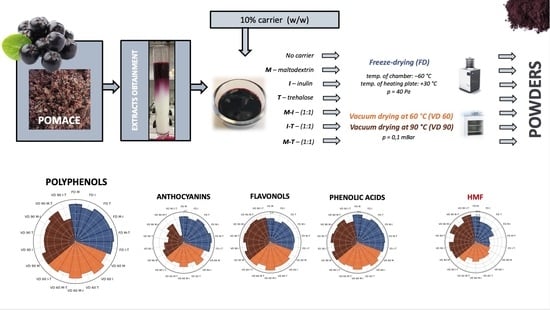
How Do the Different Types of Carrier and Drying Techniques Affect the Changes in Physico-Chemical Properties of Powders from Chokeberry Pomace Extracts?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Extraction Procedure
2.2.2. Preparation of Chokeberry Pomace Extract Powders
2.2.3. Physical Properties
Moisture Content
Water Activity
Bulk Density
Colour
2.2.4. Chemical Properties
Preparation of Extracts
Qualitative and Quantitative Determination of Polyphenolics and Hydroxymethyl-L-furfural
Antioxidant Capacity
2.2.5. Statistical Analysis and Chemometrics
3. Results and Discussion
3.1. Physical Properties
3.1.1. Moisture Content
3.1.2. Water Activity (aw)
3.1.3. Bulk Density
3.1.4. Colour
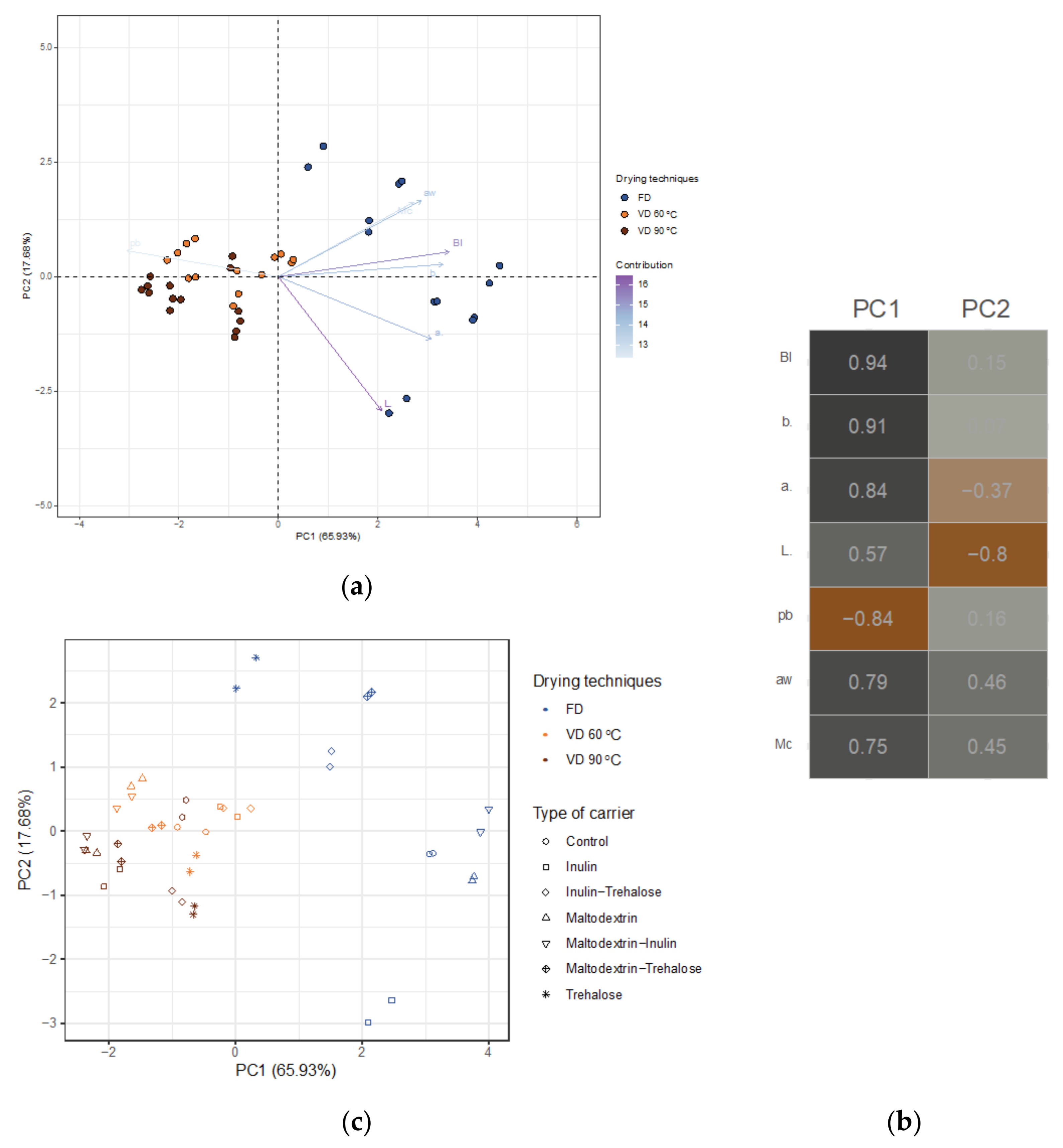
3.1.5. PCA Analysis
3.2. Chemical Properties
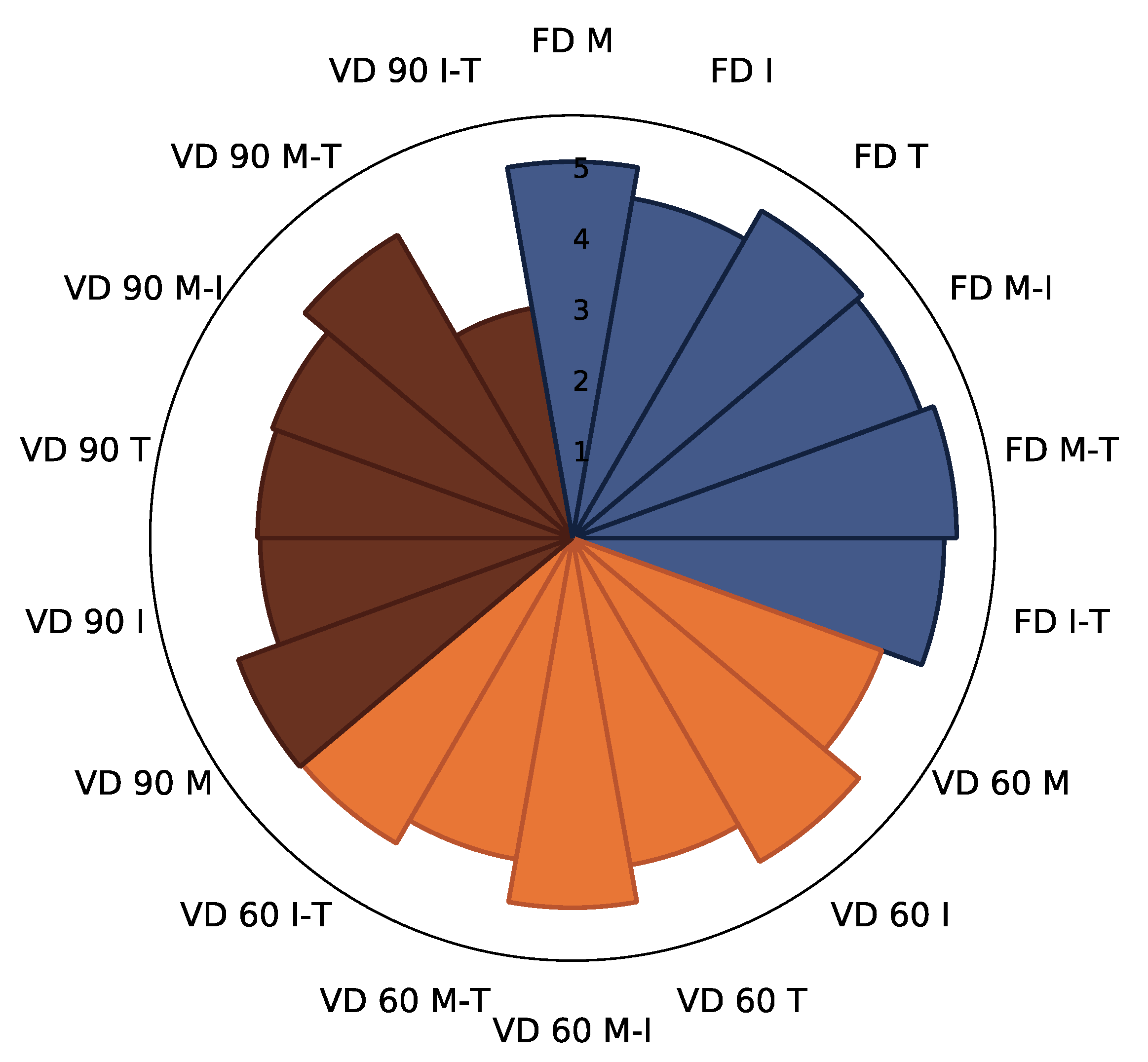
3.2.1. Polyphenols Content
3.2.2. Hydroxymethyl-L-furfural
3.2.3. Antioxidant Capacity
3.2.4. PCA Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Salehi, G.; Díaz, E.; Redondo, R. Consumers’ switching to vegan, vegetarian, and plant-based (Veggan) diets: A systematic Review of literature. In Proceedings of the 19th International Congress on Public and Nonprofit Marketing Sustainability: New Challenges for Marketing and Socioeconomic Development, Madrid, Spain, 2–4 July 2020. [Google Scholar]
- Campos, D.A.; Garcia, R.G.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of fruit industrial by-products—A case study on circular economy approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [Green Version]
- Mayer-Miebach, E.; Briviba, K.; Schiffer, C.; Geiger, L.; Behsnilian, D.; Greiner, R. Particle size of milled chokeberry pomace did not influence in vitro cellular absorption and transport efficiencies of anthocyanins, phenolic acids and flavonols. Int. J. Food Sci. Nutr. 2019, 70, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Majerska, J.; Michalska, A.; Figiel, A. A review of new directions in managing fruit and vegetable processing by-products. Trends Food Sci. Technol. 2019, 88, 207–219. [Google Scholar] [CrossRef]
- Vagiri, M.; Jensen, M. Influence of juice processing factors on quality of black chokeberry pomace as a future resource for colour extraction. Food Chem. 2017, 217, 409–417. [Google Scholar] [CrossRef]
- Sidor, A.; Drożdżyńska, A.; Gramza-Michałowska, A. Black chokeberry (Aronia melanocarpa) and its products as potential health-promoting factors—An overview. Trends Food Sci. Technol. 2019, 89, 45–60. [Google Scholar] [CrossRef]
- Witczak, T.; Stępień, A.; Gumul, D.; Witczak, M.; Fiutak, G.; Zięba, T. The influence of the extrusion process on the nutritional composition, physical properties and storage stability of black chokeberry pomaces. Food Chem. 2021, 334, 127548. [Google Scholar] [CrossRef] [PubMed]
- Comunian, T.A.; Silva, M.P.; Souza, C.J. The use of food by-products as a novel for functional foods: Their use as ingredients and for the encapsulation process. Trends Food Sci. Technol. 2021, 108, 269–280. [Google Scholar] [CrossRef]
- Roda-Serrat, M.C.; Andrade, T.; Rindom, J.; Lund, P.B.; Norddahl, B.; Errico, M. Optimization of the recovery of anthocyanins from chokeberry juice pomace by homogenization in acidified water. Waste Biomass Valorization 2020, 12, 1815–1827. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, B.; Bansal, N.; Zhang, M.; Schuck, P. Woodhead Publishing Series in Food Science, Technology and Nutrition. In Handbook of Food Powders; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Kammerer, D.; Kljusuric, J.G.; Carle, R.; Schieber, A. Recovery of anthocyanins from grape pomace extracts (Vitis vinifera L. cv. Cabernet Mitos) using a polymeric adsorber resin. Eur. Food Res. Technol. 2005, 220, 431–437. [Google Scholar] [CrossRef]
- Michalska-Ciechanowska, A.; Brzezowska, J.; Wojdyło, A.; Gajewicz-Skretna, A.; Ciska, E.; Majerska, J. Chemometric contribution for deeper understanding of thermally-induced changes of polyphenolics and the formation of hydroxymethyl-L-furfural in chokeberry powders. Food Chem. 2021, 342, 128335. [Google Scholar] [CrossRef]
- Nowak, D.; Jakubczyk, E. The Freeze-Drying of Foods—The characteristic of the process course and the effect of its parameters on the physical properties of food materials. Foods 2020, 9, 1488. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Brzezowska, J.; Majerska, J.; Ciska, E. The influence of inulin on the retention of polyphenolic compounds during the drying of blackcurrant juice. Molecules 2019, 24, 4167. [Google Scholar] [CrossRef] [Green Version]
- Sobulska, M.; Zbicinski, I. Advances in spray drying of sugar-rich products. Dry. Technol. 2020, 1–26. [Google Scholar] [CrossRef]
- Wan, X.; Guo, H.; Liang, Y.; Zhou, C.; Liu, Z.; Li, K.; Niu, F.; Zhai, X.; Wang, L. The physiological functions and pharmaceutical applications of inulin: A review. Carbohydr. Polym. 2020, 246, 116589. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J. Sposób otrzymywania barwników antocyjanowych. PL-158707. 30 July 1993. Available online: https://grab.uprp.pl/sites/WynalazkiWzoryUzytkowe/Opisy/Patenty%20i%20Wzory%20uytkowe/158707_B1.pdf (accessed on 26 July 2021). (In Polish).
- Wang, W.; Yagiz, Y.; Buran, T.J.; Nunes, C.D.N.; Gu, L. Phytochemicals from berries and grapes inhibited the formation of advanced glycation end-products by scavenging reactive carbonyls. Food Res. Int. 2011, 44, 2666–2673. [Google Scholar] [CrossRef]
- Mexis, S.; Kontominas, M. Effect of oxygen absorber, nitrogen flushing, packaging material oxygen transmission rate and storage conditions on quality retention of raw whole unpeeled almond kernels (Prunus dulcis). LWT 2010, 43, 1–11. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Bielicki, P. Polyphenolic composition, antioxidant activity, and polyphenol oxidase (PPO) activity of quince (Cydonia oblonga Miller) varieties. J. Agric. Food Chem. 2013, 61, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A.; Wojdyło, A.; Łysiak, G.P.; Figiel, A. Chemical composition and antioxidant properties of powders obtained from different plum juice formulations. Int. J. Mol. Sci. 2017, 18, 176. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data, R Package Version 1.0.7; CRAN: 2020. Available online: https://cran.r-project.org/web/packages/factoextra/index.html (accessed on 26 July 2021).
- Nakazawa, M. Fmsb: Functions for Medical Statistics Book with Some Demographic, Data Version 0.7.1; CRAN: 2019. Available online: https://cran.r-project.org/web/packages/fmsb/index.html (accessed on 26 July 2021).
- Michalska, A.; Lech, K. The Effect of carrier quantity and drying method on the physical properties of apple juice powders. Beverages 2018, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Tontul, I.; Topuz, A. Spray-drying of fruit and vegetable juices: Effect of drying conditions on the product yield and physical properties. Trends Food Sci. Technol. 2017, 63, 91–102. [Google Scholar] [CrossRef]
- Carvalho, A.G.D.S.; Machado, M.T.D.C.; da Silva, V.M.; Sartoratto, A.; Rodrigues, R.A.F.; Hubinger, M. Physical properties and morphology of spray dried microparticles containing anthocyanins of Jussara (Euterpe edulis Martius) extract. Powder Technol. 2016, 294, 421–428. [Google Scholar] [CrossRef]
- Shafiur Rahman, M. Handbook of Food Preservation, 2nd ed.; Shafiur Rahman, M., Ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Adetoro, A.O.; Opara, U.L.; Fawole, O.A. Effect of carrier agents on the physicochemical and technofunctional properties and antioxidant capacity of freeze-dried pomegranate juice (Punica granatum) powder. Foods 2020, 9, 1388. [Google Scholar] [CrossRef]
- Muhoza, B.; Xia, S.; Wang, X.; Zhang, X. The protection effect of trehalose on the multinuclear microcapsules based on gelatin and high methyl pectin coacervate during freeze-drying. Food Hydrocoll. 2020, 105, 105807. [Google Scholar] [CrossRef]
- Kilburn, D.; Townrow, S.; Meunier, V.; Richardson, R.; Alam, A.; Ubbink, J. Organization and mobility of water in amorphous and crystalline trehalose. Nat. Mater. 2006, 5, 632–635. [Google Scholar] [CrossRef]
- Michalska-Ciechanowska, A.; Majerska, J.; Brzezowska, J.; Wojdyło, A.; Figiel, A. The Influence of maltodextrin and inulin on the physico-chemical properties of cranberry juice powders. ChemEngineering 2020, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.S.; Kim, D.W.; Kim, S.S.; Jung, M.Y. Bulk flow properties of model food powder at different water activity. Int. J. Food Prop. 1998, 1, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Khalifa, I.; Li, M.; Mamet, T.; Li, C. Maltodextrin or gum Arabic with whey proteins as wall-material blends increased the stability and physiochemical characteristics of mulberry microparticles. Food Biosci. 2019, 31, 10445. [Google Scholar] [CrossRef]
- Bednarska, M.A.; Janiszewska-Turak, E. The influence of spray drying parameters and carrier material on the physico-chemical properties and quality of chokeberry juice powder. J. Food Sci. Technol. 2019, 57, 564–577. [Google Scholar] [CrossRef] [Green Version]
- Sarabandi, K.; Peighambardoust, S.H.; Mahoonak, A.R.S.; Samaei, S. Effect of different carriers on microstructure and physical characteristics of spray dried apple juice concentrate. J. Food Sci. Technol. 2018, 55, 3098–3109. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Lech, K.; Nowicka, P. Effects of different drying methods on the retention of bioactive compounds, on-line antioxidant capacity and color of the novel snack from red-fleshed apples. Molecules 2020, 25, 5521. [Google Scholar] [CrossRef]
- Pathare, P.; Opara, U.L.; Al-Said, F.A.-J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2012, 6, 36–60. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Michalska-Ciechanowska, A.; Turkiewicz, I.P.; Lech, K.; Nowicka, P. Influence carrier agents, drying methods, storage time on physico-chemical properties and bioactive potential of encapsulated sea buckthorn juice powders. Molecules 2020, 25, 3801. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Lech, K.; Łysiak, G.P.; Figiel, A. Physicochemical properties of whole fruit plum powders obtained using different drying technologies. Food Chem. 2016, 207, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-C.; Chang, H.-M.; Wu, J.S.-B. A study on the mechanism of browning in mei liqueur using model solutions. Food Res. Int. 2003, 36, 579–585. [Google Scholar] [CrossRef]
- Dorris, M.R.; Voss, D.M.; Bollom, M.; Krawiec-Thayer, M.P.; Bolling, B.W. Browning index of anthocyanin-rich fruit juice depends on pH and anthocyanin loss more than the gain of soluble polymeric pigments. J. Food Sci. 2018, 83, 911–921. [Google Scholar] [CrossRef]
- Sójka, M.; Kołodziejczyk, K.; Milala, J.; Abadias, M.; Viñas, I.; Guyot, S.; Baron, A. Composition and properties of the polyphenolic extracts obtained from industrial plum pomaces. J. Funct. Foods 2015, 12, 168–178. [Google Scholar] [CrossRef]
- Sójka, M.; Kołodziejczyk, K.; Milala, J. Polyphenolic and basic chemical composition of black chokeberry industrial by-products. Ind. Crop. Prod. 2013, 51, 77–86. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Honke, J.; Ciska, E.; Andlauer, W. Drying-induced physico-chemical changes in cranberry products. Food Chem. 2018, 240, 448–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomas, M.; Beekwilder, J.; Hall, R.; Simon, C.D.; Sagdic, O.; Capanoglu, E. Effect of dietary fiber (inulin) addition on phenolics and in vitro bioaccessibility of tomato sauce. Food Res. Int. 2018, 106, 129–135. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Kubica, P.; Banaszczak, P.; Wojtanowska-Krośniak, A.; Krosniak, M.; Marzec-Wróblewska, U.; Badura, A.; Zagrodzki, P.; Bucinski, A.; et al. Comparative analysis of different groups of phenolic compounds in fruit and leaf extracts of Aronia sp.: A. melanocarpa, A. arbutifolia, and A.×prunifolia and their antioxidant activities. Eur. Food Res. Technol. 2017, 243, 1645–1657. [Google Scholar] [CrossRef] [Green Version]
- Turkiewicz, I.; Wojdyło, A.; Tkacz, K.; Lech, K.; Michalska-Ciechanowska, A.; Nowicka, P. The influence of different carrier agents and drying techniques on physical and chemical characterization of Japanese quince (Chaenomeles japonica) microencapsulation powder. Food Chem. 2020, 323, 126830. [Google Scholar] [CrossRef]
- Hamrouni-Sellami, I.; Rahali, F.Z.; Rebey, I.B.; Bourgou, S.; Limam, F.; Marzouk, B. Total phenolics, flavonoids, and antioxidant activity of sage (Salvia officinalis L.) plants as affected by different drying methods. Food Bioprocess Technol. 2012, 6, 806–817. [Google Scholar] [CrossRef]
- Sharma, K.; Ko, E.Y.; Assefa, A.; Ha, S.; Nile, S.; Lee, E.T.; Park, S.W. Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. J. Food Drug Anal. 2015, 23, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Aktağ, I.G.; Gökmen, V. Multiresponse kinetic modelling of α-dicarbonyl compounds formation in fruit juices during storage. Food Chem. 2020, 320, 126620. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, K.; Kendrick, B.; Santos, C.; Green, P.; Zhang, B.; Hunt, D.; Ronk, M.; Luo, Y. Freeze-dry mediated formation of 5-(Hydroxylmethyl)furfural. In Developments in Biotechnology and Bioprocessing; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2013; Volume 1125, pp. 129–145. ISBN 978-0-8412-2910-5. [Google Scholar]
- Zhang, Z.; Zou, Y.; Wu, T.; Huang, C.; Pei, K.; Zhang, G.; Lin, X.; Bai, W.; Ou, S. Chlorogenic acid increased 5-hydroxymethylfurfural formation when heating fructose alone or with aspartic acid at two pH levels. Food Chem. 2016, 190, 832–835. [Google Scholar] [CrossRef]
- Zhang, Y.; An, X. Inhibitory mechanism of quercetin against the formation of 5-(hydroxymethyl)-2-furaldehyde in buckwheat flour bread by ultra-performance liquid chromatography coupled with high-resolution tandem mass spectrometry. Food Res. Int. 2017, 95, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Tenorio, M.-L.; Verkerk, R.; van Boekel, M.A.; Dekker, M. Thermal stability of phytochemicals, HMF and antioxidant activity in cape gooseberry (Physalis peruviana L.). J. Funct. Foods 2017, 32, 46–57. [Google Scholar] [CrossRef]
- Michalska-Ciechanowska, A.; Wojdyło, A.; Łysiak, G.P.; Lech, K.; Figiel, A. Functional relationships between phytochemicals and drying conditions during the processing of blackcurrant pomace into powders. Adv. Powder Technol. 2017, 28, 1340–1348. [Google Scholar] [CrossRef]
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Subj. Biochim. Biophys. Acta Gen. Subj. 2005, 1721, 174–184. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Wilkowska, A.; Ambroziak, W.; Czyżowska, A.; Adamiec, J. Effect of microencapsulation by spray drying and freeze drying technique on the antioxidant properties of blueberry (Vaccinium myrtillus) juice polyphenolic compounds. Pol. J. Food Nutr. Sci. 2016, 66, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Silva-Espinoza, M.A.; García-Martínez, E.; Martínez-Navarrete, N. Protective capacity of gum Arabic, maltodextrin, different starches, and fibers on the bioactive compounds and antioxidant activity of an orange puree (Citrus sinensis (L.) Osbeck) against freeze-drying and in vitro digestion. Food Chem. 2021, 357, 129724. [Google Scholar] [CrossRef]
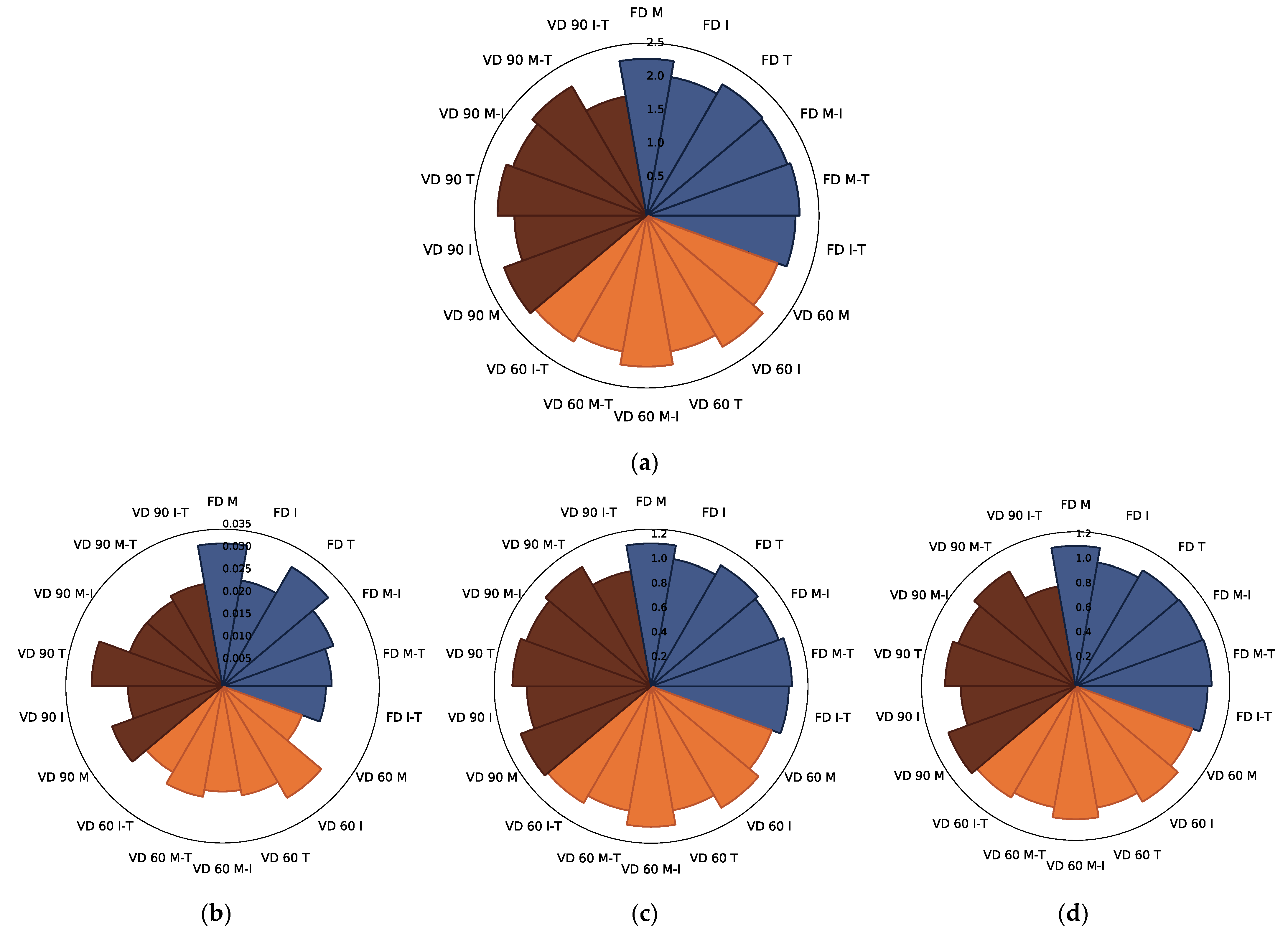
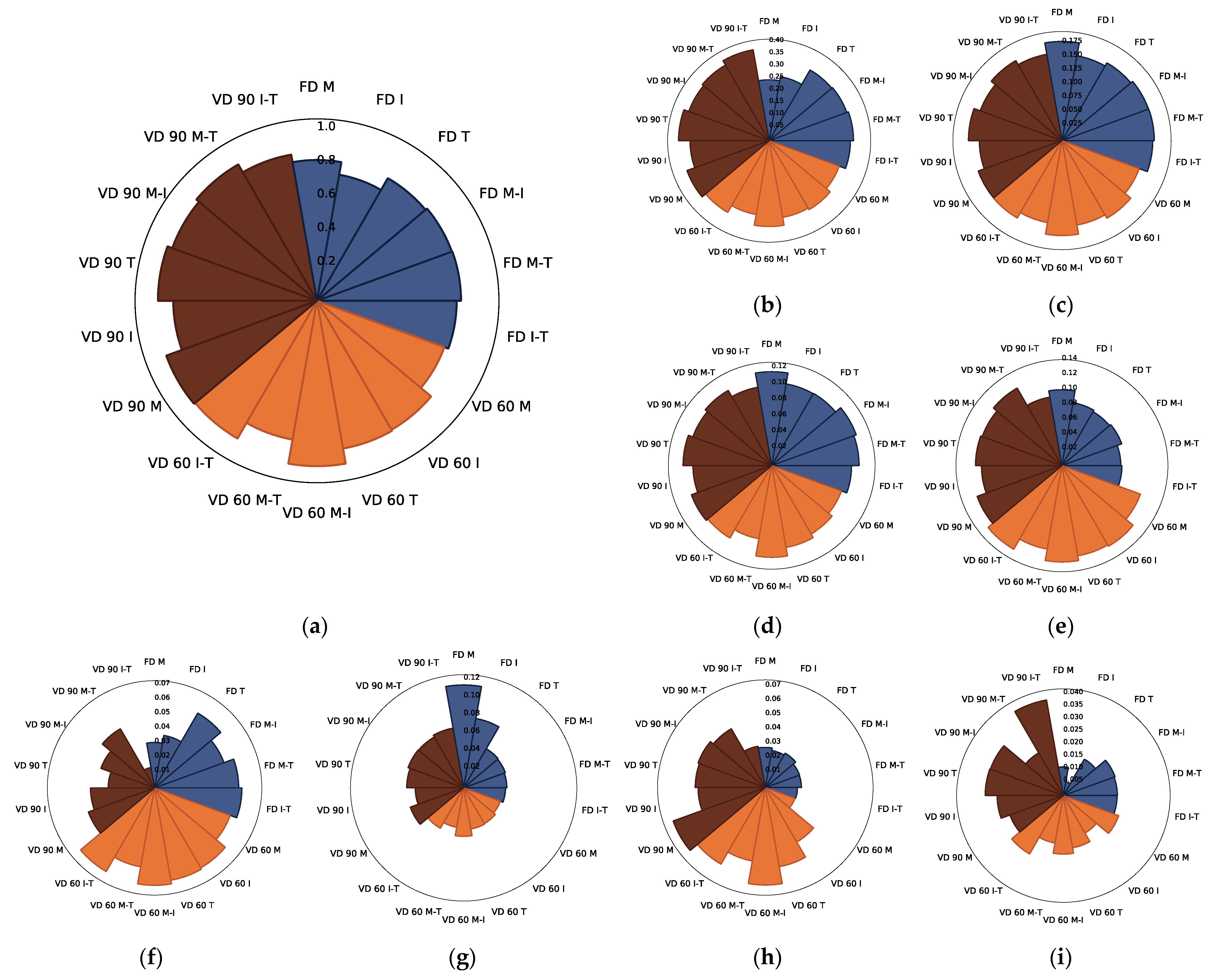

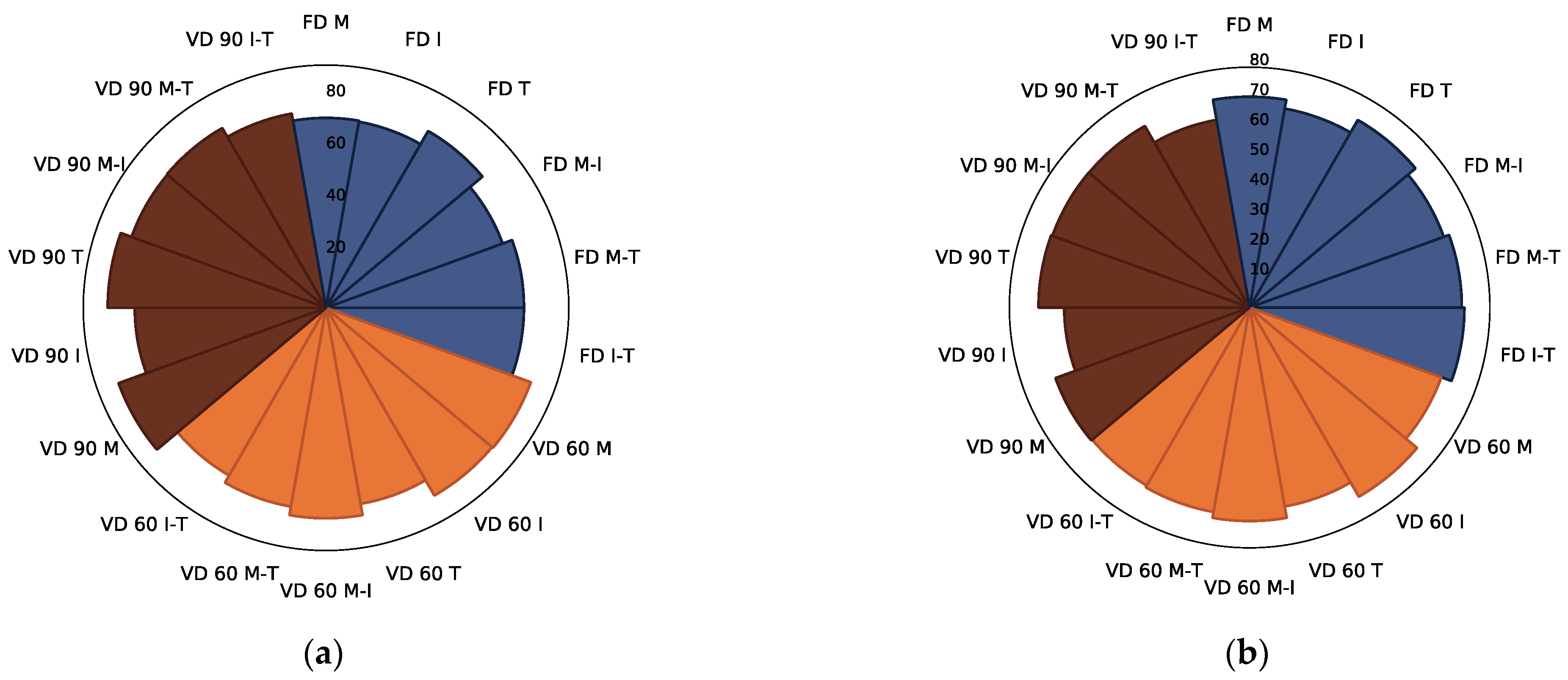
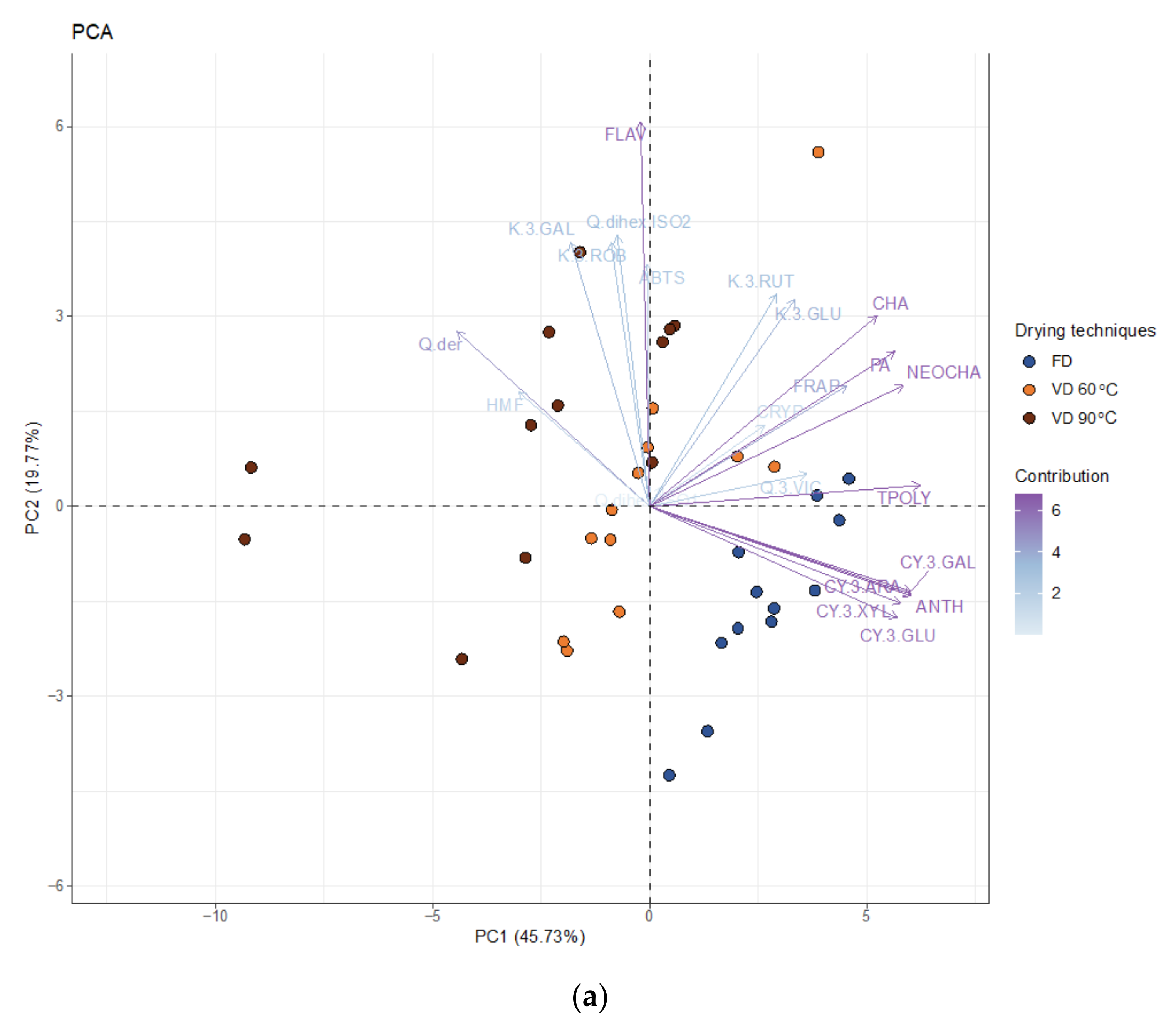
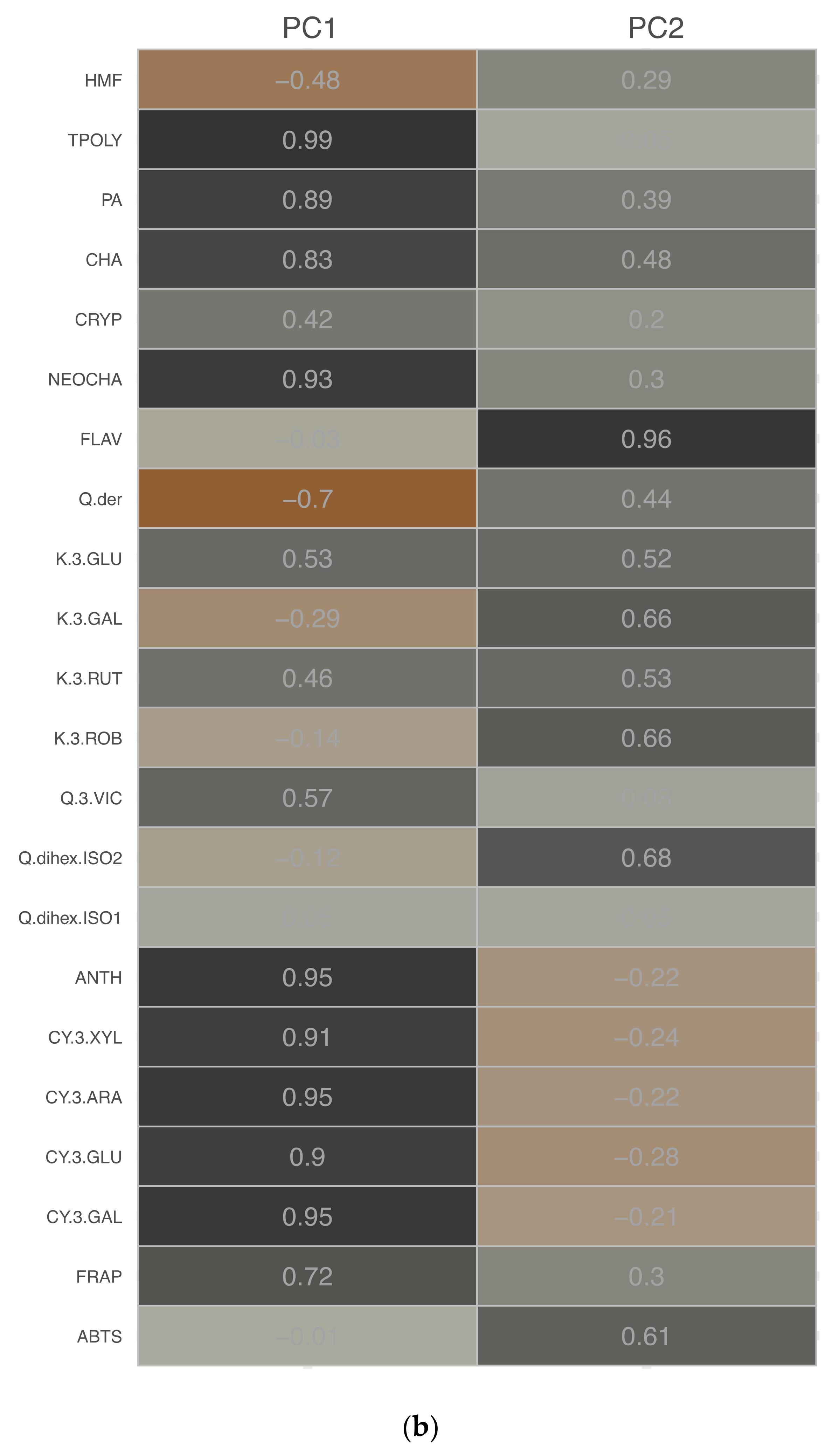

| Drying Technique | Process Conditions | Carrier | Mc | aw | ρb | Colour | BI (AU) | ||
|---|---|---|---|---|---|---|---|---|---|
| (%) | (−) | (g/cm3) | L* | a* | b* | ||||
| FD | −60 °C/24 °C | (−) | 4.38 ± 0.54 cdef | 0.34 ± 0.02 h | 0.09 ± 0.01 a | 24.20 ± 0.11 i | 16.14 ± 0.23 i | 4.65 ± 0.02 m | 0.42 ± 0.01 ij |
| Maltodextrin | 4.20 ± 0.42 cdef | 0.41 ± 0.02 i | 0.12 ± 0.01 a | 25.78 ± 0.25 j | 22.31 ± 0.07 j | 4.09 ± 0.01 l | 0.43 ± 0.01 k | ||
| Inulin | 4.74 ± 1.04 def | 0.15 ± 0.01 ab | 0.32 ± 0.02 bc | 28.59 ± 0.06 k | 25.93 ± 0.04 k | 2.26 ± 0.04 i | 0.42 ± 0.02 i | ||
| Trehalose | 7.59 ± 1.05 g | 0.29 ± 0.02 gh | 0.61 ± 0.01 defg | 13.57 ± 0.16 a | 12.43 ± 0.19 g | 1.53 ± 0.03 h | 0.43 ± 0.01 jk | ||
| Maltodextrin-Inulin | 6.15 ± 1.25 efg | 0.42 ± 0.02 i | 0.17 ± 0.01 ab | 23.44 ± 0.71 i | 22.94 ± 0.07 i | 4.35 ± 0.11 l | 0.45 ± 0.01 l | ||
| Maltodextrin-Trehalose | 6.53 ± 0.01 fg | 0.43 ± 0.01 i | 0.29 ± 0.01 bc | 17.28 ± 0.03 bc | 12.69 ± 0.05 g | 3.32 ± 0.02 k | 0.43 ± 0.01 jk | ||
| Inulin-Trehalose | 5.42 ± 0.70 efg | 0.31 ± 0.01 h | 0.32 ± 0.03 bc | 18.27 ± 0.18 cde | 12.89 ± 0.33 g | 3.37 ± 0.07 k | 0.42 ± 0.01 ij | ||
| VD | 60 °C | (−) | 1.12 ± 0.39 a | 0.24 ± 0.01 def | 0.64 ± 0.05 defgh | 18.48 ± 0.36 cdef | 9.76 ± 0.87 d | 2.46 ± 0.36 ij | 0.39 ± 0.01 g |
| Maltodextrin | 2.75 ± 0.57 abcd | 0.23 ± 0.01 def | 0.70 ± 0.01 efghi | 16.57 ± 0.11 b | 7.38 ± 0.09 c | 0.49 ± 0.02 cde | 0.37 ± 0.02 cd | ||
| Inulin | 2.65 ± 0.04 abcd | 0.32 ± 0.01 h | 0.65 ± 0.06 defgh | 18.55 ± 0.54 def | 15.51 ± 0.24 i | 1.05 ± 0.02 g | 0.41 ± 0.01 h | ||
| Trehalose | 2.74 ± 1.16 abcd | 0.19 ± 0.01 bcde | 0.53 ± 0.05 d | 20.55 ± 0.12 gh | 10.71 ± 0.03 ef | 0.81 ± 0.04 fg | 0.38 ± 0.01 ef | ||
| Maltodextrin-Inulin | 2.89 ± 0.45 abcd | 0.21 ± 0.01 cdef | 0.75 ± 0.04 fghi | 17.93 ± 0.18 cd | 6.34 ± 0.15 ab | 0.19 ± 0.03 ab | 0.35 ± 0.01 ab | ||
| Maltodextrin-Trehalose | 2.02 ± 0.42 abc | 0.25 ± 0.01 fg | 0.60 ± 0.01 def | 19.61 ± 0.19 fg | 6.12 ± 0.07 ab | 0.39 ± 0.04 bcd | 0.35 ± 0.01 a | ||
| Inulin-Trehalose | 3.91 ± 0.54 bcde | 0.32 ± 0.01 h | 0.77 ± 0.08 hi | 20.29 ± 0.36 gh | 13.79 ± 0.18 h | 1.41 ± 0.03 h | 0.40 ± 0.01 g | ||
| 90 °C | (−) | 0.98 ± 0.47 a | 0.24 ± 0.01 efg | 0.55 ± 0.03 de | 17.92 ± 0.11 cd | 6.07 ± 0.21 ab | 2.71 ± 0.06 j | 0.38 ± 0.01 f | |
| Maltodextrin | 0.89 ± 0.38 a | 0.14 ± 0.01 a | 0.65 ± 0.08 defgh | 17.97 ± 0.11 cd | 5.66 ± 0.01 a | 0.26 ± 0.01 abc | 0.35 ± 0.01 a | ||
| Inulin | 1.37 ± 0.25 a | 0.15 ± 0.02 ab | 0.76 ± 0.02 ghi | 19.31 ± 0.12 efg | 10.49 ± 0.22 de | 0.02 ± 0.04 a | 0.37 ± 0.01 de | ||
| Trehalose | 0.47 ± 0.02 a | 0.18 ± 0.01 abcd | 0.34 ± 0.02 c | 21.06 ± 0.18 h | 11.44 ± 0.03 f | 0.70 ± 0.03 ef | 0.38 ± 0.01 ef | ||
| Maltodextrin-Inulin | 1.69 ± 0.28 ab | 0.14 ± 0.02 a | 0.80 ± 0.04 hi | 17.55 ± 0.69 bcd | 7.50 ± 0.12 c | 0.07 ± 0.09 a | 0.36 ± 0.02 bc | ||
| Maltodextrin-Trehalose | 0.88 ± 0.12 a | 0.16 ± 0.02 ab | 0.57 ± 0.06 de | 18.15 ± 0.13 cde | 6.80 ± 0.25 bc | 0.52 ± 0.04 cde | 0.36 ± 0.01 b | ||
| Inulin-Trehalose | 2.62 ± 0.09 abcd | 0.17 ± 0.01 abc | 0.83 ± 0.01i | 21.75 ± 0.77 h | 15.8 ± 0.27 i | 0.67 ± 0.04 def | 0.39 ± 0.01 g | ||
| FD | VD | ||
|---|---|---|---|
| −60 °C/+24 °C | 60 °C | 90 °C | |
| Total polyphenols | 22.70 ± 0.12 c | 15.88 ± 0.44 a | 18.64 ± 0.47 b |
| Phenolic acids Neochlorogenic acid Cryptochlorogenic acid Chlorogenic acid Sum | |||
| 4.86 ± 0.18 b | 3.01 ± 0.09 a | 4.47 ± 0.67 ab | |
| 0.10 ± 0.01 ab | 0.07 ± 0.01 a | 0.14 ± 0.02 b | |
| 5.15 ± 0.15 b | 3.43 ± 0.11 a | 4.72 ± 0.37 b | |
| 10.10 ± 0.02 b | 6.51 ± 0.20 a | 9.33 ± 1.06 b | |
| Anthocyanins | |||
| Cyanidin-3-O-galactoside | 5.90 ± 0.14 b | 3.68 ± 0.09 a | 3.93 ± 0.37 a |
| Cyanidin-3-O-glucoside | 0.33 ± 0.05 b | 0.18 ± 0.01 a | 0.22 ± 0.02 ab |
| Cyanidin-3-O-arabinoside | 2.56 ± 0.05 b | 1.68 ± 0.05 a | 1.61 ± 0.13 a |
| Cyanidin-3-O-xyloside | 0.41 ± 0.01 b | 0.28 ± 0.02 a | 0.29 ± 0.03 a |
| Sum | 9.19 ± 0.13 b | 5.82 ± 0.12 a | 6.06 ± 0.56 a |
| Flavonols | |||
| Quercetin-dihexoside 1 | 0.26 ± 0.01 b | 0.17 ± 0.01 a | 0.30 ± 0.01 c |
| Quercetin-dihexoside 2 | 0.14 ± 0.01 a | 0.21 ± 0.01 a | 0.21 ± 0.04 a |
| Quercetin-3-O-vicianoside | 0.14 ± 0.01 b | 0.12 ± 0.01 b | 0.06 ± 0.02 a |
| Kaempferol-3-O-robinobioside | 0.45 ± 0.02 b | 0.24 ± 0.01 a | 0.43 ± 0.02 b |
| Kaempferol-3-O-rutinoside | 0.53 ± 0.02 b | 0.30 ± 0.01 a | 0.48 ± 0.02 b |
| Kaempferol-3-O-galactoside | 1.06 ± 0.02 a | 1.72 ± 0.07 b | 1.03 ± 0.01 a |
| Kaempferol-3-O-glucoside | 0.78 ± 0.02 b | 0.59 ± 0.02 a | 0.72 ± 0.02 b |
| Derivative of quercetin | 0.05 ± 0.01 b | 0.20 ± 0.01 c | 0.04 ± 0.01 a |
| Sum | 3.40 ± 0.01 a | 3.55 ± 0.12 a | 3.26 ± 0.03 a |
| Hydroxymethyl-L-furfural | 11.57 ± 0.23 a | 2.62 ± 0.24 b | 14.03 ± 1.59 a |
| TEAC ABTS | 364.95 ± 11.98 a | 357.33 ± 11.01 a | 374.80 ± 12.92 a |
| FRAP | 292.95 ± 7.61 a | 285.14 ± 1.02 a | 299.38 ± 1.01 a |
| Drying Technique | Process Conditions | Carrier | Anthocyanins | ||||
|---|---|---|---|---|---|---|---|
| Cyanidin-3-O-Galactoside | Cyanidin-3-O-Glucoside | Cyanidin-3-O-Arabinoside | Cyanidin-3-O-Xyloside | Sum of Anthocyanins | |||
| FD | −60 °C/24 °C | Maltodextrin | 1.36 ± 0.04 fg | 0.08 ± 0.01 g | 0.59 ± 0.01 fgh | 0.10 ± 0.01 fg | 2.12 ± 0.06 ghi |
| Inulin | 1.28 ± 0.06 efg | 0.07 ± 0.01 defg | 0.56 ± 0.03 efgh | 0.10 ± 0.01 efg | 2.00 ± 0.10 fghi | ||
| Trehalose | 1.42 ± 0.08 g | 0.07 ± 0.01 efg | 0.62 ± 0.04 gh | 0.11 ± 0.01 g | 2.22 ± 0.14 hi | ||
| Maltodextrin—Inulin | 1.38 ± 0.05 g | 0.06 ± 0.01 cdefg | 0.59 ± 0.02 fgh | 0.10 ± 0.01 fg | 2.13 ± 0.07 ghi | ||
| Maltodextrin—Trehalose | 1.45 ± 0.02 g | 0.07 ± 0.01 fg | 0.64 ± 0.02 h | 0.11 ± 0.01 g | 2.27 ± 0.04 i | ||
| Inulin—Trehalose | 1.41 ± 0.04 g | 0.07 ± 0.01 defg | 0.62 ± 0.02 gh | 0.09 ± 0.01 defg | 2.18 ± 0.08 hi | ||
| VD | 60 °C | Maltodextrin | 1.13 ± 0.03 cde | 0.05 ± 0.01 bc | 0.49 ± 0.01 de | 0.08 ± 0.01 bcd | 1.75 ± 0.05 cdef |
| Inulin | 1.37 ± 0.06 fg | 0.06 ± 0.01 cdef | 0.60 ± 0.03 fgh | 0.09 ± 0.01 efg | 2.12 ± 0.09 ghi | ||
| Trehalose | 1.12 ± 0.04 cde | 0.05 ± 0.01 bc | 0.48 ± 0.02 cde | 0.08 ± 0.01 cde | 1.73 ± 0.07 cdef | ||
| Maltodextrin—Inulin | 1.27 ± 0.10 efg | 0.06 ± 0.01 cdef | 0.55 ± 0.05 efg | 0.09 ± 0.01 cdef | 1.97 ± 0.15 efgh | ||
| Maltodextrin—Trehalose | 1.10 ± 0.04 cde | 0.05 ± 0.01 bc | 0.47 ± 0.02 cde | 0.07 ± 0.01 bc | 1.70 ± 0.07 cde | ||
| Inulin—Trehalose | 1.19 ± 0.01 def | 0.06 ± 0.01 cde | 0.52 ± 0.01 ef | 0.09 ± 0.01 cdef | 1.85 ± 0.01 defg | ||
| 90 °C | Maltodextrin | 1.16 ± 0.01 de | 0.06 ± 0.01 cdef | 0.49 ± 0.01 de | 0.08 ± 0.01 cdef | 1.80 ± 0.01 def | |
| Inulin | 1.03 ± 0.02 cd | 0.05 ± 0.01 bc | 0.43 ± 0.01 bcd | 0.07 ± 0.01 bcd | 1.58 ± 0.04 cd | ||
| Trehalose | 0.82 ± 0.02 b | 0.04 ± 0.01 b | 0.35 ± 0.01 b | 0.06 ± 0.01 b | 1.27 ± 0.03 b | ||
| Maltodextrin—Inulin | 0.95 ± 0.04 bc | 0.05 ± 0.01 bc | 0.40 ± 0.02 bc | 0.07 ± 0.01 bc | 1.47 ± 0.07 bc | ||
| Maltodextrin—Trehalose | 1.15 ± 0.02 de | 0.05 ± 0.01 bcd | 0.48 ± 0.01 cde | 0.08 ± 0.01 cdef | 1.76 ± 0.02 def | ||
| Inulin—Trehalose | 0.39 ± 0.01 a | 0.02 ± 0.01 a | 0.16 ± 0.01 a | 0.03 ± 0.01 a | 0.60 ± 0.02 a | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalska-Ciechanowska, A.; Hendrysiak, A.; Brzezowska, J.; Wojdyło, A.; Gajewicz-Skretna, A. How Do the Different Types of Carrier and Drying Techniques Affect the Changes in Physico-Chemical Properties of Powders from Chokeberry Pomace Extracts? Foods 2021, 10, 1864. https://doi.org/10.3390/foods10081864
Michalska-Ciechanowska A, Hendrysiak A, Brzezowska J, Wojdyło A, Gajewicz-Skretna A. How Do the Different Types of Carrier and Drying Techniques Affect the Changes in Physico-Chemical Properties of Powders from Chokeberry Pomace Extracts? Foods. 2021; 10(8):1864. https://doi.org/10.3390/foods10081864
Chicago/Turabian StyleMichalska-Ciechanowska, Anna, Aleksandra Hendrysiak, Jessica Brzezowska, Aneta Wojdyło, and Agnieszka Gajewicz-Skretna. 2021. "How Do the Different Types of Carrier and Drying Techniques Affect the Changes in Physico-Chemical Properties of Powders from Chokeberry Pomace Extracts?" Foods 10, no. 8: 1864. https://doi.org/10.3390/foods10081864
APA StyleMichalska-Ciechanowska, A., Hendrysiak, A., Brzezowska, J., Wojdyło, A., & Gajewicz-Skretna, A. (2021). How Do the Different Types of Carrier and Drying Techniques Affect the Changes in Physico-Chemical Properties of Powders from Chokeberry Pomace Extracts? Foods, 10(8), 1864. https://doi.org/10.3390/foods10081864