Physicochemical Properties and Antioxidant Activity of Pumpkin Polysaccharide (Cucurbita moschata Duchesne ex Poiret) Modified by Subcritical Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pumpkin Polysaccharide Preparation
2.3. Subcritical Water Hydrolysis
2.4. Total Carbohydrate and Reducing Sugar Content Measurements
2.5. Molecular Mass Determination
2.6. Rheological Measurements
2.6.1. Intrinsic Viscosity and Viscosity-Average Molecular Weight
2.6.2. Steady-State Flow Test
2.6.3. Dynamic Oscillation
2.6.4. Activation Energy
2.7. Emulsifying Properties
2.7.1. Emulsifying Activity
2.7.2. Zeta Potential and Conductivity
2.8. Fourier Transform Infrared (FTIR) Spectroscopy and Scanning Electron Microscopy (SEM) Analyses
2.9. Antioxidant Activity Analyses
2.9.1. Measurement of DPPH Radical Scavenging Activity
2.9.2. Hydroxyl Radical Scavenging Activity Measurements
2.9.3. ABTS Radical Scavenging Assay
2.9.4. Reducing Power
2.10. Statistical Analysis
3. Results and Discussion
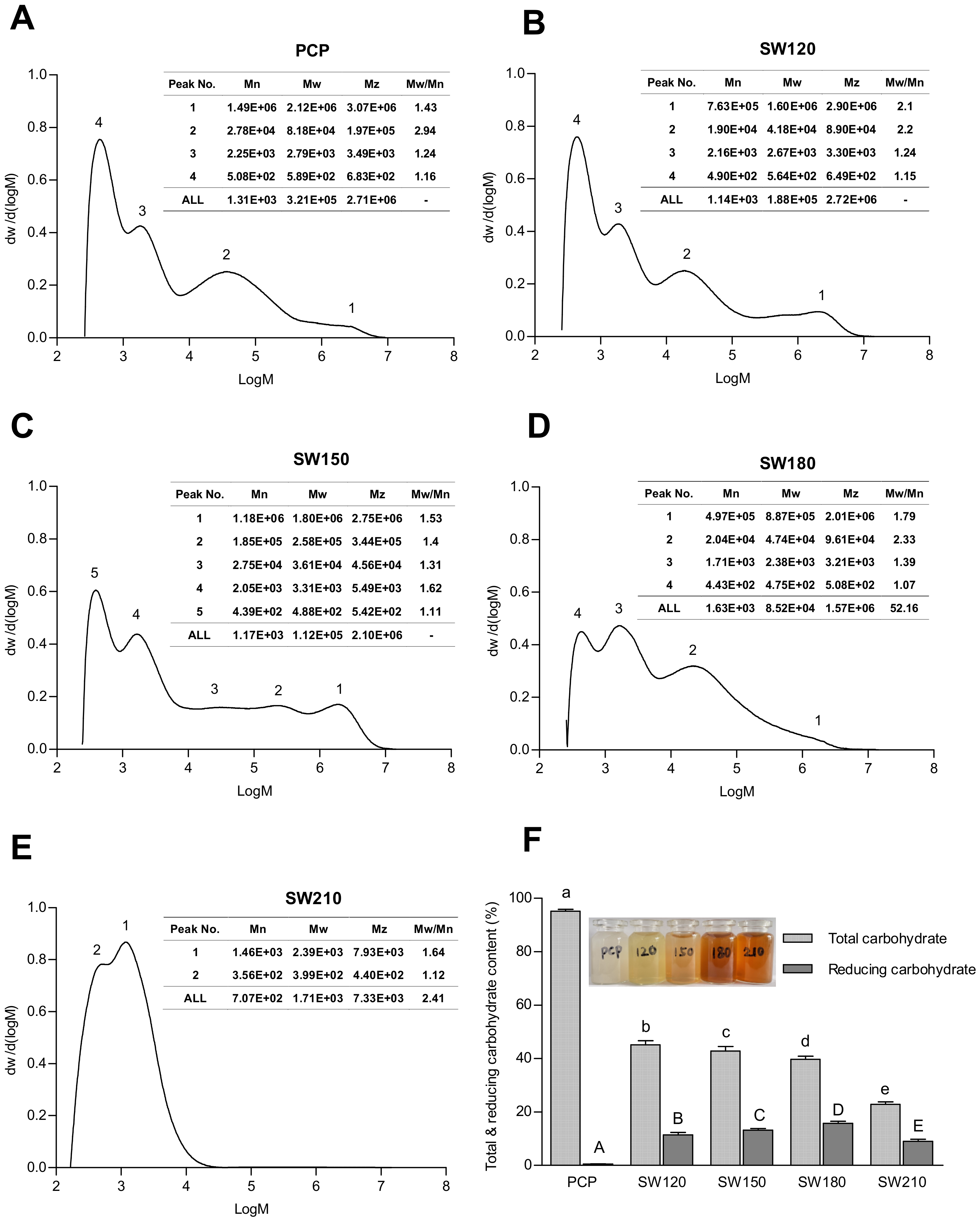
3.1. The Molecular Mass and Distribution
3.2. Rheological Analyses
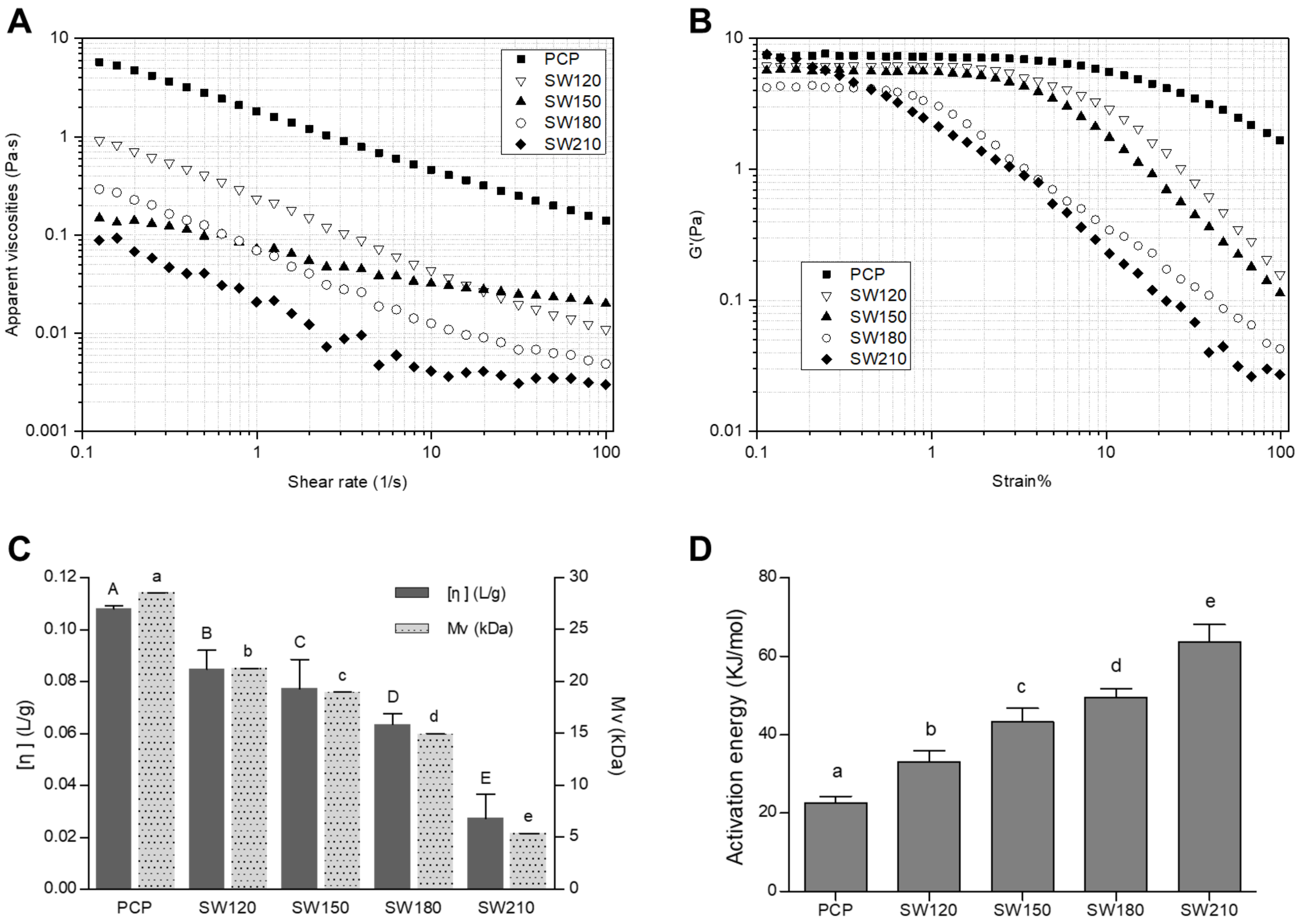
3.2.1. Viscosity-Related Analyses
3.2.2. Apparent Viscosity Analyses
3.2.3. Strain Sweep Analysis
3.2.4. Activation Energy
3.3. Emulsifying Properties
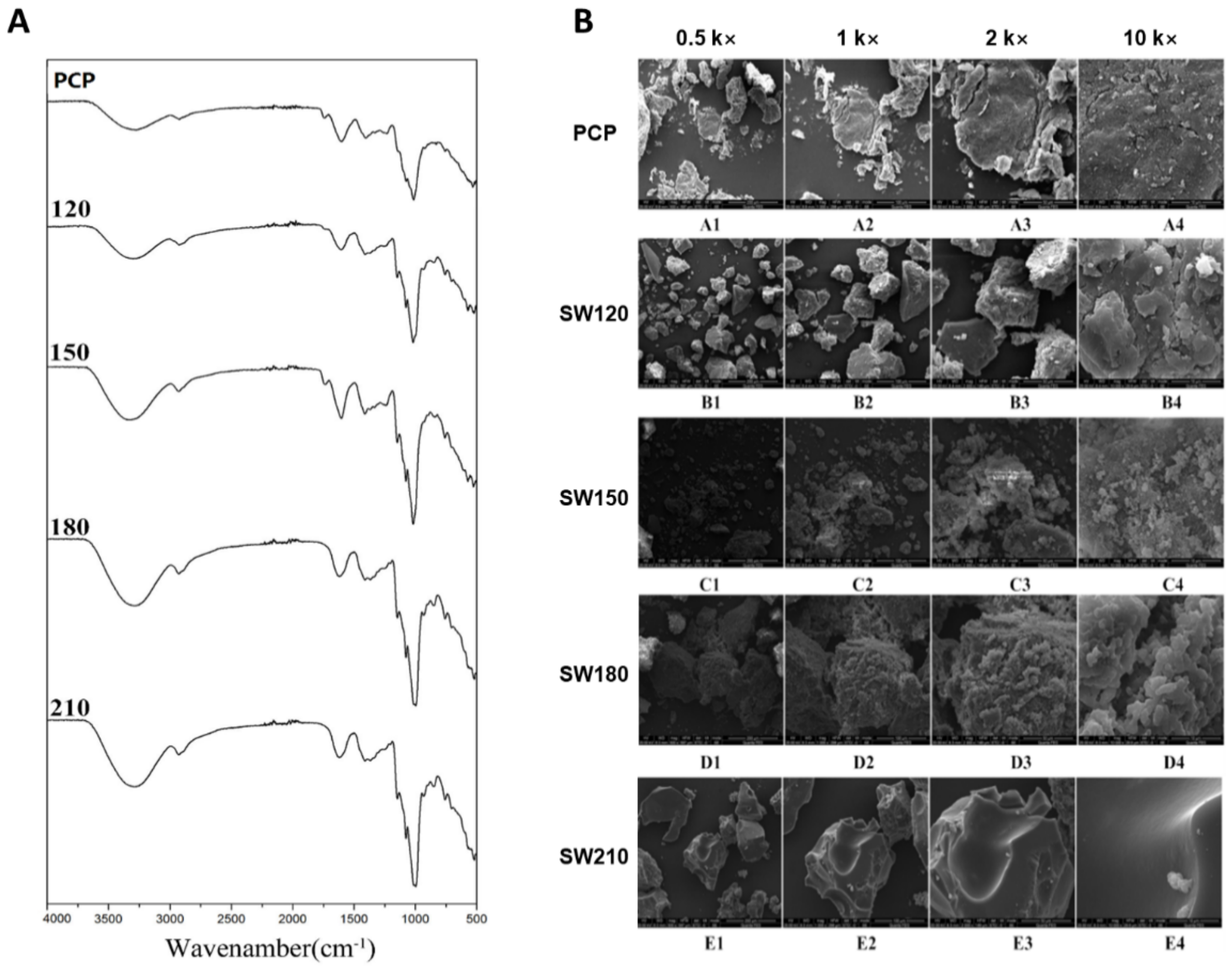
3.4. FTIR Analyses of SCW-Treated Polysaccharides
3.5. Scanning Electron Microscopy
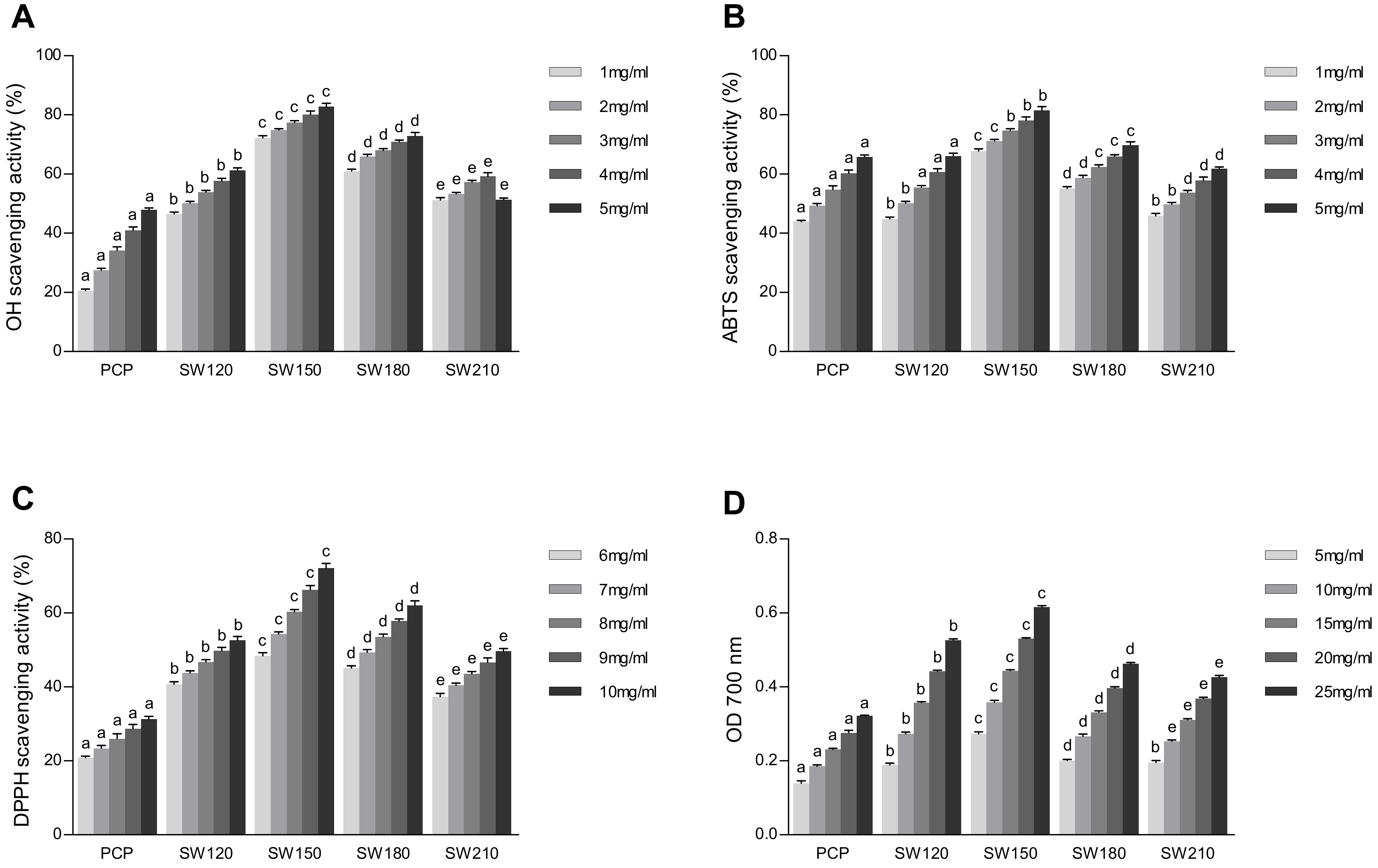
3.6. Assessment of Pumpkin Polysaccharide Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Huang, G. Antioxidant activities of phosphorylated pumpkin polysaccharide. Int. J. Biol. Macromol. 2019, 125, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Ying, L.; Qin-Lu, L.; Qian, L.U.; Ming-Hui, T.; Feng-Xia, Z. Research progress on polysaccharide extracted and antioxidant activity of pumpkin. Food Mach. 2014, 30, 239–243. [Google Scholar]
- Chen, L.; Long, R.; Huang, G.; Huang, H. Extraction and antioxidant activities in vivo of pumpkin polysaccharide. Ind. Crop. Prod. 2020, 146, 112199. [Google Scholar] [CrossRef]
- Yu, M.; Xiao, B.; Hao, X.; Tan, J.; Zhang, Y. Pumpkin polysaccharide preparation, simulated gastrointestinal digestion, and in vivo biodistribution. Int. J. Biol. Macromol. 2019, 141, 1293–1303. [Google Scholar] [CrossRef]
- Song, Y.; Ni, Y.; Hu, X.; Li, Q. Effect of phosphorylation on antioxidant activities of pumpkin (Cucurbita pepo, Lady godiva) polysaccharide. Int. J. Biol. Macromol. 2015, 81, 41–48. [Google Scholar] [CrossRef]
- Oloyede, F.M.; Adebooye, O.C.; Obuotor, E.M. Planting date and fertilizer affect antioxidants in pumpkin fruit. Entia Hortic. 2014, 168, 46–50. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, J.; Diao, W.; Wang, C. Ultrasound-assisted enzymatic extraction and antioxidant activity of polysaccharides from pumpkin (Cucurbita moschata). Carbohydr. Polym. 2014, 113, 314–324. [Google Scholar] [CrossRef]
- Jiang, Z.; Du, Q. Glucose-lowering activity of novel tetrasaccharide glyceroglycolipids from the fruits of Cucurbita moschata. Bioorganic Med. Chem. Lett. 2011, 21, 1001–1003. [Google Scholar] [CrossRef]
- Park, S.C.; Lee, J.R.; Kim, J.Y.; Hwang, I.; Nah, J.W.; Cheong, H.; Park, Y.; Hahm, K.S. Pr-1, a novel antifungal protein from pumpkin rinds. Biotechnol. Lett. 2010, 32, 125–130. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. Extraction, characterization and antioxidant activities of pumpkin polysaccharide. Int. J. Biol. Macromol. 2018, 118, 770–774. [Google Scholar] [CrossRef]
- Liang, L.; Ao, L.; Ma, T.; Ni, Y.; Liao, X.; Hu, X.; Song, Y. Sulfated modification and anticoagulant activity of pumpkin (Cucurbita pepo, Lady Godiva) polysaccharide. Int. J. Biol. Macromol. 2018, 106, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Maalej, H.; Hmidet, N.; Boisset, C.; Bayma, E.; Nasri, M. Rheological and emulsifying properties of a gel-like exopolysaccharide produced by Pseudomonas stutzeri AS22. Food Hydrocoll. 2016, 52, 634–647. [Google Scholar] [CrossRef]
- Ji, Y.; Liao, A.; Huang, J.; Thakur, K.; Li, X.; Hu, F.; Wei, Z. The rheological properties and emulsifying behavior of polysaccharides sequentially extracted from Amana edulis. Int. J. Biol. Macromol. 2019, 137, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.S.; Mody, K. Microbial Exopolysaccharides: Variety and Potential Applications; Caister Academic Press: Wymondham, UK, 2009. [Google Scholar]
- Chen, X.; Qi, Y.; Zhu, C.; Wang, Q. Effect of ultrasound on the properties and antioxidant activity of hawthorn pectin. Int. J. Biol. Macromol. 2019, 131, 273–281. [Google Scholar] [CrossRef]
- Hu, H.; Li, H.; Han, M.; Cao, Q.; Liang, H.; Yuan, R.; Sun, J.; Zhang, L.; Wu, Y. Chemical modification and antioxidant activity of the polysaccharide from Acanthopanax leucorrhizus. Carbohydr. Res. 2020, 487, 107890. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, F.; Cheng, H.; Huang, G. Modification and application of polysaccharide from traditional Chinese medicine such as Dendrobium officinale. Int. J. Biol. Macromol. 2020, 157, 385–393. [Google Scholar] [CrossRef]
- Kruse, A.; Dinjus, E. Hot compressed water as reaction medium and reactant: 2. Degradation reactions. J. Supercrit. Fluids 2007, 41, 361–379. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Varc-Gaji, J.; Morais, S.; Delerue-Matos, C.; Vieira, E.F.; Spigno, G. Valorization Potential of Oilseed Cakes by Subcritical Water Extraction. Appl. Sci. 2020, 10, 8815. [Google Scholar] [CrossRef]
- Salami, A.; Asefi, N.; Kenari, R.E.; Gharekhani, M. Extraction of pumpkin peel extract using supercritical CO2 and subcritical water technology: Enhancing oxidative stability of canola oil. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Ko, J.-Y.; Ko, M.-O.; Kim, D.-S.; Lim, S.-B. Enhanced Production of Phenolic Compounds from Pumpkin Leaves by Subcritical Water Hydrolysis. Prev. Nutr. Food Sci. 2016, 21, 132–137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salami, A.; Asefi, N.; Kenari, R.E.; Gharekhani, M. Addition of pumpkin peel extract obtained by supercritical fluid and subcritical water as an effective strategy to retard canola oil oxidation. J. Food Meas. Charact. 2020, 14, 2433–2442. [Google Scholar] [CrossRef]
- Wu, X.; Yang, L.; Zhao, T.; Zhu, B.; Zhang, B.; Qu, H.; Mao, G.; Li, F. Optimization of subcritical water extraction of polysaccharides from Grifola frondosa using response surface methodology. Pharmacogn. Mag. 2013, 9, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Ling, L.; Hongyi, S.; Shuang, S.; Zhenya, Z. Optimization of Subcritical Water Extraction of Polysaccharides from Inonotus Obliquus and their Antioxidant Activities. Int. J. Biol. 2017, 9, 38–50. [Google Scholar]
- Zhang, J.; Chen, M.; Wen, C.; Zhou, J.; Gu, J.; Duan, Y.; Zhang, H.; Ren, X.; Ma, H. Structural characterization and immunostimulatory activity of a novel polysaccharide isolated with subcritical water from Sagittaria sagittifolia L. Int. J. Biol. Macromol. 2019, 133, 11–20. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Gu, J.; Ji, C.; Duan, Y.; Zhang, H. Effects of subcritical water extraction microenvironment on the structure and biological activities of polysaccharides from Lentinus edodes. Int. J. Biol. Macromol. 2019, 123, 1002–1011. [Google Scholar] [CrossRef]
- Lu, W.; Chen, X.; Wang, J.; Yang, X.; Qi, J. Enzyme-assisted subcritical water extraction and characterization of soy protein from heat-denatured meal. J. Food Eng. 2016, 169, 250–258. [Google Scholar] [CrossRef]
- Meillisa, A.; Woo, H.C.; Chun, B. Production of monosaccharides and bio-active compounds derived from marine polysaccharides using subcritical water hydrolysis. Food Chem. 2015, 171, 70–77. [Google Scholar] [CrossRef]
- Song, Y.; Li, J.; Hu, X.; Ni, Y.; Li, Q. Structural Characterization of a Polysaccharide Isolated from Lady Godiva Pumpkins (Cucurbita pepo lady godiva). Macromol. Res. 2011, 19, 1172–1178. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Guo, X.; Han, D.; Xi, H.; Rao, L.; Liao, X.; Hu, X.; Wu, J. Extraction of pectin from navel orange peel assisted by ultra-high pressure, microwave or traditional heating: A comparison. Carbohydr. Polym. 2012, 88, 441–448. [Google Scholar] [CrossRef]
- Arias, C.; Yague, A.; Rueda, C.; Blanco, F.G. Intrinsic viscosity calculated out of single point measurements for chondroitin-4-sulfate and chondroitin-6-sulfate solutions. Biophys. Chem. 1998, 72, 307–312. [Google Scholar] [CrossRef]
- Kar, F.; Arslan, N. Effect of temperature and concentration on viscosity of orange peel pectin solutions and intrinsic viscosity-molecular weight relationship. Carbohydr. Polym. 1999, 40, 277–284. [Google Scholar] [CrossRef]
- Khalil, M.; Jan, B.M. Herschel-Bulkley rheological parameters of a novel environmentally friendly lightweight biopolymer drilling fluid from xanthan gum and starch. J. Appl. Polym. Sci. 2012, 124, 595–606. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Li, D.; Xue, J.; Mao, Z.H. Effects of drying methods on rheological properties of flaxseed gum. Carbohydr. Polym. 2009, 78, 213–219. [Google Scholar] [CrossRef]
- Wang, B.; Wang, L.; Li, D.; Adhikari, B.; Shi, J. Effect of gum Arabic on stability of oil-in-water emulsion stabilized by flaxseed and soybean protein. Carbohydr. Polym. 2011, 86, 343–351. [Google Scholar] [CrossRef]
- Wang, B.; Li, D.; Wang, L.; Adhikari, B.; Shi, J. Ability of flaxseed and soybean protein concentrates to stabilize oil-in-water emulsions. J. Food Eng. 2010, 100, 417–426. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, B.; Wang, H.; Qi, G.; Cheng, S. Characterization and antioxidant activity of polysaccharides obtained from ginger pomace using two different extraction processes. Int. J. Biol. Macromol. 2019, 139, 801–809. [Google Scholar] [CrossRef]
- Ren, B.; Chen, C.; Li, C.; Fu, X.; You, L.; Liu, R.H. Optimization of microwave-assisted extraction of Sargassum thunbergii polysaccharides and its antioxidant and hypoglycemic activities. Carbohydr. Polym. 2017, 173, 192–201. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, F.; Liu, R.; Tang, X.; Zhang, Q.; Zhang, Z. Effects of superfine grinding on physicochemical and antioxidant properties of Lycium barbarum polysaccharides. Lwt Food Sci. Technol. 2014, 58, 594–601. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Chen, M.; Gu, J.; Zhou, J.; Duan, Y.; Zhang, H.; Ma, H. Antioxidant activities of Sagittaria sagittifolia L. polysaccharides with subcritical water extraction. Int. J. Biol. Macromol. 2019, 134, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-q.; Huo, W.-k.; Dai, X.; Dang, Y.-h. Preparation of low-molecular-weight citrus pectin by recombinant Bacillus subtilis pectate lyase and promotion of growth of Bifidobacterium longum. Catal. Commun. 2018, 107, 39–42. [Google Scholar] [CrossRef]
- Li, X.; Shang, J.; Yang, X.; Xue, J.; Guo, Y. Rheology of food polysaccharides—thickening, gelling and emulsifying properties. Food Sci. 2021, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Zhang, L. Dynamic viscoelastic behavior of triple helical Lentinan in water: Effect of temperature. Carbohydr. Polym. 2008, 73, 26–34. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Hong, Y.; Jeong, G. Comparison of sulfuric and hydrochloric acids as catalysts in hydrolysis of Kappaphycus alvarezii (cottonii). Bioprocess Biosyst. Eng. 2012, 35, 123–128. [Google Scholar] [CrossRef]
- Omari, K.W.; Besaw, J.E.; Kerton, F.M. Hydrolysis of chitosan to yield levulinic acid and 5-hydroxymethylfurfural in water under microwave irradiation. Green Chem. 2012, 14, 1480–1487. [Google Scholar] [CrossRef]
- Rushing, T.S.; Hester, R.D. Intrinsic Viscosity Dependence on Polymer Molecular Weight and Fluid Temperature. J. Appl. Polym. Sci. 2003, 89, 2831–2835. [Google Scholar] [CrossRef]
- Wang, S.; He, L.; Guo, J.; Zhao, J.; Tang, H. Intrinsic viscosity and rheological properties of natural and substituted guar gums in seawater. Int. J. Biol. Macromol. 2015, 76, 262–268. [Google Scholar] [CrossRef]
- Kontogiorgos, V.; Margelou, I.; Georgiadis, N.; Ritzoulis, C. Rheological characterization of okra pectins. Food Hydrocoll. 2012, 29, 356–362. [Google Scholar] [CrossRef]
- Nep, E.I.; Conway, B.R. Physicochemical characterization of grewia polysaccharide gum: Effect of drying method. Carbohydr. Polym. 2011, 84, 446–453. [Google Scholar] [CrossRef]
- Arzandeh, A.; Razi, M.M.; Naderi, A.; Razi, F.M. Experimental Investigation of Guar Gum Aqueous Solution Rheological Behavior. Eur. J. Sci. Res. 2013, 95, 303–315. [Google Scholar]
- Anvari, M.; Tabarsa, M.; Cao, R.; You, S.; Joyner, H.S.; Behnam, S.; Rezaei, M. Compositional characterization and rheological properties of an anionic gum from Alyssum homolocarpum seeds. Food Hydrocoll. 2016, 52, 766–773. [Google Scholar] [CrossRef]
- Pongsawatmanit, R.; Temsiripong, T.; Ikeda, S.; Nishinari, K. Influence of tamarind seed xyloglucan on rheological properties and thermal stability of tapioca starch. J. Food Eng. 2006, 77, 41–50. [Google Scholar] [CrossRef]
- da Silva, J.A.L.; Goncalves, M.P.; Rao, M.A. Influence of temperature on the dynamic and steady-shear rheology of pectin dispersions. Carbohydr. Polym. 1994, 23, 77–87. [Google Scholar] [CrossRef]
- Shnoudeh, A.J.; Hamad, I.; Abdo, R.W.; Qadumii, L.; Jaber, A.Y.; Surchi, H.S.; Alkelany, S.Z. Synthesis, Characterization, and Applications of Metal Nanoparticles. In Biomaterials and Bionanotechnology; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; Chapter 15; pp. 527–612. [Google Scholar] [CrossRef]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazoyer, J. Emulsion stabilizing properties of pectin. Food Hydrocoll. 2003, 17, 455–462. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Tan, C.P.; Hamid, N.; Yusof, S.; Chern, B.H. Characterization of the influence of main emulsion components on the physicochemical properties of orange beverage emulsion using response surface methodology. Food Hydrocoll. 2009, 23, 271–280. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. The antioxidant activity of derivatized cushaw polysaccharides. Int. J. Biol. Macromol. 2019, 128, 1–4. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Qin, W.; Qin, P.; Zhang, H.; Duan, Y. Ultrasonic-enhanced subcritical water extraction of polysaccharides by two steps and its characterization from Lentinus edodes. Int. J. Biol. Macromol. 2018, 118, 2269–2277. [Google Scholar] [CrossRef]
- Getachew, A.T.; Cho, Y.J.; Chun, B. Effect of pretreatments on isolation of bioactive polysaccharides from spent coffee grounds using subcritical water. Int. J. Biol. Macromol. 2018, 109, 711–719. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. Antioxidant activities of sulfated pumpkin polysaccharides. Int. J. Biol. Macromol. 2019, 126, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, G.; Hu, J. Preparation, deproteinization, characterisation, and antioxidant activity of polysaccharide from cucumber (Cucumis saticus L.). Int. J. Biol. Macromol. 2018, 108, 408–411. [Google Scholar] [CrossRef] [PubMed]

| Sample | τ0(Pa) | K (Pa·sn) | n | R2 |
|---|---|---|---|---|
| PCP | 0.440 ± 0.039 a | 1.309 ± 0.032 a | 0.206 ± 0.005 a | 1.000 |
| SW120 | 0.153 ± 0.003 b | 0.047 ± 0.001 b | 0.416 ± 0.006 b | 1.000 |
| SW150 | 0.121 ± 0.014 c | 0.023 ± 0.012 c | 0.480 ± 0.025 c | 0.991 |
| SW180 | 0.050 ± 0.003 d | 0.013 ± 0.001 d | 0.758 ± 0.023 d | 0.995 |
| SW210 | 0.015 ± 0.002 e | 0.003 ± 0.001 e | 0.964 ± 0.033 e | 0.993 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.; Zhao, J.; Wei, Y.; Huang, L.; Li, F.; Zhang, Y.; Li, Q. Physicochemical Properties and Antioxidant Activity of Pumpkin Polysaccharide (Cucurbita moschata Duchesne ex Poiret) Modified by Subcritical Water. Foods 2021, 10, 197. https://doi.org/10.3390/foods10010197
Yu G, Zhao J, Wei Y, Huang L, Li F, Zhang Y, Li Q. Physicochemical Properties and Antioxidant Activity of Pumpkin Polysaccharide (Cucurbita moschata Duchesne ex Poiret) Modified by Subcritical Water. Foods. 2021; 10(1):197. https://doi.org/10.3390/foods10010197
Chicago/Turabian StyleYu, Guoyong, Jing Zhao, Yunlu Wei, Linlin Huang, Fei Li, Yu Zhang, and Quanhong Li. 2021. "Physicochemical Properties and Antioxidant Activity of Pumpkin Polysaccharide (Cucurbita moschata Duchesne ex Poiret) Modified by Subcritical Water" Foods 10, no. 1: 197. https://doi.org/10.3390/foods10010197
APA StyleYu, G., Zhao, J., Wei, Y., Huang, L., Li, F., Zhang, Y., & Li, Q. (2021). Physicochemical Properties and Antioxidant Activity of Pumpkin Polysaccharide (Cucurbita moschata Duchesne ex Poiret) Modified by Subcritical Water. Foods, 10(1), 197. https://doi.org/10.3390/foods10010197