High Hydrostatic Pressure vs. Thermal Pasteurization: The Effect on the Bioactive Compound Profile of a Citrus Maqui Beverage
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Ingredients
2.3. Beverage Preparation
2.4. HHP Processing and Thermal Pasteurization
2.5. Sampling
2.6. pH, Titratable Acidity, and Total Soluble Solids
2.7. Microbiology Analysis
2.8. Qualitative and Quantitative Analysis of Phenolic Compounds by RP-HPLC-DAD
2.9. Extraction and Analysis of Vitamin C
2.10. Color Measurements
2.11. Statistical Analyses
3. Results and Discussion
3.1. Initial Impact of Proccesing on the Overall Quality Parameters in Juices
3.2. Effect of HHP and TP Treatments on pH, Tritrable Acidity (TA), and Total Soluble Solids (°Brix) during Storage
3.3. Changes in the Microbiological Profiles during Storage
3.4. Effect of HHP and TP Treatments on Vitamin C during Storage
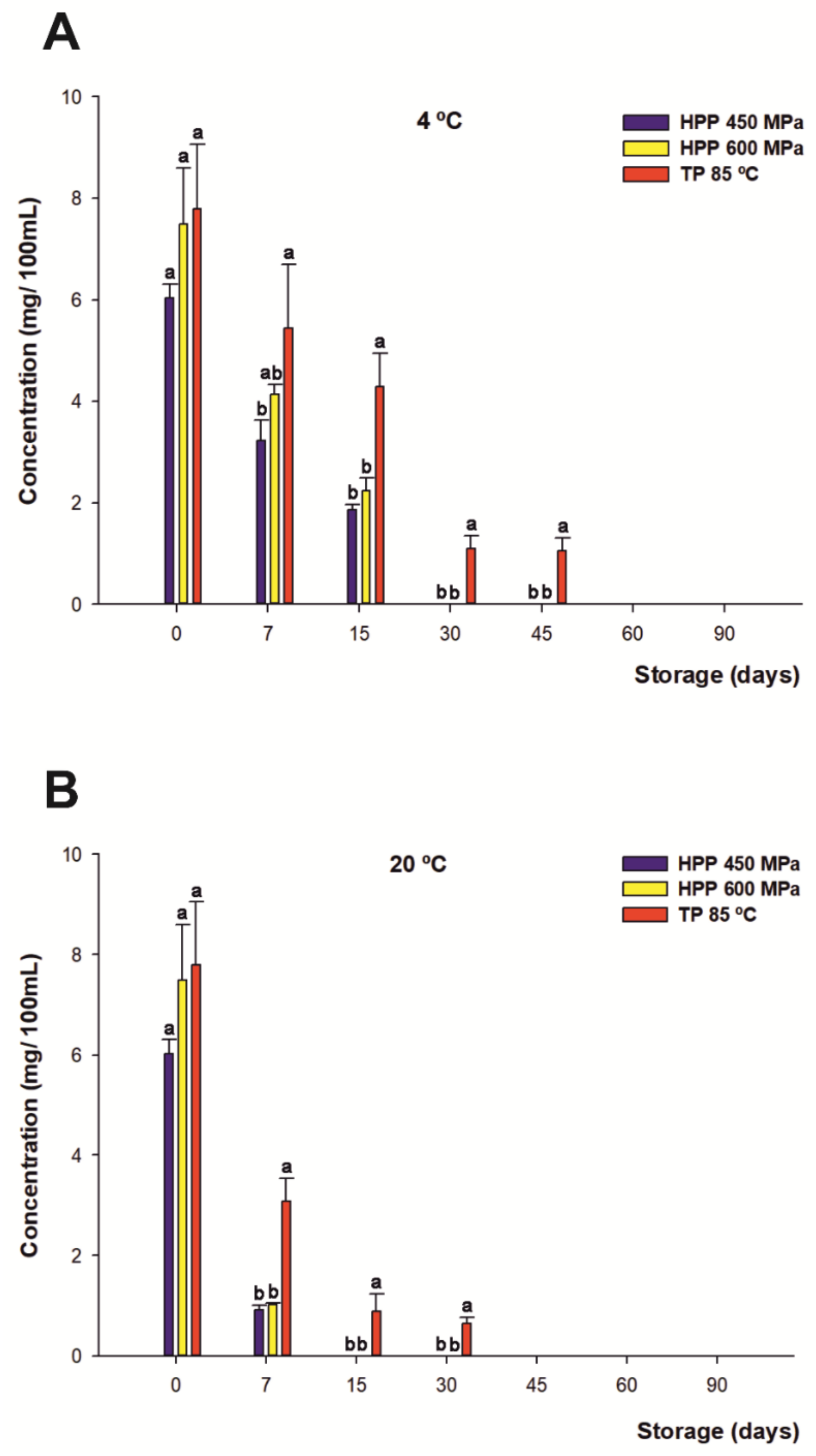
3.5. Effect of HHP and TP Treatments on Phenolic Composition during Storage
3.5.1. Flavanones
3.5.2. Anthocyanins
3.6. Color Changes of Juices during Storage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khouryieh, H.A. Novel and emerging technologies used by the U.S. food processing industry. Innov. Food Sci. Emerg. Technol. 2020, 67, 102559. [Google Scholar] [CrossRef]
- Sunil, N.C.J.S.; Chandra, S.; Chaudhary, V.; Kumar, V. “Non-thermal techniques: Application in food industries” A review. J. Pharmacogn. Phytochem. 2018, 7, 1507–1518. [Google Scholar]
- Khan, M.K.; Ahmad, K.; Hassan, S.; Imran, M.; Ahmad, N.; Xu, C. Effect of novel technologies on polyphenols during food processing. Innov. Food Sci. Emerg. Technol. 2018, 45, 361–381. [Google Scholar] [CrossRef]
- Meier, T.; Gräfe, K.; Senn, F.; Sur, P.; Stangl, G.I.; Dawczynski, C.; März, W.; Kleber, M.E.; Lorkowski, S. Cardiovascular mortality attributable to dietary risk factors in 51 countries in the WHO European Region from 1990 to 2016: A systematic analysis of the Global Burden of Disease Study. Eur. J. Epidemiol. 2018, 34, 37–55. [Google Scholar] [CrossRef]
- Rastogi, N.K.; Raghavarao, K.S.M.S.; Balasubramaniam, V.; Niranjan, K.; Knorr, D. Opportunities and Challenges in High Pressure Processing of Foods. Crit. Rev. Food Sci. Nutr. 2007, 47, 69–112. [Google Scholar] [CrossRef]
- Hooshyar, L.; Hesari, J.; Azadmard-Damirchi, S. Investigation of selected thermal and non-thermal preservative techniques to produce high quality and safe to drink sour cherry, red grape and pomegranate juices. J. Food Sci. Technol. 2020, 57, 1689–1697. [Google Scholar] [CrossRef]
- Salar, F.J.; Agulló, V.; García-Viguera, C.; Domínguez-Perles, R. Stevia vs. Sucrose: Influence on the Phytochemical Content of a Citrus–Maqui Beverage—A Shelf Life Study. Foods 2020, 9, 219. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Mena, P.; Garcia-Viguera, C.; Moreno-Fernández, D. A novel beverage rich in antioxidant phenolics: Maqui berry (Aristotelia chilensis) and lemon juice. LWT 2012, 47, 279–286. [Google Scholar] [CrossRef]
- Agulló, V.; García-Viguera, C.; Domínguez-Perles, R. Beverages Based on Second Quality Citrus Fruits and Maqui Berry, a Source of Bioactive (Poly)phenols: Sorting Out Urine Metabolites upon a Longitudinal Study. Nutrients 2021, 13, 312. [Google Scholar] [CrossRef]
- Villaño, D.; Masoodi, H.; Marhuenda, J.; García-Viguera, C.; Zafrilla, P. Stevia, sucralose and sucrose added to a maqui-Citrus beverage and their effects on glycemic response in overweight subjects: A randomized clinical trial. LWT 2021, 144, 111173. [Google Scholar] [CrossRef]
- Agulló, V.; González-Trujano, M.E.; Hernandez-Leon, A.; Estrada-Camarena, E.; Pellicer, F.; García-Viguera, C. Antinociceptive effects of maqui-berry (Aristotelia chilensis (Mol.) Stuntz). Int. J. Food Sci. Nutr. 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Araos, J.P. Aristotelia chilensis: A Possible Nutraceutical or Functional Food. Med. Chem. 2015, 5, 378–382. [Google Scholar] [CrossRef]
- Ortiz, T.; Argüelles-Arias, F.; Begines, B.; García-Montes, J.-M.; Pereira, A.; Victoriano, M.; Vázquez-Román, V.; Bernal, J.P.; Callejón, R.; De-Miguel, M.; et al. Native Chilean Berries Preservation and In Vitro Studies of a Polyphenol Highly Antioxidant Extract from Maqui as a Potential Agent against Inflammatory Diseases. Antioxidants 2021, 10, 843. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Butelli, E.; De Santis, S.; Cavalcanti, E.; Hill, L.; De Angelis, M.; Giovinazzo, G.; Chieppa, M.; Martin, C.; Santino, A. Combined Dietary Anthocyanins, Flavonols, and Stilbenoids Alleviate Inflammatory Bowel Disease Symptoms in Mice. Front. Nutr. 2018, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- Vega-Galvez, A.; Rodríguez, A.; Stucken, K. Antioxidant, functional properties and health-promoting potential of native South American berries: A review. J. Sci. Food Agric. 2020, 101, 364–378. [Google Scholar] [CrossRef]
- Bastías-Montes, J.M.; Monterrosa, K.; Muñoz-Fariña, O.; García, O.; Acuña-Nelson, S.M.; Martín, C.V.-S.; Quevedo, R.; Kubo, I.; Avila-Acevedo, J.G.; Domiguez-Lopez, M.; et al. Chemoprotective and antiobesity effects of tocols from seed oil of Maqui-berry: Their antioxidative and digestive enzyme inhibition potential. Food Chem. Toxicol. 2019, 136, 111036. [Google Scholar] [CrossRef]
- Mena, J.; Elgueta, E.; Espinola-Gonzales, F.; Cardenas, H.; Orihuela, P.A. Hydroethanolic Extracts of the Aristotelia Chilensis (Maqui) Berry Reduces Cellular Viability and Invasiveness in the Endometrial Cancer Cell Line Ishikawa. Integr. Cancer Ther. 2021, 20, 15347354211007560. [Google Scholar] [CrossRef]
- Céspedes-Acuña, C.L.; Xiao, J.; Wei, Z.-J.; Chen, L.; Bastias, J.M.; Avila, J.G.; Alarcon-Enos, J.; Werner-Navarrete, E.; Kubo, I. Antioxidant and anti-inflammatory effects of extracts from Maqui berry Aristotelia chilensis in human colon cancer cells. J. Berry Res. 2018, 8, 275–296. [Google Scholar] [CrossRef]
- Ávila, F.; Jiménez-Aspee, F.; Cruz, N.; Gómez, C.; González, M.A.; Ravello, N. Additive effect of maqui (Aristotelia chilensis) and lemon (Citrus x limon) juice in the postprandial glycemic responses after the intake of high glycemic index meals in healthy men. NFS J. 2019, 17, 8–16. [Google Scholar] [CrossRef]
- Ma, H.; Johnson, S.L.; Liu, W.; DaSilva, N.A.; Meschwitz, S.; Dain, J.A.; Seeram, N.P. Evaluation of Polyphenol Anthocyanin-Enriched Extracts of Blackberry, Black Raspberry, Blueberry, Cranberry, Red Raspberry, and Strawberry for Free Radical Scavenging, Reactive Carbonyl Species Trapping, Anti-Glycation, Anti-β-Amyloid Aggregation, and Microglial Neuroprotective Effects. Int. J. Mol. Sci. 2018, 19, 461. [Google Scholar] [CrossRef]
- Cebadera-Miranda, L.; Morales, P.; Cámara, M. Bioactive compounds in oranges from the Mediterranean climate area. In The Mediterranean Diet: An Evidence-Based Approach; Elsevier: Amsterdam, The Netherlands, 2020; pp. 293–309. ISBN 9780128186497. [Google Scholar]
- Ballistreri, G.; Fabroni, S.; Romeo, F.V.; Timpanaro, N.; Amenta, M.; Rapisarda, P. Anthocyanins and Other Polyphenols in Citrus Genus: Biosynthesis, Chemical Profile, and Biological Activity. In Polyphenols in Plants; Academic Press: Cambridge, MA, USA, 2018; pp. 191–215. ISBN 9780128137680. [Google Scholar]
- Miles, E.A.; Calder, P.C. Effects of Citrus Fruit Juices and Their Bioactive Components on Inflammation and Immunity: A Narrative Review. Front. Immunol. 2021, 12, 712608. [Google Scholar] [CrossRef]
- Martinez, P.F.; de Carvalho, M.R.; Mendonça, M.L.M.; Okoshi, M.P.; de Oliveira-Junior, S.A. Efeito Antioxidante e Anti-inflamatório do Suco de Laranja. Arq. Bras. De Cardiol. 2021, 116, 1137–1138. [Google Scholar] [CrossRef]
- ElSawy, H.; Algefare, A.I.; Alfwuaires, M.; Khalil, M.; Elmenshawy, O.M.; Sedky, A.; Abdel-Moneim, A.M. Naringin alleviates methotrexate-induced liver injury in male albino rats and enhances its antitumor efficacy in HepG2 cells. Biosci. Rep. 2020, 40, BSR20193686. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, J.; Ran, Q.; Lou, G.; Peng, C.; Gan, Q.-X.; Hu, J.; Sun, J.; Yao, R.; Huang, Q. Hesperidin: A Therapeutic Agent for Obesity. Drug Des. Dev. Ther. 2019, 13, 3855–3866. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, C.; Yan, Y.; Chen, Q.; Luo, F.; Zhu, X.; Li, X.; Chen, K. Purification of naringin and neohesperidin from Huyou (Citrus changshanensis) fruit and their effects on glucose consumption in human HepG2 cells. Food Chem. 2012, 135, 1471–1478. [Google Scholar] [CrossRef]
- Mizrahi, A.; Knekt, P.; Montonen, J.; Laaksonen, M.A.; Heliövaara, M.; Järvinen, R. Plant foods and the risk of cerebrovascular diseases: A potential protection of fruit consumption. Br. J. Nutr. 2009, 102, 1075–1083. [Google Scholar] [CrossRef]
- Testai, L.; Calderone, V. Nutraceutical Value of Citrus Flavanones and Their Implications in Cardiovascular Disease. Nutrients 2017, 9, 502. [Google Scholar] [CrossRef] [PubMed]
- Roobab, U.; Aadil, R.M.; Madni, G.M.; Bekhit, A.E.-D. The Impact of Nonthermal Technologies on the Microbiological Quality of Juices: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 437–457. [Google Scholar] [CrossRef]
- Daher, D.; Pérez-Lamela, C.; Le Gourrierec, S. Effect of High Pressure Processing on the Microbial Inactivation in Fruit Preparations and Other Vegetable Based Beverages. Agriculture 2017, 7, 72. [Google Scholar] [CrossRef]
- Patrignani, F.; Siroli, L.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. Innovative strategies based on the use of essential oils and their components to improve safety, shelf-life and quality of minimally processed fruits and vegetables. Trends Food Sci. Technol. 2015, 46, 311–319. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Wang, L.-H.; Zeng, X.-A.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: A review. Int. J. Food Sci. Technol. 2018, 54, 1–13. [Google Scholar] [CrossRef]
- Rojo, M.C.; Cristiani, M.; Szerman, N.; Gonzalez, M.L.; Lerena, M.C.; Mercado, L.A.; Combina, M. Reduction of Zygosaccharomyces rouxii Population in Concentrated Grape Juices by Thermal Pasteurization and Hydrostatic High Pressure Processing. Food Bioprocess Technol. 2019, 12, 781–788. [Google Scholar] [CrossRef]
- Abera, G. Review on high-pressure processing of foods. Cogent Food Agric. 2019, 5, 1568725. [Google Scholar] [CrossRef]
- Narjabadi Fam, S.; Khosravi-Darani, K.; Massoud, R.; Massoud, A. High-Pressure Processing in Food. Biointerface Res. Appl. Chem. 2020, 11, 11553–11561. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Zhao, F.; Sun, Z.; Liao, X. Quality comparison of carrot juices processed by high-pressure processing and high-temperature short-time processing. Innov. Food Sci. Emerg. Technol. 2015, 33, 135–144. [Google Scholar] [CrossRef]
- Muntean, M.-V.; Marian, O.; Barbieru, V.; Cătunescu, G.M.; Ranta, O.; Drocas, I.; Terhes, S. High Pressure Processing in Food Industry—Characteristics and Applications. Agric. Agric. Sci. Procedia 2016, 10, 377–383. [Google Scholar] [CrossRef]
- Balda, F.P.; Aparicio, B.V.; Samson, C.T. Industrial High Pressure Processing of Foods: Review of Evolution and Emerging Trends. J. Food Sci. Eng. 2012, 2, 543. [Google Scholar] [CrossRef][Green Version]
- De Oliveira, P.M.; Júnior, B.R.D.C.L.; Martins, E.M.F.; Cristianini, M.; Martins, M.L.; Vieira, E.N.R.; Binoti, M.L.; Paula, D.D.A.; Ramos, A.M. Impact of high pressure and thermal processing on probiotic mixed mango and carrot juices. J. Food Process. Preserv. 2020, 44, e14530. [Google Scholar] [CrossRef]
- Wongfhun, P.; Gordon, M.H.; Apichartsrangkoon, A. Flavour characterisation of fresh and processed pennywort (Centella asiatica L.) juices. Food Chem. 2010, 119, 69–74. [Google Scholar] [CrossRef]
- Huang, H.-W.; Hsu, C.-P.; Wang, C.-Y. Healthy expectations of high hydrostatic pressure treatment in food processing industry. J. Food Drug Anal. 2019, 28, 1–13. [Google Scholar] [CrossRef]
- Kaşıkcı, M.B. High Hydrostatic Pressure Treatment of Fruit, Fruit Products and Fruit Juices: A Review on Phenolic Compounds. J. Food Health Sci. 2015, 2, 27–39. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Wu, S.-J.; Chen, B.-Y.; Huang, H.-W.; Wang, C.-Y. Effect of high-pressure processing and thermal pasteurization on overall quality parameters of white grape juice. J. Sci. Food Agric. 2016, 97, 3166–3172. [Google Scholar] [CrossRef]
- Yi, J.; Kebede, B.T.; Dang, D.N.H.; Buvé, C.; Grauwet, T.; Van Loey, A.; Hu, X.; Hendrickx, M.E. Quality change during high pressure processing and thermal processing of cloudy apple juice. LWT 2017, 75, 85–92. [Google Scholar] [CrossRef]
- Conesa, R.; Andreu, S.; Fernandez, P.S.; Esnoz, A.; Palop, A. Nonisothermal heat resistance determinations with the thermoresistometer Mastia. J. Appl. Microbiol. 2009, 107, 506–513. [Google Scholar] [CrossRef]
- Baenas, N.; Salar, F.J.; Domínguez-Perles, R.; García-Viguera, C. New UHPLC-QqQ-MS/MS Method for the Rapid and Sensitive Analysis of Ascorbic and Dehydroascorbic Acids in Plant Foods. Molecules 2019, 24, 1632. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Wang, Y.-T.; Wu, S.-J.; Shyu, Y.-T. Quality changes in high hydrostatic pressure and thermal pasteurized grapefruit juice during cold storage. J. Food Sci. Technol. 2018, 55, 5115–5122. [Google Scholar] [CrossRef]
- Nayak, P.K.; Rayaguru, K.; Krishnan, K.R. Quality comparison of elephant apple juices after high-pressure processing and thermal treatment. J. Sci. Food Agric. 2016, 97, 1404–1411. [Google Scholar] [CrossRef]
- Ganje, M.; Jafari, S.M.; Farzaneh, V.; Malekjani, N. Kinetics modelling of color deterioration during thermal processing of tomato paste with the use of response surface methodology. Heat Mass Transf. 2018, 54, 3663–3671. [Google Scholar] [CrossRef]
- Kruszewski, B.; Zawada, K.; Karpiński, P. Impact of High-Pressure Homogenization Parameters on Physicochemical Characteristics, Bioactive Compounds Content, and Antioxidant Capacity of Blackcurrant Juice. Molecules 2021, 26, 1802. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Palma, P.; Tobar-Bolaños, G.; Casas-Forero, N.; Zúñiga, R.N.; Petzold, G. Quality Attributes of Cryoconcentrated Calafate (Berberis microphylla) Juice during Refrigerated Storage. Foods 2020, 9, 1314. [Google Scholar] [CrossRef] [PubMed]
- Subasi, B.; Alpas, H. Effect of high hydrostatic pressure processing and squeezing pressure on some quality properties of pomegranate juice against thermal treatment. High Press. Res. 2016, 37, 78–92. [Google Scholar] [CrossRef]
- Da Silveira, T.F.F.; Cristianini, M.; Kuhnle, G.G.; Ribeiro, A.B.; Filho, J.T.; Godoy, H.T. Anthocyanins, non-anthocyanin phenolics, tocopherols and antioxidant capacity of açaí juice (Euterpe oleracea) as affected by high pressure processing and thermal pasteurization. Innov. Food Sci. Emerg. Technol. 2019, 55, 88–96. [Google Scholar] [CrossRef]
- Ahmed, M.; Eun, J.-B. Flavonoids in fruits and vegetables after thermal and nonthermal processing: A review. Crit. Rev. Food Sci. Nutr. 2017, 58, 3159–3188. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, G.; Shu, B.; Huang, F.; Dong, L.; Zhang, R.; Su, D. Comparison of the phenolic profiles and physicochemical properties of different varieties of thermally processed canned lychee pulp. RSC Adv. 2020, 10, 6743–6751. [Google Scholar] [CrossRef]
- He, Z.; Tao, Y.; Zeng, M.; Zhang, S.; Tao, G.; Qin, F.; Chen, J. High pressure homogenization processing, thermal treatment and milk matrix affect in vitro bioaccessibility of phenolics in apple, grape and orange juice to different extents. Food Chem. 2016, 200, 107–116. [Google Scholar] [CrossRef]
- Spira, P.; Bisconsin-Junior, A.; Rosenthal, A.; Monteiro, M. Effects of high hydrostatic pressure on the overall quality of Pêra-Rio orange juice during shelf life. Food Sci. Technol. Int. 2018, 24, 507–518. [Google Scholar] [CrossRef]
- Andrés, V.; Villanueva, M.J.; Tenorio, M.D. The effect of high-pressure processing on colour, bioactive compounds, and antioxidant activity in smoothies during refrigerated storage. Food Chem. 2016, 192, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Du, B.-L.; Cui, Z.-W.; Xu, L.-P.; Li, C. Effects of high hydrostatic pressure and thermal processing on bioactive compounds, antioxidant activity, and volatile profile of mulberry juice. Food Sci. Technol. Int. 2016, 23, 119–127. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, S.; Liu, F.; Dong, P.; Huang, W.; Xiong, L.; Liao, X. Comparing the effects of high hydrostatic pressure and thermal pasteurization combined with nisin on the quality of cucumber juice drinks. Innov. Food Sci. Emerg. Technol. 2013, 17, 27–36. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Li, R.; Bi, X.; Liao, X. Effects of high hydrostatic pressure and high temperature short time on antioxidant activity, antioxidant compounds and color of mango nectars. Innov. Food Sci. Emerg. Technol. 2014, 21, 35–43. [Google Scholar] [CrossRef]
- Queirós, R.; Rainho, D.; Santos, M.D.; Fidalgo, L.; Delgadillo, I.; Saraiva, J.A. High pressure and thermal pasteurization effects on sweet cherry juice microbiological stability and physicochemical properties. High Press. Res. 2014, 35, 69–77. [Google Scholar] [CrossRef]
- Elez-Martínez, P.; Soliva-Fortuny, R.C.; Martín-Belloso, O. Comparative study on shelf life of orange juice processed by high intensity pulsed electric fields or heat treatment. Eur. Food Res. Technol. 2005, 222, 321–329. [Google Scholar] [CrossRef]
- Chen, D.; Xi, H.; Guo, X.; Qin, Z.; Pang, X.; Hu, X.; Liao, X.; Wu, J. Comparative study of quality of cloudy pomegranate juice treated by high hydrostatic pressure and high temperature short time. Innov. Food Sci. Emerg. Technol. 2013, 19, 85–94. [Google Scholar] [CrossRef]
- Hsu, K.-C.; Tan, F.-J.; Chi, H.-Y. Evaluation of microbial inactivation and physicochemical properties of pressurized tomato juice during refrigerated storage. LWT 2008, 41, 367–375. [Google Scholar] [CrossRef]
- Bull, M.K.; Zerdin, K.; Howe, E.; Goicoechea, D.; Paramanandhan, P.; Stockman, R.; Sellahewa, J.; Szabo, E.A.; Johnson, R.L.; Stewart, C.M. The effect of high pressure processing on the microbial, physical and chemical properties of Valencia and Navel orange juice. Innov. Food Sci. Emerg. Technol. 2004, 5, 135–149. [Google Scholar] [CrossRef]
- Parish, M. Orange Juice Quality After Treatment by Thermal Pasteurization or Isostatic High Pressure. LWT 1998, 31, 439–442. [Google Scholar] [CrossRef]
- Martí, N.; Mena, P.; Cánovas, J.A.; Micol, V.; Saura, D. Vitamin C and the Role of Citrus Juices as Functional Food. Nat. Prod. Commun. 2009, 4, 677–700. [Google Scholar] [CrossRef]
- Njoku, P.; Ayuk, A.; Okoye, C. Temperature Effects on Vitamin C Content in Citrus Fruits. Pak. J. Nutr. 2011, 10, 1168–1169. [Google Scholar] [CrossRef]
- Garcia-Viguera, C.; Bridle, P. Influence of structure on colour stability of anthocyanins and flavylium salts with ascorbic acid. Food Chem. 1999, 64, 21–26. [Google Scholar] [CrossRef]
- Chung, C.; Rojanasasithara, T.; Mutilangi, W.; McClements, D.J. Stabilization of natural colors and nutraceuticals: Inhibition of anthocyanin degradation in model beverages using polyphenols. Food Chem. 2016, 212, 596–603. [Google Scholar] [CrossRef]
- Monteiro, A.B.M. Effect of High Hydrostatic Pressure on Ascorbic Acid, Phenolic Compounds and Antioxidant Activity of Pera Rio Orange Juice. J. Food Process. Technol. 2015, 1–7. [Google Scholar] [CrossRef]
- El-Ishaq, A. Effect of Temperature and Storage on Vitamin C Content in Fruits Juice. Int. J. Chem. Biomol. Sci. 2015, 1, 17–21. [Google Scholar]
- Munyaka, A.W.; Makule, E.E.; Oey, I.; Van Loey, A.; Hendrickx, M. Thermal Stability of l-Ascorbic Acid and Ascorbic Acid Oxidase in Broccoli (Brassica oleracea var. italica). J. Food Sci. 2010, 75, C336–C340. [Google Scholar] [CrossRef] [PubMed]
- Zaritzky, N.E. Chemical and physical deterioration of frozen foods. In Chemical Deterioration and Physical Instability of Food and Beverages; Woodhead Publishing Limited: Sawston, UK, 2010; pp. 561–607. ISBN 9781845694951. [Google Scholar]
- Terefe, N.S.; Buckow, R. High-Pressure Processing Effects on Endogenous Enzymes in Fruits and Vegetables. In High Pressure Processing of Fruit and Vegetable Products; Houska, M., Marques da Silva, F.V., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 39–62. ISBN 9781315121123. [Google Scholar]
- Bai, J.-W.; Gao, Z.-J.; Xiao, H.-W.; Wang, X.-T.; Zhang, Q. Polyphenol oxidase inactivation and vitamin C degradation kinetics of Fuji apple quarters by high humidity air impingement blanching. Int. J. Food Sci. Technol. 2013, 48, 1135–1141. [Google Scholar] [CrossRef]
- Vishwasrao, C.; Ananthanarayan, L. Kinetics of inactivation of quality-deteriorating enzymes and degradation of selective phytoconstituents in pink guava pulp during thermal processing. J. Food Sci. Technol. 2018, 55, 3273–3280. [Google Scholar] [CrossRef]
- Polydera, A.; Stoforos, N.; Taoukis, P. Quality degradation kinetics of pasteurised and high pressure processed fresh Navel orange juice: Nutritional parameters and shelf life. Innov. Food Sci. Emerg. Technol. 2005, 6, 1–9. [Google Scholar] [CrossRef]
- Barba, F.J.; Esteve, M.J.; Frigola, A. Ascorbic Acid Is the Only Bioactive That Is Better Preserved by High Hydrostatic Pressure than by Thermal Treatment of a Vegetable Beverage. J. Agric. Food Chem. 2010, 58, 10070–10075. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Plaza, L.; de Ancos, B.; Cano, P.M. Effect of high-pressure processing on health-promoting attributes of freshly squeezed orange juice (Citrus sinensis L.) during chilled storage. Eur. Food Res. Technol. 2003, 216, 18–22. [Google Scholar] [CrossRef]
- Plaza, L.; Sánchez-Moreno, C.; de Ancos, B.; Elez-Martínez, P.; Martín-Belloso, O.; Cano, M.P. Carotenoid and flavanone content during refrigerated storage of orange juice processed by high-pressure, pulsed electric fields and low pasteurization. LWT 2011, 44, 834–839. [Google Scholar] [CrossRef]
- González-Molina, E.; Moreno-Fernández, D.; García-Viguera, C. A new drink rich in healthy bioactives combining lemon and pomegranate juices. Food Chem. 2009, 115, 1364–1372. [Google Scholar] [CrossRef]
- Zhang, L.; Ling, W.; Yan, Z.; Liang, Y.; Guo, C.; Ouyang, Z.; Wang, X.; Kumaravel, K.; Ye, Q.; Zhong, B.; et al. Effects of storage conditions and heat treatment on the hesperidin concentration in Newhall navel orange (Citrus sinensis Osbeck cv. Newhall) juice. J. Food Compos. Anal. 2019, 85, 103338. [Google Scholar] [CrossRef]
- Kouniaki, S.; Kajda, P.; Zabetakis, I. The effect of high hydrostatic pressure on anthocyanins and ascorbic acid in blackcurrants(Ribes nigrum). Flavour Fragr. J. 2004, 19, 281–286. [Google Scholar] [CrossRef]
- Suthanthangjai, W.; Kajda, P.; Zabetakis, I. The effect of high hydrostatic pressure on the anthocyanins of raspberry (Rubus idaeus). Food Chem. 2005, 90, 193–197. [Google Scholar] [CrossRef]
- Chaikham, P. Comparison of high hydrostatic pressure and thermal processing on physicochemical and antioxidant properties of Maoberry (Antidesma thwaitesianum Müell. Arg.) juice. Int. Food Res. J. 2015, 22, 1993–2001. [Google Scholar]
- Roobha, J.J.; Saravanakumar, M.; Aravindhan, K.M.; Suganya, P. The effect of light, temperature, pH on stability of anthocyanin pigments in Musa acuminata bract. Res. Plant Biol. 2011, 1, 5–12. [Google Scholar]
- Hellström, J.; Mattila, P.; Karjalainen, R. Stability of anthocyanins in berry juices stored at different temperatures. J. Food Compos. Anal. 2013, 31, 12–19. [Google Scholar] [CrossRef]
- Ertan, K.; Türkyılmaz, M.; Ozkan, M. Effect of sweeteners on anthocyanin stability and colour properties of sour cherry and strawberry nectars during storage. J. Food Sci. Technol. 2018, 55, 4346–4355. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lin, Y.; Zhan, Y.; He, J.; Zhu, S. Effect of high pressure processing on the stability of anthocyanin, ascorbic acid and color of Chinese bayberry juice during storage. J. Food Eng. 2013, 119, 701–706. [Google Scholar] [CrossRef]
- Sipahli, S.; Mohanlall, V.; Mellem, J. Stability and degradation kinetics of crude anthocyanin extracts from H. sabdariffa. Food Sci. Technol. 2017, 37, 209–215. [Google Scholar] [CrossRef]
- Song, H.-N.; Ji, S.-A.; Park, H.-R.; Kim, H.-H.; Hogstrand, C. Impact of Various Factors on Color Stability of Fresh Blueberry Juice during Storage. Prev. Nutr. Food Sci. 2018, 23, 46–51. [Google Scholar] [CrossRef]
- Poei-Langston, M.S.; Wrolstad, R.E. Color Degradation in an Ascorbic Acid-Anthocyanin-Flavanol Model System. J. Food Sci. 1981, 46, 1218–1236. [Google Scholar] [CrossRef]
- Torres, B.; Tiwari, B.; Patras, A.; Cullen, P.; Brunton, N.; O’Donnell, C. Stability of anthocyanins and ascorbic acid of high pressure processed blood orange juice during storage. Innov. Food Sci. Emerg. Technol. 2011, 12, 93–97. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Hernandez, M.D.L.P.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Martynenko, A.; Chen, Y. Degradation kinetics of total anthocyanins and formation of polymeric color in blueberry hydrothermodynamic (HTD) processing. J. Food Eng. 2016, 171, 44–51. [Google Scholar] [CrossRef]
- Martín, C.V.-S.; Bastías-Montes, J.; Villagra-Jorquera, C.; Salinas-Huenchulao, G.; Flores-Ríos, A.; Gonzáles-Díaz, N.; Tamarit-Pino, Y.; Muñoz-Fariña, O.; Quevedo-León, R. Effect of Cryoconcentration Assisted by Centrifugation-Filtration on Bioactive Compounds and Microbiological Quality of Aqueous Maqui (Aristotelia chilensis (Mol.) Stuntz) and Calafate (Berberis microphylla G. Forst) Extracts Pretreated with High-Pressure Homogenization. Processes 2021, 9, 692. [Google Scholar] [CrossRef]
- Ferrari, G.; Maresca, P.; Ciccarone, R. The Effects of High Hydrostatic Pressure on the Polyphenols and Anthocyanins in Red Fruit Products. Procedia Food Sci. 2011, 1, 847–853. [Google Scholar] [CrossRef][Green Version]
- Benjamin, O.; Gamrasni, D. Microbial, nutritional, and organoleptic quality of pomegranate juice following high-pressure homogenization and low-temperature pasteurization. J. Food Sci. 2020, 85, 592–599. [Google Scholar] [CrossRef]
- Feng, X.; Zhou, Z.; Wang, X.; Bi, X.; Ma, Y.; Xing, Y. Comparison of High Hydrostatic Pressure, Ultrasound, and Heat Treatments on the Quality of Strawberry–Apple–Lemon Juice Blend. Foods 2020, 9, 218. [Google Scholar] [CrossRef]
- Cao, X.; Bi, X.; Huang, W.; Wu, J.; Hu, X.; Liao, X. Changes of quality of high hydrostatic pressure processed cloudy and clear strawberry juices during storage. Innov. Food Sci. Emerg. Technol. 2012, 16, 181–190. [Google Scholar] [CrossRef]
- Huang, W.; Bi, X.; Zhang, X.; Liao, X.; Hu, X.; Wu, J. Comparative study of enzymes, phenolics, carotenoids and color of apricot nectars treated by high hydrostatic pressure and high temperature short time. Innov. Food Sci. Emerg. Technol. 2013, 18, 74–82. [Google Scholar] [CrossRef]
- Aghajanzadeh, S.; Kashaninejad, M.; Ziaiifar, A.M. Cloud stability of sour orange juice as affected by pectin methylesterase during come up time: Approached through fractal dimension. Int. J. Food Prop. 2017, 20, S2508–S2519. [Google Scholar] [CrossRef]
- Croak, S.; Corredig, M. The role of pectin in orange juice stabilization: Effect of pectin methylesterase and pectinase activity on the size of cloud particles. Food Hydrocoll. 2006, 20, 961–965. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kaushik, N.; Rao, P.S.; Mishra, H.N. High-Pressure Inactivation of Enzymes: A Review on Its Recent Applications on Fruit Purees and Juices. Compr. Rev. Food Sci. Food Saf. 2014, 13, 578–596. [Google Scholar] [CrossRef]
- Kopjar, M.; Piližota, V.; Tiban, N.; Subaric, D.; Babić, J.; Ačkar, Đ.; Sajdl, M. Strawberry jams: Influence of different pectins on colour and textural properties. Czech. J. Food Sci. 2009, 27, 20–28. [Google Scholar] [CrossRef]
- Timmermans, R.; Mastwijk, H.; Knol, J.; Quataert, M.; Vervoort, L.; Van der Plancken, I.; Hendrickx, M.E.; Matser, A. Comparing equivalent thermal, high pressure and pulsed electric field processes for mild pasteurization of orange juice. Part I: Impact on overall quality attributes. Innov. Food Sci. Emerg. Technol. 2011, 12, 235–243. [Google Scholar] [CrossRef]
- Baker, R.A.; Cameron, R.G. Clouds of citrus juices and juice drinks. Food Technol. 1999, 53, 64–69. [Google Scholar]
- Weber, F.; Boch, K.; Schieber, A. Influence of copigmentation on the stability of spray dried anthocyanins from blackberry. LWT 2017, 75, 72–77. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Chatham, L.A.; Howard, J.E.; Juvik, J.A. A natural colorant system from corn: Flavone-anthocyanin copigmentation for altered hues and improved shelf life. Food Chem. 2019, 310, 125734. [Google Scholar] [CrossRef]
- Zheng, X.; Yu, Y.; Xiao, G.; Xu, Y.; Wu, J.; Tang, D.; Zhang, Y. Comparing product stability of probiotic beverages using litchi juice treated by high hydrostatic pressure and heat as substrates. Innov. Food Sci. Emerg. Technol. 2014, 23, 61–67. [Google Scholar] [CrossRef]
- Barba, F.J.; Jäger, H.; Meneses, N.; Esteve, M.; Frígola, A.; Knorr, D. Evaluation of quality changes of blueberry juice during refrigerated storage after high-pressure and pulsed electric fields processing. Innov. Food Sci. Emerg. Technol. 2012, 14, 18–24. [Google Scholar] [CrossRef]
- Aaby, K.; Grimsbo, I.H.; Hovda, M.B.; Rode, T.M. Effect of high pressure and thermal processing on shelf life and quality of strawberry purée and juice. Food Chem. 2018, 260, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Stinco, C.M.; Szczepańska, J.; Marszałek, K.; Pinto, C.; Inácio, R.S.; Mapelli-Brahm, P.; Barba, F.J.; Lorenzo, J.M.; Saraiva, J.; Melendez-Martinez, A.J. Effect of high-pressure processing on carotenoids profile, colour, microbial and enzymatic stability of cloudy carrot juice. Food Chem. 2019, 299, 125112. [Google Scholar] [CrossRef]
- Gao, G.; Zhao, L.; Ma, Y.; Wang, Y.; Sun, Z.; Liao, X. Microorganisms and Some Quality of Red Grapefruit Juice Affected by High Pressure Processing and High Temperature Short Time. Food Bioprocess. Technol. 2015, 8, 2096–2108. [Google Scholar] [CrossRef]



| Code | Beverage and Storage Conditions |
|---|---|
| Control | Untreated sample |
| HHP—450 MPa 4 | Beverage subjected to high hydrostatic pressure (450 MPa) stored at 4 °C under darkness conditions |
| HHP— 450 MPa 20 | Beverage subjected to high hydrostatic pressure (450 MPa) stored at 20 °C under darkness conditions |
| HHP— 600 MPa 4 | Beverage subjected to high hydrostatic pressure (600 MPa) stored at 4 °C under darkness conditions |
| HHP— 600 MPa 20 | Beverage subjected to high hydrostatic pressure (600 MPa) stored at 20 °C under darkness conditions |
| TP—85 °C 4 | Beverage subjected to thermal pasteurization stored at 4 °C under darkness conditions |
| TP—85 °C 20 | Beverage subjected to thermal pasteurization stored at 20 °C under darkness conditions |
| Condition Z | Physicochemical Parameters | Bioactive Compounds (mg/100 mL) | |||||
|---|---|---|---|---|---|---|---|
| Color (ΔE) | TSS (°Brix) | pH | TA (g CA/100 mL) | Anthocyanins | Flavanones | Vitamin C | |
| Non-processed | 0.00c Y | 13.60 a | 3.41 b | 2.95 a | 16.54 b | 14.99 b | 10.90 a |
| HHP—450 MPa | 1.39bc | 13.60 a | 3.45 a | 2.91 a | 15.98 c | 15.11 b | 6.03 b |
| HHP—600 MPa | 1.82b | 13.60 a | 3.40 b | 2.95 a | 16.1 c | 15.14 b | 7.49 b |
| TP—85 °C | 3.59a | 13.60 a | 3.41 b | 2.95 a | 18.37 a | 23.33 a | 7.79 b |
| LSD (p < 0.05) | 1.02 | <0.01 | 0.03 | <0.01 | 0.31 | 0.48 | 1.70 |
| p-value | *** X | N.s. | * | N.s. | *** | *** | ** |
| Condition Z | Microbiological Count (CFU/mL) | ||||
|---|---|---|---|---|---|
| Aerobic Mesophilic Bacteria | Aerobic Psycrophilic Bacteria | Molds and Yeast | Enterobacteriae | Lactic Acid Bacteria | |
| Non-processed | 80 ± 5 | <10 Y | <100 Y | <10 Y | <10 Y |
| HHP—450 MPa | <10 Y | <10 Y | <100 Y | <10 Y | <10 Y |
| HHP—600 MPa | <10 Y | <10 Y | <100 Y | <10 Y | <10 Y |
| TP—85°C | <10 Y | <10 Y | <100 Y | <10 Y | <10 Y |
| Condition Z | TSS (°Brix) | pH | TA (g CA/100 mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | Final | p-Value | Initial | Final | p-Value | Initial | Final | p-Value | |
| HHP—450 MPa 4 | 13.60 a Y | 14.20 a | *** X | 3.45 a | 3.41 a | * | 2.91 a | 3.00 a | * |
| HHP—600 MPa 4 | 13.60 a | 14.20 a | *** | 3.40 b | 3.41 a | N.s. | 2.95 a | 3.00 a | N.s. |
| TP—85°C 4 | 13.60 a | 14.20 a | *** | 3.41 b | 3.41 a | N.s. | 2.95 a | 2.98 a | N.s. |
| LSD (p <0.05) | <0.01 | <0.01 | 0.03 | <0.01 | <0.01 | <0.01 | |||
| p-value | N.s. | N.s. | * | N.s. | N.s. | N.s. | |||
| HHP—450 MPa 20 | 13.60 a | 14.20 a | *** | 3.45 a | 3.40 a | * | 2.91 a | 2.98 a | * |
| HHP —450 MPa 20 | 13.60 a | 14.20 a | *** | 3.40 b | 3.40 a | N.s. | 2.95 a | 3.00 a | * |
| TP— 85°C 20 | 13.60 a | 14.20 a | *** | 3.41 b | 3.41 a | N.s. | 2.95 a | 2.99 a | * |
| LSD (p <0.05) | <0.01 | <0.01 | 0.03 | <0.01 | <0.01 | <0.01 | |||
| p-value | N.s. | N.s. | * | N.s. | N.s. | N.s. | |||
| Parameter | Storage (Days) | HHP-450 MPa | HHP-600 MPa | TP-85 °C | LSD (p < 0.001) |
|---|---|---|---|---|---|
| w CIEL* | 0 | 23.39 aB Z | 22.55 aAB | 21.38 deA | 1.04 |
| 7 | 23.53 aB | 24.74 bC | 19.57 abA | 0.80 | |
| 15 | 24.64 bB | 33.54 cC | 19.72 aA | 0.51 | |
| 30 | 30.96 cB | 38.13 dC | 20.54 bcA | 0.49 | |
| 45 | 33.58 dB | 39.08 eC | 20.31 abA | 0.13 | |
| 60 | 33.49 dB | 40.31 fC | 21.23 cA | 0.41 | |
| 90 | 34.11 dB | 41.12 gC | 21.97 eA | 0.63 | |
| LSD (p < 0.001) | 0.69 | 0.41 | 0.46 | ||
| CIEa* | 0 | 47.87 aB | 47.48 aAB | 46.78 bA | 0.86 |
| 7 | 47.86 aB | 48.93 bC | 44.80 aA | 0.79 | |
| 15 | 48.40 aB | 56.53 cC | 45.06 aA | 0.50 | |
| 30 | 53.48 cB | 59.01 eC | 45.26 aA | 0.49 | |
| 45 | 54.83 dB | 58.50 eC | 44.60 aA | 0.40 | |
| 60 | 53.35 cB | 57.49 dC | 45.03 aA | 0.21 | |
| 90 | 52.39 bB | 56.42 cC | 44.89 aA | 0.59 | |
| LSD (p < 0.001) | 0.57 | 0.41 | 0.43 | ||
| CIEb* | 0 | 37.15 aB | 36.30 cAB | 34.82 cdA | 1.41 |
| 7 | 37.39 aB | 38.54 eC | 32.41 aA | 0.80 | |
| 15 | 38.52 bB | 37.85 dB | 32.63 aA | 0.59 | |
| 30 | 40.95 deC | 32.68 aA | 33.85 abcB | 0.28 | |
| 45 | 39.73 cC | 32.73 aA | 33.59 abB | 0.53 | |
| 60 | 40.29 cdC | 32.81 aA | 34.71 bcdB | 0.60 | |
| 90 | 41.70 eC | 33.95 bA | 35.79 dB | 0.49 | |
| LSD (p < 0.001) | 0.68 | 0.41 | 0.76 | ||
| Chroma | 0 | 60.60 aB | 59.76 aAB | 58.31 cA | 1.52 |
| 7 | 60.73 abB | 62.28 bC | 55.30 aA | 1.10 | |
| 15 | 61.86 bB | 68.03 dfC | 55.63 aA | 0.73 | |
| 30 | 67.36 cB | 67.46 efB | 56.52 abA | 0.25 | |
| 45 | 67.71 cC | 67.04 deB | 55.84 aA | 0.14 | |
| 60 | 66.85 cC | 66.20 cdB | 56.86 abA | 0.52 | |
| 90 | 66.96 cA | 65.85 cB | 57.41 bcA | 0.32 | |
| LSD (p < 0.001) | 0.77 | 0.54 | 0.78 | ||
| Hue angle | 0 | 37.81 bcB | 37.40 fAB | 36.66 bcA | 0.62 |
| 7 | 38.00 cdB | 38.22 eB | 35.88 abA | 0.19 | |
| 15 | 38.51 bdC | 33.80 dA | 35.91 aB | 0.27 | |
| 30 | 37.44 bcC | 28.98 aA | 36.79 bcB | 0.02 | |
| 45 | 35.92 aB | 29.23 aA | 36.95 cC | 0.14 | |
| 60 | 37.06 bB | 29.71 bA | 37.62 dB | 0.66 | |
| 90 | 38.52 dB | 31.04 cA | 38.56 eB | 0.59 | |
| LSD (p < 0.001) | 0.51 | 0.18 | 0.39 | ||
| ΔE | 0 | 0.00 a | 0.00 a | 0.00 a | <0.01 |
| 7 | 0.47 aA | 3.45 bB | 2.86 cdB | 1.26 | |
| 15 | 1.93 bA | 14.32 cC | 3.24 dB | 0.90 | |
| 30 | 10.16 cB | 19.72 dC | 1.99 bcA | 0.28 | |
| 45 | 12.61 dB | 20.18 dC | 2.72 cdA | 0.13 | |
| 60 | 12.34 dB | 20.68 dC | 1.76 bA | 0.31 | |
| 90 | 12.49 dB | 20.74 dC | 2.22 bcA | 0.64 | |
| LSD (p < 0.001) | 0.48 | 0.66 | 0.57 |
| Parameter | Storage (Days) | HHP-450 MPa | HHP-600 MPa | TP-85 °C | LSD (p < 0.001) |
|---|---|---|---|---|---|
| CIEL* | 0 | 23.39 aB Z | 22.55 aAB | 21.38 bA | 1.04 |
| 7 | 24.84 bB | 31.80 bC | 20.51 aA | 0.74 | |
| 15 | 28.84 cB | 37.44 cC | 22.23 bA | 0.51 | |
| 30 | 32.55 dB | 40.13 dC | 22.31 bA | 0.64 | |
| 45 | 37.06 eB | 43.61 eC | 23.86 cA | 0.43 | |
| 60 | 40.07 gB | 45.71 fC | 23.66 cA | 1.59 | |
| 90 | 38.52 fB | 47.81 gC | 24.13 cA | 0.11 | |
| LSD (p < 0.001) | 0.69 | 0.92 | 0.66 | ||
| CIEa* | 0 | 47.87 dB | 47.48 dAB | 46.78 eA | 0.86 |
| 7 | 47.08 cdB | 51.89 fC | 44.36 dA | 0.68 | |
| 15 | 47.75 dB | 53.61 gC | 44.15 dA | 0.38 | |
| 30 | 46.34 cB | 49.76 eC | 41.51 cA | 0.24 | |
| 45 | 45.19 bB | 46.88 cC | 40.19 bA | 0.30 | |
| 60 | 44.60 bB | 45.12 bC | 39.41 aA | 0.18 | |
| 90 | 42.19 aB | 43.92 aC | 38.92 aA | 0.32 | |
| LSD (p < 0.001) | 0.51 | 0.19 | 0.49 | ||
| CIEb* | 0 | 37.17 aB | 36.30 aAB | 34.82 abA | 1.41 |
| 7 | 39.14 bB | 41.84 cC | 33.76 aA | 0.86 | |
| 15 | 43.15 cB | 40.34 bC | 36.19 bcA | 0.64 | |
| 30 | 46.56 dC | 44.07 dB | 36.57 cA | 0.73 | |
| 45 | 48.31 eC | 44.76 dB | 38.78 dA | 0.55 | |
| 60 | 48.68 eC | 45.63 eB | 38.64 dA | 0.91 | |
| 90 | 50.36 fC | 46.23 eB | 39.24 dA | 0.14 | |
| LSD (p < 0.001) | 0.67 | 0.54 | 0.92 | ||
| Chroma | 0 | 60.60 aB | 59.76 aAB | 58.31 cA | 1.52 |
| 7 | 61.22 aB | 66.66 deC | 55.75 abA | 1.05 | |
| 15 | 64.35 bB | 67.10 eC | 57.09 bcA | 0.69 | |
| 30 | 65.69 cB | 66.48 dB | 55.32 aA | 0.63 | |
| 45 | 66.15 cA | 64.81 cB | 55.85 abA | 0.58 | |
| 60 | 66.03 cC | 64.17 bcB | 55.19 aA | 0.60 | |
| 90 | 65.70 cC | 63.77 bB | 55.26 aA | 0.32 | |
| LSD (p < 0.001) | 0.81 | 0.39 | 0.93 | ||
| Hue angle | 0 | 37.81 aB | 37.40 aAB | 36.66 aA | 0.62 |
| 7 | 39.74 bC | 38.88 bB | 37.27 aA | 0.28 | |
| 15 | 42.10 cC | 36.96 aA | 39.45 bB | 0.66 | |
| 30 | 45.14 dB | 41.53 cA | 41.38 cA | 0.46 | |
| 45 | 46.92 eB | 43.67 dA | 43.98 dA | 0.25 | |
| 60 | 47.50 fC | 45.32 eB | 44.43 dA | 0.63 | |
| 90 | 50.04 gC | 46.46 fB | 45.24 eA | 0.17 | |
| LSD (p < 0.001) | 0.21 | 0.37 | 0.47 | ||
| ΔE | 0 | 0.00 a | 0.00 a | 0.00 a | <0.01 |
| 7 | 2.63 bA | 11.64 bB | 2.80 bA | 0.97 | |
| 15 | 8.10 cB | 16.60 cC | 3.14 bA | 0.33 | |
| 30 | 13.23 dB | 19.35 dC | 5.67 cA | 0.40 | |
| 45 | 17.85 eB | 22.70 eC | 8.09 dA | 0.19 | |
| 60 | 20.53 fB | 25.10 fC | 8.61 dA | 1.10 | |
| 90 | 20.88 fB | 27.37 gC | 9.46 eA | 0.19 | |
| LSD (p < 0.001) | 0.47 | 0.64 | 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salar, F.J.; Periago, P.M.; Agulló, V.; García-Viguera, C.; Fernández, P.S. High Hydrostatic Pressure vs. Thermal Pasteurization: The Effect on the Bioactive Compound Profile of a Citrus Maqui Beverage. Foods 2021, 10, 2416. https://doi.org/10.3390/foods10102416
Salar FJ, Periago PM, Agulló V, García-Viguera C, Fernández PS. High Hydrostatic Pressure vs. Thermal Pasteurization: The Effect on the Bioactive Compound Profile of a Citrus Maqui Beverage. Foods. 2021; 10(10):2416. https://doi.org/10.3390/foods10102416
Chicago/Turabian StyleSalar, Francisco J., Paula M. Periago, Vicente Agulló, Cristina García-Viguera, and Pablo S. Fernández. 2021. "High Hydrostatic Pressure vs. Thermal Pasteurization: The Effect on the Bioactive Compound Profile of a Citrus Maqui Beverage" Foods 10, no. 10: 2416. https://doi.org/10.3390/foods10102416
APA StyleSalar, F. J., Periago, P. M., Agulló, V., García-Viguera, C., & Fernández, P. S. (2021). High Hydrostatic Pressure vs. Thermal Pasteurization: The Effect on the Bioactive Compound Profile of a Citrus Maqui Beverage. Foods, 10(10), 2416. https://doi.org/10.3390/foods10102416







