Kinetics of Colour Development during Frying of Potato Pre-Treated with Pulsed Electric Fields and Blanching: Effect of Cultivar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Characterisation of Their Chemical Composition
2.2. Pulsed Electric Fields (PEF) Treatment
2.3. Kinetic Study on the Colour Changes of Non-PEF and PEF-Treated Potato Slices during Frying
2.3.1. Kinetic Frying Experiment
2.3.2. Estimation of the Time Dependence of the Colour Change of Potato Slices during Frying
2.3.3. Estimation of the Temperature Dependence of Rate Constant for Changes in L* Value during Frying
2.4. Statistical Analysis
3. Results and Discussion
3.1. Comparison on the Chemical Composition of Four Different Potato Cultivars
3.2. Colour Evaluation of Fried Potato Slices Produced from Non-PEF and PEF-Treated Potatoes
3.2.1. The Effect of PEF Pre-Treatment Alone (without Blanching) on the Colour Characteristics of Fried Potato Slices
3.2.2. The Effect of Sequential PEF and Blanching Pre-Treatment on the Colour Characteristics of Fried Potato Slices
3.3. Kinetic Study on the Colour Changes of Sequential PEF and Blanching Pre-Treated Potatoes during Frying
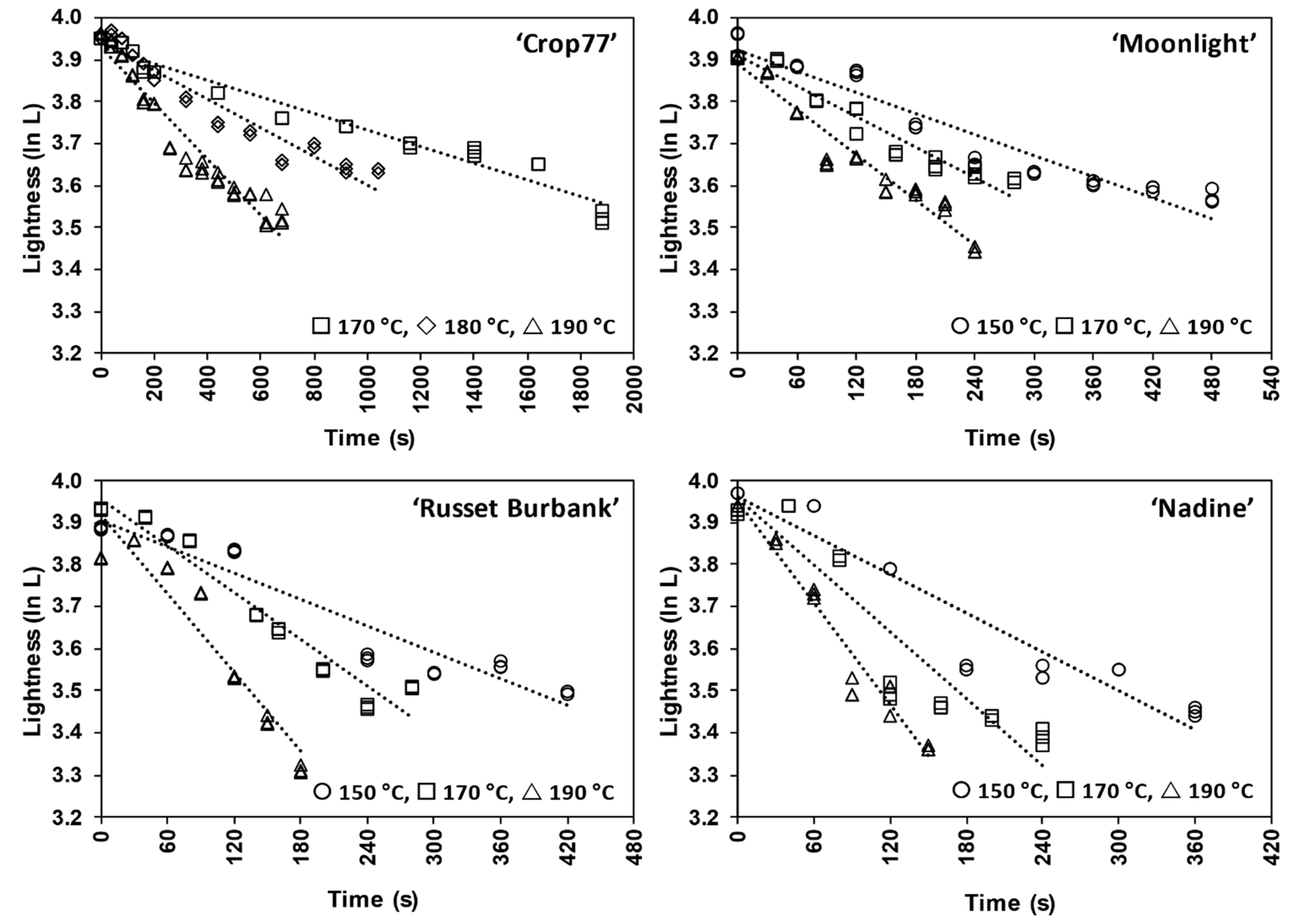
3.3.1. Time and Temperature Dependences of L* Value Change for Non-PEF (Blanching Only) Pre-Treated Potatoes during Frying
3.3.2. Time Dependency of L* Value for PEF-Treated Potatoes during Frying
3.3.3. Temperature Dependency of k for L* Value for PEF-Treated Potatoes during Frying
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toepfl, S.; Heinz, V.; Knorr, D. Applications of pulsed electric fields technology for the food industry. In Pulsed Electric Fields Technology for the Food Industry: Fundamentals and Applications; Raso, J., Heinz, V., Eds.; Springer: Boston, MA, USA, 2006; pp. 197–221. [Google Scholar]
- Zimmermann, U.; Pilwat, G.; Riemann, F. Dielectric breakdown of cell membranes. Biophys. J. 1974, 14, 881–899. [Google Scholar] [CrossRef] [Green Version]
- Botero-Uribe, M.; Fitzgerald, M.; Gilbert, R.G.; Midgley, J. Effect of pulsed electrical fields on the structural properties that affect french fry texture during processing. Trends Food Sci. Technol. 2017, 67, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fauster, T.; Schlossnikl, D.; Rath, F.; Ostermeier, R.; Teufel, F.; Toepfl, S.; Jaeger, H. Impact of pulsed electric field (PEF) pretreatment on process performance of industrial French fries production. J. Food Eng. 2018, 235, 16–22. [Google Scholar] [CrossRef]
- Ignat, A.; Manzocco, L.; Brunton, N.P.; Nicoli, M.C.; Lyng, J.G. The effect of pulsed electric field pre-treatments prior to deep-fat frying on quality aspects of potato fries. Innov. Food Sci. Emerg. Technol. 2015, 29, 65–69. [Google Scholar] [CrossRef]
- Schouten, M.A.; Genovese, J.; Tappi, S.; Di Francesco, A.; Baraldi, E.; Cortese, M.; Caprioli, G.; Angeloni, S.; Vittori, S.; Rocculi, P.; et al. Effect of innovative pre-treatments on the mitigation of acrylamide formation in potato chips. Innov. Food Sci. Emerg. Technol. 2020, 64, 102397. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Wrolstad, R.E. Influence of potato composition on chip color quality. Am. Potato J. 1997, 74, 87–106. [Google Scholar] [CrossRef]
- Cullen, E. Blending Potato Varieties on the Rise. Available online: https://food.tomra.com/blog/blending-potato-varieties-on-the-rise (accessed on 14 July 2021).
- Califano, A.N.; Calvelo, A. Adjustment of surface concentration of reducing sugars before frying of potato strips. J. Food Process. Preserv. 1988, 12, 1–9. [Google Scholar] [CrossRef]
- Pedreschi, F.; Travisany, X.; Reyes, C.; Troncoso, E.; Pedreschi, R. Kinetics of extraction of reducing sugar during blanching of potato slices. J. Food Eng. 2009, 91, 443–447. [Google Scholar] [CrossRef]
- Viklund, G.Å.I.; Olsson, K.M.; Sjöholm, I.M.; Skog, K.I. Acrylamide in crisps: Effect of blanching studied on long-term stored potato clones. J. Food Compos. Anal. 2010, 23, 194–198. [Google Scholar] [CrossRef]
- Moscetti, R.; Raponi, F.; Monarca, D.; Bedini, G.; Ferri, S.; Massantini, R. Effects of hot-water and steam blanching of sliced potato on polyphenol oxidase activity. Int. J. Food Sci. Technol. 2019, 54, 403–411. [Google Scholar] [CrossRef]
- Elfnesh, F.; Tekalign, T.; Solomon, W. Processing quality of improved potato (Solanum tuberosum L.) cultivars as influenced by growing environment and blanching. Afr. J. Food Sci. 2011, 5, 324–332. [Google Scholar]
- Singh, J.; Kaur, L.; Mccarthy, O.J.; Moughan, P.J.; Singh, H. Rheological and textural characteristics of raw and par-cooked taewa (Maori potatoes) of New Zealand. J. Texture Stud. 2008, 39, 210–230. [Google Scholar] [CrossRef]
- Anderson, J.A.D.; Lewthwaite, S.L.; Genet, R.A.; Braam, W.F. ‘Moonlight’: A new dual-purpose main crop potato (Solanum tuberosum) cultivar. N. Z. J. Crop Hortic. Sci. 2004, 32, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Bethke, P.C.; Nassar, A.M.K.; Kubow, S.; Leclerc, Y.N.; Li, X.-Q.; Haroon, M.; Molen, T.; Bamberg, J.; Martin, M.; Donnelly, D.J. History and origin of Russet Burbank (Netted Gem) a sport of Burbank. Am. J. Potato Res. 2014, 91, 594–609. [Google Scholar] [CrossRef]
- IP Australia. Potato (Solanum tuberosum)-‘Crop77’. Plant Var. J. Off. J. Plant Breeder’s Rights Office 2018, 31, 77. [Google Scholar]
- Faridnia, F.; Burritt, D.J.; Bremer, P.J.; Oey, I. Innovative approach to determine the effect of pulsed electric fields on the microstructure of whole potato tubers: Use of cell viability, microscopic images and ionic leakage measurements. Food Res. Int. 2015, 77, 556–564. [Google Scholar] [CrossRef]
- Liu, T.; Dodds, E.; Leong, S.Y.; Eyres, G.T.; Burritt, D.J.; Oey, I. Effect of pulsed electric fields on the structure and frying quality of “kumara” sweet potato tubers. Innov. Food Sci. Emerg. Technol. 2017, 39, 197–208. [Google Scholar] [CrossRef]
- Leong, S.Y.; Richter, L.K.; Knorr, D.; Oey, I. Feasibility of using pulsed electric field processing to inactivate enzymes and reduce the cutting force of carrot (Daucus carota var. Nantes). Innov. Food Sci. Emerg. Technol. 2014, 26, 159–167. [Google Scholar] [CrossRef]
- Bazhal, M.I.; Lebovka, N.I.; Vorobiev, E. Optimisation of pulsed electric field strength for electroplasmolysis of vegetable tissues. Biosys. Eng. 2003, 86, 339–345. [Google Scholar] [CrossRef]
- Ammar, J.B.; Lanoisellé, J.L.; Lebovka, N.I.; Van Hecke, E.; Vorobiev, E. Impact of a pulsed electric field on damage of plant tissues: Effects of cell size and tissue electrical conductivity. J. Food Sci. 2011, 76, E90–E97. [Google Scholar] [CrossRef]
- Lebovka, N.I.; Shynkaryk, N.V.; Vorobiev, E. Pulsed electric field enhanced drying of potato tissue. J. Food Eng. 2007, 78, 606–613. [Google Scholar] [CrossRef]
- Lebovka, N.I.; Praporscic, I.; Ghnimi, S.; Vorobiev, E. Temperature enhanced electroporation under the pulsed electric field treatment of food tissue. J. Food Eng. 2005, 69, 177–184. [Google Scholar] [CrossRef]
- Lebovka, N.I.; Praporscic, I.; Vorobiev, E. Effect of moderate thermal and pulsed electric field treatments on textural properties of carrots, potatoes and apples. Innov. Food Sci. Emerg. Technol. 2004, 5, 9–16. [Google Scholar] [CrossRef]
- Liu, C.; Grimi, N.; Lebovka, N.; Vorobiev, E. Effects of pulsed electric fields treatment on vacuum drying of potato tissue. LWT 2018, 95, 289–294. [Google Scholar] [CrossRef]
- Pedreschi, F.; Moyano, P.; Kaack, K.; Granby, K. Color changes and acrylamide formation in fried potato slices. Food Res. Int. 2005, 38, 1–9. [Google Scholar] [CrossRef]
- Pedreschi, F.; Bustos, O.; Mery, D.; Moyano, P.; Kaack, K.; Granby, K. Color kinetics and acrylamide formation in NaCl soaked potato chips. J. Food Eng. 2007, 79, 989–997. [Google Scholar] [CrossRef]
- Moyano, P.C.; Ríoseco, V.K.; González, P.A. Kinetics of crust color changes during deep-fat frying of impregnated french fries. J. Food Eng. 2002, 54, 249–255. [Google Scholar] [CrossRef]
- Krokida, M.K.; Oreopoulou, V.; Maroulis, Z.B.; Marinos-Kouris, D. Colour changes during deep fat frying. J. Food Eng. 2001, 48, 219–225. [Google Scholar] [CrossRef]
- Nourian, F.; Ramaswamy, H.D. Kinetics of quality change during cooking and frying of potatoes: Part II. Color. J. Food Process Eng. 2003, 26, 395–411. [Google Scholar] [CrossRef]
- van Boekel, M.A.J.S. Kinetic aspects of the Maillard reaction: A critical review. Mol. Nutr. Food Res. 2001, 45, 150–159. [Google Scholar]
- Martins, S.I.; Jongen, W.M.; Van Boekel, M.A. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Kita, A. The influence of potato chemical composition on crisp texture. Food Chem. 2002, 76, 173–179. [Google Scholar] [CrossRef]
- Šimková, D.; Lachman, J.; Hamouz, K.; Vokál, B. Effect of cultivar, location and year on total starch, amylose, phosphorus content and starch grain size of high starch potato cultivars for food and industrial processing. Food Chem. 2013, 141, 3872–3880. [Google Scholar] [CrossRef] [PubMed]
- Ngobese, N.Z.; Workneh, T.S.; Alimi, B.A.; Tesfay, S. Nutrient composition and starch characteristics of eight European potato cultivars cultivated in South Africa. J. Food Compos. Anal. 2017, 55, 1–11. [Google Scholar] [CrossRef]
- Bartlett, L.; Montague, G.; McNamara, G.; Davies, B.; Fiore, A.; Sturrock, K.; Ledbetter, M.; Hein, I.; Mantelin, S.; Harrower, B.; et al. Operational considerations for hot-washing in potato crisp manufacture. Food Bioprod. Process 2020, 124, 387–396. [Google Scholar] [CrossRef]
- Pedreschi, F.; Kaack, K.; Granby, K. Acrylamide content and color development in fried potato strips. Food Res. Int. 2006, 39, 40–46. [Google Scholar] [CrossRef]
- Janositz, A.; Noack, A.K.; Knorr, D. Pulsed electric fields and their impact on the diffusion characteristics of potato slices. LWT 2011, 44, 1939–1945. [Google Scholar] [CrossRef]
- Zhang, C.; Ye, J.; Lyu, X.; Zhao, W.; Mao, J.; Yang, R. Effects of pulse electric field pretreatment on the frying quality and pore characteristics of potato chips. Food Chem. 2022, 369, 130516. [Google Scholar] [CrossRef]
- Zhang, Y.; Kahl, D.H.W.; Bizimungu, B.; Lu, Z.-X. Effects of blanching treatments on acrylamide, asparagine, reducing sugars and colour in potato chips. J. Food Sci. Technol. 2018, 55, 4028–4041. [Google Scholar] [CrossRef]
- Moens, L.G.; Van Wambeke, J.; De Laet, E.; Van Ceunebroeck, J.-C.; Goos, P.; Van Loey, A.M.; Hendrickx, M.E.G. Effect of postharvest storage on potato (Solanum tuberosum L.) texture after pulsed electric field and thermal treatments. Innov. Food Sci. Emerg. Technol. 2021, 8, 102826. [Google Scholar] [CrossRef]
- Genovese, J.; Tappi, S.; Luo, W.; Tylewicz, U.; Marzocchi, S.; Marziali, S.; Romani, S.; Ragni, L.; Rocculi, P. Important factors to consider for acrylamide mitigation in potato crisps using pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2019, 55, 18–26. [Google Scholar] [CrossRef]
- Oey, I.; Faridnia, F.; Leong, S.Y.; Burritt, D.J.; Liu, T. Determination of pulsed electric fields effects on the structure of potato tubers. In Handbook of Electroporation; Miklavcic, D., Ed.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Obretenov, T.; Vernin, G. Melanoidins in the Maillard reaction. In Developments in Food Science: Food Flavors: Formation, Analysis and Packaging Influences; Contis, E.T., Ho, C.-T., Mussinan, C.J., Parliament, T.H., Shahidi, F., Spanier, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 1998; Volume 40, pp. 455–482. [Google Scholar]
- Yaylayan, V.A.; Kaminsky, E. Isolation and structural analysis of maillard polymers: Caramel and melanoidin formation in glycine/glucose model system. Food Chem. 1998, 63, 25–31. [Google Scholar] [CrossRef]
- Arevalo, P.; Ngadi, M.O.; Bazhal, M.I.; Raghavan, G.S.V. Impact of pulsed electric fields on the dehydration and physical properties of apple and potato slices. Dry. Technol. 2004, 22, 1233–1246. [Google Scholar] [CrossRef]
- Xu, Z.; Leong, S.Y.; Farid, M.; Silcock, P.; Bremer, P.; Oey, I. Understanding the frying process of plant-based foods pretreated with Pulsed Electric Fields using frying models. Foods 2020, 9, 949. [Google Scholar] [CrossRef] [PubMed]
- Gholamibozanjani, G.; Leong, S.Y.; Oey, I.; Bremer, P.; Silcock, P.; Farid, M. Heat and mass transfer modeling to predict temperature distribution during potato frying after pre-treatment with pulsed electric field. Foods 2021, 10, 1679. [Google Scholar] [CrossRef]

| Tuber Characteristics and Chemical Contents | Potato Cultivar | |||
|---|---|---|---|---|
| ‘Crop77’ †# | ‘Moonlight’ † | ‘Russet Burbank’ | ‘Nadine’ ## | |
| Shape of tuber | Short-oval | Oval | Long | Oval |
| Colour of skin | Cream | Cream | Cream | Cream |
| Colour of flesh | White | White | White | Cream |
| Dry matter (%) | 24.61 ± 1.30 a | 20.57 ± 0.60 b | 24.09 ± 0.51 a | 14.92 ± 0.38 c |
| Total starch (mg/g DW) | 731.04 ± 69.96 a | 587.34 ± 88.72 a | 708.95 ± 27.21 a | 722.87 ± 46.60 a |
| Amylose (mg/g DW) | 75.53 ± 7.84 b | 147.23 ± 5.38 a | 81.73 ± 3.61 b | 172.68 ± 10.17 a |
| Glucose (mg/g DW) | 0.75 ± 0.09 c | 20.68 ± 1.46 b | 22.35 ± 1.60 b | 104.64 ± 2.77 a |
| Total amino acids (mmol/g DW) | 0.99 ± 0.07 b | 1.78 ± 0.20 a | 1.64 ± 0.19 a | 1.64 ± 0.04 a |
| Colour Parameters | Non-PEF and No Blanching | PEF Low (1 kV/cm, 50 kJ/kg) without Blanching | PEF High (1 kV/cm, 150 kJ/kg) without Blanching |
|---|---|---|---|
| ‘Crop77’ | |||
| L* | 34.27 ± 2.09 aA | 33.77 ± 2.19 aA | 33.87 ± 2.40 aA |
| a* | 3.45 ± 1.17 aC | 3.41 ± 1.14 aB | 3.29 ± 0.99 aC |
| b* | 18.37 ± 2.45 aA | 18.48 ± 2.37 aA | 17.52 ± 2.49 aA |
| ‘Moonlight’ | |||
| L* | 29.21 ± 2.48 abC | 29.75 ± 2.46 aB | 27.96 ± 2.32 bB |
| a* | 5.37 ± 1.17 aA | 5.05 ± 0.99 aA | 5.52 ± 1.65 aA |
| b* | 17.47 ± 2.53 abA | 18.55 ± 2.68 aA | 16.97 ± 2.67 bA |
| ‘Russet Burbank’ | |||
| L* | 31.49 ± 1.84 aB | 32.33 ± 2.14 aA | 32.31 ± 1.74 aA |
| a* | 5.62 ± 1.01 aA | 4.47 ± 1.25 bA | 4.23 ± 0.73 bB |
| b* | 15.37 ± 1.62 aB | 14.88 ± 1.48 aB | 14.85 ± 1.11 aB |
| ‘Nadine’ | |||
| L* | 17.44 ± 3.38 bD | 19.31 ± 2.29 aC | 19.99 ± 3.58 aC |
| a* | 4.46 ± 1.59 aB | 3.62 ± 0.92 bB | 3.54 ± 1.26 bBC |
| b* | 9.21 ± 1.99 aC | 8.40 ± 1.31 aC | 8.58 ± 1.38 aC |
| Colour Parameters | Non-PEF (Blanching Only) | PEF Low (1 kV/cm, 50 kJ/kg) Followed by Blanching | PEF High (1 kV/cm, 150 kJ/kg) Followed by Blanching |
|---|---|---|---|
| ‘Crop77’ | |||
| L* | 35.04 ± 2.26 bA | 37.06 ± 1.80 aA* | 35.78 ± 1.54 bA* |
| a* | 2.66 ± 1.08 aC* | 1.52 ± 0.46 bD* | 2.23 ± 0.78 aBC* |
| b* | 17.31 ± 1.87 aA* | 17.59 ± 1.20 aA | 17.39 ± 2.39 aA |
| ‘Moonlight’ | |||
| L* | 32.94 ± 2.68 aB* | 33.15 ± 1.77 aB* | 33.72 ± 1.25 aB* |
| a* | 3.83 ± 1.04 aB* | 3.27 ± 0.85 bB* | 2.78 ± 0.57 bB* |
| b* | 18.03 ± 1.81 aA | 17.60 ± 2.06 aA | 16.28 ± 1.37 bAB |
| ‘Russet Burbank’ | |||
| L* | 33.96 ± 2.07 bAB* | 36.71 ± 0.96 aA* | 36.86 ± 1.12 aA* |
| a* | 3.85 ± 1.31 aB* | 2.23 ± 0.53 bC* | 2.02 ± 0.60 bC* |
| b* | 16.11 ± 1.38 aB* | 16.30 ± 0.76 aB* | 15.73 ± 1.00 aB* |
| ‘Nadine’ | |||
| L* | 22.34 ± 2.60 aC* | 24.12 ± 2.14 aC* | 23.71 ± 2.96 aC* |
| a* | 5.37 ± 1.42 aA* | 4.34 ± 1.26 bA* | 5.15 ± 0.61 abA* |
| b* | 12.88 ± 2.52 aC* | 11.50 ± 2.32 aC* | 13.03 ± 0.82 aC* |
| PEF Treatment | Frying Temperature (°C) | k (×10−3 s−1) * | Range of R2 for k Estimation | Ea (kJ·mol−1) ** | Range of R2 for Ea Estimation |
|---|---|---|---|---|---|
| ‘Crop77’ | |||||
| Non-PEF | 170 | 0.24 ± 0.04 | 0.90–0.96 | 105.53 ± 1.16 aA | 0.90–0.99 |
| (blanching only) | 180 | 0.37 ± 0.03 | 0.92–0.93 | ||
| 190 | 0.83 ± 0.16 | 0.93–0.96 | |||
| PEF Low | 170 | 0.30 ± 0.07 | 0.84–0.91 | 86.78 ± 1.19 cA | 0.99–1.00 |
| (1 kV/cm, | 180 | 0.49 ± 0.12 | 0.93–0.98 | ||
| 50 kJ/kg) + blanching | 190 | 0.82 ± 0.20 | 0.82–0.98 | ||
| PEF High | 170 | 0.22 ± 0.03 | 0.86–0.87 | 102.41 ± 0.32 bA | 1.00–1.00 |
| (1 kV/cm, | 180 | 0.40 ± 0.07 | 0.89–0.98 | ||
| 150 kJ/kg) + blanching | 190 | 0.72 ± 0.11 | 0.95–0.98 | ||
| ‘Moonlight’ | |||||
| Non-PEF | 150 | 0.86 ± 0.05 | 0.89–0.93 | 31.73 ± 1.03 D | 0.95–1.00 |
| (blanching only) | 170 | 1.27 ± 0.12 | 0.94–0.96 | ||
| 190 | 1.87 ± 0.17 | 0.93–0.95 | |||
| PEF Low | 150 | 0.77 ± 0.14 | 0.87–0.94 | 32.82 ± 0.23 D | 0.99–0.99 |
| (1 kV/cm, | 170 | 1.22 ± 0.22 | 0.88–0.97 | ||
| 50 kJ/kg) + blanching | 190 | 1.72 ± 0.30 | 0.88–0.95 | ||
| PEF High | 150 | 0.84 ± 0.03 | 0.81–0.97 | 31.09 ± 0.81 C | 0.91–1.00 |
| (1 kV/cm, | 170 | 1.23 ± 0.17 | 0.50–0.86 | ||
| 150 kJ/kg) + blanching | 190 | 1.81 ± 0.03 | 0.87–0.95 | ||
| ‘Russet Burbank’ | |||||
| Non-PEF | 150 | 0.94 ± 0.13 | 0.85–0.92 | 45.37 ± 0.85 aB | 0.96–1.00 |
| (blanching only) | 170 | 1.63 ± 0.21 | 0.84–0.95 | ||
| 190 | 2.87 ± 0.37 a | 0.87–0.90 | |||
| PEF Low | 150 | 0.76 ± 0.07 | 0.86–0.86 | 35.37 ± 0.76 bC | 0.97–0.98 |
| (1 kV/cm, | 170 | 1.16 ± 0.26 | 0.83–0.83 | ||
| 50 kJ/kg) + blanching | 190 | 1.82 ± 0.15 b | 0.80–0.93 | ||
| PEF High | 150 | 0.66 ± 0.23 | 0.85–0.98 | 31.80 ± 1.08 cC | 0.91–1.00 |
| (1 kV/cm, | 170 | 1.11 ± 0.43 | 0.85–0.90 | ||
| 150 kJ/kg) + blanching | 190 | 1.45 ± 0.53 b | 0.81–0.91 | ||
| ‘Nadine’ | |||||
| Non-PEF | 150 | 1.09 ± 0.41 | 0.82–0.89 | 41.15 ± 1.87 C | 0.99–1.00 |
| (blanching only) | 170 | 1.93 ± 0.66 | 0.84–0.93 | ||
| 190 | 2.96 ± 0.99 | 0.91–0.96 | |||
| PEF Low | 150 | 0.95 ± 0.45 | 0.84–0.97 | 39.92 ± 1.12 B | 0.88–0.96 |
| (1 kV/cm, | 170 | 1.69 ± 0.99 | 0.75–0.81 | ||
| 50 kJ/kg) + blanching | 190 | 2.50 ± 1.12 | 0.92–0.94 | ||
| PEF High | 150 | 1.15 ± 0.31 | 0.84–0.88 | 38.85 ± 1.46 B | 0.87–1.00 |
| (1 kV/cm, | 170 | 2.06 ± 0.63 | 0.88–0.89 | ||
| 150 kJ/kg) + blanching | 190 | 2.95 ± 0.72 | 0.82–0.93 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abduh, S.B.M.; Leong, S.Y.; Zhao, C.; Baldwin, S.; Burritt, D.J.; Agyei, D.; Oey, I. Kinetics of Colour Development during Frying of Potato Pre-Treated with Pulsed Electric Fields and Blanching: Effect of Cultivar. Foods 2021, 10, 2307. https://doi.org/10.3390/foods10102307
Abduh SBM, Leong SY, Zhao C, Baldwin S, Burritt DJ, Agyei D, Oey I. Kinetics of Colour Development during Frying of Potato Pre-Treated with Pulsed Electric Fields and Blanching: Effect of Cultivar. Foods. 2021; 10(10):2307. https://doi.org/10.3390/foods10102307
Chicago/Turabian StyleAbduh, Setya Budi Muhammad, Sze Ying Leong, Chun Zhao, Samantha Baldwin, David J. Burritt, Dominic Agyei, and Indrawati Oey. 2021. "Kinetics of Colour Development during Frying of Potato Pre-Treated with Pulsed Electric Fields and Blanching: Effect of Cultivar" Foods 10, no. 10: 2307. https://doi.org/10.3390/foods10102307







