ingallina, C.; Sobolev, A.P.; Circi, S.; Spano, M.; Fraschetti, C.; Filippi, A.; Di Sotto, A.; Di Giacomo, S.; Mazzoccanti, G.; Gasparrini, F.; et al. Cannabis sativa L. inflorescences from monoecious cultivars grown in central Italy: An untargeted chemical characterization from early flowering to ripening. Molecules 2020, 25, 1908. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, A.P.; Segre, A.; Lamanna, R. Proton high-field NMR study of tomato juice. Magn. Reson. Chem. 2003, 41, 237–245. [Google Scholar] [CrossRef]
- Sobolev, A.P.; Mannina, L.; Capitani, D.; Sanzò, G.; Ingallina, C.; Botta, B.; Fornarini, S.; Crestoni, M.E.; Chiavarino, B.; Carradori, S.; et al. A multi-methodological approach in the study of Italian PDO “Cornetto di Pontecorvo” red sweet pepper. Food Chem. 2018, 255, 120–131. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Rapa, M.; Ciano, S.; Gobbi, L.; Ruggieri, R.; Vinci, G. Quality and safety evaluation of new tomato cultivars. Ital. J. Food Sci. 2021, 33, 35–45. [Google Scholar] [CrossRef]
- Rapa, M.; Ciano, S.; Ruggieri, R.; Vinci, G. Bioactive compounds in cherry tomatoes (Solanum lycopersicum var. Cerasiforme): Cultivation techniques classification by multivariate analysis. Food Chem. 2021, 355, 129630. [Google Scholar]
- Preti, R.; Rapa, M.; Vinci, G. Effect of streaming and boiling on the antioxidant properties and biogenic amines content in green bean (Phaselous vulgaris) varieties of different colours. J. Food Qual. 2017, 5329070. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.X.; Fu, S.F.; Bi, X.F.; Chen, F.; Liao, X.J.; Hu, X.S.; Wu, J.H. Physico-chemical and antioxidant properties of four mango (Mangifera indica L.) species in China. Food Chem. 2013, 138, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Blümich, B.; Perlo, J.; Casanova, F. Mobile single-sided NMR. Progr. Nucl. Magn. Reson. Spectrosc. 2008, 52, 197–269. [Google Scholar] [CrossRef]
- Farrar, T.C.; Becker, E.D. Pulse and Fourier Transform NMR; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Stark, N.M. Opportunities for cellulose nanomaterials in packaging films: A review and future trends. J. Renew. Mater. 2016, 4, 313–326. [Google Scholar] [CrossRef]
- Siracusa, V.; Blanco, I.; Romani, S.; Tylewicz, U.; Rocculi, P.; Rosa, M.D. Poly (lactic acid)-modified films for food packaging application: Physical, mechanical, and barrier behavior. J. Appl. Polym. Sci. 2012, 125 (Suppl. S2), E390–E401. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Ferrándiz, S.; Peltzer, M.A. Characterization of PLA-limonene blends for food packaging applications. Polym. Test. 2013, 32, 760–768. [Google Scholar] [CrossRef]
- Embleni, A. Modified atmosphere packaging and other active packaging systems for food, beverages and other fast-moving consumer goods. In Trends in Packaging of Food, Beverages and Other Fast-Moving Consumer Goods (FMCG); Woodhead Publishing: Cambridge, UK, 2013; pp. 22–34. [Google Scholar] [CrossRef]
- Siracusa, V. Food packaging permeability behaviour: A report. Int. J. Polym. Sci. 2012, 2012, 302029. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G.; Syverud, K. On the structure and oxygen transmission rate of biodegradable cellulose nanobarriers. Nanoscale Res. Lett. 2012, 7, 192. [Google Scholar] [CrossRef]
- Available online: http://www.ecosign-project.eu/wp-content/uploads/2018/09/FOOD_UNIT11_IT_Lecture.pdf (accessed on 10 July 2021).
- Piscopo, A.; Zappia, A.; De Bruno, A.; Pozzo, S.; Limbo, S.; Piergiovanni, L.; Poiana, M. Use of biodegradable materials as alternative packaging of typical Calabrian Provola cheese. Food Packag. Shelf Life 2019, 21, 100351. [Google Scholar] [CrossRef] [Green Version]
- Peelman, N.; Ragaert, P.; Vandemoortele, A.; Verguldt, E.; Devlieghere, F.; De Meulenaer, B. Application of biobased materials for packing short, medium and long shelf life food products. In Proceedings of the 26th IAPRI symposium on Packaging 2013, Espoo, Finland, 10–13 June 2013; VTT Technical Research Centre of Finland. pp. 494–498. [Google Scholar]
- Available online: https://thevacuumpouch.co.uk/wp-content/uploads/2021/03/VPC-Eco-Pouch-Spec-Sheet-50mu.pdf (accessed on 10 July 2021).
- Panseri, S.; Martino, P.A.; Cagnardi, P.; Celano, G.; Tedesco, D.; Castrica, M.; Chiesa, L.M. Feasibility of biodegradable based packaging used for red meat storage during shelf-life: A pilot study. Food Chem. 2018, 249, 22–29. [Google Scholar] [CrossRef]
- Tripathi, N.; Katiyar, V. PLA/functionalized-gum arabic based bionanocomposite films for high gas barrier applications. J. Appl. Polym. Sci. 2016, 133–139. [Google Scholar] [CrossRef]
- Ingallina, C.; Sobolev, A.P.; Circi, S.; Spano, M.; Giusti, A.M.; Mannina, L. New hybrid tomato cultivars: An NMR-based chemical characterization. Appl. Sci. 2020, 10, 1887. [Google Scholar] [CrossRef] [Green Version]
- Sobolev, A.P.; Brosio, E.; Gianferri, R.; Segre, A.L. Metabolic profile of lettuce leaves by high-field NMR spectra. Magn. Reson. Chem. 2005, 43, 625–638. [Google Scholar] [CrossRef] [Green Version]
- Schneider, T.; Butz, P.; Ludwig, H.; Tauscher, B. Pressure-induced formation of pyroglutamic acid from glutamine in neutral and alkaline solutions. LWT—Food Sci. Technol. 2003, 36, 365–367. [Google Scholar] [CrossRef]
- Liang, T.; Wei, F.; Lu, Y.; Kodani, Y.; Nakada, M.; Miyakawa, T.; Tanokura, M. Comprehensive NMR analysis of compositional changes of black garlic during thermal processing. J. Agric. Food Chem. 2015, 63, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 10, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Kulczyński, B.; Gramza-Michałowska, A. The Profile of Secondary Metabolites and Other Bioactive Compounds in Cucurbita pepo L. and Cucurbita moschata Pumpkin Cultivars. Molecules 2019, 24, 2945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Can-Cauich, C.A.; Sauri-Duch, E.; Victor, M.M.; Betancur-Ancona, D.; Cuevas-Glory, L.F. Effect of extraction method and specie on the content of bioactive compounds and antioxidant activity of pumpkin oil from Yucatan, Mexico. Food Chem. 2019, 285, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Świder, O.; Roszko, M.L.; WòJcicki, M.; Szymczyk, K. Biogenic amines and free amino acids in traditional fermented vegetables –dietary risk evaluation. J. Agric. Food Chem. 2020, 68, 856–868. [Google Scholar] [CrossRef]
- Sánchez-Pérez, S.; Comas-Basté, O.; Rabell-González, J.; Veciana-Nogués, M.; Latorre-Moratalla, M.; Vidal-Carou, M. Biogenic amines in plant-origin foods: Are they frequently underestimated in low-histamine diets? Foods 2018, 7, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavizzari, T.; Veciana-Nogués, M.T.; Weingart, O.; Bover-Cid, S.; Mariné-Font, A.; Vidal-Carou, M.C. Occurrence of biogenic amines and polyamines in spinach and changes during storage under refrigeration. J. Agric. Food Chem. 2007, 55, 9514–9519. [Google Scholar] [CrossRef] [PubMed]
- Jay, J.M.; Golden, D.A.; Loessner, M.J. Modern Food Microbiology, 7th ed.; Springer: New York, NY, USA, 2005. [Google Scholar]
- Ministere de l’Economie des Finances et du Budget. Marché consommation, Produits vegetaux prets a l’emploi dits de la ‘IVemme Gamme’: Guide de bonnes pratique hygieniques. J. Off. De La Repub. Fr. 1988, 1621, 1–29. Available online: https://catalogue.bnf.fr/ark:/12148/cb350090367 (accessed on 13 October 2021).
- Corbo, M.R.; Altieri, C.; D’Amato, D.; Campaniello, D.; Del Nobile, M.A.; Sinigaglia, M. Effect of temperature on shelf life and microbial population of lightly processed cactus pear fruit. Postharvest Biol. Technol. 2004, 31, 93–104. [Google Scholar] [CrossRef]
- Gutiérrez-López, G.; Barbosa-Canóvas, G.; WeltiChanes, J.; Parada-Arias, E. Use of Tapioca Starch Edible Film Containing Potassium Sorbate to Extend the Shelf Life of Minimally Processed Pumpkin. In Food Engineering: Integrated Approaches; Springer: New York, NY, USA, 2008; pp. 226–229. [Google Scholar]
- Guzman Norleyn, M.N.; Orellana, L.E.; Obregon Quinones, L.G. Shelf Life of a Mixture of Pumpkin Puree (Cucurbita moschata) During Storage at 4 °C. Adv. J. Food Sci. Technol. 2018, 15, 152–157. [Google Scholar] [CrossRef]
- Lucera, A.; Costa, C.; Mastromatteo, M.; Conte, A.; Del Nobile, M.A. Influence of different packaging systems on fresh-cut zucchini (Cucurbita pepo). Innov. Food Sci. Emerg. Technol. 2010, 11, 361–368. [Google Scholar] [CrossRef]
- Zhou, C.L.; Mi, L.; Hu, X.Y.; Zhu, B.H. Evaluation of three pumpkin species: Correlation with physicochemical, antioxidant properties and classification using SPME-GC–MS and E-nose methods. J. Food Sci. Technol. 2017, 54, 3118–3131. [Google Scholar] [CrossRef]
- Snaar, J.E.M.; Van As, H. Probing water compartments and membrane permeability in plantcells by 1H NMR relaxation measurements. Biophys. J. 1992, 63, 1654–1658. [Google Scholar] [CrossRef] [Green Version]
- Hills, B.P.; Remigereau, B. NMR studies of changes in subcellular water compartmentation in parenchyma apple tissue during drying and freezing. Int. J. Food Sci. Tech. 1997, 32, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.H.; Wellard, R.M.; Nagy, S.A.; Joardder, M.U.H.; Karim, M.A. Investigation of bound and free water in plat-based food material using NMR T2 relaxometry. Innov. Food Sci. Emerg. 2016, 38, 252–261. [Google Scholar] [CrossRef]
- Proietti, N.; Adiletta, G.; Russo, P.; Buonocore, R.; Mannina, L.; Crescitelli, V.; Capitani, D. Evolution of physicochemical properties of pear during drying by conventional techniques, portable-NMR, and modelling. J. Food Eng. 2018, 230, 82–98. [Google Scholar] [CrossRef]






| Biofilm Commercial | Biofilm Composition | Thickness (µm) (Measured Value) | OTR (cm3⸱m−2⸱day−1) (Measured Value) | CO2TR (cm3⸱m−2⸱day−1) (Measured Value) |
|---|---|---|---|---|
| A | Biodegradable cellophane from regenerated cellulose pulp | 20 | 4.91 ± 0.27 | 10.78 ± 0.50 |
| B | Corn starch, cassava and eucalyptus | 40 | 4.30 ± 0.20 | 13.42 ± 0.71 |
| C | Polylactic acid from corn starch | 30 | 646.41 ± 32.33 | 2162.51 ± 110.13 |
| ppm | Compound | ppm | Compound |
|---|---|---|---|
| 0.94 | Isoleucine | 4.18 | Pyro-glutamate |
| 0.97 | Leucine | 4.30 | Malic acid |
| 1.05 | Valine | 4.60 | β-Galactose |
| 1.34 | Threonine | 4.66 | β-Glucose |
| 1.49 | Alanine | 5.25 | α-Glucose |
| 1.70 | Arginine | 5.28 | α-Galactose |
| 2.30 | GABA | 5.42 | Sucrose |
| 2.35 | Glutamic acid | 6.53 | Fumaric acid |
| 2.46 | Glutamine | 6.91 | Tyrosine |
| 2.54 | Citric acid | 7.44 | Phenylalanine |
| 2.81 | Aspartic acid | 7.55 | Tryptophan |
| 2.89 | Asparagine | 8.15 | Istidine |
| 3.21 | Choline | 8.47 | Formic acid |
| 3.30 | Myo-inositol | 8.84 | Trigonelline |
| 4.12 | Fructose |
| FP | A | B | C | REF | |
|---|---|---|---|---|---|
| Carbohydrates | |||||
| Sucrose | 759.42 ± 21.09 | 1425.34 ± 44.62 | 1026.61 ± 20.09 | 989.41 ± 26.60 | 1113.97 ± 87.08 |
| Fructose | 1095.69 ± 34.88 | 823.90 ± 26.82 | 1116.69 ± 36.09 | 1005.36 ± 9.47 | 1169.32 ± 135.70 |
| Glucose | 987.47 ± 36.58 | 641.06 ± 31.04 | 1053.60 ± 9.43 | 879.11 ± 15.30 | 1025.42 ± 125.89 |
| Mio-inositol | 170.66 ± 9.00 | 165.97 ± 2.98 | 147.79 ± 6.82 | 156.90 ± 5.16 | 162.15 ± 15.43 |
| Galactose | 80.97 ± 6.46 | 53.74 ± 2.68 | 67.18 ± 1.46 | 63.35 ± 0.58 | 70.46 ± 7.06 |
| Organic acids | |||||
| Malic acid | 340.84 ± 1.41 | 301.09 ± 5.97 | 255.01 ± 2.20 | 277.07 ± 1.38 | 178.78 ± 1.05 |
| Citric acid | 20.52 ± 0.77 | 34.76 ± 0.72 | 18.82 ± 0.24 | 36.14 ± 4.27 | 22.54 ± 0.11 |
| Fumaric acid | 0.94 ± 0.09 | 1.10 ± 0.04 | 1.81 ± 0.14 | 0.80 ± 0.05 | 1.35 ± 0.06 |
| Formic acid | 0.59 ± 0.19 | 0.69 ± 0.12 | 0.89 ± 0.06 | 0.73 ± 0.06 | 0.78 ± 0.10 |
| Amino acids | |||||
| Aspartate | 491.06 ± 16.94 | 456.09 ± 5.84 | 322.02 ± 2.76 | 457.88 ± 19.53 | 532.23 ± 47.67 |
| Glutamine | 272.77 ± 15.89 | 259.64 ± 4.08 | 196.37 ± 6.30 | 240.50 ± 5.44 | 286.30 ± 32.78 |
| Asparagine | 297.37 ± 8.98 | 284.24 ± 5.11 | 256.66 ± 2.86 | 238.47 ± 0.27 | 249.83 ± 20.12 |
| Arginine | 71.17 ± 0.20 | 70.62 ± 1.36 | 51.75 ± 0.78 | 63.40 ± 1.15 | 44.85 ± 4.30 |
| Pyro-glutamate | 57.24 ± 5.45 | 51.39 ± 2.01 | 37.13 ± 0.87 | 58.49 ± 8.72 | 40.31 ± 3.40 |
| Tyrosine | 55.17 ± 1.02 | 48.70 ± 0.70 | 35.85 ± 0.24 | 48.02 ± 0.45 | 46.05 ± 4.60 |
| Alanine | 12.47 ± 0.58 | 8.79 ± 0.07 | 43.42 ± 2.31 | 35.32 ± 1.35 | 9.47 ± 0.92 |
| Glutamate | 19.83 ± 1.58 | 15.46 ± 0.08 | 18.37 ± 1.80 | 36.07 ± 3.02 | 17.52 ± 1.33 |
| GABA | 29.99 ± 1.38 | 30.80 ± 1.94 | 28.03 ± 0.04 | 26.34 ± 0.05 | 24.13 ± 2.54 |
| Phenylalanine | 21.90 ± 0.63 | 20.26 ± 0.32 | 15.88 ± 0.17 | 20.90 ± 0.21 | 20.65 ± 1.82 |
| Isoleucine | 16.00 ± 1.04 | 18.07 ± 0.50 | 14.87 ± 0.59 | 12.71 ± 0.13 | 12.78 ± 1.71 |
| Histidine | 14.44 ± 0.13 | 15.99 ± 0.04 | 11.64 ± 0.76 | 13.07 ± 0.07 | 11.64 ± 1.40 |
| Valine | 16.22 ± 0.90 | 16.77 ± 0.46 | 13.82 ± 0.15 | 13.52 ± 0.24 | 13.54 ± 1.59 |
| Threonine | 15.87 ± 0.78 | 15.81 ± 0.22 | 14.83 ± 0.65 | 14.18 ± 0.01 | 15.18 ± 1.57 |
| Tryptophan | 12.31 ± 0.20 | 12.69 ± 0.75 | 8.50 ± 0.04 | 12.94 ± 0.10 | 10.33 ± 1.14 |
| Leucine | 2.04 ± 0.14 | 2.35 ± 0.11 | 2.02 ± 0.05 | 1.54 ± 0.05 | 1.12 ± 0.24 |
| Miscellaneous metabolites | |||||
| Trigonelline | 10.99 ± 0.30 | 9.49 ± 0.46 | 9.01 ± 0.43 | 14.65 ± 0.18 | 8.39 ± 0.68 |
| Choline | 9.99 ± 0.49 | 7.98 ± 0.14 | 6.23 ± 0.02 | 9.50 ± 0.24 | 7.66 ± 0.75 |
| FP | A | B | C | REF | |
|---|---|---|---|---|---|
| Tot SFA | 63.47 ± 0.92 | 58.90 ± 1.84 | 62.56 ± 0.78 | 62.69 ± 0.79 | 63.97 ± 3.51 |
| Tot UFA | 36.53 ± 0.92 | 41.10 ± 1.84 | 37.44 ± 0.78 | 37.31 ± 0.79 | 36.04 ± 3.51 |
| TUFA | 23.31 ± 0.17 | 25.52 ± 0.37 | 25.44 ± 0.25 | 25.23 ± 0.17 | 27.81 ± 1.26 |
| MUFA | 5.19 ± 1.13 | 7.19 ± 2.60 | 3.45 ± 1.10 | 4.88 ± 0.56 | 2.79 ± 2.20 |
| DUFA | 8.02 ± 0.04 | 8.40 ± 0.39 | 8.56 ± 0.07 | 7.20 ± 0.05 | 5.43 ± 0.05 |
| Compound | Retention Time (min) | Δ Area, % |
|---|---|---|
| Ethanol | 2.560 | 22.23 ± 0.12 |
| Hexanal | 3.951 | −31.50 ± 1.23 |
| Ethyl acetate | 4.998 | 1.05 ± 0.24 |
| 1-Hexanol | 5.347 | −15.03 ± 2.87 |
| 1-Octen-3-ol | 8.162 | −14.00 ± 2.26 |
| 3-Hexen-1-ol | 9.032 | −11.71 ± 1.58 |
| 2 cyclohexen-1-ol-2.4.4-trimethyl | 10.223 | −5.10 ± 1.47 |
| 2-octenal | 10.388 | 14.47 ± 1.51 |
| iso-phorone | 10.445 | 8.90 ± 1.04 |
| Eucalyptol | 10.765 | −12.36 ± 1.78 |
| 6-nonenal | 11.681 | 17.40 ± 1.24 |
| 2–6 nonadienal | 13.123 | −22.47 ± 0.60 |
| 6-nonen-1-ol | 13.621 | −27.11 ± 0.41 |
| Decanal | 14.616 | 13.49 ± 0.17 |
| 1-cyclohexene-1-carboxaldehyde-2,6,6-trimethyl | 15.068 | −6.32 ± 0.04 |
| 1-cyclohexene-1-acetaldehyde-2,6,6-trimethyl | 16.081 | −7.45 ± 0.02 |
| Tetradecane | 19.806 | 6.64 ± 0.05 |
| Naftalene 1,4-dimethyl | 20.281 | −0.50 ± 0.06 |
| 3-buten-2-one,4-(2,6,6-trimethyl-1-cyclohexen-1-yl) | 20.544 | −26.14 ± 0.01 |
| 5,9-undecadien-2-one,6,10-dimethyl | 21.071 | −11.47 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Matteo, G.; Di Matteo, P.; Sambucci, M.; Tirillò, J.; Giusti, A.M.; Vinci, G.; Gobbi, L.; Prencipe, S.A.; Salvo, A.; Ingallina, C.; et al. Commercial Bio-Packaging to Preserve the Quality and Extend the Shelf-Life of Vegetables: The Case-Study of Pumpkin Samples Studied by a Multimethodological Approach. Foods 2021, 10, 2440. https://doi.org/10.3390/foods10102440
Di Matteo G, Di Matteo P, Sambucci M, Tirillò J, Giusti AM, Vinci G, Gobbi L, Prencipe SA, Salvo A, Ingallina C, et al. Commercial Bio-Packaging to Preserve the Quality and Extend the Shelf-Life of Vegetables: The Case-Study of Pumpkin Samples Studied by a Multimethodological Approach. Foods. 2021; 10(10):2440. https://doi.org/10.3390/foods10102440
Chicago/Turabian StyleDi Matteo, Giacomo, Paola Di Matteo, Matteo Sambucci, Jacopo Tirillò, Anna Maria Giusti, Giuliana Vinci, Laura Gobbi, Sabrina Antonia Prencipe, Andrea Salvo, Cinzia Ingallina, and et al. 2021. "Commercial Bio-Packaging to Preserve the Quality and Extend the Shelf-Life of Vegetables: The Case-Study of Pumpkin Samples Studied by a Multimethodological Approach" Foods 10, no. 10: 2440. https://doi.org/10.3390/foods10102440
APA StyleDi Matteo, G., Di Matteo, P., Sambucci, M., Tirillò, J., Giusti, A. M., Vinci, G., Gobbi, L., Prencipe, S. A., Salvo, A., Ingallina, C., Spano, M., Sobolev, A. P., Proietti, N., Di Tullio, V., Russo, P., Mannina, L., & Valente, M. (2021). Commercial Bio-Packaging to Preserve the Quality and Extend the Shelf-Life of Vegetables: The Case-Study of Pumpkin Samples Studied by a Multimethodological Approach. Foods, 10(10), 2440. https://doi.org/10.3390/foods10102440





















