Determination of Post-Harvest Biochemical Composition, Enzymatic Activities, and Oxidative Browning in 14 Apple Cultivars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apple Cultivars and Sampling
2.2. Extraction and Enzymatic Activity of POD and PPO
2.3. Extraction of Apple Phenolics and Quantification by High-Performance Liquid Chromatography (HPLC)
2.4. Statistical Analyses
3. Results and Discussion
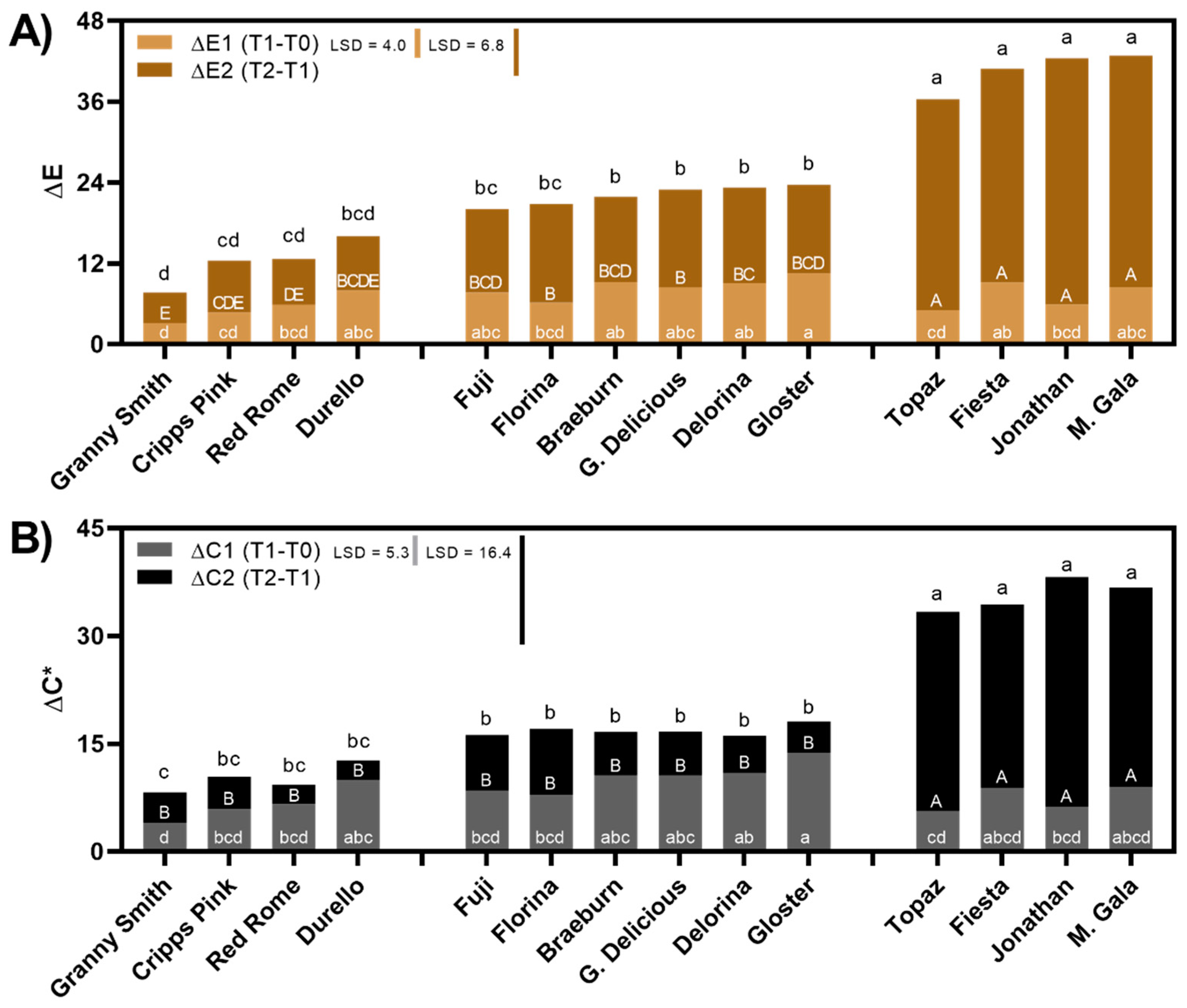
3.1. Apple Flesh Color Changes with Respect to Time Post-Slicing and Cultivar
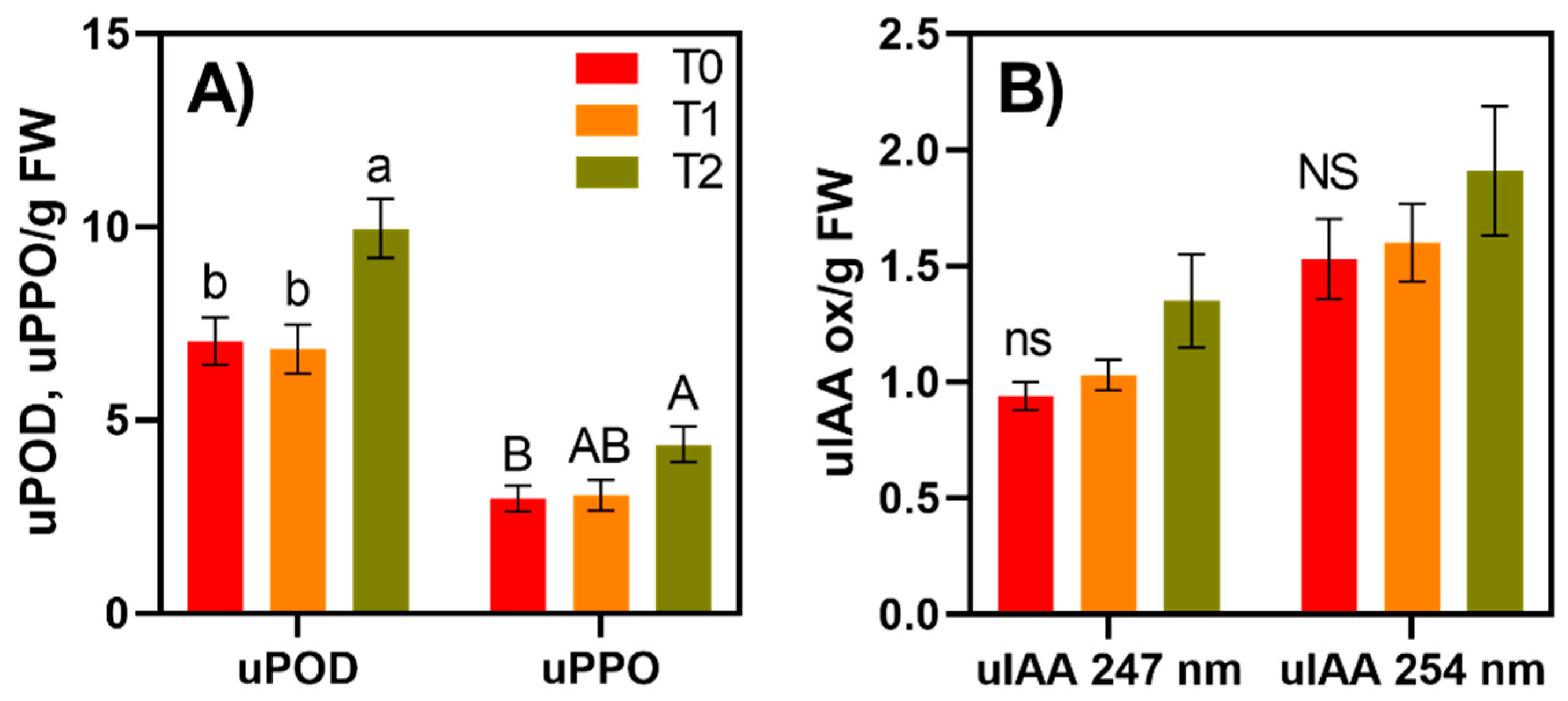
3.2. Enzyme Activity Varies across Time and Cultivar
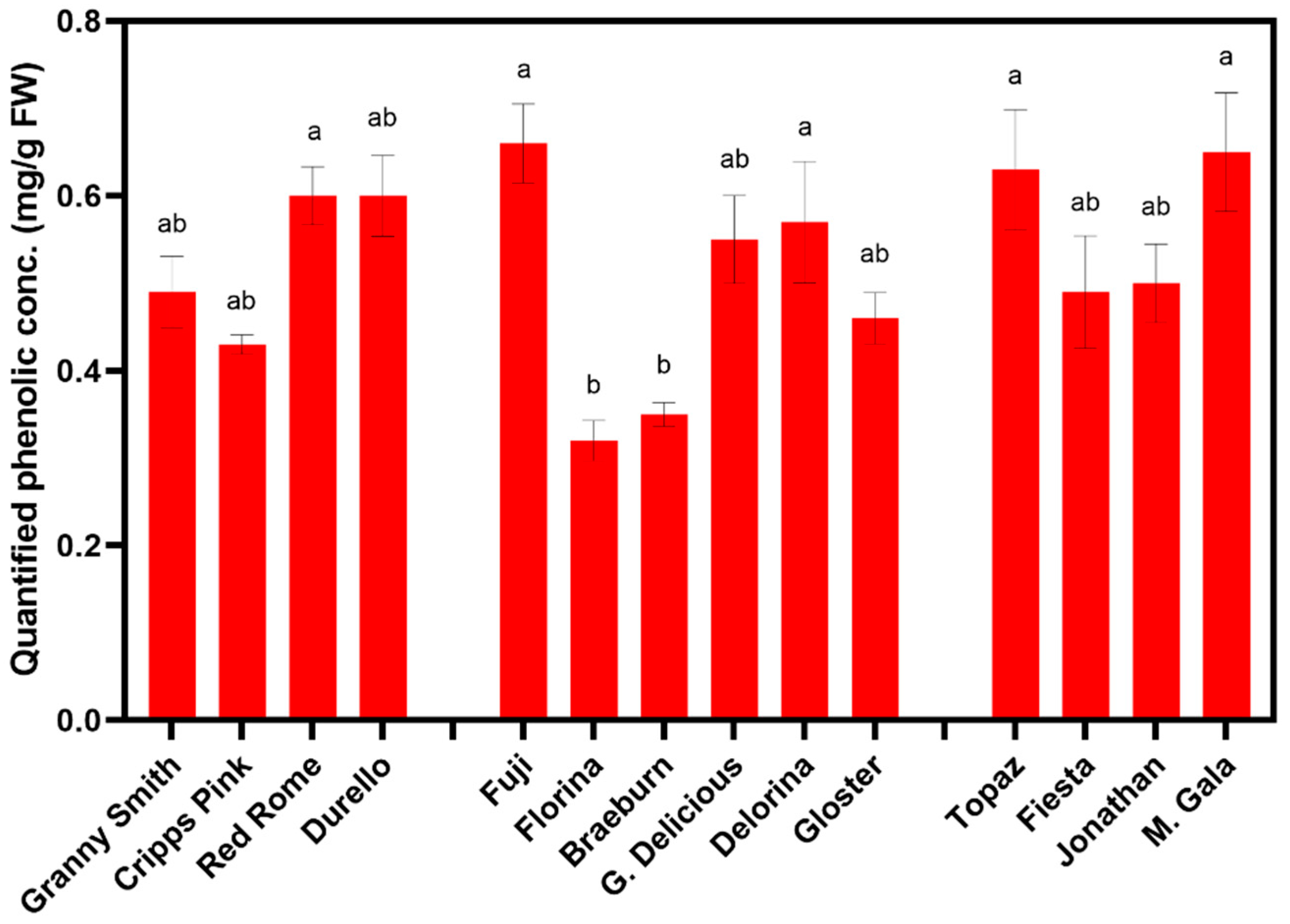
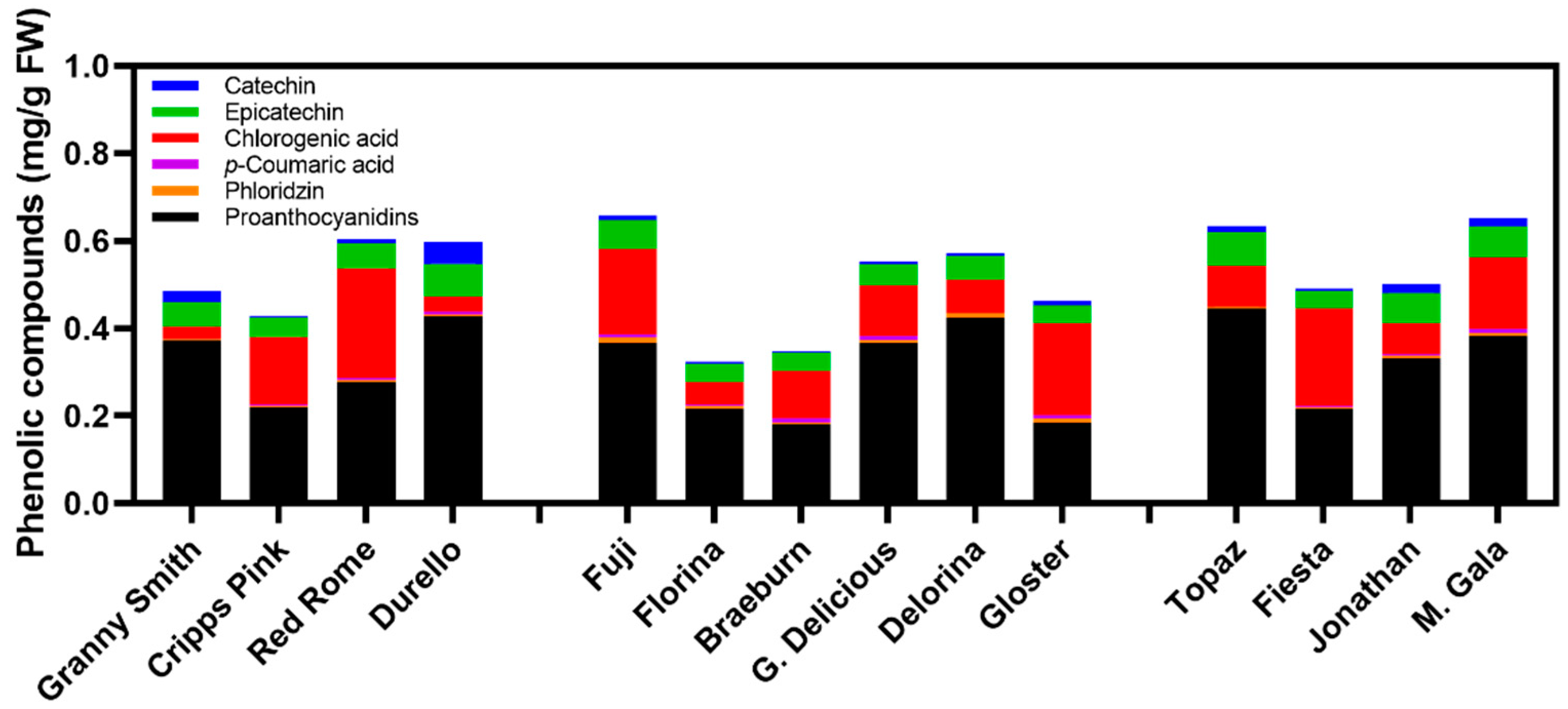
3.3. Phenolic Compound Concentration Influenced by Time and Apple Cultivar
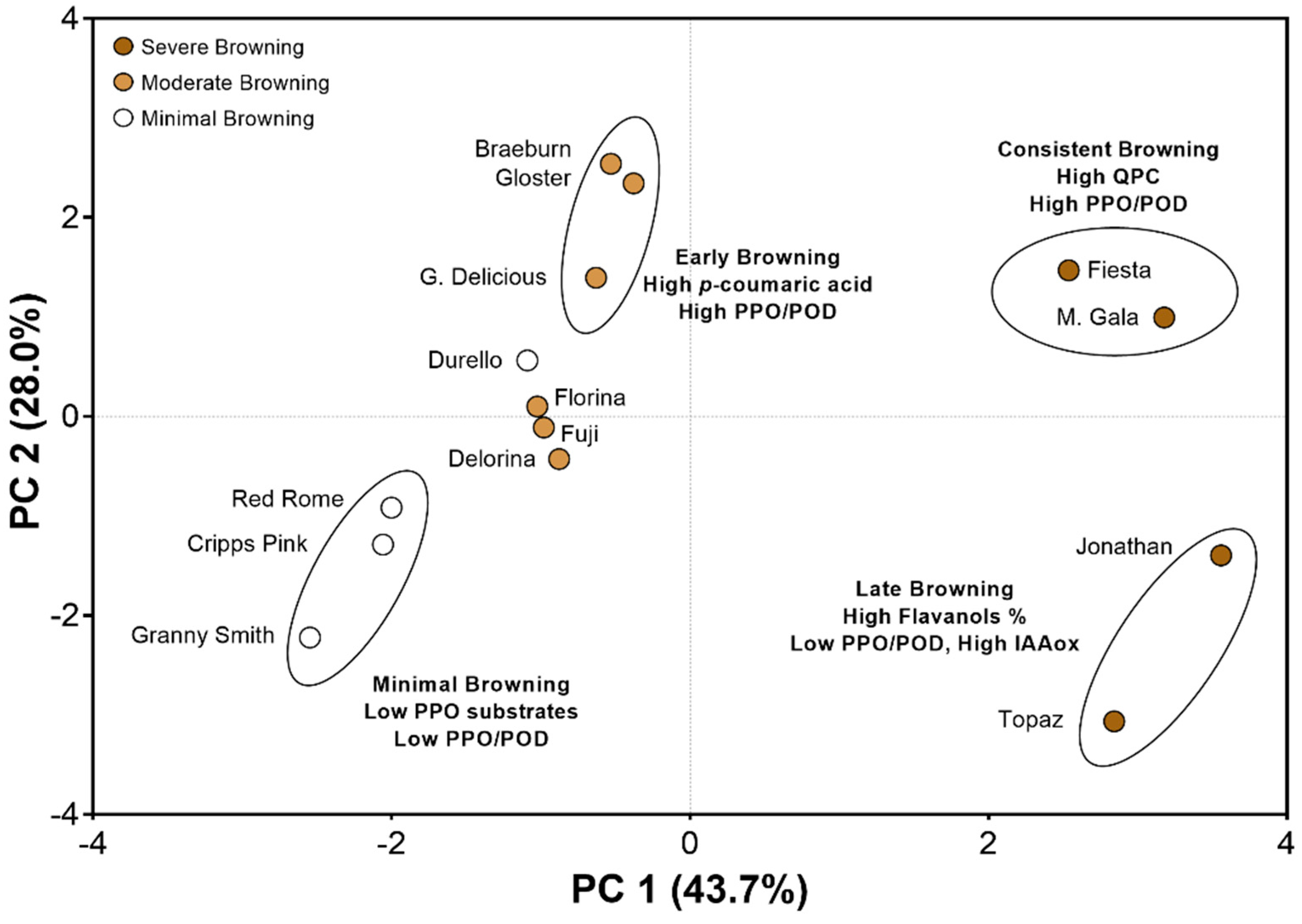
3.4. Principal Component Analysis Reveals Cultivar “Clusters” Based on Biochemical Characteristics and Browning Behaviors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robards, K. Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J. Chromatogr. A 2003, 1000, 657–691. [Google Scholar] [CrossRef]
- Devic, E.; Guyot, S.; Daudin, J.-D.; Bonazzi, C. Kinetics of Polyphenol Losses During Soaking and Drying of Cider Apples. Food Bioprocess Technol. 2010, 3, 867–877. [Google Scholar] [CrossRef]
- Serra, S.; Anthony, B.; Masia, A.; Giovannini, D.; Musacchi, S. Determination of Biochemical Composition in Peach (Prunus persica L. Batsch) Accessions Characterized by Different Flesh Color and Textural Typologies. Foods 2020, 9, 1452. [Google Scholar] [CrossRef]
- Holderbaum, D.F.; Kon, T.; Kudo, T.; Guerra, M.P. Enzymatic Browning, Polyphenol Oxidase Activity, and Polyphenols in Four Apple Cultivars: Dynamics during Fruit Development. HortScience 2010, 45, 1150–1154. [Google Scholar] [CrossRef] [Green Version]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J. Antioxidant Activity Modulated by Polyphenol Contents in Apple and Leaves during Fruit Development and Ripening. Antioxidants 2020, 9, 567. [Google Scholar] [CrossRef]
- Lau, F.C.; Shukitt-Hale, B.; Joseph, J.A. The beneficial effects of fruit polyphenols on brain aging. Neurobiol. Aging 2005, 26, 128–132. [Google Scholar] [CrossRef]
- Hertog, M.G.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in the Netherlands. Nutr. Cancer 1993, 20, 21–29. [Google Scholar] [CrossRef]
- Guthrie, N.; Kurowska, E.M.N. Anticancer and cholesterol-lowering activities of citrus flavonoids. In Handbook of Nutraceuticals and Functional Foods; Wildman, R.E., Ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 113–126. [Google Scholar]
- Giovannini, D.; Liverani, A.; Merli, M.; Brandi, F. Breeding strategies to improve peach fruit quality. Acta Hortic. 2006, 713, 107–112. [Google Scholar] [CrossRef]
- Nunes, C.; Guyot, S.; Marnet, N.; Barros, A.S.; Saraiva, J.A.; Renard, C.M.; Coimbra, M.A. Characterization of plum procyanidins by thiolytic depolymerization. J. Agric. Food Chem. 2008, 56, 5188–5196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, T.; Xie, X.; Ren, X.; Wang, W.; Tang, X.; Zhang, J.; Wang, Z. A difference of enzymatic browning unrelated to PPO from physiology, targeted metabolomics and gene expression analysis in Fuji apples. Post. Biol. Tech. 2020, 170, 111323. [Google Scholar] [CrossRef]
- Deutch, C.E. Browning in apples: Exploring the biochemical basis of an easily-observable phenotype. Biochem. Mol. Biol. Ed. 2018, 46, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.V.; Whitaker, J.R. The biochemistry and control of enzymatic browning. Trends Food Sci. Tech. 1995, 6, 195–200. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Sobrino-López, A.; Soledad Tapia, M.; Martín-Belloso, O. Browning inhibition in fresh-cut ‘Fuji’ apple slices by natural antibrowning agents. J. Food Sci. 2006, 71, S59–S65. [Google Scholar] [CrossRef]
- Vaughn, K.C.; Lax, A.R.; Duke, S.O. Polyphenol oxidase. the chloroplast oxidase with no established function. Phys. Plant. 1988, 72, 659–665. [Google Scholar] [CrossRef]
- Glagoleva, A.Y.; Shoeva, O.Y.; Khlestkina, E.K. Melanin pigment in plants: Current knowledge and future perspectives. Frontiers Plant Sci. 2020, 11, 770. [Google Scholar] [CrossRef]
- Di Guardo, M.; Tadiello, A.; Farneti, B.; Lorenz, G.; Masuero, D.; Vrhovsek, U.; Costa, G.; Velasco, R.; Costa, F. A multidisciplinary approach providing new insight into fruit flesh browning physiology in apple (Malus × domestica Borkh.). PLoS ONE 2013, 8, e78004. [Google Scholar] [CrossRef]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D.; et al. The genome of the domesticated apple (Malus × domestica Borkh.). Nat. Genet. 2010, 42, 833–839. [Google Scholar] [CrossRef]
- Xu, K. The next generation biotechnology for apple improvement and beyond: The CRISP/cas9 story. N. Y. Fruit Q. 2013, 21, 19–22. [Google Scholar]
- Ma, Y.; Lu, X.; Nock, J.F.; Watkins, C.B. Peroxidase and polyphenoloxidase activities in relation to flesh browning of stem-end and calyx-end tissues of ‘Empire’ apples during controlled atmosphere storage. Post. Biol. Tech. 2015, 108, 1–7. [Google Scholar] [CrossRef]
- Whitaker, J.R. Mechanisms of oxidoreductases important in food component modification. In Chemical Changes in Food During Processing; Springer: Boston, MA, USA, 1985; pp. 121–176. [Google Scholar]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Yokotsuka, K.; Singleton, V.L. Glycoproteins: Characterization in a hybrid grape variety (Muscat Bailey A) juice, fermenting must, and resultant red wine. Am. J. Enol. Vitic. 1997, 48, 100–114. [Google Scholar]
- Valentines, M.C.; Vilaplana, R.; Torres, R.; Usall, J.; Larrigaudiere, C. Specific roles of enzymatic browning and lignification in apple disease resistance. Post. Biol. Tech. 2005, 36, 227–234. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Takahama, U. Changes in peroxidase activity and in peroxidase isoenzymes in carrot callus. Physiol. Planta 1993, 88, 167–171. [Google Scholar] [CrossRef]
- Biles, C.L.; Martyn, R.D. Peroxidase, polphenoloxidase, and shikimate dehydrogenase isozymes in relation to tissue type, maturity and pathogen induction of watermelon seedlings. Plant Physiol. Biochem. 1993, 31, 499–506. [Google Scholar]
- Chinnici, F.; Gaiani, A.; Natali, N.; Riponi, C.; Galassi, S. Improved HPLC determination of phenolic compounds in cv. Golden Delicious apples using a monolithic column. J. Agric. Food Chem. 2004, 52, 3–7. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of polyphenols in different apple varieties. J. Agric. Food Chem. 2004, 52, 6532–6538. [Google Scholar] [CrossRef]
- Kindt, M.; Orsini, M.C.; Costantini, B. Improved high-performance liquid chromatography-diode array detection method for the determination of phenolic compounds in leaves and peels from different apple varieties. J. Chrom. Sci. 2007, 45, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Podsędek, A.; Wilska-Jeszka, J.; Anders, B.; Markowski, J. Compositional characterisation of some apple varieties. Eur. Food Res. Tech. 2000, 210, 268–272. [Google Scholar] [CrossRef]
- Mayr, U.; Batzdorfer, R.; Treutter, D.; Feucht, W. Surfactant-induced changes in phenol content of apple leaves and fruit skins. Acta Hortic. 1994, 381, 479–487. [Google Scholar] [CrossRef]
- Wang, S.Y.; Jiao, H.J.; Faust, M. Changes in the activities of catalase, peroxidase, and polyphenol oxidase in apple buds during bud break induced by thidiazuron. J. Plant Growth Reg. 1991, 10, 33. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Le Quéré, J.M.; Sanoner, P.; Drilleau, J.F.; Guyot, S. Inhibition of apple polyphenol oxidase activity by procyanidins and polyphenol oxidation products. J. Agric. Food Chem. 2004, 52, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Delicado, E.; Serrano-Megías, M.; Pérez-López, A.J.; López-Nicolás, J.M. Polyphenol oxidase from Dominga table grape. J. Agric. Food Chem. 2005, 53, 6087–6093. [Google Scholar] [CrossRef]
- Alcobendas, R.; Mirás-Avalos, J.M.; Alarcón, J.J.; Pedrero, F.; Nicolás, E. Combined effects of irrigation, crop load and fruit position on size, color and firmness of fruits in an extra-early cultivar of peach. Sci. Hortic. 2012, 142, 128–135. [Google Scholar] [CrossRef]
- Kim, J.Y.; Seo, Y.S.; Kim, J.E.; Sung, S.K.; Song, K.J.; An, G.; Kim, W.T. Two polyphenol oxidases are differentially expressed during vegetative and reproductive development and in response to wounding in the Fuji apple. Plant Sci. 2001, 161, 1145–1152. [Google Scholar] [CrossRef]
- Careri, M.; Elviri, L.; Mangia, A.; Musci, M. Spectrophotometric and coulometric detection in the high performance liquid chromatography of flavonoids and optimization of sample treatment for the determination of quercetin in orange juice. J. Chromatogr. A 2000, 881, 449–460. [Google Scholar] [CrossRef]
- Mattivi, F.; Tonon, D.; Sanchez, C. Gli antiossidanti polifenolici naturali. Laboratorio 2000, 2002, 46–56. [Google Scholar]
- Masia, A.; Ventura, M.; Gemma, H.; Sansavini, S. Effect of some plant growth regulator treatments on apple fruit ripening. Plant Growth Reg. 1998, 25, 127–134. [Google Scholar] [CrossRef]
- Ushimaru, T.; Maki, Y.; Sano, S.; Koshiba, K.; Asada, K.; Tsuji, H. Induction of enzymes involved in the ascorbate-dependent antioxidative system, namely, ascorbate peroxidase, monodehydroascorbate reductase and dehydroascorbate reductase, after exposure to air of rice (Oryza sativa) seedlings germinated under water. Plant Cell Phys. 1997, 38, 541–549. [Google Scholar] [CrossRef] [Green Version]
- Cañal, M.J.; Tamés, R.S.; Fernández, B. Peroxidase and polyphenol oxidase activities in Cyperus esculentus leaves following glyphosate applications. Physiol. Plant. 1988, 74, 125–130. [Google Scholar] [CrossRef]
- Persic, M.; Mikulic-Petkovsek, M.; Slatnar, A.; Veberic, R. Chemical composition of apple fruit, juice and pomace and the correlation between phenolic content, enzymatic activity and browning. LWT Food Sci. Tech. 2017, 82, 23–31. [Google Scholar] [CrossRef]
- Łata, B.; Trampczynska, A.; Paczesna, J. Cultivar variation in apple peel and whole fruit phenolic composition. Sci. Hortic. 2009, 121, 176–181. [Google Scholar] [CrossRef]
- Kołodziejczyk, K.; Milala, J.; Sójka, M.; Kosmala, M.; Markowski, J. Polyphenol oxidase activity in selected apple cultivars. J. Fruit Ornam. Plant Res. 2010, 18, 51–61. [Google Scholar]
- Liu, F.; Han, Q.; Ni, Y. Comparison of biochemical properties and thermal inactivation of membrane-bound polyphenol oxidase from three apple cultivars (Malus domestica Borkh). Int. J. Food Sci. Tech. 2018, 53, 1005–1012. [Google Scholar] [CrossRef]
- Yu, T.; Chen, J.; Lu, H.; Zheng, X. Indole-3-acetic acid improves post-harvest biological control of blue mold rot of apple by Cryptococcus laurentii. Phytopathology 2009, 99, 258–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiot, M.J.; Tacchini, M.; Aubert, S.; Nicolas, J. Phenolic composition and browning susceptibility of various apple cultivars at maturity. J. Food Sci. 1992, 57, 958–962. [Google Scholar] [CrossRef]
- Szalay, L.; Hegedűs, A.; Stefanovits-Bányai, É. Presumable protective role of peroxidase and polyphenol oxidase enzymes against freezing stress in peach (Prunus persica L. Batsch). Acta Biol. Szeged. 2005, 49, 121–122. [Google Scholar]
- Markowski, J.; Płocharski, W. Determination of phenolic compounds in apples and processed apple products. J. Fruit Ornam. Plant Res. 2006, 14, 2. [Google Scholar]
- Tsao, R.; Yang, R.; Young, J.C.; Zhu, H. Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC). J. Agric. Food Chem. 2003, 51, 6347–6353. [Google Scholar] [CrossRef]
- Vanzani, P.; Rossetto, M.; Rigo, A.; Vrhovsek, U.; Mattivi, F.; D’Amato, E.; Scarpa, M. Major phytochemicals in apple cultivars: Contribution to peroxyl radical trapping efficiency. J. Agric. Food Chem. 2005, 53, 3377–3382. [Google Scholar] [CrossRef] [PubMed]
- Chinnici, F.; Bendini, A.; Gaiani, A.; Riponi, C. Radical scavenging activities of peels and pulps from cv. Golden Delicious apples as related to their phenolic composition. J. Agric. Food Chem. 2004, 52, 4684–4689. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, V.A.; Glories, Y.; Laguerre, M. Incidence of molecular structure in oxidation of grape seed procyanidins. J. Agric. Food Chem. 1998, 46, 376–382. [Google Scholar] [CrossRef] [PubMed]

| Time | Catechin (mg/g FW) | Epicatechin (mg/g FW) | Chlorogenic Acid (mg/g FW) | p-Coumaric Acid (mg/g FW) | Phloridzin (mg/g FW) | Proanthocyanidins (mg/g FW) | Quant. Phenolic Conc. (mg/g FW) |
|---|---|---|---|---|---|---|---|
| T0 | 0.014 | 0.05 b | 0.11 | 0.005 | 0.005 | 0.30 | 0.49 b |
| T1 | 0.010 | 0.05 b | 0.12 | 0.005 | 0.006 | 0.28 | 0.47 b |
| T2 | 0.016 | 0.07 a | 0.15 | 0.005 | 0.007 | 0.36 | 0.60 a |
| LSD | nd | 0.02 | nd | nd | nd | nd | 0.11 |
| Cultivar | Catechin (mg/g FW) | Epicatechin (mg/g FW) | Chlorogenic Acid (mg/g FW) | p-Coumaric Acid (mg/g FW) | Phloridzin (mg/g FW) | Proanthocyanidis (mg/g FW) | Quant. phenolic Conc. (mg/g FW) |
|---|---|---|---|---|---|---|---|
| ‘Granny Smith’ | 0.026 b | 0.06 ab | 0.03 f | 0.002 c | 0.004 cd | 0.37 abc | 0.49 abc |
| ‘Cripps Pink’ | 0.003 d | 0.04 b | 0.16 bcd | 0.003 c | 0.004 d | 0.22 bc | 0.43 abc |
| ‘Red Rome’ | 0.010 cd | 0.06 ab | 0.25 a | 0.005 bc | 0.005 bcd | 0.28 abc | 0.60 a |
| ‘Durello’ | 0.049 a | 0.08 a | 0.03 ef | 0.006 ab | 0.006 abcd | 0.43 a | 0.60 ab |
| ‘Fuji’ | 0.010 cd | 0.07 ab | 0.20 abc | 0.006 abc | 0.014 a | 0.37 abc | 0.66 a |
| ‘Florina’ | 0.005 cd | 0.04 b | 0.05 ef | 0.001 d | 0.006 abc | 0.22 bc | 0.32 c |
| ‘Braeburn’ | 0.003 d | 0.04 b | 0.11 def | 0.011 a | 0.005 abcd | 0.18 c | 0.35 bc |
| ‘G. Delicious’ | 0.004 d | 0.05 ab | 0.12 cde | 0.008 a | 0.008 ab | 0.37 abc | 0.55 abc |
| ‘Delorina’ | 0.007 cd | 0.05 ab | 0.08 def | - | 0.011 a | 0.42 a | 0.57 abc |
| ‘Gloster’ | 0.011 bcd | 0.04 b | 0.21 ab | 0.008 ab | 0.009 ab | 0.18 c | 0.46 abc |
| ‘Topaz’ | 0.013 bcd | 0.08 a | 0.09 def | - | 0.003 d | 0.45 a | 0.63 a |
| ‘Fiesta’ | 0.005 d | 0.04 b | 0.22 ab | 0.003 c | 0.003 bcd | 0.22 bc | 0.49 abc |
| ‘Jonathan’ | 0.019 bc | 0.07 ab | 0.07 ef | 0.005 bc | 0.005 abcd | 0.33 abc | 0.50 abc |
| ‘M. Gala’ | 0.020 bcd | 0.07 ab | 0.16 bcd | 0.010 bc | 0.006 abcd | 0.38 ab | 0.65 a |
| LSD | 0.015 | 0.03 | 0.09 | 0.005 | 0.007 | 0.20 | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, S.; Anthony, B.; Boscolo Sesillo, F.; Masia, A.; Musacchi, S. Determination of Post-Harvest Biochemical Composition, Enzymatic Activities, and Oxidative Browning in 14 Apple Cultivars. Foods 2021, 10, 186. https://doi.org/10.3390/foods10010186
Serra S, Anthony B, Boscolo Sesillo F, Masia A, Musacchi S. Determination of Post-Harvest Biochemical Composition, Enzymatic Activities, and Oxidative Browning in 14 Apple Cultivars. Foods. 2021; 10(1):186. https://doi.org/10.3390/foods10010186
Chicago/Turabian StyleSerra, Sara, Brendon Anthony, Francesca Boscolo Sesillo, Andrea Masia, and Stefano Musacchi. 2021. "Determination of Post-Harvest Biochemical Composition, Enzymatic Activities, and Oxidative Browning in 14 Apple Cultivars" Foods 10, no. 1: 186. https://doi.org/10.3390/foods10010186
APA StyleSerra, S., Anthony, B., Boscolo Sesillo, F., Masia, A., & Musacchi, S. (2021). Determination of Post-Harvest Biochemical Composition, Enzymatic Activities, and Oxidative Browning in 14 Apple Cultivars. Foods, 10(1), 186. https://doi.org/10.3390/foods10010186







