Effect of Several Pretreatments on the Lactic Acid Production from Exhausted Sugar Beet Pulp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Pretreatment of ESBP
2.2.1. Biological Pretreatment
2.2.2. Alkaline Hydrogen Peroxide Pretreatment
2.2.3. Thermochemical Pretreatment
2.3. Enzymatic Hydrolysis of Pretreated Solid
2.4. Lactic Acid Fermentation of Pretreated ESBP Hydrolysate
2.5. Analytical Techniques
2.5.1. Determination of Fibre Composition
2.5.2. Determination of Moisture Content of ESBP
2.5.3. Determination of Sugar Concentration
2.5.4. Determination of Organic Acid Concentration
2.5.5. Determination of Cell Growth in Lactic Acid Fermentation
2.5.6. Statistical Analysis
3. Results and Discussion
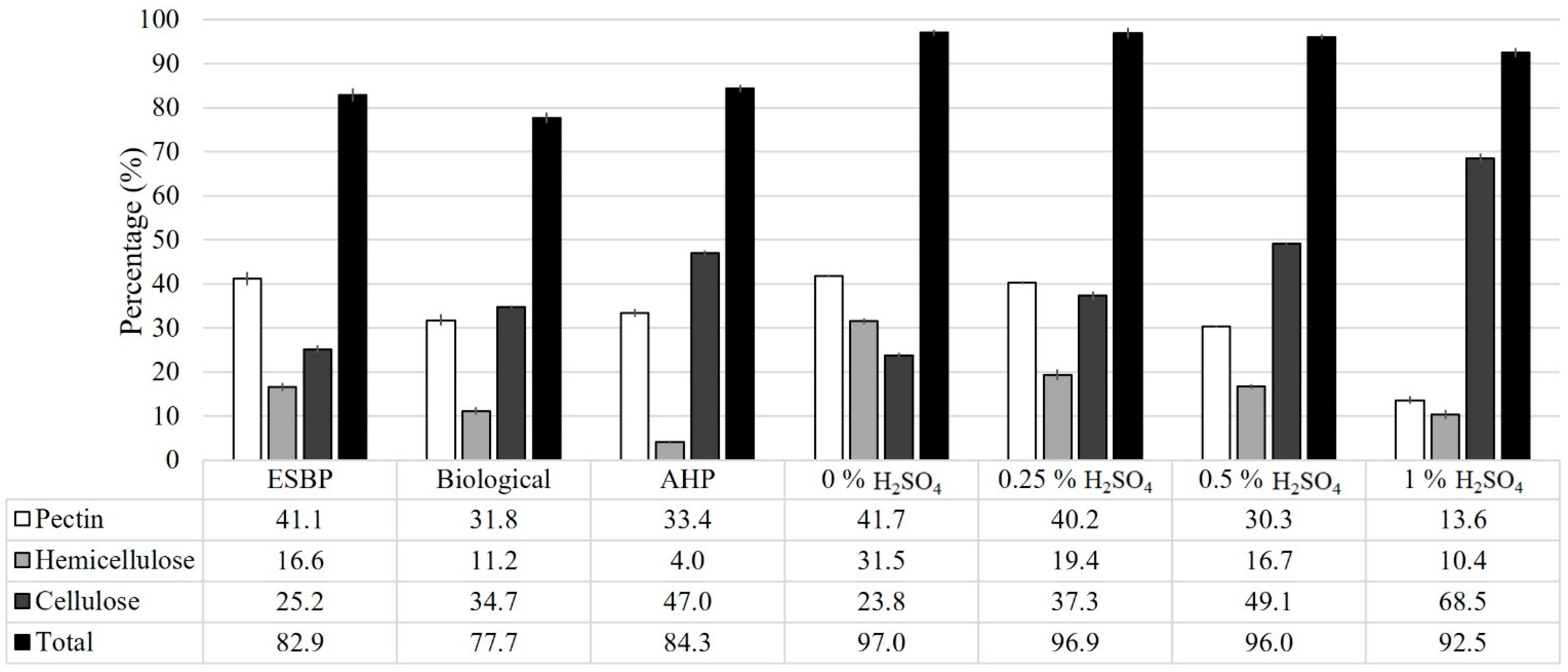
3.1. Pretreatment Effects on the Fibre Composition
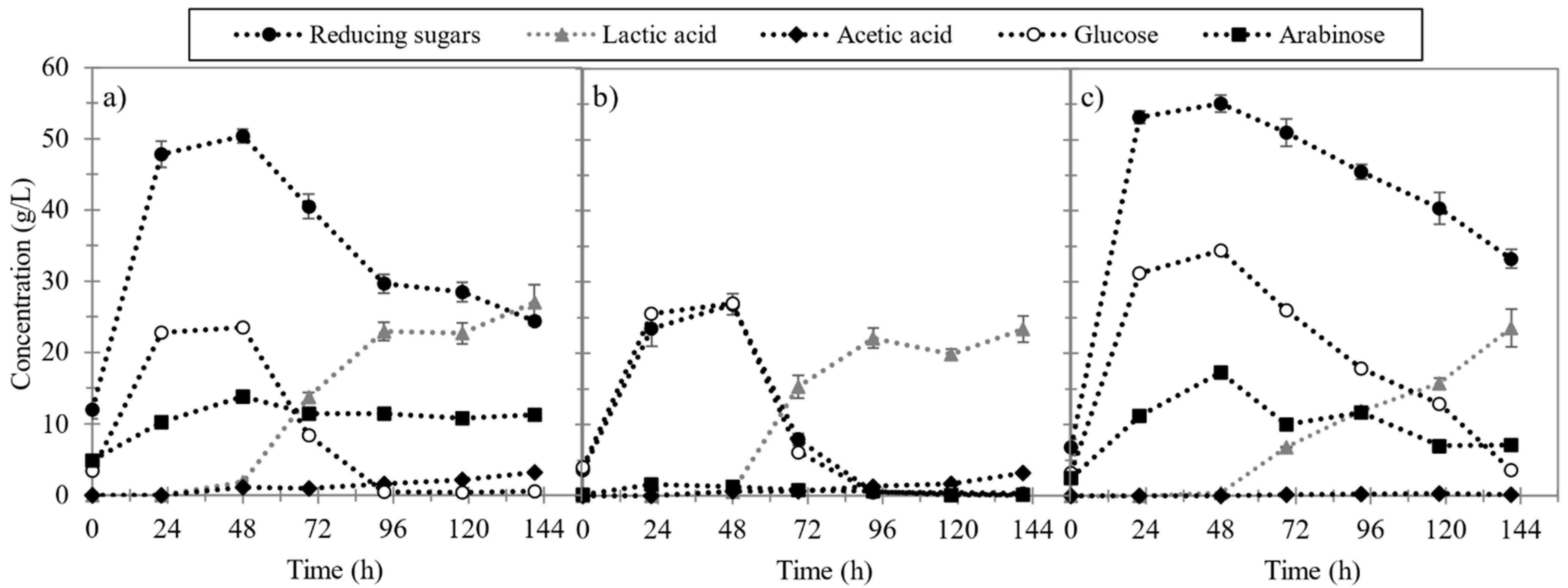
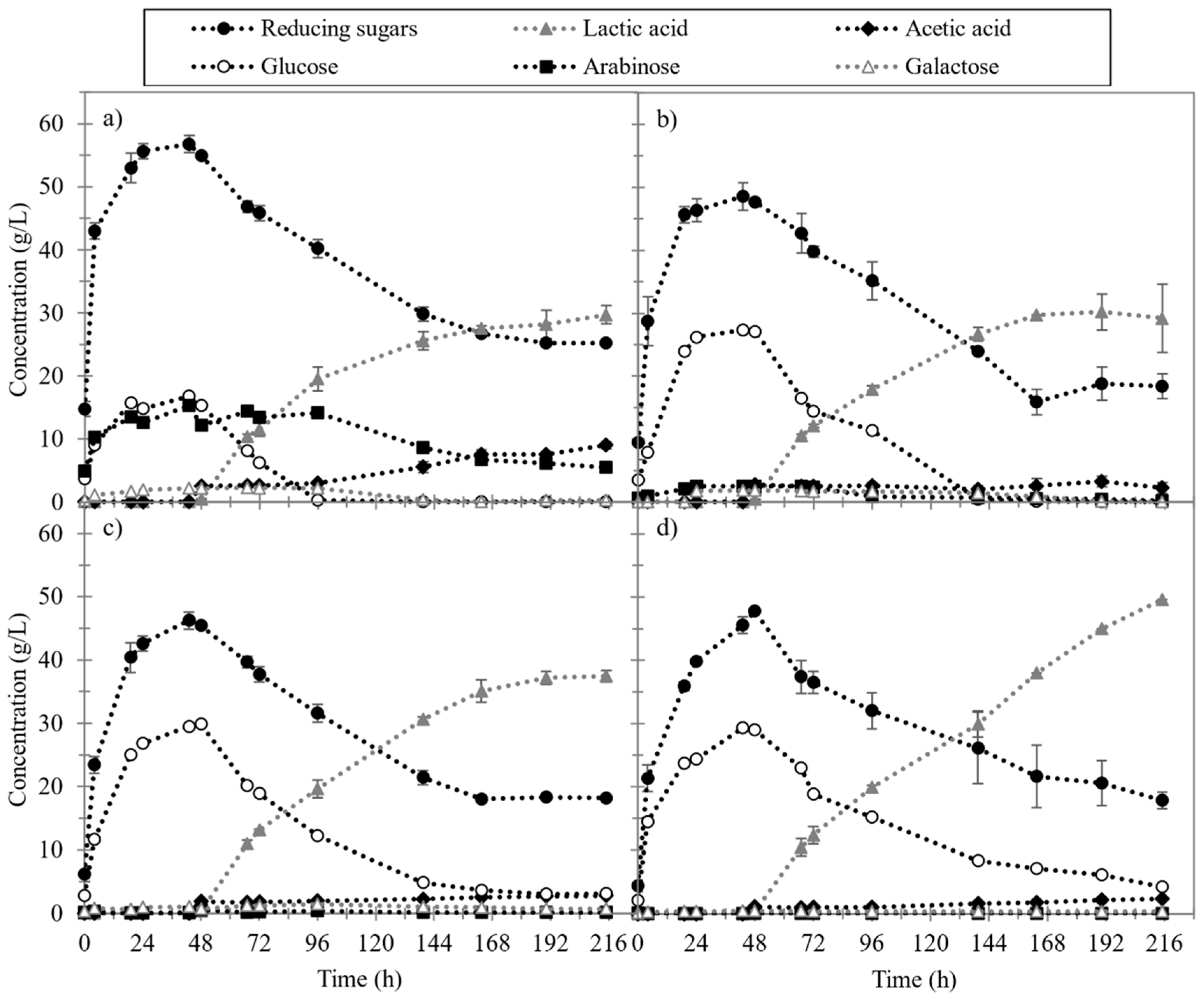
3.2. Pretreatment Effects on Enzymatic Hydrolysis and Lactic Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Gómez, J.P.; Pérez-Rivero, C.; Venus, J. Valorisation of solid biowastes: The lactic acid alternative. Process Biochem. 2020, 99, 222–235. [Google Scholar] [CrossRef]
- García, C.; Bautista, L.; Rendueles, M.; Díaz, M. A new synbiotic dairy food containing lactobionic acid and Lactobacillus casei. Int. J. Dairy Technol. 2019, 72, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Shafi, A.; Naeem Raja, H.; Farooq, U.; Akram, K.; Hayat, Z.; Naz, A.; Nadeem, H.R. Antimicrobial and antidiabetic potential of synbiotic fermented milk: A functional dairy product. Int. J. Dairy Technol. 2019, 72, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Silva, H.L.A.; Balthazar, C.F.; Silva, R.; Vieira, A.H.; Costa, R.G.B.; Esmerino, E.A.; Freitas, M.Q.; Cruz, A.G. Sodium reduction and flavor enhancer addition in probiotic prato cheese: Contributions of quantitative descriptive analysis and temporal dominance of sensations for sensory profiling. J. Dairy Sci. 2018, 101, 8837–8846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cubas-Cano, E.; González-Fernández, C.; Ballesteros, M.; Tomás-Pejó, E. Biotechnological advances in lactic acid production by lactic acid bacteria: Lignocellulose as novel subtrate. Biofuels Bioprod. Biorefining 2018, 12, 290–303. [Google Scholar] [CrossRef]
- Ahmed, T.; Shahid, M.; Azeem, F.; Rasul, I.; Shah, A.A.; Noman, M.; Hameed, A.; Manzoor, N.; Manzoor, I.; Muhammad, S. Biodegradation of plastics: Current scenario and future prospects for environmental safety. Environ. Sci. Pollut. Res. 2018, 25, 7287–7298. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Gao, M.; Wang, N.; Liu, S.; Wang, Q.; Sun, X. Lactic acid production from co-fermentation of food waste and spent mushroom substance with Aspergillus niger cellulase. Bioresour. Technol. 2021, 337, 125365. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.Y.; Tan, I.S.; Foo, H.C.Y.; Lam, M.K.; Tong, K.T.X.; Lee, K.T. Sustainable and green pretreatment strategy of Eucheuma denticulatum residues for third-generation L-lactic acid production. Bioresour. Technol. 2021, 330, 124930. [Google Scholar] [CrossRef] [PubMed]
- Camesasca, L.; de Mattos, J.A.; Vila, E.; Cebreiros, F.; Lareo, C. Lactic acid production by Carnobacterium sp. isolated from a maritime Antarctic lake using eucalyptus enzymatic hydrolysate. Biotechnol. Rep. 2021, 31, e00643. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, M.; Sobrinho, M.A.M.; Kraume, M. The use of biomagnetism for biogas production from sugar beet pulp. Biochem. Eng. J. 2020, 164, 107770. [Google Scholar] [CrossRef]
- Cieciura-Włoch, W.; Borowski, S.; Domański, J. Dark fermentative hydrogen production from hydrolyzed sugar beet pulp improved by iron addition. Bioresour. Technol. 2020, 314, 123713. [Google Scholar] [CrossRef]
- Achinas, S.; Leenders, N.; Krooneman, J.; Euverink, G.J.W. Feasibility assessment of a bioethanol plant in the Northern Netherlands. Appl. Sci. 2019, 9, 4586. [Google Scholar] [CrossRef] [Green Version]
- Alexandri, M.; Schneider, R.; Papapostolou, H.; Ladakis, D.; Koutinas, A.; Venus, J. Restructuring the conventional sugar beet industry into a novel biorefinery: Fractionation and bioconversion of sugar beet pulp into succinic acid and value-added coproducts. ACS Sustain. Chem. Eng. 2019, 7, 6569–6579. [Google Scholar] [CrossRef]
- Oliveira, R.A.; Schneider, R.; Lunelli, B.H.; Rossell, C.E.V.; Filho, R.M.; Venus, J. A simple biorefinery concept to produce 2G-lactic acid from sugar beet pulp (SBP): A high-value target approach to valorize a waste stream. Molecules 2020, 25, 2113. [Google Scholar] [CrossRef] [PubMed]
- Marzo, C.; Díaz, A.B.; Caro, I.; Blandino, A. Valorization of agro-industrial wastes to produce hydrolytic enzymes by fungal solid-state fermentation. Waste Manag. Res. 2018, 32, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Berlowska, J.; Binczarski, M.; Dziugan, P.; Wilkowska, A.; Kregiel, D.; Witonska, I. Sugar Beet Pulp as a Source of Valuable Biotechnological Products; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 14, ISBN 9780128114957. [Google Scholar]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Haldar, D.; Purkait, M.K. A review on the environment-friendly emerging techniques for pretreatment of lignocellulosic biomass: Mechanistic insight and advancements. Chemosphere 2021, 264, 128523. [Google Scholar] [CrossRef]
- Abo, B.O.; Gao, M.; Wang, Y.; Wu, C.; Ma, H.; Wang, Q. Lignocellulosic biomass for bioethanol: An overview on pretreatment, hydrolysis and fermentation processes. Rev. Environ. Health 2019, 34, 57–68. [Google Scholar] [CrossRef]
- Donkoh, E.; Degenstein, J.; Tucker, M.; Ji, Y. Optimization of enzymatic hydrolysis of dilute acid pretreated sugar beet pulp using response surface design. J. Sugar Beet Res. 2012, 49, 26–38. [Google Scholar] [CrossRef] [Green Version]
- El-gendy, N.S.; Madian, H.R.; Nassar, H.N. Response surface optimization of the thermal acid pretreatment of sugar beet pulp for bioethanol production using Trichoderma viride and Saccharomyces cerevisiae. Recent Pat. Biotechnol. 2015, 9, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Lee, C.; Yu, C.; Cheng, Y.S.; Zhang, R.; Jenkins, B.M.; Vandergheynst, J.S. Dilute acid pretreatment and fermentation of sugar beet pulp to ethanol. Appl. Energy 2013, 105, 1–7. [Google Scholar] [CrossRef]
- Díaz, A.B.; Blandino, A.; Belleli, C.; Caro, I. An effective process for pretreating rice husk to enhance enzyme hydrolysis. Ind. Eng. Chem. Res. 2014, 53, 10870–10875. [Google Scholar] [CrossRef]
- Díaz, A.; Le Toullec, J.; Blandino, A.; De Ory, I.; Caro, I.; Diaz, A.; Le Toullec, J.; Blandino, A.; De Ory, I.; Caro, I. Pretreatment of rice hulls with alkaline peroxide to enhance enzyme hydrolysis for ethanol production. Chem. Eng. Trans. 2013, 32, 949–954. [Google Scholar] [CrossRef]
- Cabrera, E.; Muñoz, M.J.; Martín, R.; Caro, I.; Curbelo, C.; Díaz, A.B. Alkaline and alkaline peroxide pretreatments at mild temperature to enhance enzymatic hydrolysis of rice hulls and straw. Bioresour. Technol. 2014, 167, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Gonçalves, C.; Rodriguez-Jasso, R.M.; Gomes, N.; Teixeira, J.A.; Belo, I. Adaptation of dinitrosalicylic acid method to microtiter plates. Anal. Methods 2010, 2, 2046–2048. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Sharma-Shivappa, R.R.; Chinn, M.; Howell, N. Effect of microbial pretreatment on enzymatic hydrolysis and fermentation of cotton stalks for ethanol production. Biomass Bioenergy 2009, 33, 88–96. [Google Scholar] [CrossRef]
- Wan, C.; Li, Y. Microbial pretreatment of corn stover with Ceriporiopsis subvermispora for enzymatic hydrolysis and ethanol production. Bioresour. Technol. 2010, 101, 6398–6403. [Google Scholar] [CrossRef] [PubMed]
- Dutra, E.D.; Santos, F.A.; Alencar, B.R.A.; Reis, A.L.S.; de Souza, R.d.F.R.; Aquino, K.A.d.S.; Morais, M.A.; Menezes, R.S.C. Alkaline hydrogen peroxide pretreatment of lignocellulosic biomass: Status and perspectives. Biomass Convers. Biorefinery 2018, 8, 225–234. [Google Scholar] [CrossRef]
- Park, Y.C.; Kim, J.S. Comparison of various alkaline pretreatment methods of lignocellulosic biomass. Energy 2012, 47, 31–35. [Google Scholar] [CrossRef]
- Canilha, L.; Santos, V.T.O.; Rocha, G.J.M.; Almeida, E.; Silva, J.B.; Giulietti, M.; Silva, S.S.; Felipe, M.G.A.; Ferraz, A.; Milagres, A.M.F.; et al. A study on the pretreatment of a sugarcane bagasse sample with dilute sulfuric acid. J. Ind. Microbiol. Biotechnol. 2011, 38, 1467–1475. [Google Scholar] [CrossRef]
- Tsoutsos, T. Modelling hydrolysis and fermentation processes in lignocelluloses-to-bioalcohol production. In Bioalcohol Production: Biochemical Conversion of Lignocellulosic Biomass; Elsevier Inc.: Amsterdam, The Netherlands, 2010; pp. 340–362. ISBN 9781845695101. [Google Scholar]
- Torget, R.W.; Kim, J.S.; Lee, Y.Y. Fundamental aspects of dilute acid hydrolysis/fractionation kinetics of hardwood carbohydrates. 1. Cellulose hydrolysis. Ind. Eng. Chem. Res. 2000, 39, 2817–2825. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Marzo, C.; Díaz, A.B.; Caro, I.; Blandino, A. Valorisation of fungal hydrolysates of exhausted sugar beet pulp for lactic acid production. J. Sci. Food Agric. 2021, 101, 4108–4117. [Google Scholar] [CrossRef]
- Ho, M.C.; Ong, V.Z.; Wu, T.Y. Potential use of alkaline hydrogen peroxide in lignocellulosic biomass pretreatment and valorization—A review. Renew. Sustain. Energy Rev. 2019, 112, 75–86. [Google Scholar] [CrossRef]
- Wei, W.; Wu, S.; Liu, L. Enzymatic saccharification of dilute acid pretreated eucalyptus chips for fermentable sugar production. Bioresour. Technol. 2012, 110, 302–307. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Huang, C.; Ling, Z.; Lai, C.; Yong, Q. Natural surfactant-aided dilute sulfuric acid pretreatment of waste wheat straw to enhance enzymatic hydrolysis efficiency. Bioresour. Technol. 2021, 324, 124651. [Google Scholar] [CrossRef] [PubMed]
- Komesu, A.; Martins, P.F.; Lunelli, B.H.; Rocha, J.O.; Filho, R.M.; Maciel, M.R.W. The Effect of Evaporator Temperature on Lactic Acid Purity and Recovery by Short Path Evaporation. Sep. Sci. Technol. 2015, 50, 1548–1553. [Google Scholar] [CrossRef]
- Biddy, M.J.; Scarlata, C.; Kinchin, C. Chemicals from Biomass: A Market Assessment of Bioproducts with Near-Term Potential; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2016.
- Komesu, A.; de Oliveira, J.A.R.; Martins, L.H.d.S.; Maciel, M.R.W.; Filho, R.M. Lactic acid production to purification: A review. BioResources 2017, 12, 4364–4383. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzo, C.; Díaz, A.B.; Caro, I.; Blandino, A. Effect of Several Pretreatments on the Lactic Acid Production from Exhausted Sugar Beet Pulp. Foods 2021, 10, 2414. https://doi.org/10.3390/foods10102414
Marzo C, Díaz AB, Caro I, Blandino A. Effect of Several Pretreatments on the Lactic Acid Production from Exhausted Sugar Beet Pulp. Foods. 2021; 10(10):2414. https://doi.org/10.3390/foods10102414
Chicago/Turabian StyleMarzo, Cristina, Ana Belén Díaz, Ildefonso Caro, and Ana Blandino. 2021. "Effect of Several Pretreatments on the Lactic Acid Production from Exhausted Sugar Beet Pulp" Foods 10, no. 10: 2414. https://doi.org/10.3390/foods10102414
APA StyleMarzo, C., Díaz, A. B., Caro, I., & Blandino, A. (2021). Effect of Several Pretreatments on the Lactic Acid Production from Exhausted Sugar Beet Pulp. Foods, 10(10), 2414. https://doi.org/10.3390/foods10102414








