The Comprehensive Study of TiO2 Blocking Layer with Complementary Electrochemical and SPM Methods for the Application in Photovoltaics
Abstract
1. Introduction
2. Results and Discussion
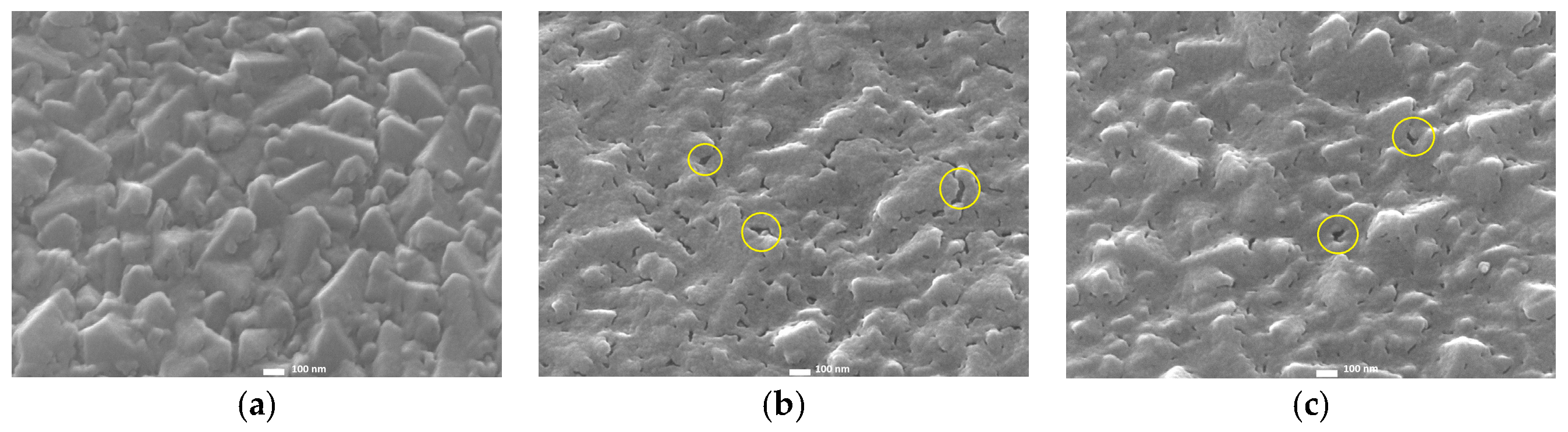
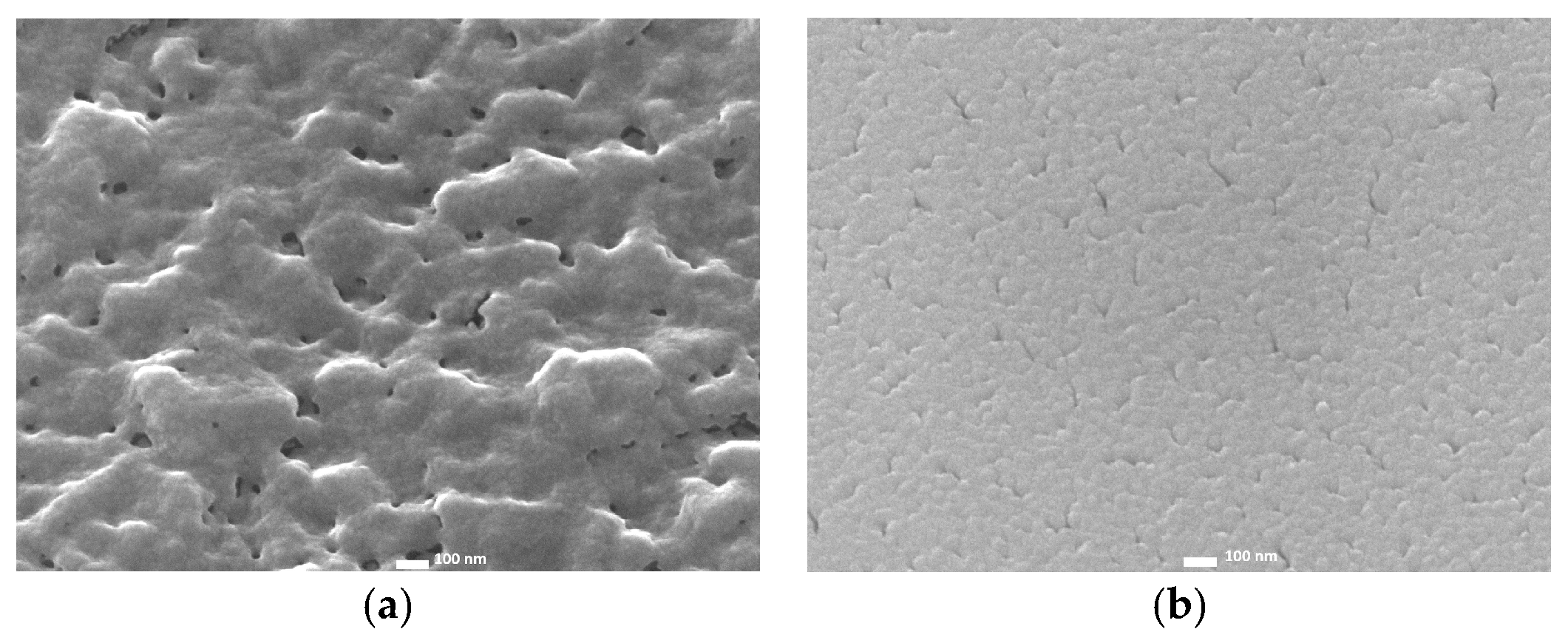
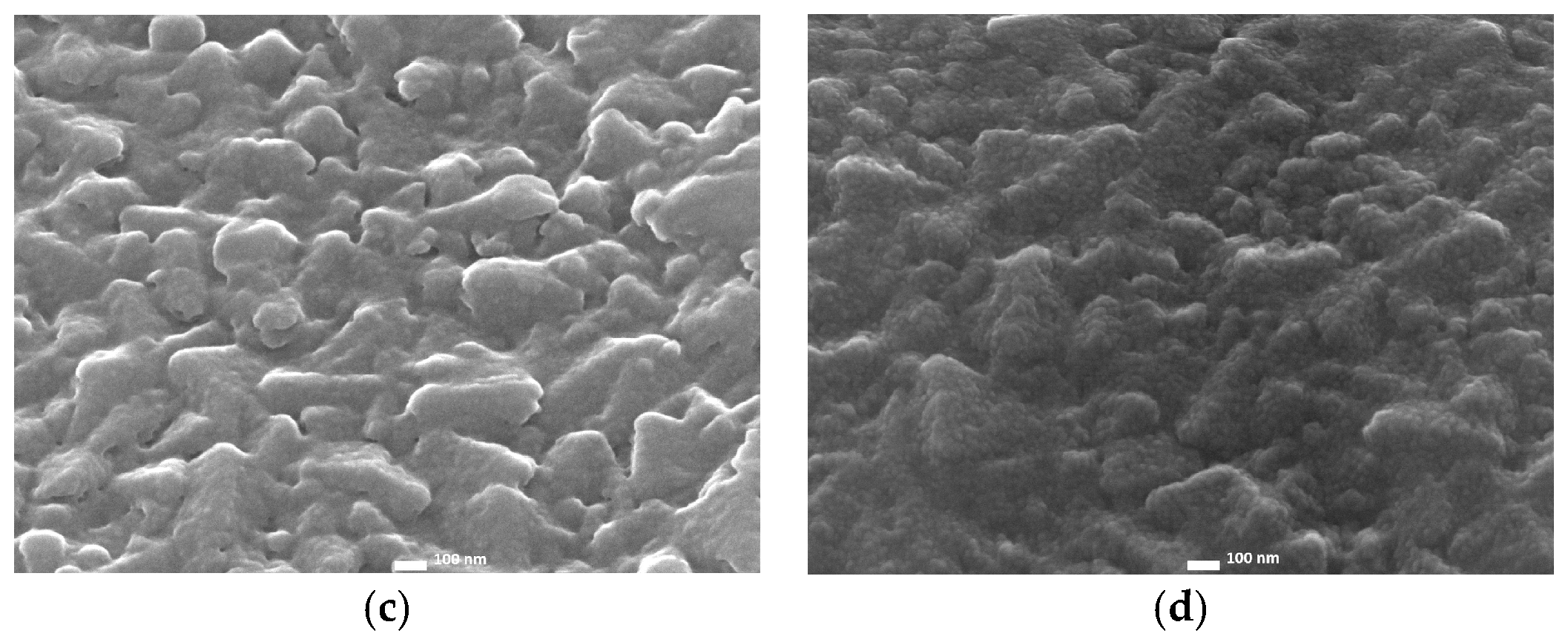
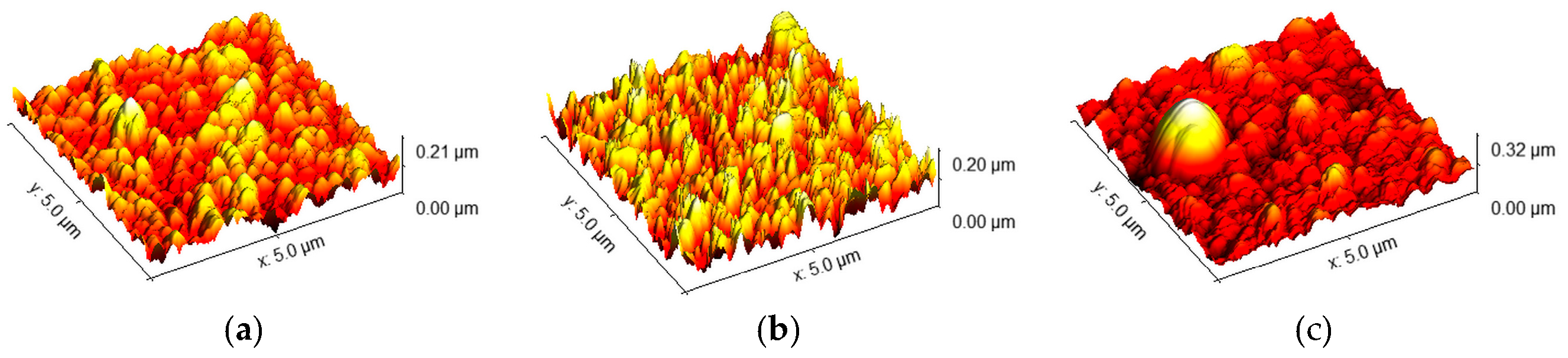
2.1. Radio-Frequency Sputtering of TiO2 Layers—SP Method
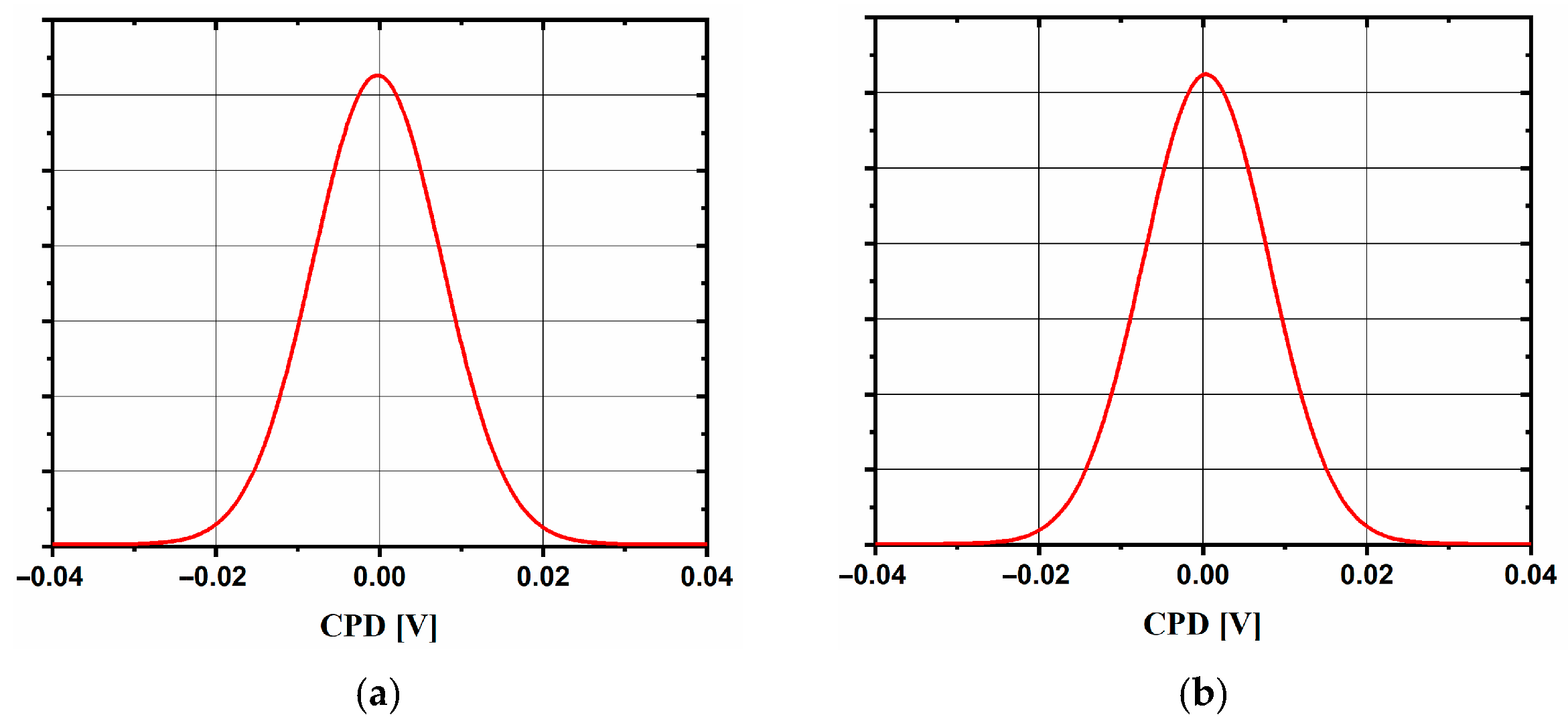
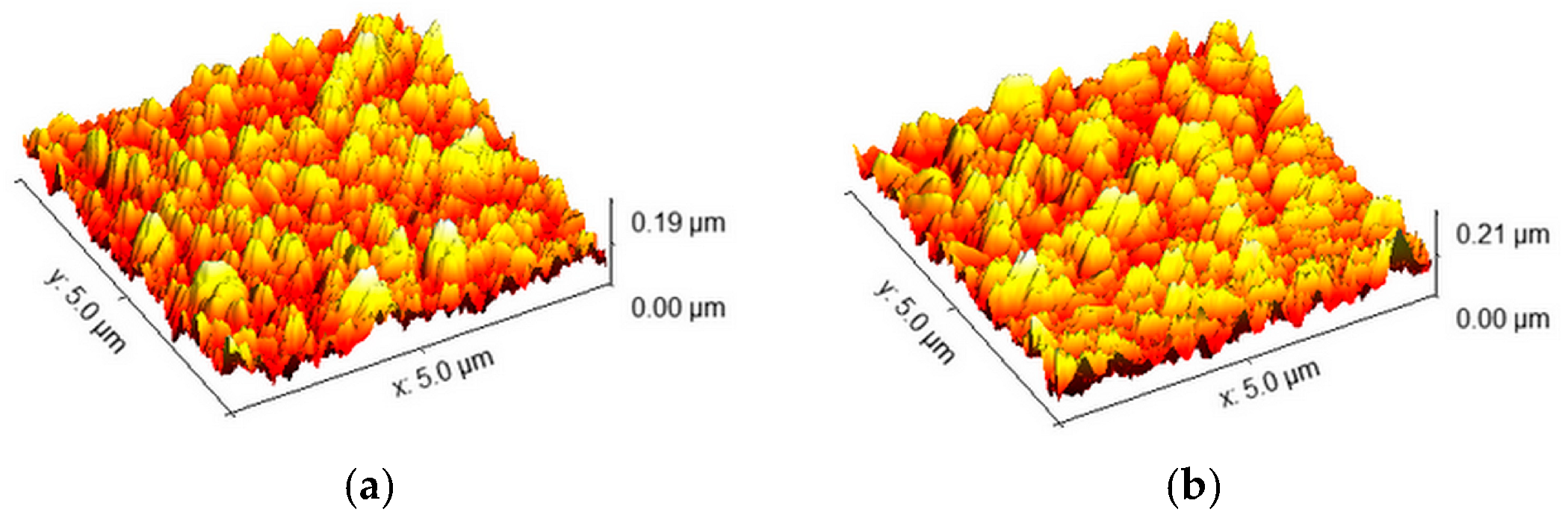
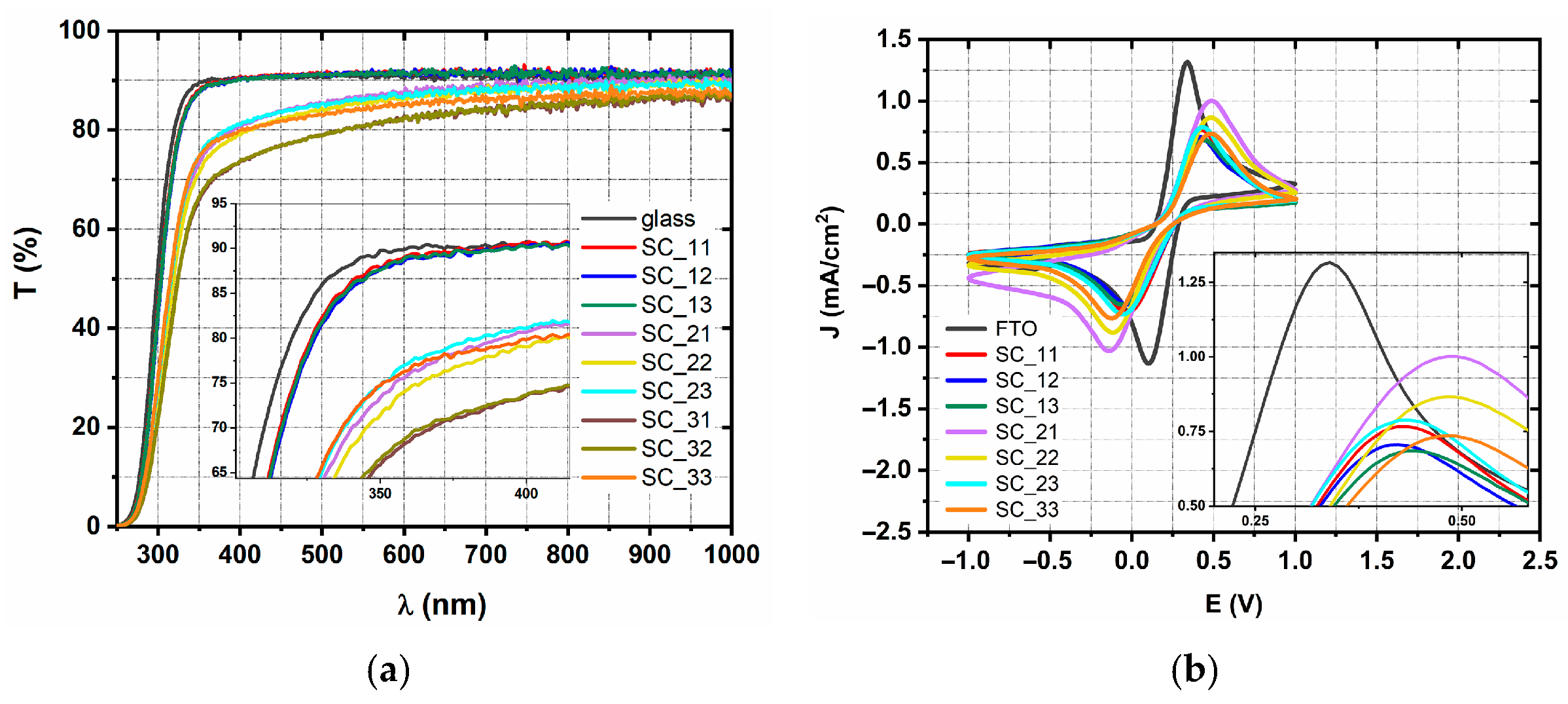
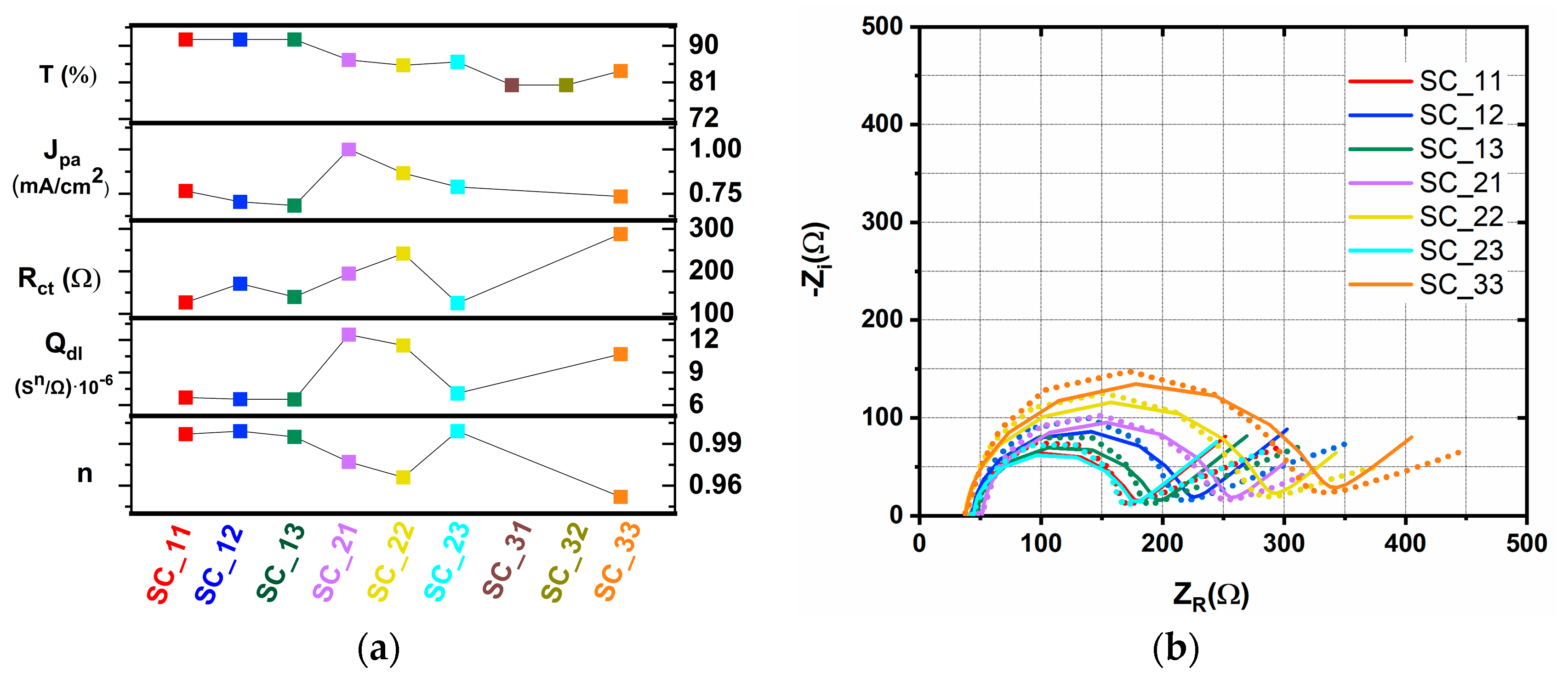
2.2. Spin-Coated TiO2 Layers—SC Method
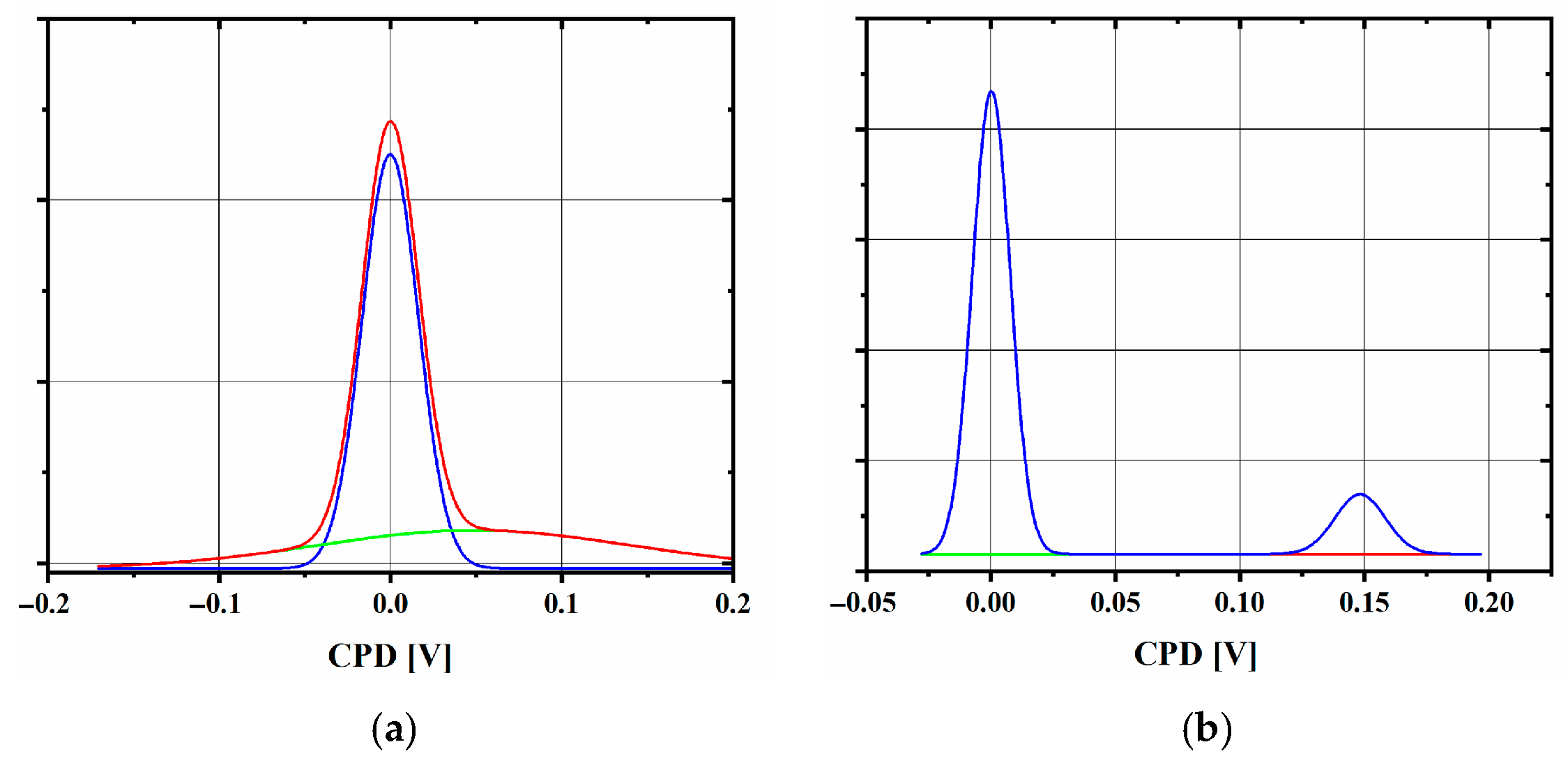
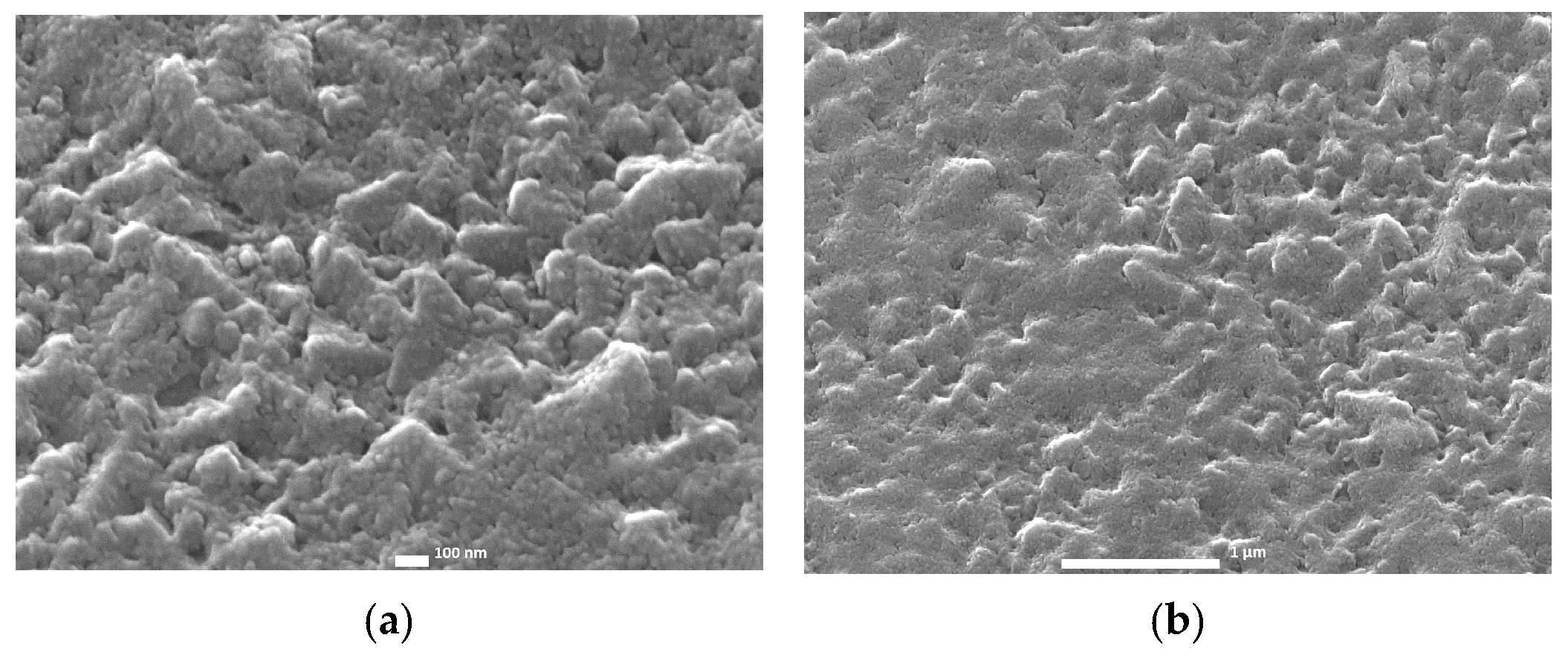
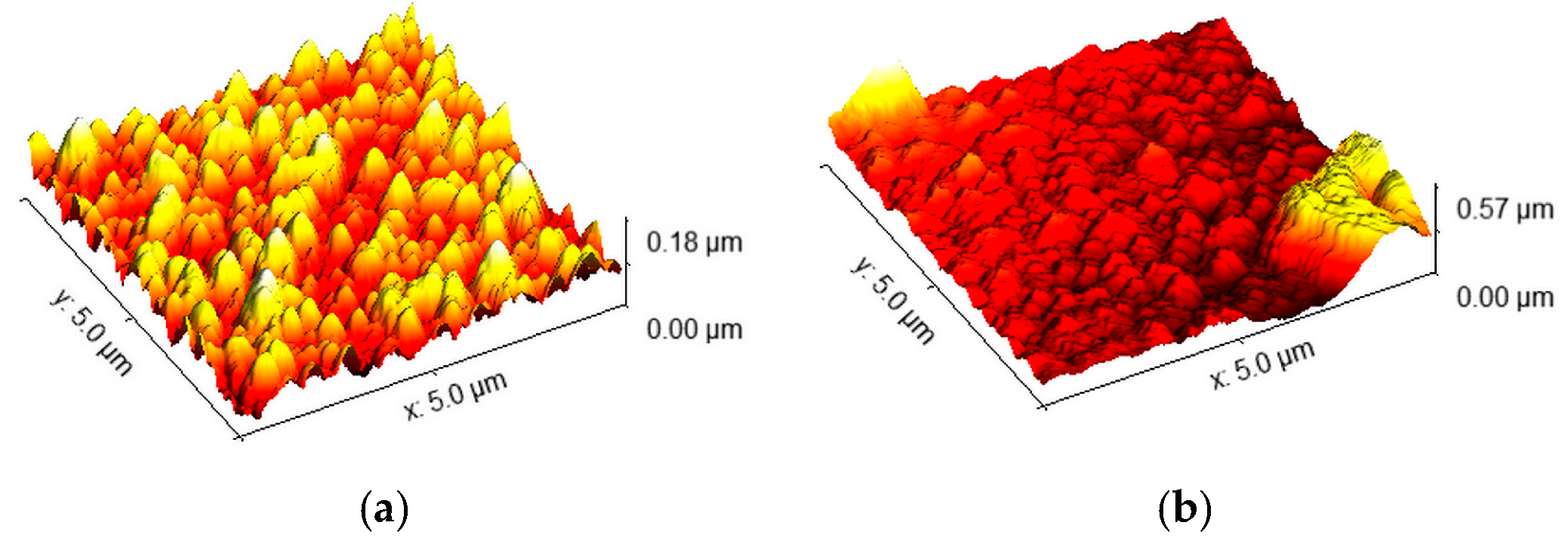
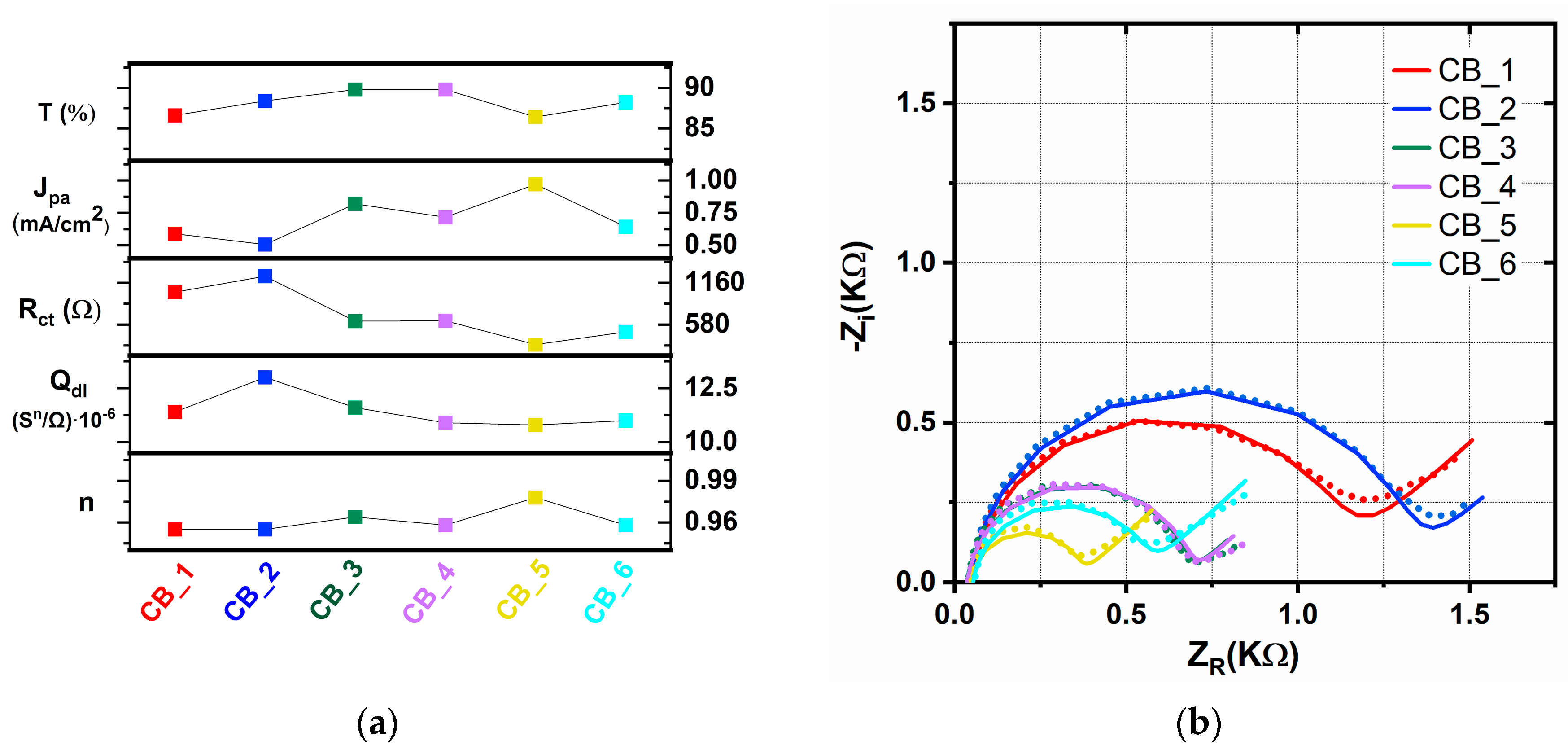
2.3. Chemical Bath-Deposited TiO2 Layers—CB Method
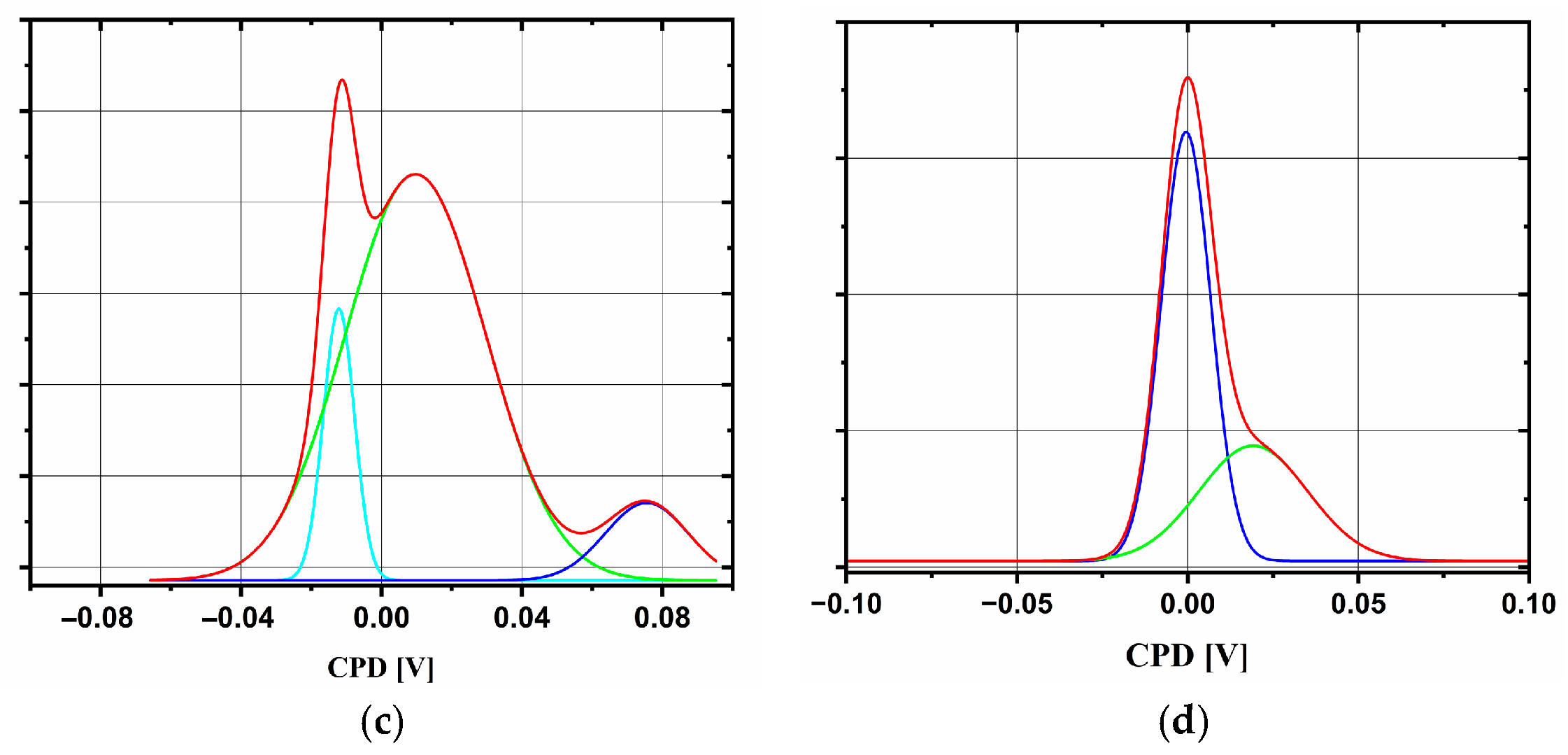
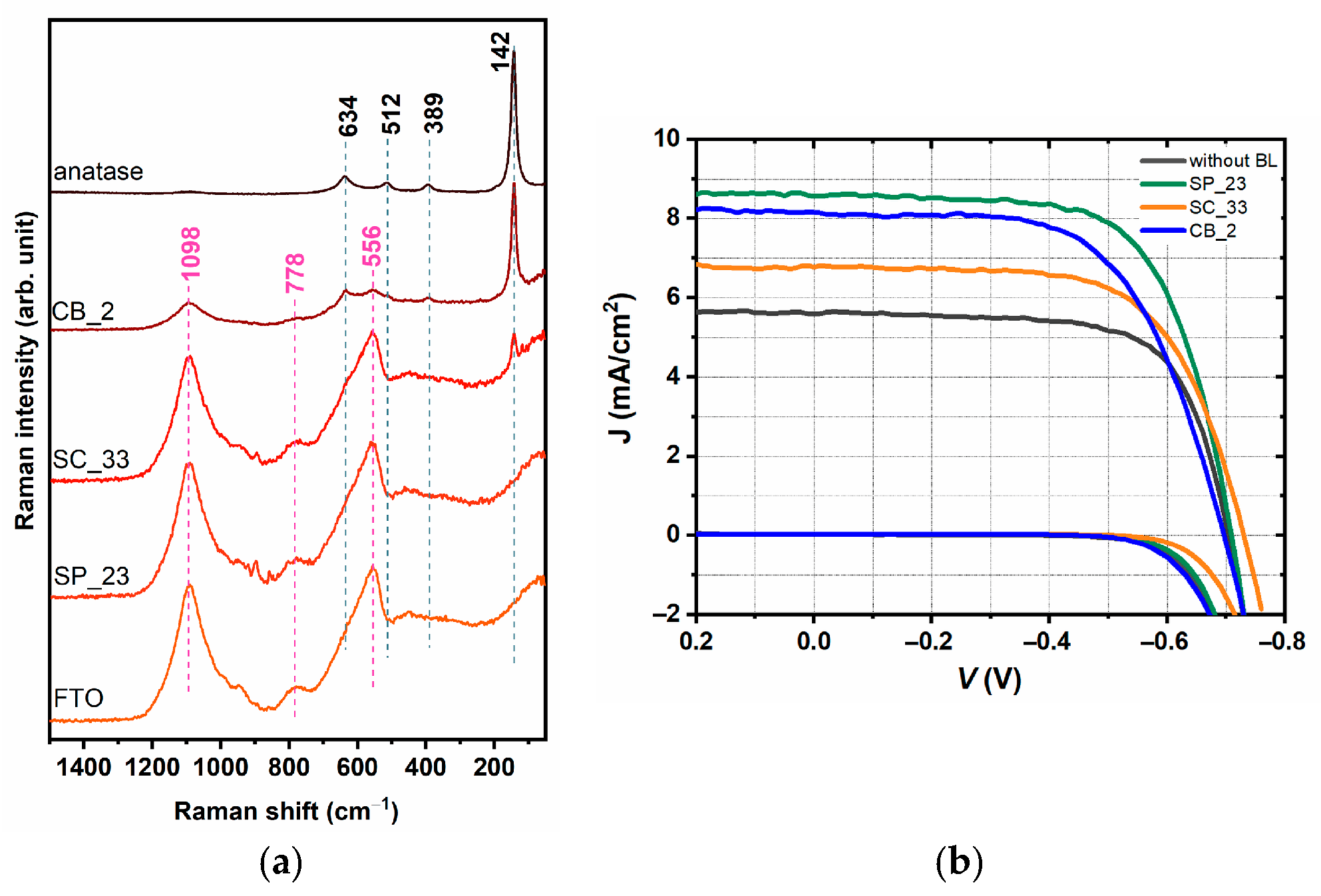
2.4. Comparison of Three Deposition Methods for the Application in DSSCs
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Preparation of TiO2 Blocking Layers
3.3. Preparation of DSSCs
3.4. Characterization and Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Y.; Li, C.; Tian, Z.; Sun, J. Solar-Driven Integrated Energy Systems: State of the Art and Challenges. J. Power Sources 2020, 478, 228762. [Google Scholar] [CrossRef]
- Green, M.A. Third Generation Photovoltaics: Solar Cells for 2020 and Beyond. Phys. E Low Dimens. Syst. Nanostruct. 2002, 14, 65–70. [Google Scholar] [CrossRef]
- Badawy, W.A. A Review on Solar Cells from Si-Single Crystals to Porous Materials and Quantum Dots. J. Adv. Res. 2015, 6, 123–132. [Google Scholar] [CrossRef]
- Sharma, S.; Jain, K.K.; Sharma, A. Solar Cells: In Research and Applications—A Review. MSA 2015, 6, 1145–1155. [Google Scholar] [CrossRef]
- Shah, N.; Shah, A.A.; Leung, P.K.; Khan, S.; Sun, K.; Zhu, X.; Liao, Q. A Review of Third Generation Solar Cells. Processes 2023, 11, 1852. [Google Scholar] [CrossRef]
- Zhang, D.; Stojanovic, M.; Ren, Y.; Cao, Y.; Eickemeyer, F.T.; Socie, E.; Vlachopoulos, N.; Moser, J.-E.; Zakeeruddin, S.M.; Hagfeldt, A.; et al. A Molecular Photosensitizer Achieves a Voc of 1.24 V Enabling Highly Efficient and Stable Dye-Sensitized Solar Cells with Copper(II/I)-Based Electrolyte. Nat. Commun. 2021, 12, 1777. [Google Scholar] [CrossRef]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.-E.; Grätzel, M.; et al. Dye-Sensitized Solar Cells for Efficient Power Generation under Ambient Lighting. Nat. Photon. 2017, 11, 372–378. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Omar, A.; Ali, M.S.; Abd Rahim, N. Electron Transport Properties Analysis of Titanium Dioxide Dye-Sensitized Solar Cells (TiO2-DSSCs) Based Natural Dyes Using Electrochemical Impedance Spectroscopy Concept: A Review. Sol. Energy 2020, 207, 1088–1121. [Google Scholar] [CrossRef]
- Toyoda, T.; Shen, Q. Quantum-Dot-Sensitized Solar Cells: Effect of Nanostructured TiO2 Morphologies on Photovoltaic Properties. J. Phys. Chem. Lett. 2012, 3, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.-L.; Wu, W.-Q.; Rao, H.-S.; Wan, Q.; Li, L.-B.; Kuang, D.-B.; Su, C.-Y. Three-Dimensional TiO2/ZnO Hybrid Array as a Heterostructured Anode for Efficient Quantum-Dot-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 5199–5205. [Google Scholar] [CrossRef]
- Yao, D.; Hu, Z.; Zheng, L.; Chen, S.; Lü, W.; Xu, H. Laser-Engineered Black Rutile TiO2 Photoanode for CdS/CdSe-Sensitized Quantum Dot Solar Cells with a Significant Power Conversion Efficiency of 9.1%. Appl. Surf. Sci. 2023, 608, 155230. [Google Scholar] [CrossRef]
- Schmidt-Mende, L.; Kraner, S.; Fakharuddin, A. Organic and Hybrid Solar Cells, 2nd ed.; De Gruyter: Berlin, Germany, 2022. [Google Scholar]
- Yodyingyong, S.; Zhou, X.; Zhang, Q.; Triampo, D.; Xi, J.; Park, K.; Limketkai, B.; Cao, G. Enhanced Photovoltaic Performance of Nanostructured Hybrid Solar Cell Using Highly Oriented TiO2 Nanotubes. J. Phys. Chem. C 2010, 114, 21851–21855. [Google Scholar] [CrossRef]
- Lee, J.; Jho, J.Y. Fabrication of Highly Ordered and Vertically Oriented TiO2 Nanotube Arrays for Ordered Heterojunction Polymer/Inorganic Hybrid Solar Cell. Sol. Energy Mater. Sol. Cells 2011, 95, 3152–3156. [Google Scholar] [CrossRef]
- Hu, W.; Yang, S.; Yang, S. Surface Modification of TiO2 for Perovskite Solar Cells. Trends Chem. 2020, 2, 148–162. [Google Scholar] [CrossRef]
- Ke, W.; Fang, G.; Wang, J.; Qin, P.; Tao, H.; Lei, H.; Liu, Q.; Dai, X.; Zhao, X. Perovskite Solar Cell with an Efficient TiO2 Compact Film. ACS Appl. Mater. Interfaces 2014, 6, 15959–15965. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.M.; Yadav, P.; Tavakoli, R.; Kong, J. Surface Engineering of TiO2 ETL for Highly Efficient and Hysteresis-Less Planar Perovskite Solar Cell (21.4%) with Enhanced Open-Circuit Voltage and Stability. Adv. Energy Mater. 2018, 8, 1800794. [Google Scholar] [CrossRef]
- Huang, K.-W.; Chen, Y.-H.; Li, M.-H.; Wu, Y.-S.; Chiu, P.-T.; Lin, Y.-P.; Tung, Y.-L.; Tsai, S.-Y.; Chen, P. Low-Temperature Growth of Uniform Ultrathin TiO2 Blocking Layer for Efficient Perovskite Solar Cell. Org. Electron. 2019, 75, 105379. [Google Scholar] [CrossRef]
- Shalan, A.E.; Elseman, A.M.; Rashad, M.M. Controlling the Microstructure and Properties of Titanium Dioxide for Efficient Solar Cells. In Titanium Dioxide-Material for a Sustainable Environment, 1st ed.; InTech: London, UK, 2018. [Google Scholar]
- Zhang, W.; He, H.; Li, H.; Duan, L.; Zu, L.; Zhai, Y.; Li, W.; Wang, L.; Fu, H.; Zhao, D. Visible-Light Responsive TiO2 -Based Materials for Efficient Solar Energy Utilization. Adv. Energy Mater. 2021, 11, 2003303. [Google Scholar] [CrossRef]
- Cameron, P.J.; Peter, L.M. Characterization of Titanium Dioxide Blocking Layers in Dye-Sensitized Nanocrystalline Solar Cells. J. Phys. Chem. B 2003, 107, 14394–14400. [Google Scholar] [CrossRef]
- Ito, S.; Liska, P.; Comte, P.; Charvet, R.; Péchy, P.; Bach, U.; Schmidt-Mende, L.; Zakeeruddin, S.M.; Kay, A.; Nazeeruddin, M.K.; et al. Control of Dark Current in Photoelectrochemical (TiO2/I––I3–) and Dye-Sensitized Solar Cells. Chem. Commun. 2005, 34, 4351. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, M. Performance Enhancement of Dye-Sensitized Solar Cell with a TiCl4-Treated TiO2 Compact Layer. Electron. Mater. Lett. 2015, 11, 271–275. [Google Scholar] [CrossRef]
- Zhang, S.; Lan, Z.; Wu, J.; Chen, X.; Zhang, C. Preparation of Novel TiO2 Quantum Dot Blocking Layers at Conductive Glass/TiO2 Interfaces for Efficient CdS Quantum Dot Sensitized Solar Cells. J. Alloys Compd. 2016, 656, 253–258. [Google Scholar] [CrossRef]
- Su, T.-S.; Hsieh, T.-Y.; Hong, C.-Y.; Wei, T.-C. Electrodeposited Ultrathin TiO2 Blocking Layers for Efficient Perovskite Solar Cells. Sci. Rep. 2015, 5, 16098. [Google Scholar] [CrossRef]
- Zatirostami, A. Increasing the Efficiency of TiO2-Based DSSC by Means of a Double Layer RF-Sputtered Thin Film Blocking Layer. Optik 2020, 207, 164419. [Google Scholar] [CrossRef]
- Burke, A.; Ito, S.; Snaith, H.; Bach, U.; Kwiatkowski, J.; Grätzel, M. The Function of a TiO2 Compact Layer in Dye-Sensitized Solar Cells Incorporating “Planar” Organic Dyes. Nano Lett. 2008, 8, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Kavan, L.; Zukalova, M.; Vik, O.; Havlicek, D. Sol–Gel Titanium Dioxide Blocking Layers for Dye-Sensitized Solar Cells: Electrochemical Characterization. ChemPhysChem 2014, 15, 1056–1061. [Google Scholar] [CrossRef]
- Jiang, C.; Koh, W.L.; Leung, M.Y.; Hong, W.; Li, Y.; Zhang, J. Influences of Alcoholic Solvents on Spray Pyrolysis Deposition of TiO2 Blocking Layer Films for Solid-State Dye-Sensitized Solar Cells. J. Solid State Chem. 2013, 198, 197–202. [Google Scholar] [CrossRef]
- Lim, S.P.; Huang, N.M.; Lim, H.N.; Mazhar, M. Aerosol Assisted Chemical Vapour Deposited (AACVD) of TiO2 Thin Film as Compact Layer for Dye-Sensitised Solar Cell. Ceram. Int. 2014, 40, 8045–8052. [Google Scholar] [CrossRef]
- Sangiorgi, A.; Bendoni, R.; Sangiorgi, N.; Sanson, A.; Ballarin, B. Optimized TiO2 Blocking Layer for Dye-Sensitized Solar Cells. Ceram. Int. 2014, 40, 10727–10735. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Chen, K.-T.; Huang, P.-H.; Wu, W.-Y.; Zhang, X.-Y.; Wang, C.; Liang, L.-S.; Gao, P.; Qiu, Y.; Lien, S.-Y.; et al. Effect of Annealing Temperature on Spatial Atomic Layer Deposited Titanium Oxide and Its Application in Perovskite Solar Cells. Nanomaterials 2020, 10, 1322. [Google Scholar] [CrossRef]
- Kment, S.; Krysova, H.; Hubicka, Z.; Kmentova, H.; Kavan, L.; Zboril, R. Very Thin Thermally Stable TiO2 Blocking Layers with Enhanced Electron Transfer for Solar Cells. Appl. Mater. Today 2017, 9, 122–129. [Google Scholar] [CrossRef]
- Choi, H.; Nahm, C.; Kim, J.; Moon, J.; Nam, S.; Jung, D.-R.; Park, B. The Effect of TiCl4-Treated TiO2 Compact Layer on the Performance of Dye-Sensitized Solar Cell. Curr. Appl. Phys. 2012, 12, 737–741. [Google Scholar] [CrossRef]
- Vairale, P.; Sharma, V.; Waghmare, A.; Shinde, P.; Pandharkar, S.; Punde, A.; Doiphode, V.; Hase, Y.; Aher, R.; Nair, S.; et al. Study of Structural, Optical, Morphology and Photoelectrochemical Properties of Melanin Sensitized TiO2 Thin Films Prepared by Chemical Bath Deposition Method. ES Mater. Manuf. 2020, 10, 5–15. [Google Scholar] [CrossRef]
- Liu, B.; Aydil, E.S. Growth of Oriented Single-Crystalline Rutile TiO2 Nanorods on Transparent Conducting Substrates for Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2009, 131, 3985–3990. [Google Scholar] [CrossRef] [PubMed]
- Yella, A.; Heiniger, L.-P.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Nanocrystalline Rutile Electron Extraction Layer Enables Low-Temperature Solution Processed Perovskite Photovoltaics with 13.7% Efficiency. Nano Lett. 2014, 14, 2591–2596. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.-H.; Lee, K.-M.; Suryanarayanan, V.; Hsu, J.-F.; Wu, M.-C. Controlled Photoanode Properties for Large-Area Efficient and Stable Dye-Sensitized Photovoltaic Modules. Nanomaterials 2021, 11, 2125. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Bhatt, N.; Bajpai, A.K. Nanostructured Superhydrophobic Coatings for Solar Panel Applications. In Nanomaterials-Based Coatings; Elsevier: Amsterdam, The Netherlands, 2019; pp. 397–424. [Google Scholar]
- Vyas, S.; Tiwary, R.; Shubham, K.; Chakrabarti, P. Study the Target Effect on the Structural, Surface and Optical Properties of TiO2 Thin Film Fabricated by RF Sputtering Method. Superlattices Microstruct. 2015, 80, 215–221. [Google Scholar] [CrossRef]
- Güzelçimen, F.; Tanören, B.; Çetinkaya, Ç.; Kaya, M.D.; Efkere, H.İ.; Özen, Y.; Bingöl, D.; Sirkeci, M.; Kınacı, B.; Ünlü, M.B.; et al. The Effect of Thickness on Surface Structure of Rf Sputtered TiO2 Thin Films by XPS, SEM/EDS, AFM and SAM. Vacuum 2020, 182, 109766. [Google Scholar] [CrossRef]
- Kavan, L.; Tétreault, N.; Moehl, T.; Grätzel, M. Electrochemical Characterization of TiO2 Blocking Layers for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2014, 118, 16408–16418. [Google Scholar] [CrossRef]
- Endrődi, B.; Kecsenovity, E.; Rajeshwar, K.; Janáky, C. One-Step electrodeposition of Nanocrystaline TiO2 Films with Enhanced Photoelectrochemical Performance and Charge Storage. ACS Appl. Energy Mater. 2018, 1, 851–858. [Google Scholar] [CrossRef]
- Liu, Y.; Bag, M.; Renna, L.A.; Page, Z.A.; Kim, P.; Emrick, T.; Venkataraman, D.; Russell, T.P. Understanding Interface Engineering for High-Performance Fullerene/Perovskite Planar Heterojunction Solar Cells. Adv. Energy Mater. 2016, 6, 1501606. [Google Scholar] [CrossRef]
- Wu, F.; Pathak, R.; Chen, C.; Tong, Y.; Xu, H.; Zhang, T.; Jian, R.; Li, X.; Qiao, Q. Reduced Hysteresis in Perovskite Solar Cells Using Metal Oxide/Organic Hybrid Hole Transport Layer with Generated Interfacial Dipoles. Electrochim. Acta 2020, 354, 136660. [Google Scholar] [CrossRef]
- Najm, A.S.; Alwash, S.A.; Sulaiman, N.H.; Chowdhury, M.S.; Techato, K. N719 Dye as a Sensitizer for Dye-sensitized Solar Cells (DSSCs): A Review of Its Functions and Certain Rudimentary Principles. Env. Prog. Sustain. Energy 2023, 42, e13955. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, X. The Improved Performance of Solar Cells Co-Sensitized with CdS/Ag2S Quantum Dots and N719 Dye. J. Alloys Compd. 2021, 886, 161331. [Google Scholar] [CrossRef]
- Liu, I.-P.; Lin, W.-H.; Tseng-Shan, C.-M.; Lee, Y.-L. Importance of Compact Blocking Layers to the Performance of Dye-Sensitized Solar Cells under Ambient Light Conditions. ACS Appl. Mater. Interfaces 2018, 10, 38900–38905. [Google Scholar] [CrossRef] [PubMed]
- Löbl, P.; Huppertz, M.; Mergel, D. Nucleation and Growth in TiO2 Films Prepared by Sputtering and Evaporation. Thin Solid Films 1994, 251, 72–79. [Google Scholar] [CrossRef]
- Balkan, T.; Guler, Z.; Morozova, M.; Dytrych, P.; Solcova, O.; Sarac, A.S. The Effect of Deposition on Electrochemical Impedance Properties of TiO2/FTO Photoanodes. J. Electroceram. 2016, 36, 102–111. [Google Scholar] [CrossRef]
- Bredar, A.R.C.; Chown, A.L.; Burton, A.R.; Farnum, B.H. Electrochemical Impedance Spectroscopy of Metal Oxide Electrodes for Energy Applications. ACS Appl. Energy Mater. 2020, 3, 66–98. [Google Scholar] [CrossRef]
- Bisquert, J.; Fabregat-Santiago, F.; Mora-Seró, I.; Garcia-Belmonte, G.; Giménez, S. Electron Lifetime in Dye-Sensitized Solar Cells: Theory and Interpretation of Measurements. J. Phys. Chem. C 2009, 113, 17278–17290. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Mora-Seró, I.; Garcia-Belmonte, G.; Bisquert, J. Cyclic Voltammetry Studies of Nanoporous Semiconductors. Capacitive and Reactive Properties of Nanocrystalline TiO2 Electrodes in Aqueous Electrolyte. J. Phys. Chem. B 2003, 107, 758–768. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Y.; Liu, W. Efficient Dye-Sensitized Solar Cells Based on Nanoflower-like ZnO Photoelectrode. Molecules 2017, 22, 1284. [Google Scholar] [CrossRef] [PubMed]
- Córdoba-Torres, P.; Mesquita, T.J.; Nogueira, R.P. Relationship between the Origin of Constant-Phase Element Behavior in Electrochemical Impedance Spectroscopy and Electrode Surface Structure. J. Phys. Chem. C 2015, 119, 4136–4147. [Google Scholar] [CrossRef]
- Pajkossy, T. Impedance of Rough Capacitive Electrodes. J. Electroanal. Chem. 1994, 364, 111–125. [Google Scholar] [CrossRef]
- Zhang, G.; Roy, B.K.; Allard, L.F.; Cho, J. Titanium Oxide Nanoparticles Precipitated from Low-Temperature Aqueous Solutions: I.; Nucleation, Growth, and Aggregation. J. Am. Ceram. Soc. 2008, 91, 3875–3882. [Google Scholar] [CrossRef]
- Pellitero, M.A.; Curtis, S.D.; Arroyo-Currás, N. Interrogation of Electrochemical Aptamer-Based Sensors via Peak-to-Peak Separation in Cyclic Voltammetry Improves the Temporal Stability and Batch-to-Batch Variability in Biological Fluids. ACS Sens. 2021, 6, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Mansfeldova, V.; Zlamalova, M.; Tarabkova, H.; Janda, P.; Vorokhta, M.; Piliai, L.; Kavan, L. Work Function of TiO2 (Anatase, Rutile, and Brookite) Single Crystals: Effects of the Environment. J. Phys. Chem. C 2021, 125, 1902–1912. [Google Scholar] [CrossRef]
- Chen, T.; Hu, W.; Song, J.; Hong Guai, G.; Ming Li, C. Interface Functionalization of Photoelectrodes with Graphene for High Performance Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2012, 22, 5245–5250. [Google Scholar] [CrossRef]
- Kavan, L. Electrochemistry and band structure of semiconductors (TiO2, SnO2, ZnO): Avoiding pitfalls and textbook errors. J. Solid State Electr. 2024, 28, 829–845. [Google Scholar] [CrossRef]
- Hvojnik, M.; Vida, J.; Homola, T.; Pavlickova, M.; Hatala, M.; Tomanova, K.; Mikula, M.; Gemeiner, P. The effect of rapid atmospheric plasma treatment of FTO substrates on the quality of TiO2 blocking layers for printed perovskite solar cells. Mater. Sci. Semicon. Proc. 2021, 131, 105850. [Google Scholar] [CrossRef]
- Frank, O.; Zukalova, M.; Laskova, B.; Kurti, J.; Koltai, J.; Kavan, L. Raman spectra of titanium dioxide (anatase, rutile) with identified oxygen isotopes (16, 17, 18). PCCP 2012, 14, 14567–14572. [Google Scholar] [CrossRef]
- Tang, H.; Prasad, K.; Sanjines, R.; Schmid, P.E.; Levy, F. Electrical and optical properties of TiO2 anatase thin films. J. Appl. Phys. 1994, 75, 2042–2047. [Google Scholar] [CrossRef]
- Jubu, P.R.; Danladi, E.; Ndeze, U.I.; Adedokun, O.; Landi, S., Jr.; Haider, A.J.; Adepoju, A.T.; Yusof, Y.; Obaseki, O.S.; Yam, F.K. Comment about the use of unconventional Tauc plot for bandgap energy determination of semiconductors using UV-Vis spectroscopy. Results Opt 2024, 14, 100606. [Google Scholar]
- Ma’arifah, A.; Sholeha, N.; Pujiarti, H.; Diantoro, M.; Hidayat, A.; Osman, Z. The effect of RF sputtering power variation on blocking layer TiO2 on the performance of DSSC. Result Surf. Interfaces 2025, 18, 100456. [Google Scholar] [CrossRef]
- Tian, G.; He, H.; Shao, J. Effect of Microstructure of TiO2 Thin Films on Optical Band Gap Energy. Chin. Phys. Lett. 2005, 22, 1787. [Google Scholar]
- Pappas, S.D.; Grammtikopoulos, S.; Poulopoulos, P.; Trachylis, D.; Velgakis, M.J.; Politis, C. Growth and Optical Properites of Thin NiO Films. J. Surf. Interfaces Mater. 2014, 2, 233–237. [Google Scholar] [CrossRef]
- Zulkefle, M.A.; Abdul Rahman, R.; Yusof, K.A.; Abdullah, W.F.H.; Rusop, M.; Herman, S.H. Spin Speed and Duration Dependence of TiO2 Thin Films pH Sensing Behavior. J. Sens. 2016, 2016, 9746156. [Google Scholar] [CrossRef]
- Ito, S.; Chen, P.; Comte, P.; Nazeeruddin, M.K.; Liska, P.; Péchy, P.; Grätze, M. Fabrication of screen-printing pastes from TiO2 powders for dye-sensitized solar cells. Prog. Photovolt. Res. Appl. 2007, 15, 603–612. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar]



| Deposition Method | Sample Name and Conditions | |||||
|---|---|---|---|---|---|---|
| SPUTTERING | SP_11 | 250 W 15 min | SP_21 | 200 W 5 min | ||
| SP_12 | 350 W 15 min | SP_22 | 250 W 5 min | |||
| SP_13 | 450 W 15 min | SP_23 | 300 W 5 min | |||
| SP_14 | 250 W 30 min | SP_24 | 200 W 10 min | |||
| SP_15 | 350 W 30 min | |||||
| SP_16 | 450 W 30 min | |||||
| SC_11 | 40 mM 70 °C 20 min | SC_21 | 120 mM 70 °C 20 min | SC_31 | 240 mM 70°C 20 min | |
| SPIN-COATING | SC_12 | 40 mM 70 °C 40 min | SC_22 | 120 mM 70 °C 40 min | SC_32 | 240 mM 70 °C 40 min |
| SC_13 | 40 mM 70 °C 60 min | SC_23 | 120 mM 70 °C 60 min | SC_33 | 240 mM 70 °C 60 min | |
| CHEMICAL BATH DEPOSITION | CB_1 | horizontally 30 min | ||||
| CB_2 | horizontally 60 min | |||||
| CB_3 | horizontally + homogenization 30 min | |||||
| CB_4 | horizontally + homogenization 60 min | |||||
| CB_5 | vertically 30 min | |||||
| CB_6 | vertically 60 min | |||||
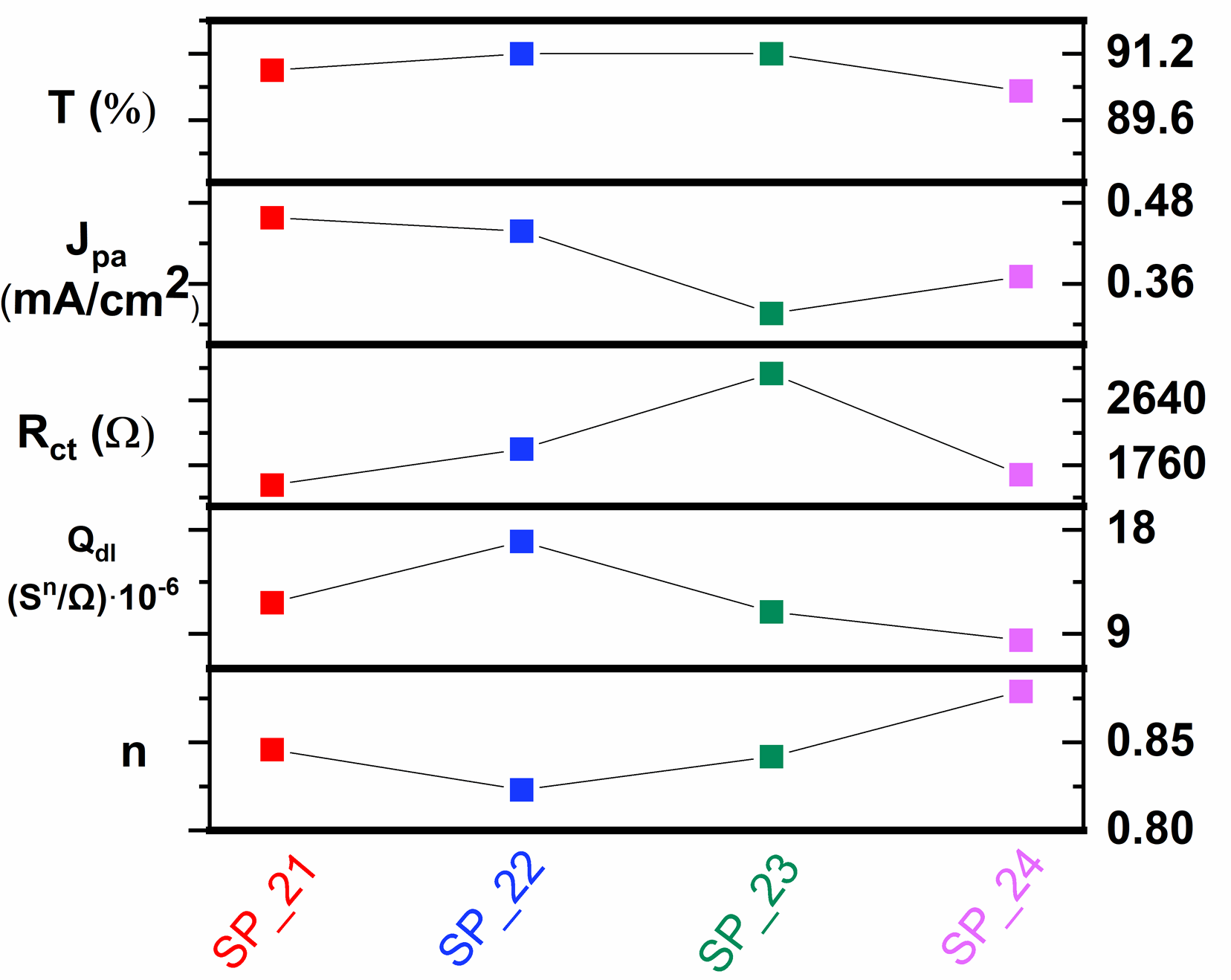
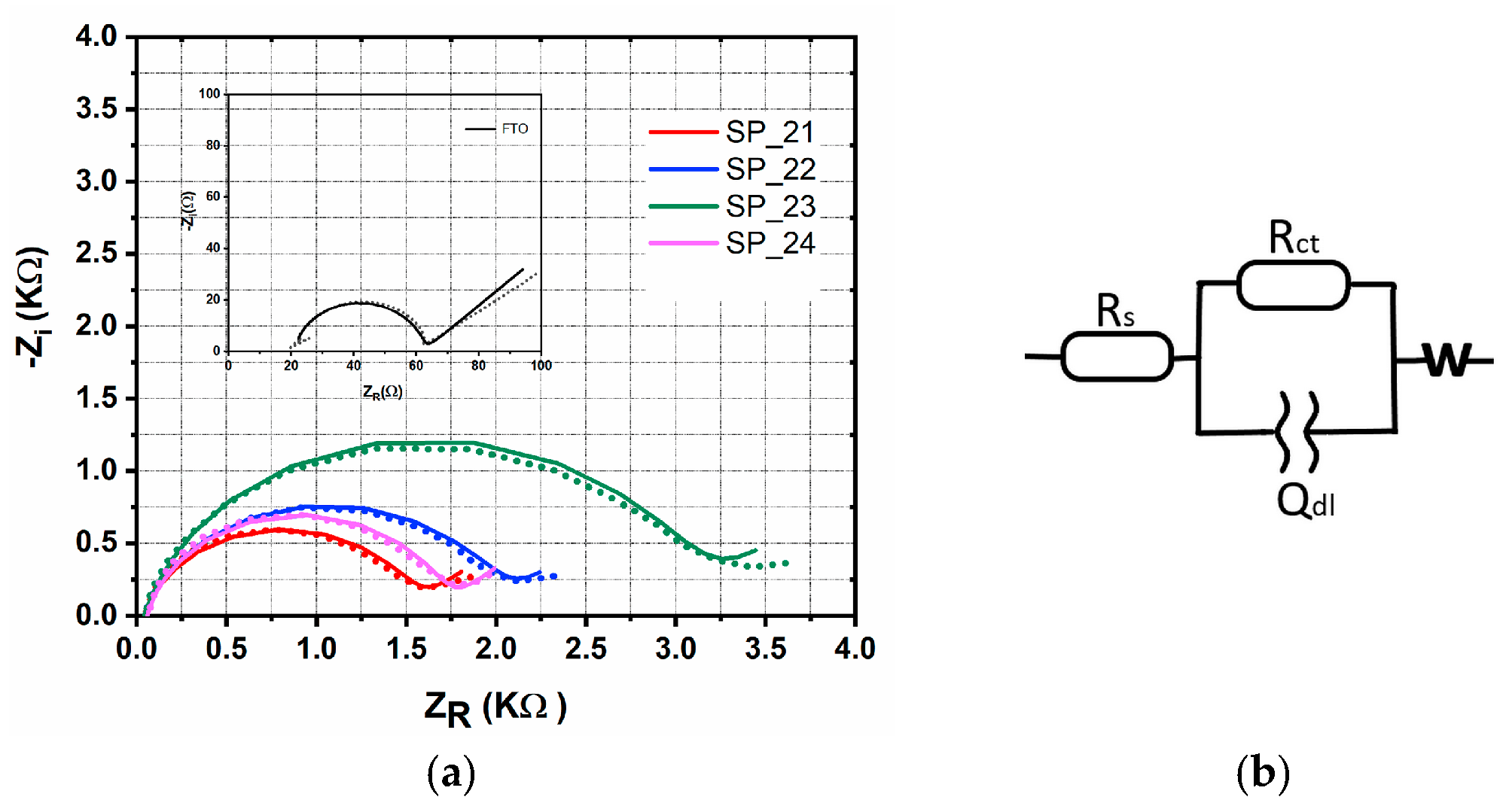
| Sample | Rs (Ω) | Rct (Ω) | Qdl (sn/Ω)∙10−6 | n | W (s1/2/Ω)∙10−2 | τ (ms) |
|---|---|---|---|---|---|---|
| Bare FTO | 30.0 | 76 | 3.80 | 0.994 | 1.76 | 0.16 |
| SP_21 | 44.6 | 1490 | 11.70 | 0.846 | 0.315 | 8.36 |
| SP_22 | 40.9 | 1980 | 17.00 | 0.823 | 0.359 | 14.13 |
| SP_23 | 41.9 | 3007 | 10.90 | 0.842 | 0.239 | 23.89 |
| SP_24 | 57.9 | 1630 | 8.46 | 0.879 | 0.285 | 8.36 |
| Sample | Rs (Ω) | Rct (Ω) | Qdl (sn/Ω)∙10−6 | n | W (s1/2/Ω)∙10−2 | τ (ms) |
|---|---|---|---|---|---|---|
| SC_11 | 44.0 | 127 | 6.70 | 0.997 | 1.10 | 0.75 |
| SC_12 | 44.1 | 171 | 6.54 | 0.999 | 1.01 | 1.30 |
| SC_13 | 47.4 | 140 | 6.52 | 0.995 | 1.09 | 0.75 |
| SC_21 | 51.6 | 195 | 12.50 | 0.977 | 1.62 | 2.25 |
| SC_22 | 37.4 | 242 | 11.50 | 0.966 | 1.40 | 2.25 |
| SC_23 | 44.7 | 125 | 7.08 | 0.999 | 1.18 | 0.75 |
| SC_33 | 37.8 | 288 | 10.70 | 0.952 | 1.12 | 2.25 |
| Sample | Rs (Ω) | Rct (Ω) | Qdl (sn/Ω)∙10−6 | n | W (s1/2/Ω)∙10−2 | τ (ms) |
|---|---|---|---|---|---|---|
| CB_1 | 45.5 | 1030 | 11.4 | 0.955 | 0.200 | 8.36 |
| CB_2 | 41.7 | 1250 | 13.0 | 0.955 | 0.354 | 14.13 |
| CB_3 | 35.8 | 629 | 11.6 | 0.964 | 0.685 | 7.31 |
| CB_4 | 37.0 | 633 | 10.9 | 0.958 | 0.629 | 7.31 |
| CB_5 | 47.0 | 302 | 10.8 | 0.978 | 0.395 | 2.93 |
| CB_6 | 52.6 | 479 | 11.0 | 0.958 | 0.282 | 4.95 |
| Sample | Jpa (mA/cm2) | Rct (Ω) | CPD Distribution | φ (eV) | Eg (eV) | d (nm) |
|---|---|---|---|---|---|---|
| FTO | 1.317 | 76 | single | 4.6 | / | / |
| SP_23 | 0.316 | 3007 | single | 4.6 | 3.80 | 13 |
| SC_33 | 0.735 | 288 | bimodal | 4.4 | 3.32 | 50 |
| CB_2 | 0.506 | 1250 | single | 4.4 | 3.41 | 130 |
| Cell | Jsc (mA/cm2) | Voc (V) | FF | η (%) |
|---|---|---|---|---|
| without blocking layer | 5.601 | 0.704 | 0.69 | 2.74 |
| SP_23 | 8.562 | 0.709 | 0.66 | 4.00 |
| SC_33 | 6.805 | 0.730 | 0.64 | 3.20 |
| CB_2 | 8.166 | 0.697 | 0.60 | 3.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milinković, E.; Cvetanović, K.; Bošković, M.V.; Conić, N.; Jovanov, V.; Stanisavljev, D.; Vasiljević-Radović, D. The Comprehensive Study of TiO2 Blocking Layer with Complementary Electrochemical and SPM Methods for the Application in Photovoltaics. Inorganics 2025, 13, 270. https://doi.org/10.3390/inorganics13080270
Milinković E, Cvetanović K, Bošković MV, Conić N, Jovanov V, Stanisavljev D, Vasiljević-Radović D. The Comprehensive Study of TiO2 Blocking Layer with Complementary Electrochemical and SPM Methods for the Application in Photovoltaics. Inorganics. 2025; 13(8):270. https://doi.org/10.3390/inorganics13080270
Chicago/Turabian StyleMilinković, Evgenija, Katarina Cvetanović, Marko V. Bošković, Nastasija Conić, Vladislav Jovanov, Dragomir Stanisavljev, and Dana Vasiljević-Radović. 2025. "The Comprehensive Study of TiO2 Blocking Layer with Complementary Electrochemical and SPM Methods for the Application in Photovoltaics" Inorganics 13, no. 8: 270. https://doi.org/10.3390/inorganics13080270
APA StyleMilinković, E., Cvetanović, K., Bošković, M. V., Conić, N., Jovanov, V., Stanisavljev, D., & Vasiljević-Radović, D. (2025). The Comprehensive Study of TiO2 Blocking Layer with Complementary Electrochemical and SPM Methods for the Application in Photovoltaics. Inorganics, 13(8), 270. https://doi.org/10.3390/inorganics13080270








