The Meat Quality Characteristics of Holstein Calves: The Story of Israeli ‘Dairy Beef’
Abstract
:1. Introduction
2. Materials & Methods
2.1. Collaboration
2.2. Selection of Animals and Meat Samples
2.3. Muscle Preparation
2.4. pH and Color
2.5. Chemical Composition
2.6. Water Holding Capacity
2.7. Warner Bratzler Shear Force
2.7.1. Sample Preparation
2.7.2. Coring and SF Measurement
2.8. Cooking Loss and Thawing Loss
2.9. Sarcomere Length (SL)
2.10. Total Collagen Content
2.11. Fatty Acid Profile
2.12. Statistical Analysis
3. Results & Discussion
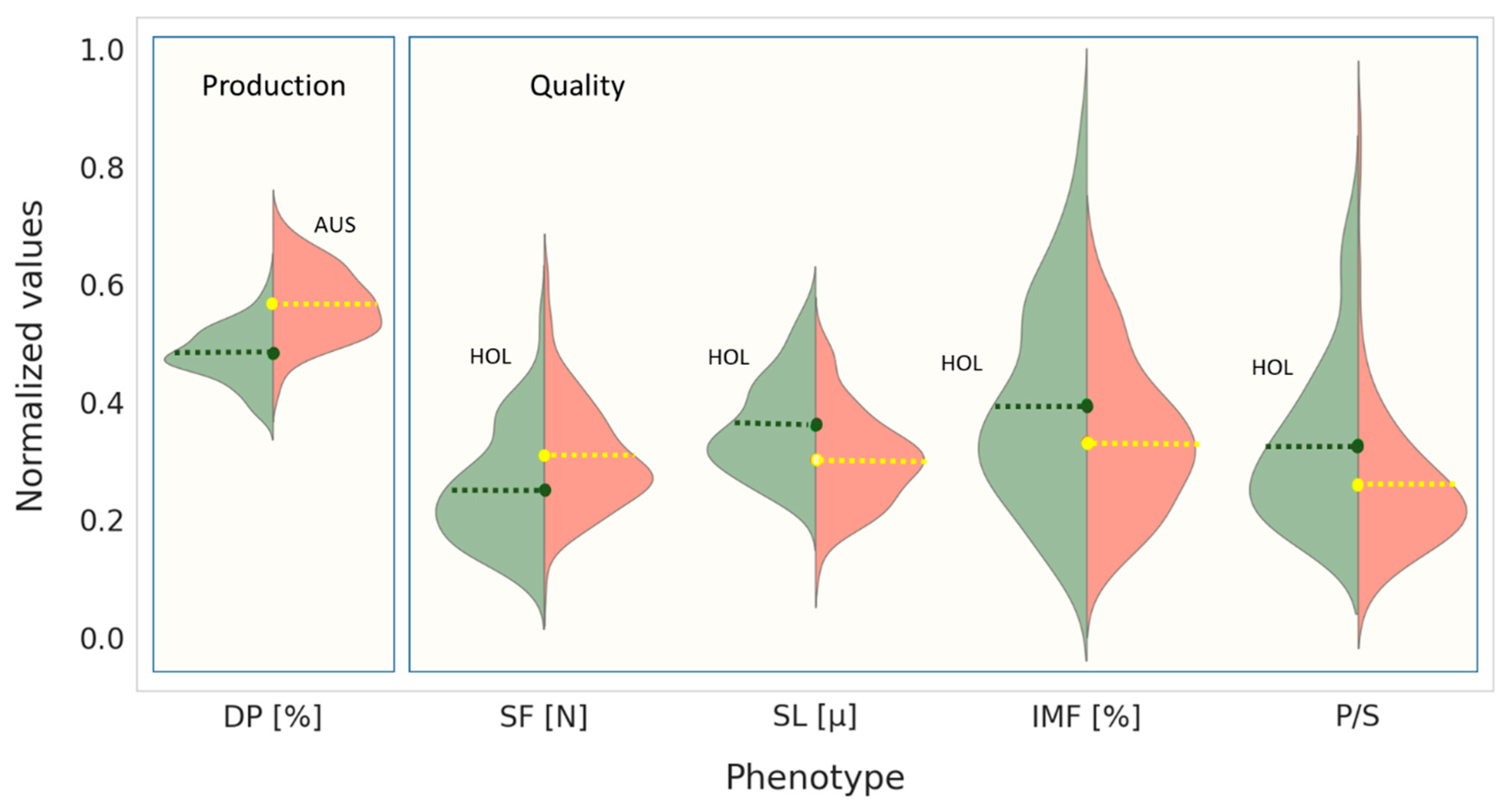
3.1. Carcass Production
3.2. Technological Parameters of Raw and Cooked Meat
3.2.1. pH and Color
3.2.2. Thawing Loss and Cooking Loss
3.2.3. Water-Holding Capacity
3.2.4. Chemical Composition
3.3. Characteristics of Meat Tenderness: Shear Force, Sarcomere Length and Total Collagen
3.4. Fatty Acid Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Meat Market Review; FAO: Rome, Italy, 2020. [Google Scholar]
- European Commission. Food Safety: A Farm to Fork Strategy for a Fair, Healthy and Environmentally Friendly Food System; European Comission: Brussels, Belgium, 2020. [Google Scholar]
- Hershkowitch, R.; Osman, E. The Beef Market of Israel—A Report, Summary of 2018; State of Israel, Ministry of Agriculture and Rural Development, Division of Research, Economy and Strategy: Jerusalem, Israel, 2019.
- Moon, S.S.; Seong, P.N.; Jeong, J.Y. Evaluation of meat color and physiochemical characteristics in forequarter muscles of holstein steers. Korean J. Food Sci. Anim. Res. 2015, 35, 646–652. [Google Scholar] [CrossRef] [Green Version]
- Drouillard, J.S. Current situation and future trends for beef production in the United States of America—A review. Asian Aust. J. Anim. Sci. 2018, 31, 1007–1016. [Google Scholar] [CrossRef] [Green Version]
- Teagasc Ireland. Sustainable Grass-Based Production—Advancing Knowledge for an Evolving Industry; Teagasc Ireland: Carlow, Ireland, 2019. [Google Scholar]
- Buege, D.R. Pricing and value of Holstein beef. Meat Facts Anal. 1988, 88, 7. [Google Scholar]
- Nogalski, Z. Effect of slaughter weight on the carcass value of young crossbred (’Polish Holstein Friesian’ x ’Limousin’) steers and bulls. Chil. J. Agric. Res. 2014, 74, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Nour, A.Y.M.; Thonney, M.L.; Stouffer, J.R.; White, W.R.C. Changes in primal cut yield with increasing weight of large and small cattle. J. Anim. Sci. 1983, 57, 1166–1172. [Google Scholar] [CrossRef]
- Reynolds, C.K.; Durst, B.; Lupoli, B.; Humphries, D.J.; Beever, D.E. Visceral Tissue Mass and Rumen Volume in Dairy Cows During the Transition from Late Gestation to Early Lactation. J. Dairy Sci. 2004, 87, 961–971. [Google Scholar] [CrossRef] [Green Version]
- Armbruster, G.; Nour, A.Y.M.; Thonney, M.L.; Stouffer, J.R. Changes in cooking losses and sensory attributes of Angus and Holstein beef with increasing carcass weight, marbling score or longissimus ether extract. J. Food Sc. 1983, 18, 835–840. [Google Scholar] [CrossRef]
- Schaefer, D.M. Yield and Quality of Holsten Beef. 1986. Available online: https://fyi.extension.wisc.edu/wbic/files/2010/11/Yield-and-Quality-of-Holstein-Beef.pdf (accessed on 5 August 2021).
- Thonney, M.L.; Perry, T.C.; Armbruster, G.; Beermann, D.H.; Fox, D.G. Comparison of steaks from Holstein and Simmental x Angus steers. J. Anim. Sci. 1991, 69, 4866–4870. [Google Scholar] [CrossRef]
- McKenna, D.R.; Roebert, D.L.; Bates, P.K.; Schmidt, T.B.; Hale, D.S.; Griffin, D.B.; Savell, J.W.; Brooks, J.C.; Morgan, J.B.; Montgomery, T.H.; et al. National Beef Quality Audit-2000: Survey of targeted cattle and carcass characteristics related to quality, quantity, and value of fed steers and heifers. J. Anim. Sci. 2002, 80, 1212–1222. [Google Scholar] [CrossRef]
- Santos, P.V.; Wagner, P.; Glasenapp de Menezes, L.F.; Vonz, D.; da Silveira, M.F.; Tubin, J. Carcass physical composition and meat quality of Holstein calves, terminated in different finishing systems and slaughter weights. Ciênc. Agrotec. Lavras 2013, 37, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Jurie, C.; Picard, B.; Hocquette, J.-F.; Dransfield, E.; Micol, D.; Listrat, A. Muscle and meat quality characteristics of Holstein and Salers cull cows. Meat Sci. 2007, 77, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Animal QTL Database. Available online: https://www.animalgenome.org/cgi-bin/QTLdb/BT/index (accessed on 5 August 2021).
- Cole, J.B.; Wiggans, G.R.; Ma, L.; Sonstegard, T.S.; Lawlor, T.J.; Crooker, B.A.; Van Tassell, C.P.; Yang, J.; Wang, S.; Matukumalli, L.K.; et al. Genome-wide association analysis of thirty one production, health, reproduction and body conformation traits in contemporary US Holstein cows. BMC Genom. 2011, 12, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesar, A.S.; Regitano, L.C.; Mourão, G.B.; Tullio, R.R.; Lanna, D.P.; Nassu, R.T.; A Mudado, M.; Oliveira, P.S.; Nascimento, M.L.D.; Chaves, A.S.; et al. Genome-wide association study for intramuscular fat deposition and composition in Nellore cattle. BMC Genet. 2014, 15, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClure, M.C.; Ramey, H.R.; Rolf, M.M.; McKay, S.D.; Decker, J.E.; Chapple, R.H.; Kim, J.W.; Taxis, T.M.; Weaber, R.L.; Schnabel, R.D.; et al. Genome-wide association analysis for quantitative trait loci influencing Warner-Bratzler shear force in five taurine cattle breeds. Anim. Genet. 2012, 43, 662–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lund, M.; Guldbrandtsen, B.; Buitenhuis, A.; Thomsen, B.; Bendixen, C. Detection of Quantitative Trait Loci in Danish Holstein Cattle Affecting Clinical Mastitis, Somatic Cell Score, Udder Conformation Traits, and Assessment of Associated Effects on Milk Yield. J. Dairy Sci. 2008, 91, 4028–4036. [Google Scholar] [CrossRef] [Green Version]
- AOAC. 991.36. Fat (Crude) in Meat and Meat Products. In Official Methods of Analysis, 18th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2006. [Google Scholar]
- AOAC. 992.15. Proximate Analysis and Calculations Crude Protein Meat and Meat Products. In Official Methods of Analysis, 18th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2006. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Grau, R.; Hamm, E. Eine einfache Methode zur Bestimmung der Wasserbindung im Fleisch. Fleischwirt 1952, 4, 295–297. [Google Scholar]
- Kovalenok, V.A.; Gilman, Z.D.; Orlov, A. Metodicheskie Recomendazii po ozenke myasnoi produktivnosti, kachestvu myasa I podkozhnogo zhira swinei. Moscow Vashniil. 1987, 7, 17. [Google Scholar]
- AMSA. Research Guidelines Cookery, Sensory Evaluation and Instrumental Tenderness Measurements of Fresh Meat; AMSA: Savoy, IL, USA, 1995. [Google Scholar]
- Wheeler, T.L.; Shackelford, S.D.; Koohmaraie, M. Sampling, cooking, and coring effects on Warner-Bratzler shear force values in beef. J. Anim. Sci. 1996, 74, 1553–1562–1562. [Google Scholar] [CrossRef]
- Shackelford, S.; Koohmaraie, M.; Whipple, G.; Wheeler, T.; Miller, M.; Crouse, J.; Reagan, J. Predictors of Beef Tenderness: Development and Verification. J. Food Sci. 1991, 56, 1130–1135. [Google Scholar] [CrossRef]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Cross, H.; West, R.; Dutson, T. Comparison of methods for measuring sarcomere length in beef semitendinosus muscle. Meat Sci. 1981, 5, 261–266. [Google Scholar] [CrossRef]
- Koolmees, P.A.; Korteknie, F.; Smulders, F.J.M. Accuracy and utility of sarcomere-length assessment by laser diffraction. Food Struct. 1986, 5, 9. [Google Scholar]
- AOAC. Hydroxyproline in Meat and Meat Products, 990.26. In Official Methods of Analysis, 14th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1993. [Google Scholar]
- Starkey, C.P.; Geesink, G.H.; Oddy, V.H.; Hopkins, D. Explaining the variation in lamb longissimus shear force across and within ageing periods using protein degradation, sarcomere length and collagen characteristics. Meat Sci. 2015, 105, 32–37. [Google Scholar] [CrossRef]
- Cohen-Zinder, M.; Orlov, A.; Trofimyuk, O.; Agmon, R.; Kabiya, R.; Shor-Shimoni, E.; Wagner, E.K.; Hussey, K.; Leibovich, H.; Miron, J.; et al. Dietary supplementation of Moringa oleifera silage increases meat tenderness of Assaf lambs. Small Rumin. Res. 2017, 151, 110–116. [Google Scholar] [CrossRef]
- Hara, A.; Radin, N.S. Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 1978, 90, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Christie, W.W. A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid Res. 1982, 23, 1072–1075. [Google Scholar] [CrossRef]
- Chouinard, P.Y.; Corneau, L.; Barbano, D.M.; Metzger, L.E.; Bauman, D.E. Conjugated Linoleic Acids Alter Milk Fatty Acid Composition and Inhibit Milk Fat Secretion in Dairy Cows. J. Nutr. 1999, 129, 1579–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Story, M.; Hamm, M.W.; Wallinga, D. Food Systems and Public Health: Linkages to Achieve Healthier Diets and Healthier Communities. J. Hunger. Environ. Nutr. 2009, 4, 219–224. [Google Scholar] [CrossRef] [PubMed]
- United States, FAO. Sustainable Food Systems—Concept and Framework. 2018. Available online: http://www.fao.org/3/ca2079en/CA2079EN.pdf (accessed on 5 August 2021).
- Allen, T.; Prosperi, P. Modeling Sustainable Food Systems. Environ. Manag. 2016, 57, 956–975. [Google Scholar] [CrossRef] [Green Version]
- Morris, S.T.; Parker, W.J.; Purchas, R.W.; McCutcheon, S.N. Dairy crossbreeding alternatives to improve New Zealand beef production. Proc. N. Z. Grassl. Assoc. 1992, 54, 19–22. [Google Scholar]
- Zotto, R.D.; Penasa, M.; De Marchi, M.; Cassandro, M.; López-Villalobos, N.; Bittante, G. Use of crossbreeding with beef bulls in dairy herds: Effect on age, body weight, price, and market value of calves sold at livestock auctions1,2. J. Anim. Sci. 2009, 87, 3053–3059. [Google Scholar] [CrossRef] [Green Version]
- Martín, N.; Schreurs, N.; Morris, S.; López-Villalobos, N.; McDade, J.; Hickson, R. Sire Effects on Carcass of Beef-Cross-Dairy Cattle: A Case Study in New Zealand. Animals 2021, 11, 636. [Google Scholar] [CrossRef]
- Keane, M.G. Beef Cross Breeding of Dairy and Beef Cows; Occasional Series No. 8; Grange Beef Research Centre, Teagasc, Dunsany, Co.: Meath, Ireland, 2011; Available online: https://core.ac.uk/download/pdf/84886318.pdf (accessed on 5 August 2021).
- Taylor, S.C.S.; Murray, J.I. Effect of feeding level, breed and milking potential on body tissues and organs of mature, non-lactating cows. Anim. Sci. 1991, 53, 27–38. [Google Scholar] [CrossRef]
- Baldwin, R.; McLeod, K.; Capuco, A. Visceral Tissue Growth and Proliferation During the Bovine Lactation Cycle. J. Dairy Sci. 2004, 87, 2977–2986. [Google Scholar] [CrossRef] [Green Version]
- Pfuhl, R.; Bellmann, O.; Kühn, C.; Teuscher, F.; Ender, K.; Wegner, J. Beef versus dairy cattle: A comparison of feed conversion, carcass composition, and meat quality. Arch. Anim. Breed. 2007, 50, 59–70. [Google Scholar] [CrossRef]
- Warner, R.D.; Ferguson, D.M.; McDonagh, M.B.; Channon, H.A.; Cottrell, J.; Dunshea, F. Acute exercise stress and electrical stimulation influence the consumer perception of sheep meat eating quality and objective quality traits. Aust. J. Exp. Agric. 2005, 45, 553–560. [Google Scholar] [CrossRef]
- Hughes, J.; Oiseth, S.; Purslow, P.; Warner, R. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci. 2014, 98, 520–532. [Google Scholar] [CrossRef]
- Klont, R.E.; Barnier, V.M.H.; Van Dijk, A.; Smulders, F.J.M.; Hoving-Bolink, A.H.; Hulsegge, B.; Eikelenboom, G. Effects of rate of pH fall, time of deboning, aging period, and their interaction on veal quality characteristics. J. Anim. Sci. 2000, 78, 1845–1851. [Google Scholar] [CrossRef]
- Nian, Y.; Kerry, J.P.; Prendiville, R.; Allen, P. The eating quality of beef from young dairy bulls derived from two breed types at three ages from two different production systems. Ir. J. Agric. Food Res. 2017, 56, 31–44. [Google Scholar] [CrossRef]
- Modzelewska-Kapituła, M.; Tkacz, K.; Nogalski, Z.; Karpińska-Tymoszczyk, M.; Draszanowska, A.; Pietrzak-Fiecko, R.; Purwin, C.; Lipiński, K. Addition of herbal extracts to the Holstein-Friesian bulls’ diet changes the quality of beef. Meat Sci. 2018, 145, 163–170. [Google Scholar] [CrossRef]
- Cho, S.; Kang, S.M.; Seong, P.; Kang, G.; Choi, S.; Kwon, E.; Moon, S.; Kim, D.; Park, B. Physico-chemical Meat Qualities of Loin and Top Round Beef from Holstein Calves with Different Slaughtering Ages. Food Sci. Anim. Resour. 2014, 34, 674–682. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.M.; Hopkins, D.L.; Zhao, X.X.; van de Ven, R.; Mao, Y.W.; Zhu, L.X.; Han, G.X.; Luo, X. Characterization of pH decline and meat color development of beef carcasses during the early postmortem period in a Chinese beef cattle abattoir. J. Integr. Agric. 2018, 17, 1691–1695. [Google Scholar] [CrossRef]
- Jama, N.; Muchenje, V.; Chimonyo, M.; Strydom, P.E.; Dzama, K.; Raats, J.G. Cooking loss components of beef from Nguni, Bonsmara and Angus steers. Afr. J. Agric. Res. 2008, 3, 416–420. [Google Scholar]
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—A review. Meat Sci. 2011, 89, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Aaslyng, M.D. Quality indicators for raw meat. In Meat Processing; Kerry, J.P., Kerry, J.F., Ledward, D., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2002; pp. 157–174. [Google Scholar]
- Woelfel, R.L.; Owens, C.M.; Hirschler, E.M.; Martinez-Dawson, R.; Sams, A.R. The characterization and incidence of pale, soft, and exudative broiler meat in a commercial processing plant. Poult. Sci. 2002, 81, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Offer, G.; Trinick, J. On the mechanism of water holding in meat: The swelling and shrinking of myofibrils. Meat Sci. 1983, 8, 245–281. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Fischer, K. Drip loss in pork: Influencing factors and relation to further meat quality traits. J. Anim. Breed. Genet. 2007, 124, 12–18. [Google Scholar] [CrossRef]
- Hocquette, J.F.; Gondret, F.; Baéza, E.; Médale, F.; Jurie, C.; Pethick, D.W. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Animal 2010, 4, 303–319. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Beak, S.H.; Da Jin Sol Jung, S.Y.; Kim, I.H.J.; Piao, M.Y.; Kang, H.J.; Fassah, D.M.; Na, S.W.; Yoo, S.P.; Maik, M. Genetic, management, and nutritional factors affecting intramuscular fat deposition in beef cattle—A review. Asian-Australas. J. Anim. Sci. 2018, 31, 1043–1061. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, R.T.D.S.; Chizzotti, M.L.; Vital, C.; Baracat-Pereira, M.C.; Barros, E.; Busato, K.; Gomes, R.A.; Ladeira, M.; Martins, T. Differences in Beef Quality between Angus (Bos taurus taurus) and Nellore (Bos taurus indicus) Cattle through a Proteomic and Phosphoproteomic Approach. PLoS ONE 2017, 12, e0170294. [Google Scholar] [CrossRef] [PubMed]
- Krone, K.G.; Ward, A.K.; Madder, K.M.; Hendrick, S.; McKinnon, J.J.; Buchanan, F.C. Interaction of vitamin A supplementation level with ADH1C genotype on intramuscular fat in beef steers. Animal 2016, 10, 403–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinh, T.; Blanton, J.R.; Riley, D.G.; Chase, C.C.; Coleman, S.W.; Phillips, W.A.; Brooks, J.C.; Miller, M.F.; Thompson, L.D. Intramuscular fat and fatty acid composition of longissimus muscle from divergent pure breeds of cattle. J. Anim. Sci. 2010, 88, 756–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwood, P.L.; Siddell, J.P.; Walmsley, B.J.; Geesink, G.H.; Pethick, D.W.; McPhee, M.J. Postweaning substitution of grazed forage with a high-energy concentrate has variable long-term effects on subcutaneous fat and marbling in Bos taurus genotypes1. J. Anim. Sci. 2015, 93, 4132–4143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lbrecht, E.; Gotoh, T.; Ebara, F.; Xu, J.; Viergutz, T.; Nürnberg, G.; Maak, S.; Wegner, J. Cellular conditions for intramuscular fat deposition in Japanese Black and Holstein steers. Meat Sci. 2011, 89, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Irie, M.; Kouda, M.; Matono, H. Effect of ursodeoxycholic acid supplementation on growth, carcass characteristics, and meat quality of Wagyu heifers (Japanese Black cattle). J. Anim. Sci. 2011, 89, 4221–4226. [Google Scholar] [CrossRef] [Green Version]
- Robelin, J. Growth of adipose tissues in cattle; partitioning between depots, chemical composition and cellularity. A review. Livest. Prod. Sci. 1986, 14, 349–364. [Google Scholar] [CrossRef]
- Maltin, C.; Balcerzak, D.; Tilley, R.; Delday, M. Determinants of meat quality: Tenderness. Proc. Nutr. Soc. 2003, 62, 337–347. [Google Scholar] [CrossRef]
- Destefanis, G.; Brugiapaglia, A.; Barge, M.; Molin, E.D. Relationship between beef consumer tenderness perception and Warner–Bratzler shear force. Meat Sci. 2008, 78, 153–156. [Google Scholar] [CrossRef]
- Gadisa, B.; Yusuf, Y.; Yousuf, M. Evaluation of eating quality in sensory panelist and instrumental tenderness of beef from Harar, Arsi and Bale cattle breeds in Oromia, Ethiopia. Int. J. Agric. Sc. Food Technol. 2019, 5, 35–42. [Google Scholar]
- Cierach, M.; Majewska, K. Comparison of instrumental and sensory evaluation of texture of cured and cooked beef meat. Nahr. Food 1997, 41, 366–369. [Google Scholar] [CrossRef]
- O’Connor, S.F.; Tatum, J.D.; Wulf, D.M.; Green, R.D.; Smith, G.C. Genetic effects on beef tenderness in Bos indicus composite and Bos taurus cattle. J. Anim. Sci. 1997, 75, 2826. [Google Scholar] [CrossRef]
- Whipple, G.; Koohmaraie, M.; Dikeman, M.E.; Crouse, J.D.; Hunt, M.C.; Klemm, R.D. Evaluation of attributes that affect longissimus muscle tenderness in Bos taurus and Bos indicus cattle. J. Anim. Sci. 1990, 68, 2716–2728. [Google Scholar] [CrossRef] [Green Version]
- Shackelford, S.D.; Koohmaraie, M.; Miller, M.F.; Crouse, J.D.; Reagan, J.O. An evaluation of tenderness of the longissimus muscle of Angus by Hereford versus Brahman crossbred heifers. J. Anim. Sci. 1991, 69, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Curi, R.A.; Chardulo, L.A.L.; Giusti, J.; Silveira, A.C.; Martins, C.L.; de Oliveira, H.N. Assessment of GH1, CAPN1 and CAST polymorphisms as markers of carcass and meat traits in Bos indicus and Bos taurus–Bos indicus cross beef cattle. Meat Sci. 2010, 86, 915–920. [Google Scholar] [CrossRef]
- Wulf, D.M.; Tatum, J.D.; Green, R.D.; Morgan, J.B.; Golden, B.L.; Smith, G.C. Genetic influences on beef longissimus palatability in charolais- and limousin-sired steers and heifers. J. Anim. Sci. 1996, 74, 2394–2405. [Google Scholar] [CrossRef] [PubMed]
- Monsón, F.; Sañudo, C.; Sierra, I. Influence of cattle breed and ageing time on textural meat quality. Meat Sci. 2004, 68, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Weaver, A.D.; Bowker, B.C.; Gerrard, D.E. Sarcomere length influences postmortem proteolysis of excised bovine semitendinosus muscle. J. Anim. Sci. 2008, 86, 1925–1932. [Google Scholar] [CrossRef]
- Ertbjerg, P.; Puolanne, E. Muscle structure, sarcomere length and influences on meat quality: A review. Meat Sci. 2017, 132, 139–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weston, A.; Rogers, R.; Althen, T. Review: The Role of Collagen in Meat Tenderness. Prof. Anim. Sci. 2002, 18, 107–111. [Google Scholar] [CrossRef]
- Subramaniyan, S.A.; Hwang, I. Biological Differences between Hanwoo longissimus dorsi and semimembranosus Muscles in Collagen Synthesis of Fibroblasts. Food Sci. Anim. Resour. 2017, 37, 392–401. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.S. The Effect of Quality Grade and Muscle on Collagen Contents and Tenderness of Intramuscular Connective Tissue and Myofibrillar Protein for Hanwoo Beef. Asian-Australasian J. Anim. Sci. 2006, 19, 1059–1064. [Google Scholar] [CrossRef]
- Christensen, M.; Ertbjerg, P.; Failla, S.; Sañudo, C.; Richardson, R.I.; Nute, G.R.; Olleta, J.L.; Panea, B.; Albertí, P.; Juárez, M.; et al. Relationship between collagen characteristics, lipid content and raw and cooked texture of meat from young bulls of fifteen European breeds. Meat Sci. 2011, 87, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Rego, O.; Simões, E.; Rosa, H.J.D. Consumption of high energy maize diets is associated with increased soluble collagen in muscle of Holstein bulls. Meat Sci. 2010, 86, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Jurie, C.; Martin, J.-F.; Listrat, A.; Jailler, R.; Culioli, J.; Picard, B. Effects of age and breed of beef bulls on growth parameters, carcass and muscle characteristics. Anim. Sci. 2005, 80, 257–263. [Google Scholar] [CrossRef]
- Archile-Contreras, A.; Mandell, I.; Purslow, P. Disparity of dietary effects on collagen characteristics and toughness between two beef muscles. Meat Sci. 2010, 86, 491–497. [Google Scholar] [CrossRef]
- Dubost, A.; Micol, D.; Meunier, B.; Lethias, C.; Listrat, A. Relationships between structural characteristics of bovine intramuscular connective tissue assessed by image analysis and collagen and proteoglycan content. Meat Sci. 2013, 93, 378–386. [Google Scholar] [CrossRef]
- De Smet, S.; Raes, K.; Demeyer, D. Meat fatty acid composition as affected by fatness and genetic factors: A review. Anim. Res. 2004, 53, 81–98. [Google Scholar] [CrossRef]
- Wood, J.; Richardson, I.; Nute, G.; Fisher, A.; Campo, M.M.; Kasapidou, E.; Sheard, P.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G. Modification of fatty acid composition in meat through diet: Effect on lipid peroxidation and relationship to nutritional quality—A review. Lipid Peroxidation 2012, 12, 239–258. [Google Scholar]
- Costa, A.S.; Silva, M.P.; Alfaia, C.P.; Pires, V.M.; Fontes, C.M.; Bessa, R.J.; Prates, J.A. Genetic background and diet impact beef fatty acid composition and stearoyl-CoA desaturase mRNA expression. Lipids 2013, 48, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Jéssica, L.O.; Suellen, M.G.; Josevan, S.; Ana, S.M.; Figueirêdo, R.D.; Rita, C.R.; Marta, S.M. Conjugated linoleic acid (Cla) concentration and fatty acid composition of brazilian fermented dairy products. J. Nutr. Food Sci. 2015, 5, 1–3. [Google Scholar] [CrossRef]
- Vahmani, P.; Mapiye, C.; Prieto, N.; Rolland, D.C.; McAllister, T.A.; Aalhus, J.L.; Dugan, M.E. The scope for manipulating the polyunsaturated fatty acid content of beef: A review. J. Anim. Sci. Biotechnol. 2015, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minihane, A.M.; Lovegrove, J.A. Health benefits of polyunsaturated fatty acids (PUFAs). In Improving the Fat Content of Foods; Woodhead Publishing: Sawston, UK, 2006; pp. 107–140. [Google Scholar]
| FARM | BREED | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Trait | F1 | F2 | F3 | HOL | AUS | FARM | BREED |
| Live BW weight (kg) | 519.6 ± 38.0 a | 534.8 ± 58.8 a | 536.9 ± 91.8 a | 530.2 ± 55.5 a | 536.9 ± 91.8 a | 0.269 | 0.380 |
| Carcass weight (kg) | 279.1 ± 22.6 a | 293.0 ± 32.2 b | 309.5 ± 58.3 c | 288.8 ± 30.3 a | 309.5 ± 58.3 b | <0.0001 | <0.0001 |
| Dressing percentage (%) | 53.7 ± 1.2 a | 54.8 ± 2.5 b | 57.5 ± 2.1 c | 54.5 ± 2.2 a | 57.5 ± 2.1 b | <0.0001 | <0.0001 |
| FARM | BREED | p-Value | |||||
|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | HOL | AUS | FARM | BREED | |
| Raw Beef | |||||||
| pHu † | 5.88 ± 0.28 a | 5.82 ± 0.21 a | 5.74 ± 0.12 b | 5.85 ± 0.24 a | 5.74 ± 0.12 b | <0.0001 | 0.0002 |
| pH48h †† | 5.70 ± 0.40 a | 5.69 ± 0.20 a | 5.53 ± 0.16 b | 5.69 ± 0.28 a | 5.53 ± 0.16 b | <0.0001 | <0.0001 |
| Color | |||||||
| L* | 38.87 ± 3.34 a | 38.68 ± 4.14 a | 40.97 ± 3.50 b | 38.70 ± 3.91 a | 40.97 ± 3.50 b | <0.0001 | <0.0001 |
| a* | 15.85 ± 1.73 a | 15.83 ± 1.97 a | 14.63 ± 1.93 b | 15.83 ± 1.90 a | 14.63 ± 1.93 b | <0.0001 | <0.0001 |
| b* | 3.25 ± 0.98 a | 3.02 ± 1.18 a | 2.59 ± 1.28 b | 3.08 ± 1.10 a | 2.59 ± 1.28 b | 0.0002 | <0.0001 |
| TL (%) | 3.30 ± 1.05 a | 4.14 ± 2.00 b | 5.82 ± 2.84 c | 3.88 ± 1.05 a | 5.82 ± 2.84 b | <0.0001 | <0.0001 |
| WHC (%) | 45.01 ± 4.65 a | 43.57 ± 3.13 b | 43.11 ± 3.40 b | 43.68 ± 3.75 a | 43.11 ± 3.40 a | 0.002 | |
| Thermally treated beef | |||||||
| CKL (%) | 22.14 ± 4.40 a | 22.12 ± 3.30 a | 23.26 ± 3.18 b | 22.12 ± 3.66 a | 23.26 ± 3.18 b | 0.007 | 0.0017 |
| FARM | BREED | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Trait (%) | F1 | F2 | F3 | HOL | AUS | FARM | BREED |
| Moisture | 73.90 ± 0.76 a | 73.59 ± 1.00 a | 73.20 ± 1.10 b | 73.7± 0.94 a | 73.20 ± 1.10 b | <0.0001 | <0.0001 |
| Protein | 22.92 ± 1.05 a | 22.37 ± 0.85 b | 22.27 ± 0.85 b | 22.47 ± 0.96 | 22.27 ± 0.85 | <0.0001 | 0.305 |
| IMF | 2.78 ± 0.96 a,b | 2.82 ± 1.02 a | 2.51 ± 0.80 b | 2.80 ± 1.00 a | 2.51 ± 0.80 b | 0.009 | 0.002 |
| Ash | 1.20 ± 0.07 a | 1.30 ± 0.18 b | 1.27 ± 0.11 b | 1.27 ± 0.16 | 1.27 ± 0.11 | <0.0001 | 0.955 |
| FARM | BREED | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Trait | F1 | F2 | F3 | HOL | AUS | FARM | BREED |
| SF (N) | 41.3 ± 10.76 a | 41.6 ± 9.17 a | 46.5 ± 9.27 b | 41.5 ± 9.66 a | 46.5 ± 9.27 b | <0.0001 | <0.0001 |
| SL (µM) | 2.22 ± 0.30 a | 2.10 ± 0.27 b | 1.98 ± 0.32 c | 2.14 ± 0.29 a | 1.98 ± 0.32 b | <0.0001 | <0.0001 |
| Total collagen (mg/g) | 2.88 ± 0.64 a | 2.81 ± 0.84 a | 2.70 ± 0.83 a | 2.82 ± 0.82 a | 2.70 ± 0.83 a | 0.410 | 0.220 |
| Proportion of Fatty Acids | HOL | S.D. | AUS | S.D. | p-Value |
|---|---|---|---|---|---|
| C10:0 | 0.040 | 0.0002 | 0.035 | 0.0003 | 1.6 × 10−1 |
| C12:0 | 0.042 | 0.0003 | 0.058 | 0.0004 | 1.5 × 10−3 |
| C14:0 | 2.716 | 0.0047 | 3.242 | 0.0074 | 9.2 × 10−9 |
| C14:1 | 0.488 | 0.0013 | 0.632 | 0.0023 | 1.4 × 10−7 |
| C15:0 | 0.304 | 0.0006 | 0.398 | 0.0012 | 2.7 × 10−11 |
| C16:0 | 25.56 | 0.0180 | 26.27 | 0.0222 | 1.2 × 10−2 |
| C16:1 | 3.266 | 0.0047 | 3.539 | 0.0063 | 4.8 × 10−4 |
| C17:0 | 0.802 | 0.0028 | 1.175 | 0.0024 | 2.2 × 10−20 |
| C17:1 | 0.428 | 0.0016 | 0.723 | 0.0019 | 4.9 × 10−26 |
| C18:0 | 17.13 | 0.0189 | 16.32 | 0.0244 | 7.4 × 10−3 |
| C18:1n9t | 3.376 | 0.0193 | 2.372 | 0.0093 | 2.6 × 10−6 |
| C18:1n9c | 35.02 | 0.0302 | 36.12 | 0.0343 | 1.5 × 10−2 |
| C18:1n10c | 1.715 | 0.0032 | 1.952 | 0.0031 | 1.2 × 10−7 |
| C18:1n11c | 0.553 | 0.0019 | 0.323 | 0.0016 | 3.0 × 10−18 |
| C18:1n12c | 0.317 | 0.0013 | 0.387 | 0.0018 | 1.6 × 10−3 |
| C18:2n6t | 0.130 | 0.0015 | 0.109 | 0.0010 | 2.2 × 10−1 |
| C18:2n6c | 5.862 | 0.0174 | 4.467 | 0.0172 | 1.9 × 10−8 |
| C20:0 | 0.041 | 0.0006 | 0.000 | 0.0000 | 4.5 × 10−12 |
| C18:3n3 | 0.277 | 0.0008 | 0.358 | 0.0015 | 3.5 × 10−6 |
| CLA c9,t11 | 0.239 | 0.0015 | 0.273 | 0.0011 | 5.7 × 10−2 |
| C22:0 | 0.237 | 0.0017 | 0.000 | 0.0000 | 1.7 × 10−28 |
| C20:3n6 | 0.067 | 0.0016 | 0.198 | 0.0015 | 9.8 × 10−9 |
| C20:4n6 | 1.017 | 0.0073 | 0.837 | 0.0047 | 3.4 × 10−2 |
| C22:2 | 0.061 | 0.0013 | 0.000 | 0.0000 | 3.1 × 10−6 |
| C24:0 | 0.000 | 0.0000 | 0.086 | 0.0010 | 1.0 × 10−12 |
| C22:6n3 | 0.202 | 0.0026 | 0.117 | 0.0022 | 1.1 × 10−2 |
| Short FA | 0.082 | 0.0004 | 0.093 | 0.0006 | 1.2 × 10−1 |
| SFA | 46.88 | 0.0217 | 47.58 | 0.0415 | 1.3 × 10−1 |
| MUFA | 45.16 | 0.0256 | 45.75 | 0.0440 | 2.5 × 10−1 |
| PUFA | 7.854 | 0.0262 | 6.359 | 0.0229 | 1.6 × 10−5 |
| PUFA/SFA | 0.170 | 0.0610 | 0.135 | 0.0530 | 2.7 × 10−5 |
| C18:0 (stearic): C18:2n6c (linoleic) | 3.150 | 0.8561 | 3.973 | 1.0693 | 7.33 × 10−9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabtay, A.; Shor-Shimoni, E.; Orlov, A.; Agmon, R.; Trofimyuk, O.; Tal, O.; Cohen-Zinder, M. The Meat Quality Characteristics of Holstein Calves: The Story of Israeli ‘Dairy Beef’. Foods 2021, 10, 2308. https://doi.org/10.3390/foods10102308
Shabtay A, Shor-Shimoni E, Orlov A, Agmon R, Trofimyuk O, Tal O, Cohen-Zinder M. The Meat Quality Characteristics of Holstein Calves: The Story of Israeli ‘Dairy Beef’. Foods. 2021; 10(10):2308. https://doi.org/10.3390/foods10102308
Chicago/Turabian StyleShabtay, Ariel, Einav Shor-Shimoni, Ala Orlov, Rotem Agmon, Olena Trofimyuk, Ofir Tal, and Miri Cohen-Zinder. 2021. "The Meat Quality Characteristics of Holstein Calves: The Story of Israeli ‘Dairy Beef’" Foods 10, no. 10: 2308. https://doi.org/10.3390/foods10102308
APA StyleShabtay, A., Shor-Shimoni, E., Orlov, A., Agmon, R., Trofimyuk, O., Tal, O., & Cohen-Zinder, M. (2021). The Meat Quality Characteristics of Holstein Calves: The Story of Israeli ‘Dairy Beef’. Foods, 10(10), 2308. https://doi.org/10.3390/foods10102308





