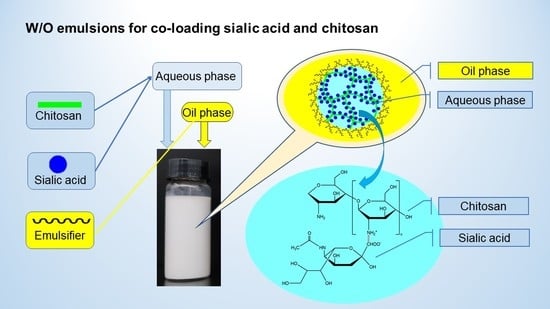
Novel Water-in-Oil Emulsions for Co-Loading Sialic Acid and Chitosan: Formulation, Characterization, and Stability Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Aqueous Phase and W/O Emulsions
2.3. High-Resolution Field Emission Scanning Electron Microscope (HRFESEM)
2.4. Fourier Transformed Infrared Spectroscopy (FT-IR)
2.5. X-ray Diffraction (XRD)
2.6. Thermogravimetric Analysis (TG)
2.7. Microstructure Measurement
2.8. Droplet Size Distribution
2.9. Rheological Characterization
2.10. Storage Stability
2.11. Statistical Analysis
3. Results and Discussion
3.1. HRFESEM of the SA-Chitosan Complex
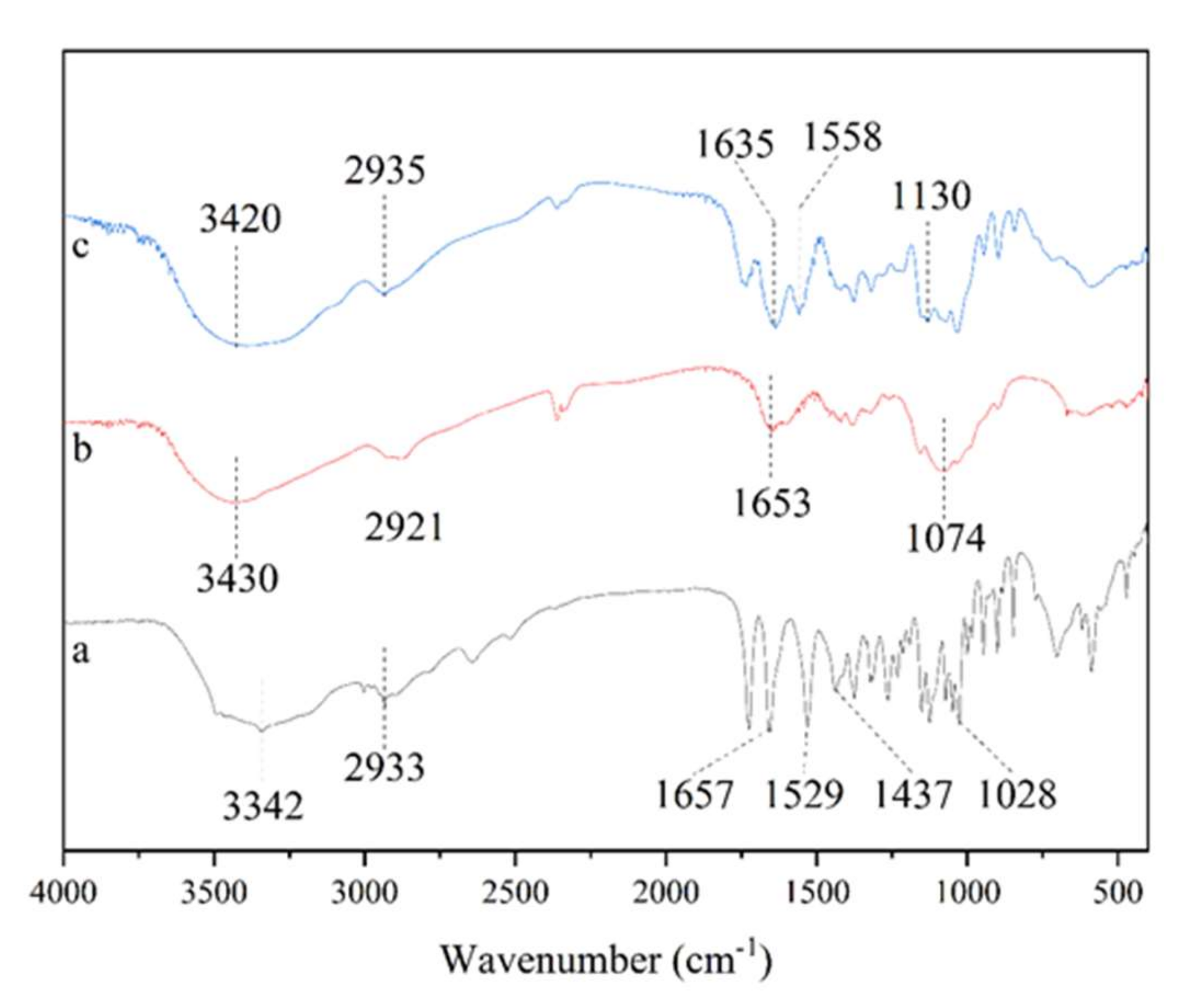
3.2. FT-IR Analysis of the SA-Chitosan Complex
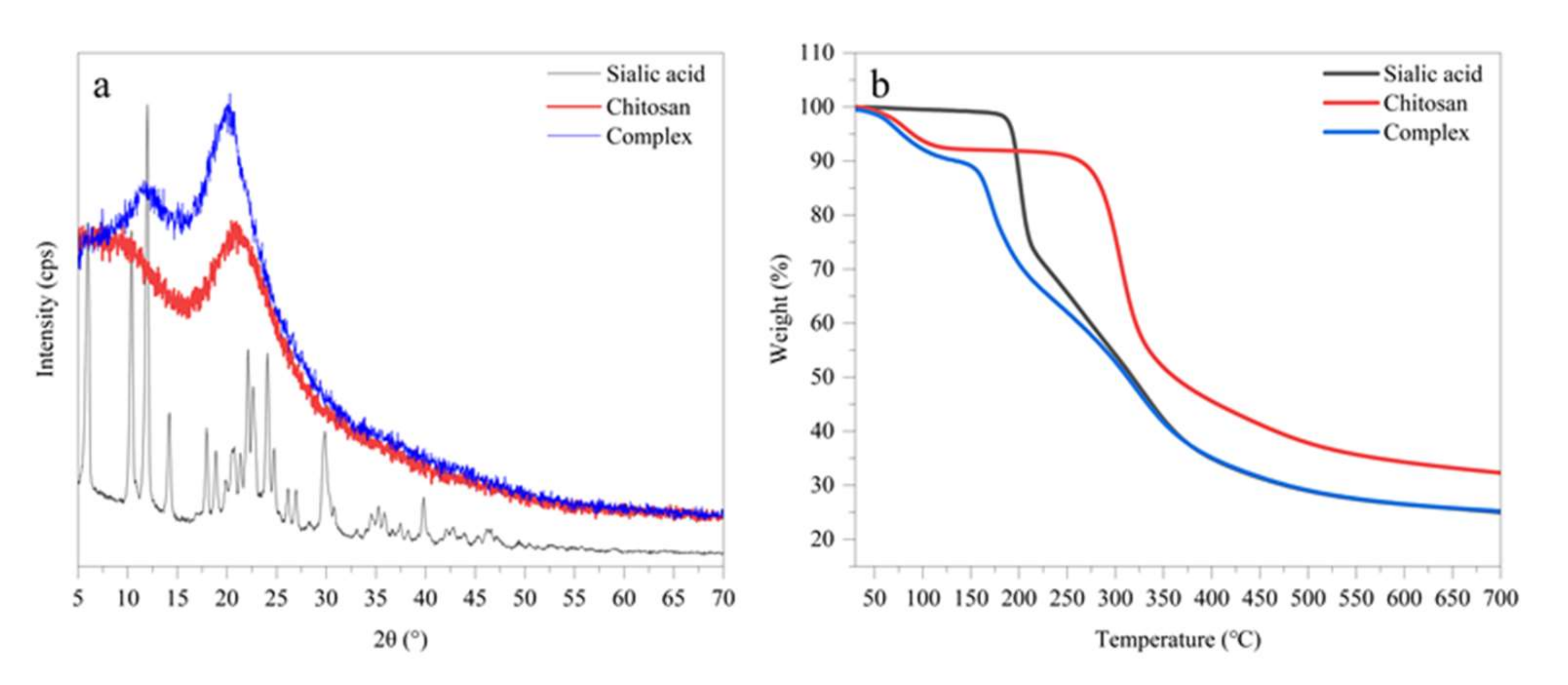
3.3. Polymorphism of the SA-Chitosan Complex
3.4. Thermogravimetric Analysis of the SA-Chitosan Complex
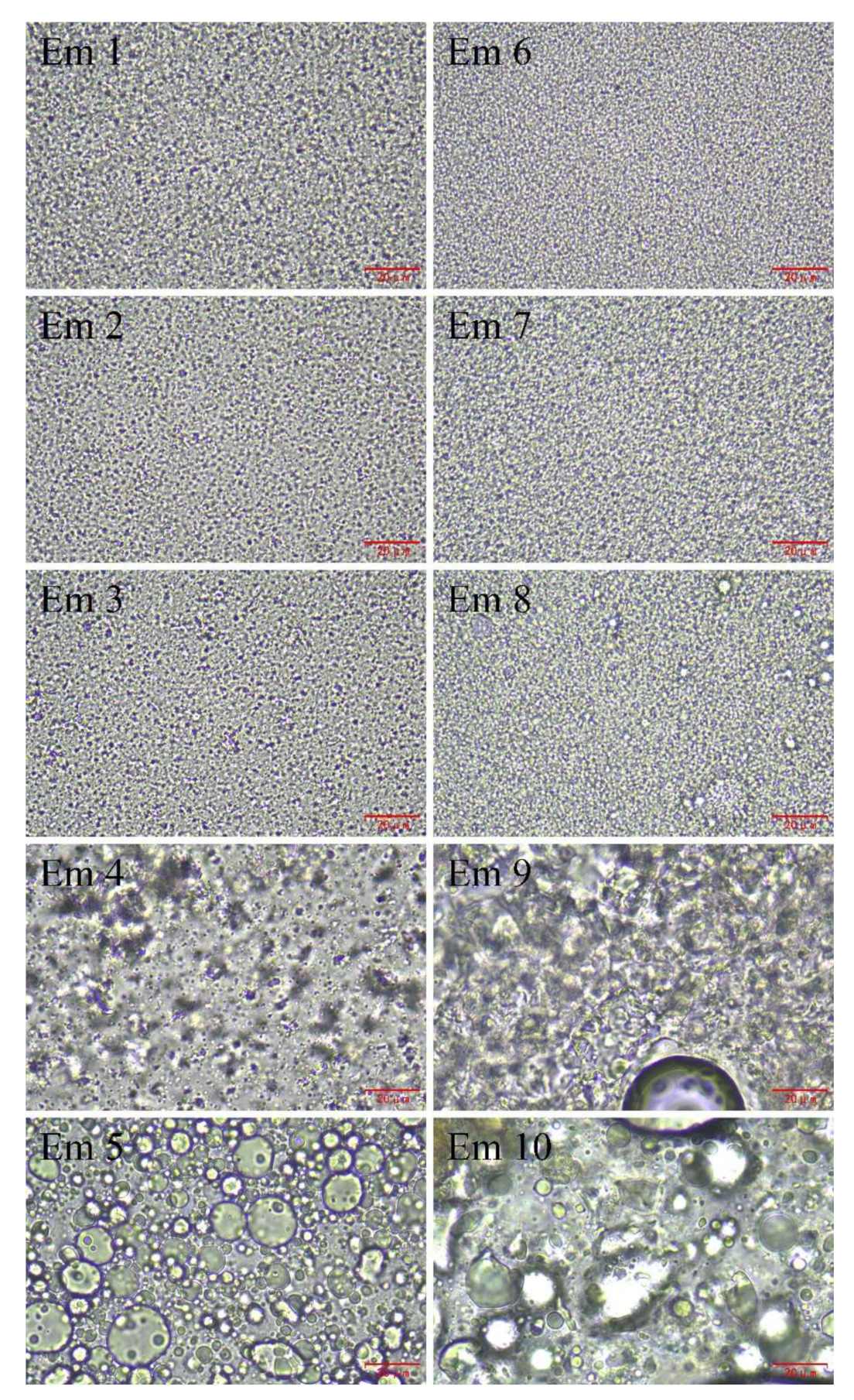
3.5. Optical Microscopy Imaging of W/O Emulsions
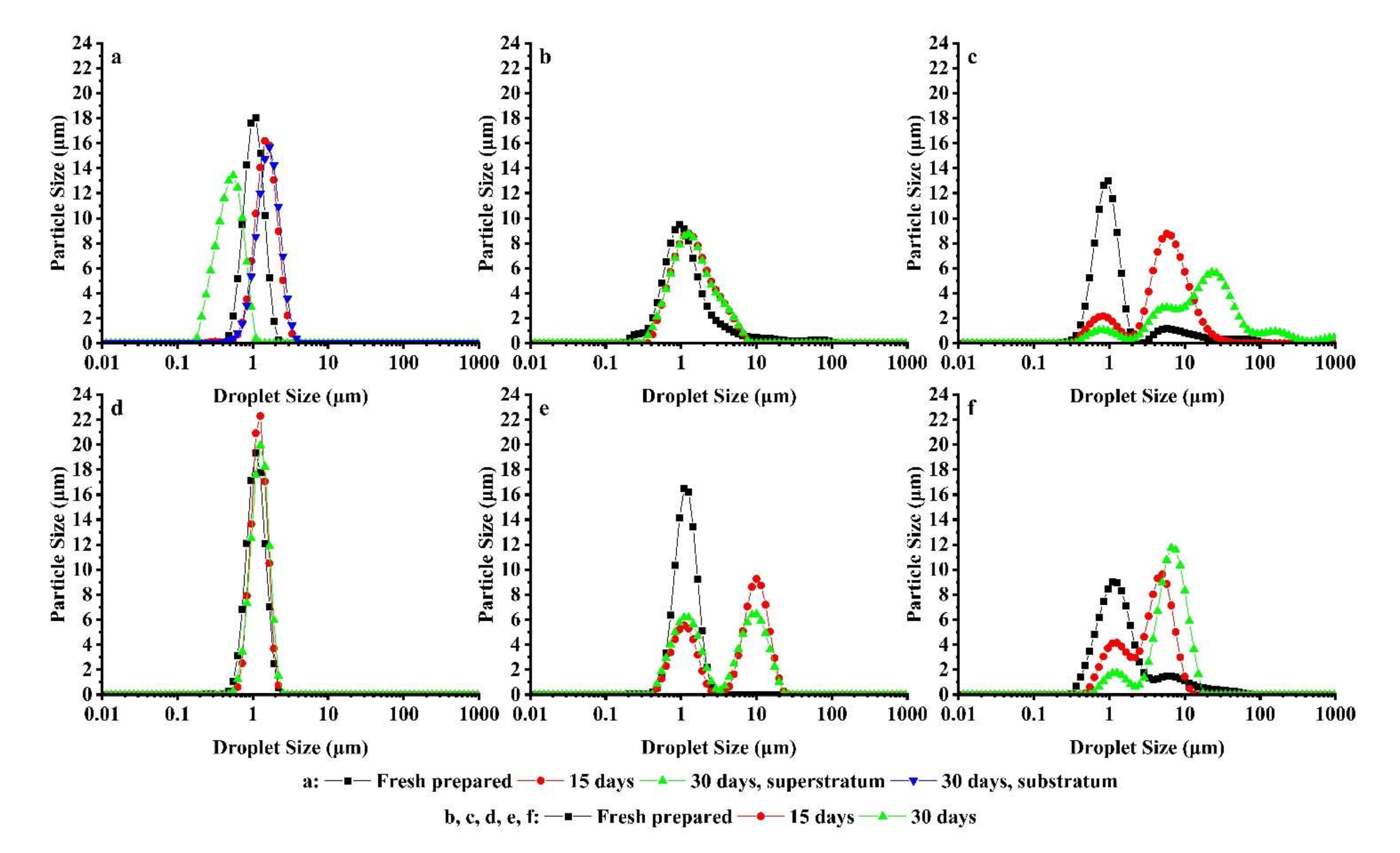
3.6. Droplet Size Distribution Analysis of W/O Emulsions
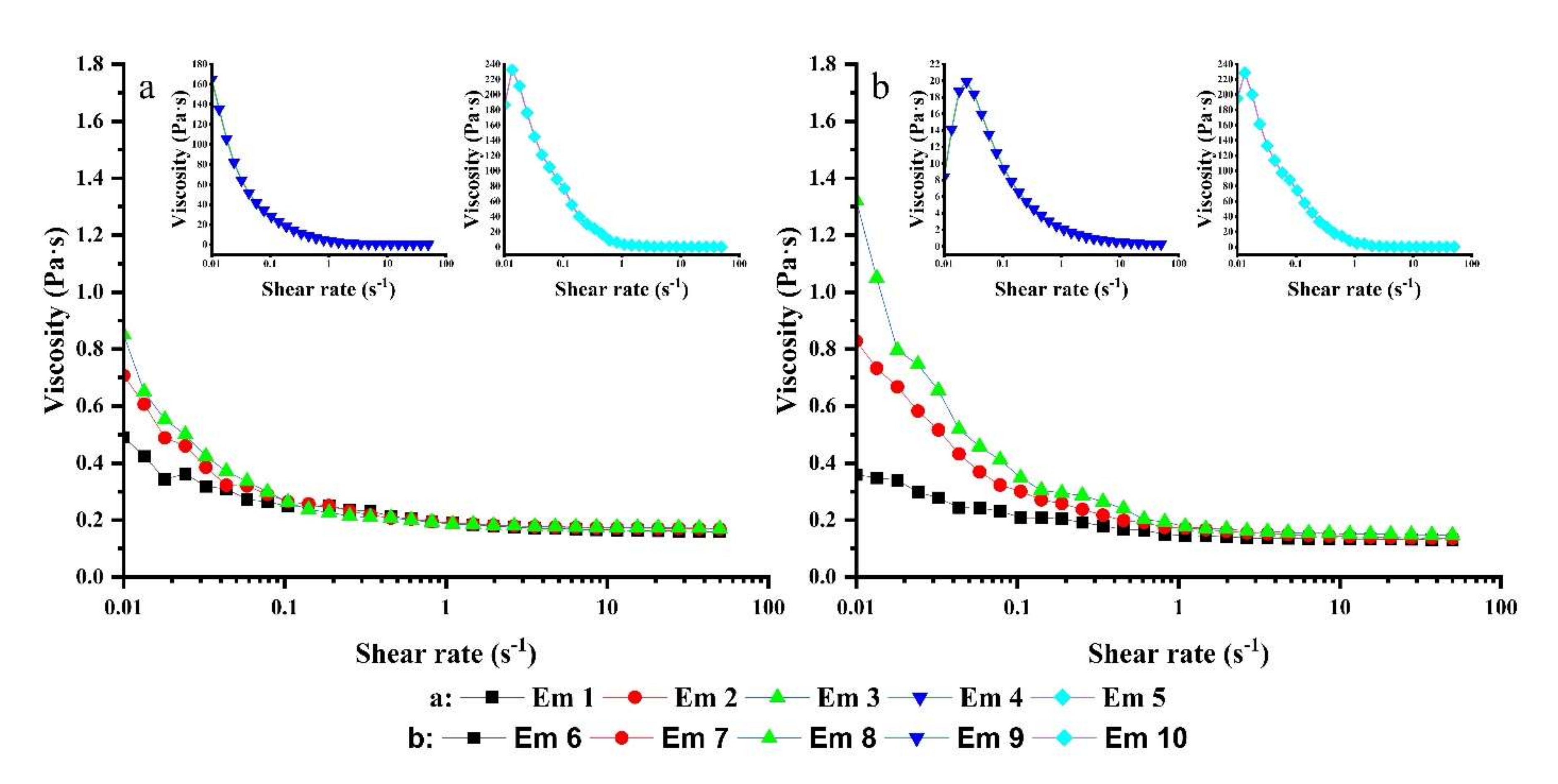
3.7. Rheological Analysis of the W/O Emulsions
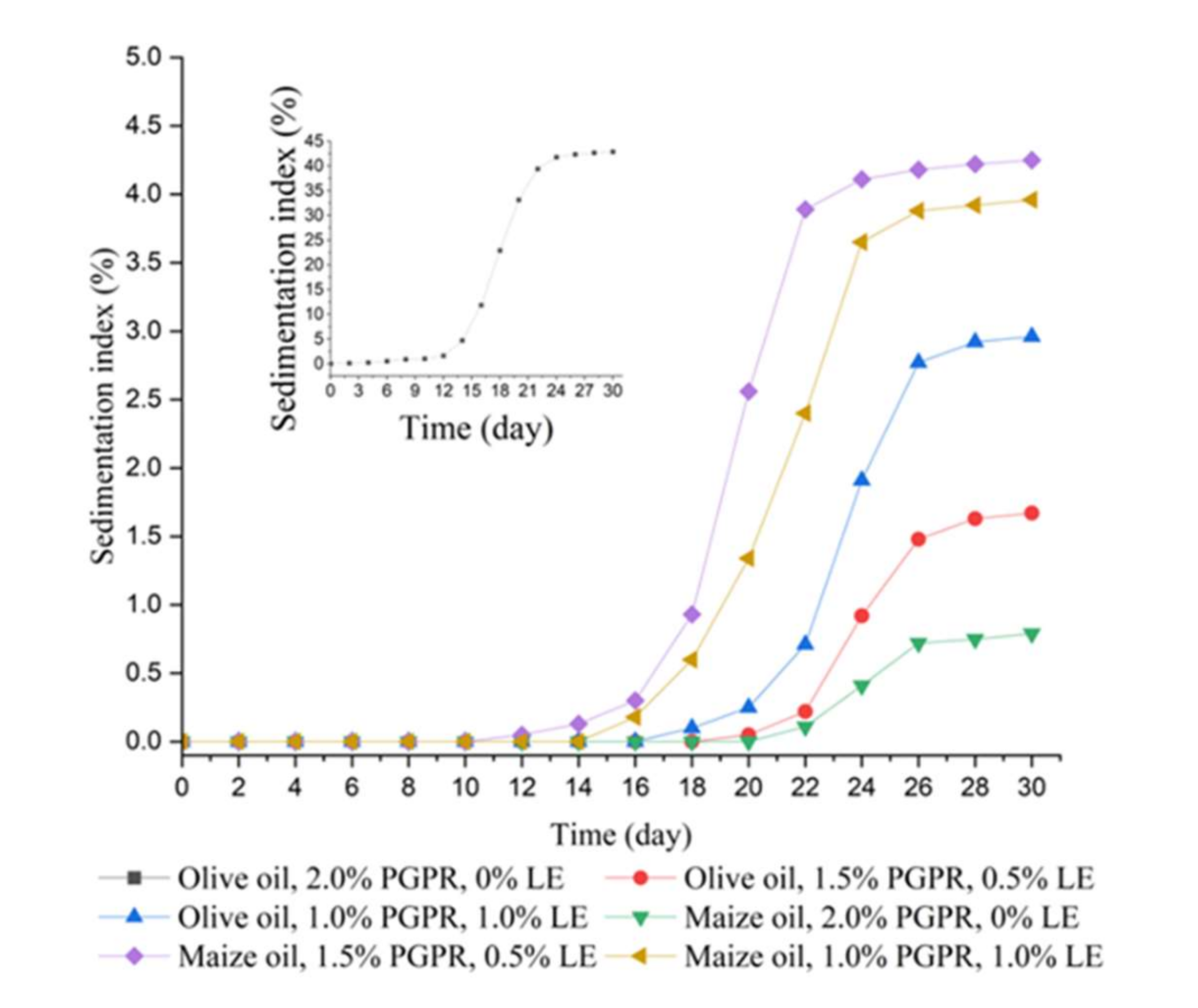
3.8. Storage Stability Evaluation of the W/O Emulsions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bande, R.M.; Prasad, B.; Mishra, I.M.; Wasewar, K.L. Oil field effluent water treatment for safe disposal by electroflotation. Chem. Eng. J. 2008, 137, 503–509. [Google Scholar] [CrossRef]
- Ushikubo, F.Y.; Cunha, R.L. Stability mechanisms of liquid water-in-oil emulsions. Food Hydrocolloid. 2014, 34, 145–153. [Google Scholar] [CrossRef]
- Chen, B.C.; Li, H.J.; Ding, Y.P.; Suo, H.Y. Formation and microstructural characterization of whey protein isolate/beet pectin coacervations by laccase catalyzed cross-linking. Lwt.-Food Sci. Technol. 2012, 47, 31–38. [Google Scholar] [CrossRef]
- Knoth, A.; Sdherze, I.; Muschiolik, G. Effect of lipid type on water-in-oil-emulsions stabilized by phosphatidylcholine-depleted lecithin and polyglycerol polyricinoleate. Eur. J. Lipid. Sci. Tech. 2005, 107, 857–863. [Google Scholar] [CrossRef]
- Li, J.L.; Qiao, Z.H.; Tatsumi, E.; Saito, M.; Cheng, Y.Q.; Yin, L.J. A Novel Approach to Improving the Quality of Bittern-Solidified Tofu by W/O Controlled-Release Coagulant. 1: Preparation of W/O Bittern Coagulant and Its Controlled-Release Property. Food Bioprocess Tech. 2013, 6, 1790–1800. [Google Scholar] [CrossRef]
- Wan, J.; Bick, A.; Sullivan, M.; Stone, H.A. Controllable microfluidic production of microbubbles in water-in-oil emulsions and the formation of porous microparticles. Adv. Mater. 2008, 20, 3314–3318. [Google Scholar] [CrossRef]
- Raviadaran, R.; Ng, M.H.; Manickam, S.; Chandran, D. Ultrasound-assisted water-in-palm oil nano-emulsion: Influence of polyglycerol polyricinoleate and NaCl on its stability. Ultrason. Sonochem. 2019, 52, 353–363. [Google Scholar] [CrossRef]
- Kralova, I.; Sjoblom, J. Surfactants Used in Food Industry: A Review. J. Disper. Sci. Technol. 2009, 30, 1363–1383. [Google Scholar] [CrossRef]
- Christiansen, K. PGPR, Polyglycerolpolyricinoleate, E476; Emulsifiers in Food Technology; John Wiley&Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Atik, D.S.; Boluk, E.; Toker, O.S.; Palabiyik, I.; Konar, N. Investigating the effects of lLecithin-PGPR mixture on physical properties of milk chocolate. Lwt.-Food Sci. Technol. 2020, 129, 109548. [Google Scholar] [CrossRef]
- Blix, F.G.; Gottschalk, A.; Klenk, E. Proposed nomenclature in the field of neuraminic and sialic acids. Nature 1957, 179, 1088. [Google Scholar] [CrossRef]
- Chen, X.; Varki, A. Advances in the biology and chemistry of sialic acids. ACS Chem. Biol. 2010, 5, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M.; Loo, D.D.; Hirayama, B.A.; Turk, E. Surprising versatility of Na+-glucose cotransporters: SLC5. Physiology 2004, 19, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; She, Z.; Huang, Z.; Li, J.; Luo, X.; Deng, Y. Application of sialic acid/polysialic acid in the drug delivery systems. Sian. J. Pharm. Sci. 2014, 9, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Sato, C.; Kitajima, K. Disialic, oligosialic and polysialic acids: Distribution, functions and related disease. J. Biochem. 2013, 154, 115–136. [Google Scholar] [CrossRef] [Green Version]
- Tao, F.; Zhang, Y.; Ma, C.; Xu, P. Biotechnological production and applications of N-acetyl-D-neuraminic acid: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 87, 1281–1289. [Google Scholar] [CrossRef]
- Traving, C.; Schauer, R. Structure, function and metabolism of sialic acids. Cell Mol. Life Sci. 1998, 54, 1330–1349. [Google Scholar] [CrossRef]
- Rohrig, C.H.; Choi, S.S.; Baldwin, N. The nutritional role of free sialic acid, a human milk monosaccharide, and its application as a functional food ingredient. Crit. Rev. Food Sci. Nutr. 2017, 57, 1017–1038. [Google Scholar] [CrossRef]
- Oetke, C.; Hinderlich, S.; Brossmer, R.; Reutter, W.; Pawlita, M.; Keppler, O.T. Evidence for efficient uptake and incorporation of sialic acid by eukaryotic cells. Eur. J. Biochem. 2001, 268, 4553–4561. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Martinez, A.; Chornet, E.; Rodrigue, D. Steady-shear rheology of concentrated chitosan solutions. J. Texture Stud. 2004, 35, 53–74. [Google Scholar] [CrossRef]
- Kasaai, M.R.; Arul, J.; Charlet, G. Intrinsic viscosity–molecular weight relationship for chitosan. J. Polym. Sci. Part B (Polym. Phys.) 2000, 38, 2591–2598. [Google Scholar] [CrossRef]
- Mekhail, M.; Tabrizian, M. Injectable Chitosan-Based Scaffolds in Regenerative Medicine and their Clinical Translatability. Adv. Healthc. Mater. 2014, 3, 1529–1545. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S. Chitosan Based Nanomaterials and Its Applications. In Systems for Drug Delivery: Safety, Animal, and Microbial Polysaccharides; Bhatia, S., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 55–117. [Google Scholar]
- Zhang, K.M.; Mao, Z.J.; Huang, Y.C.; Xu, Y.; Huang, C.G.; Guo, Y.; Ren, X.; Liu, C.Y. Ultrasonic assisted water-in-oil emulsions encapsulating macro-molecular polysaccharide chitosan: Influence of molecular properties, emulsion viscosity and their stability. Ultrason. Sonochem. 2020, 64, 105018. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, C.A.S.; Taarji, N.; Khalid, N.; Kobayashi, I.; Nakajima, M.; Neves, M.A. Formulation and characterization of water-in-oil nanoemulsions loaded with acai berry anthocyanins: Insights of degradation kinetics and stability evaluation of anthocyanins and nanoemulsions. Food Res. Int. 2018, 106, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.Z.; Hu, X.F.; Jia, Y.J.; Pan, L.H.; Zheng, Z.; Zhao, Y.Y.; Mu, D.D.; Zhong, X.Y.; Jiang, S.T. Camellia oil-based oleogels structuring with tea polyphenol-palmitate particles and citrus pectin by emulsion-templated method: Preparation, characterization and potential application. Food Hydrocolloid. 2019, 95, 76–87. [Google Scholar] [CrossRef]
- Amid, B.T.; Mirhosseini, H. Emulsifying Activity, Particle Uniformity and Rheological Properties of a Natural Polysaccharide-Protein Biopolymer from Durian Seed. Food Biophys. 2012, 7, 317–328. [Google Scholar] [CrossRef]
- Almeida, M.L.; Charin, R.M.; Nele, M.; Tavares, F.W. Stability studies of high-stable water-in-oil model emulsions. J. Disper. Sci. Technol. 2017, 38, 82–88. [Google Scholar] [CrossRef]
- Iqbal, S.; Xu, Z.C.; Huang, H.; Chen, X.D. Structuring of water-in-oil emulsions using controlled aggregation of polysaccharide in aqueous phases. J. Food Eng. 2019, 258, 34–44. [Google Scholar] [CrossRef]
- Sekeri, S.H.; Ibrahim, M.N.M.; Umar, K.; Yaqoob, A.A.; Azmi, M.N.; Hussin, M.H.; Othman, M.B.H.; Malik, M.F.I.A. Preparation and characterization of nanosized lignin fromoil palm (Elaeis guineensis) biomass as a novel emulsifying agent. Int. J. Biol. Macromol. 2020, 164, 3114–3124. [Google Scholar] [CrossRef]
- Ogutcu, M.; Arifoglu, N.; Yilmaz, E. Preparation and Characterization of Virgin Olive Oil-Beeswax Oleogel Emulsion Products. J. Am. Oil. Chem. Soc. 2015, 92, 459–471. [Google Scholar] [CrossRef]
- Kačuráková, M.; Wellner, N.; Ebringerová, A.; Hromádková, Z.; Belton, P.S. Characterisation of xylan-type polysaccharides and associated cell wall components by FT-IR and FT-Raman spectroscopies. Food Hydrocolloid. 1999, 13, 35–41. [Google Scholar] [CrossRef]
- Sorokin, A.; Lavlinskaya, M. Synthesis of the superabsobents enriched in chitosan derivatives with excellent water absorption properties. Polym. Bull. 2021, 79, 407–427. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Jafari, S.M.; Assadpoor, E.; Mohammadi, A. Nano-encapsulation of saffron extract through double-layered multiple emulsions of pectin and whey protein concentrate. J. Food Eng. 2015, 165, 149–155. [Google Scholar] [CrossRef]
- Yang, J.; Shen, M.Y.; Wu, T.; Luo, Y.; Li, M.Y.; Wen, H.L.; Xie, J.H. Role of salt ions and molecular weights on the formation of Mesona chinensis polysaccharide-chitosan polyelectrolyte complex hydrogel. Food Chem. 2020, 333, 127493. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Y.; Wang, Y.M.; Zhang, L.L.; Xu, M. Design of a novel polysaccharide-based cryogel using triallyl cyanurate as crosslinker for cell adhesion and proliferation. Int. J. Biol. Macromol. 2019, 126, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Y.; Wang, Y.M.; Zhang, L.L.; Xu, M. Formation of self-assembled polyelectrolyte complex hydrogel derived from salecan and chitosan for sustained release of Vitamin C. Carbohyd. Polym. 2020, 234, 115920. [Google Scholar] [CrossRef]
- Lindenstruth, K.; Muller, B.W. Parameters with influence on the droplet size of w/o emulsions. Pharmazie 2004, 59, 187–190. [Google Scholar]
- Kowalska, M. Physical Stability and the Droplet Distribution of Rice Oil-in-Water Emulsion. J. Disper. Sci. Technol. 2016, 37, 222–230. [Google Scholar] [CrossRef]
- Hasan, W.; Khan, M.N. Rheological characterization of vegetable oil blends: Effect of shear rate, temperature, and short-term heating. J. Food Process. Eng. 2020, 43, e13396. [Google Scholar] [CrossRef]
- Rietberg, M.R.; Rousseau, D.; Duizer, L. Sensory Evaluation of Sodium Chloride-Containing Water-in-Oil Emulsions. J. Agric. Food Chem. 2012, 60, 4005–4011. [Google Scholar] [CrossRef]
- Clements, D. Food Emulsions: Principles, Practice and Techniques, 2nd ed.; CRC Academic Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Zafimahova-Ratisbonne, A.; Wardhono, E.Y.; Lanoiselle, J.L.; Saleh, K.; Clausse, D. Stability of W/O Emulsions Encapsulating Polysaccharides. J. Disper. Sci. Technol. 2014, 35, 38–47. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Jafari, S.M.; Assadpour, E. Preparation of a multiple emulsion based on pectin-whey protein complex for encapsulation of saffron extract nanodroplets. Food Chem. 2017, 221, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Sommerling, J.H.; de Matos, M.B.C.; Hildebrandt, E.; Dessy, A.; Kok, R.J.; Nirschl, H.; Leneweit, G. Instability Mechanisms of Water-in-Oil Nanoemulsions with Phospholipids: Temporal and Morphological Structures. Langmuir 2018, 34, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Balcaen, M.; Steyls, J.; Schoeppe, A.; Nelis, V.; Van der Meeren, P. Phosphatidylcholine-depleted lecithin: A clean-label low-HLB emulsifier to replace PGPR in w/o and w/o/w emulsions. J. Colloid. Interf. Sci. 2021, 581, 836–846. [Google Scholar] [CrossRef]



| PGPR (W/%) | LE (W/%) | Olive Oil | Maize Oil | ||
|---|---|---|---|---|---|
| D3,2 (Μm) | Uniformity | D3,2 (Μm) | Uniformity | ||
| 2.0 | 0 | 0.915 ± 0.001 | 0.232 ± 0.001 | 0.972 ± 0.001 | 0.229 ± 0.002 |
| 1.5 | 0.5 | 0.877 ± 0.017 | 2.140 ± 0.145 | 0.998 ± 0.003 | 0.266 ± 0.003 |
| 1.0 | 1.0 | 0.894 ± 0.003 | 2.990 ± 0.266 | 1.101 ± 0.002 | 1.790 ± 0.026 |
| 0.5 | 1.5 | 2.850 ± 0.215 | 1.620 ± 0.047 | 2.816 ± 0.081 | 0.712 ± 0.017 |
| 0 | 2.0 | 11.328 ± 0.098 | 0.677 ± 0.015 | 15.968 ± 0.282 | 0.860 ± 0.019 |
| PGPR (W/%) | LE(W/%) | Olive Oil (Pa·S) | Maize Oil (Pa·S) |
|---|---|---|---|
| 2.0 | 0 | 0.49 | 0.82 |
| 1.5 | 0.5 | 0.71 | 0.85 |
| 1.0 | 1.0 | 0.85 | 1.00 |
| 0.5 | 1.5 | 164.90 | 124.20 |
| 0 | 2.0 | 186.60 | 556.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, M.; Zheng, D.; Jia, P.; Cao, L. Novel Water-in-Oil Emulsions for Co-Loading Sialic Acid and Chitosan: Formulation, Characterization, and Stability Evaluation. Foods 2022, 11, 873. https://doi.org/10.3390/foods11060873
Pang M, Zheng D, Jia P, Cao L. Novel Water-in-Oil Emulsions for Co-Loading Sialic Acid and Chitosan: Formulation, Characterization, and Stability Evaluation. Foods. 2022; 11(6):873. https://doi.org/10.3390/foods11060873
Chicago/Turabian StylePang, Min, Donglei Zheng, Pengpeng Jia, and Lili Cao. 2022. "Novel Water-in-Oil Emulsions for Co-Loading Sialic Acid and Chitosan: Formulation, Characterization, and Stability Evaluation" Foods 11, no. 6: 873. https://doi.org/10.3390/foods11060873
APA StylePang, M., Zheng, D., Jia, P., & Cao, L. (2022). Novel Water-in-Oil Emulsions for Co-Loading Sialic Acid and Chitosan: Formulation, Characterization, and Stability Evaluation. Foods, 11(6), 873. https://doi.org/10.3390/foods11060873