Abstract
The electrochemical reduction of CO2 (eCO2RR) has emerged as a promising route for carbon-neutral fuel and chemical production, offering a sustainable alternative to fossil-based processes. This article begins with an overview of conventional CO2 conversion methods, highlighting their limitations and the advantages of electrochemical approaches under ambient conditions. We focus on recent advancements in electrocatalyst development for the eCO2RR, including metal-based, Cu-based, and metal-free catalysts. Metal-based catalysts are categorized by product selectivity (formate, CO, and multicarbon products), emphasizing their structures and practical performance. Cu-based catalysts are discussed in detail due to their unique capability to produce multicarbon products, with emphasis on design strategies, material types, and performance trends. Additionally, we review emerging metal-free catalysts, including their synthesis, mechanisms, and potential applications. This article provides a comparative analysis to guide future research toward efficient, selective, and durable catalysts for CO2 electroreduction, aiming to accelerate the deployment of carbon capture and utilization technologies.
1. Introduction
The rising concentration of atmospheric carbon dioxide (CO2), primarily driven by human industrial activities, has disrupted the Earth’s natural carbon balance and thus contributed to a measurable increase in global average temperature [1]. The natural carbon cycle—comprising biological processes, like photosynthesis, and geological mechanisms, such as terrestrial silicate weathering—has historically maintained a stable climate. However, the current rate of anthropogenic CO2 emissions has surpassed the Earth’s inherent capacity to reabsorb and convert CO2, prompting urgent calls for artificial intervention strategies. To address this challenge, researchers have turned to engineered CO2 conversion technologies that can complement or enhance natural carbon sinks. Among the available approaches, such as thermochemical conversion, photocatalysis, and biological fixation, the electrochemical CO2 reduction reaction (electrochemical CO2RR, or eCO2RR) has emerged as a particularly promising route due to its scalability, tunability, and compatibility with renewable electricity [2,3].
Electrochemical CO2 reduction offers a sustainable platform for simultaneously mitigating carbon emissions and generating value-added products, including fuels and industrial chemicals. Operating under ambient conditions and powered by electricity—ideally sourced from renewables—this approach facilitates the direct transformation of CO2 into chemicals such as carbon monoxide (CO), formate (HCOO−) or formic acid (HCOOH), methanol (CH3OH), methane (CH4), and ethylene (C2H4) [4,5]. Moreover, eCO2RR systems can be designed for integration into existing energy infrastructures or decentralized carbon utilization setups. Despite these advantages, several challenges remain. Product selectivity is a major concern due to the complexity of competing reaction pathways and the prevalence of the hydrogen evolution reaction (HER), which often dominates under aqueous electrochemical conditions [6]. The outcome of the eCO2RR depends on multiple interrelated factors, including electrode composition, electrolyte identity, electrolyzer configuration, operating conditions, and the structure–activity relationship of the catalyst [4,5,7,8,9]. Addressing these challenges requires the development of efficient, stable, and cost-effective electrocatalysts that can guide the reaction toward desired products while suppressing undesired side reactions.
This review article focuses on the development of electrocatalysts for the eCO2RR. While noble and transition metal catalysts (e.g., Au, Ag, Bi, Sn) have demonstrated high activity and selectivity, especially for two-electron products like CO and formate, their high cost and limited availability pose challenges for widespread adoption [2,7,10]. In contrast, metal-free carbon-based catalysts offer a sustainable and abundant alternative. These materials include defect-engineered carbons, which leverage intrinsic structural irregularities as active sites, and heteroatom-doped carbons, where non-metallic elements, such as nitrogen, boron, sulfur, or phosphorus, are incorporated to modulate electronic structure and catalytic behavior [4,7,9]. Biomass-derived carbons, wood-templated porous structures, and covalent organic frameworks (COFs) also fall within this class [9]. Copper (Cu)-based catalysts, notable for their ability to generate multicarbon (C2+) products such as hydrocarbons and oxygenates, are discussed extensively in the literature and form a distinct category due to their unique reaction profiles [11,12,13].
We highlight recent advances in catalyst design, synthesis strategies, and structure–activity relationships. Special attention is given to product distributions, mechanistic insights, and the roles of catalyst architecture in tuning reaction selectivity. By critically analyzing the current landscape, this review aims to provide a roadmap for the rational development of next-generation, sustainable electrocatalysts for CO2 electroreduction.
2. Mechanisms of the Electrochemical CO2 Reduction Reaction (eCO2RR)
2.1. Reaction Pathways
The electrochemical reduction of carbon dioxide is a surface-bound, multi-step process involving proton-coupled electron transfer reactions [5,7]. It typically begins with the adsorption and activation of the chemically inert CO2 molecule on an electrocatalyst surface [13]. The initial step involves the formation of either a *CO2− radical anion or a bent *CO2 intermediate. Once activated, CO2 can follow different mechanistic pathways depending on the local electronic environment, the nature of the catalyst, and the reaction conditions. These pathways can be broadly categorized based on the number of electrons involved in the final product.
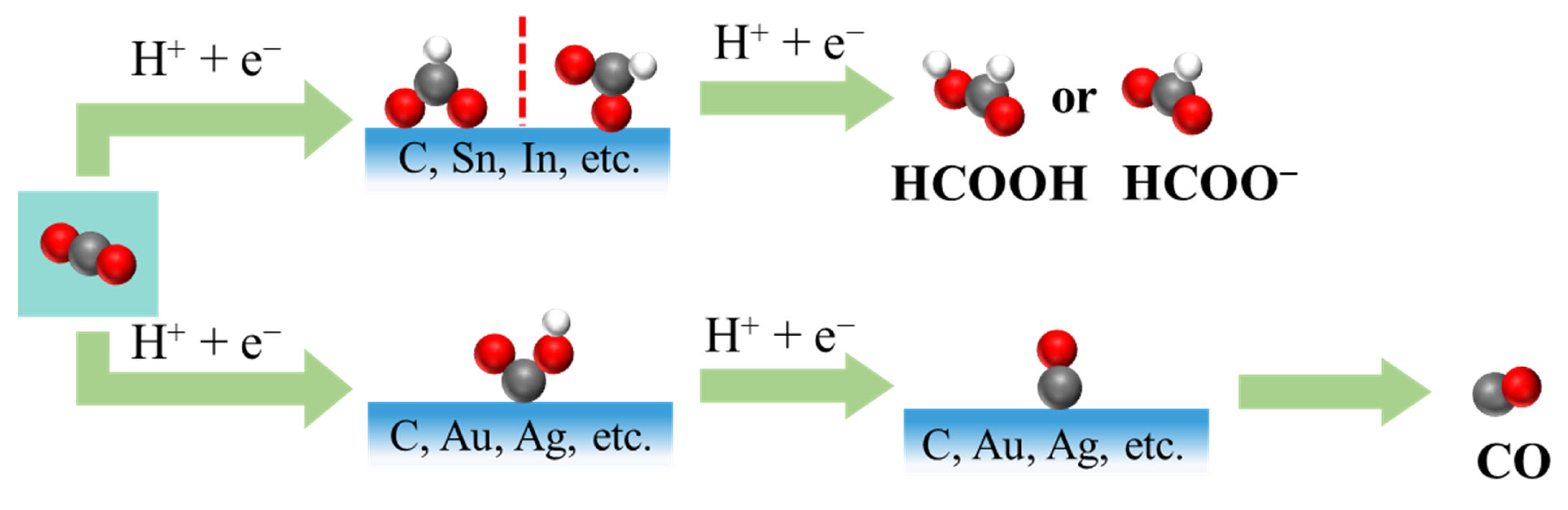
Two-electron reduction pathways lead primarily to the formation of CO or formate, depending on whether the *COOH or *OCHO intermediate is stabilized on the catalyst surface (Figure 1) [5,14]. For example, metal electrocatalysts can be broadly categorized into three groups based on the predominant CO2RR products they yield under specific operating conditions [15,16,17]: CO-producing metals, such as gold (Au), silver (Ag), zinc (Zn), gallium (Ga), platinum (Pt), and nickel (Ni); formate-producing metals, such as tin (Sn), indium (In), lead (Pb), bismuth (Bi), cadmium (Cd), and mercury (Hg); and multicarbon-producing metals, notably copper (Cu), which is uniquely capable of facilitating C-C coupling reactions under electrochemical conditions. Each group exhibits distinct catalytic characteristics that arise from their intrinsic binding affinities to key reaction intermediates, as well as from their electronic structure, particularly the position and occupancy of the d-band center relative to the Fermi level [18]. A d-band center that is closer to the Fermi level typically correlates with stronger adsorption of eCO2RR intermediates such as *COOH, *CO, and *OCOH, which, in turn, impacts product selectivity [19]. This concept has become a central design principle in electrocatalysis, guiding strategies such as alloying [10], doping [4], and nanostructuring [5,17] to optimize performance. In addition, metal-free catalysts, namely, carbon-based materials, have gained increasing attention as a cost-effective alternative to traditional noble metal catalysts.
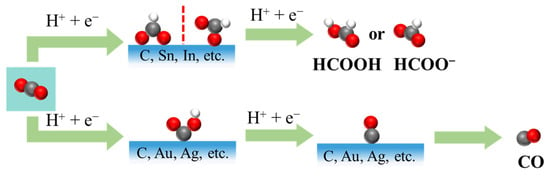
Figure 1.
Possible two-electron eCO2RR pathways for formate and CO.
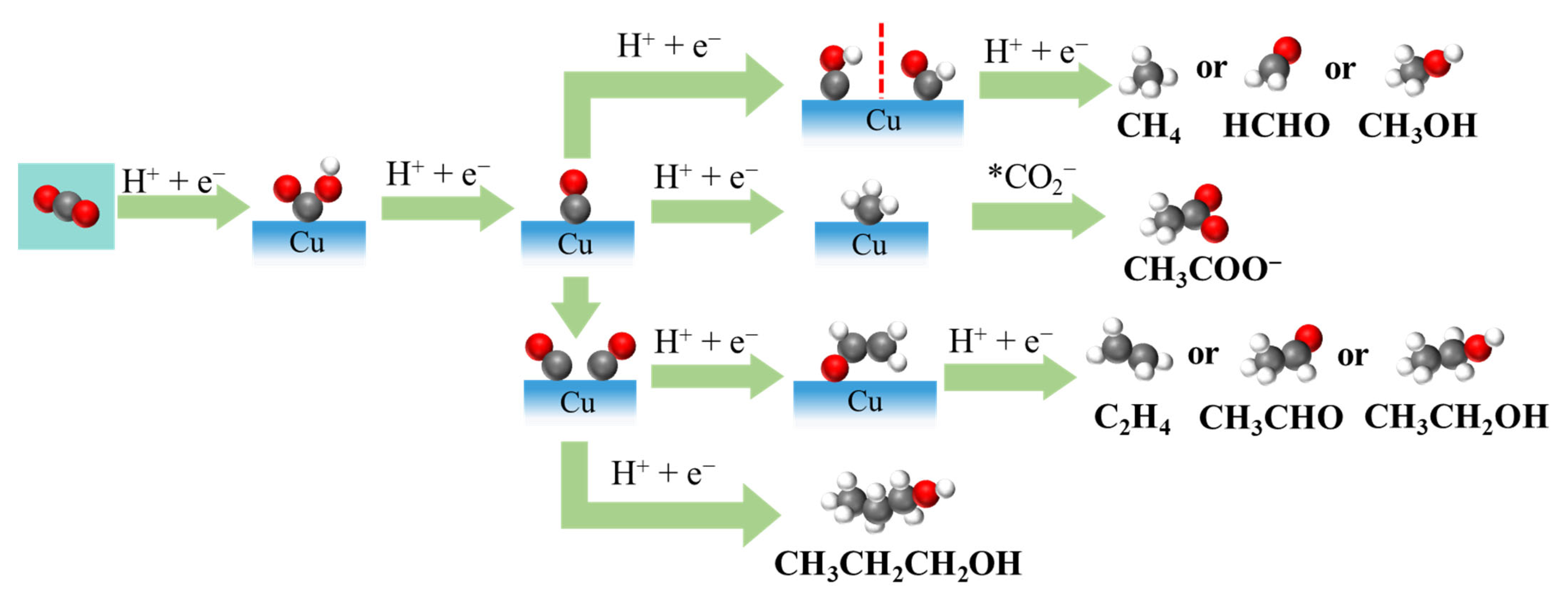
In contrast, multielectron pathways involve further hydrogenation and C-C coupling steps to produce hydrocarbons (such as methane and ethylene) and oxygenates (such as methanol or ethanol) (Figure 2) [12,13]. These complex pathways typically require more intricate active sites and well-optimized reaction environments. Alongside these desirable eCO2RRs, the HER often occurs as a competitive side reaction, especially under acidic or neutral conditions, consuming electrons that would otherwise contribute to CO2 conversion [6]. The ability to suppress the HER is thus a key performance target in catalyst design.
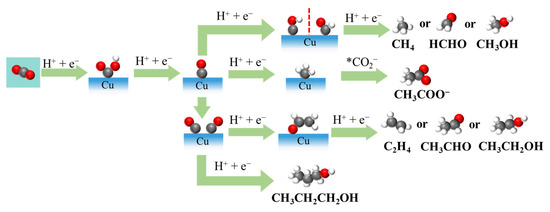
Figure 2.
Possible eCO2RR pathways for C1 and C2+ products on Cu-based electrocatalysts.
2.2. Crucial Performance Indicators
To evaluate the efficiency and viability of eCO2RR electrocatalysts, several key performance indicators are commonly used. Faradaic efficiency (FE) is one of the most critical indicators, representing the proportion of charge used to form a specific product relative to the total charge passed through the system. FE is calculated as follows [20]:
where Qprod refers to the charge consumed to generate a particular product and Qtotal denotes the total charge passed during the reaction. A high FE toward a desired product reflects both the selectivity and effectiveness of the catalyst.
Overpotential is another important metric, defined as the additional voltage required beyond the thermodynamic equilibrium potential to drive the desired reaction at a measurable rate. Catalysts that can operate at low overpotentials are considered more energy efficient.
Current density, typically reported in milliamperes per square centimeter, indicates the reaction rate and is vital for assessing the practicality of the catalyst in scaled-up systems. A good electrocatalyst should be able to achieve high current densities at low overpotentials while maintaining high FE.
In addition, long-term stability and durability are indispensable for real-world applications. A catalyst that retains its activity and selectivity over extended periods (often measured over dozens or even hundreds of hours) demonstrates structural resilience and chemical robustness under operating conditions. For mechanistic investigations, turnover frequency (TOF) and turnover number (TON) are also employed. TOF refers to the number of product molecules generated per active site per unit time, while TON tracks the total number of reaction cycles a catalyst can complete before deactivation.
2.3. Key Influence Factors
The outcome of CO2 electroreduction depends on multiple interconnected factors, the most critical of which are the properties of the electrocatalyst itself. The surface morphology, including porosity, roughness, and edge density, affects reactant accessibility and intermediate stabilization [5]. The electronic structure, particularly the distribution of the density of states and Fermi level alignment, governs the adsorption strength of intermediates like *COOH, *CO, and *OCHO [2]. The nature and spatial distribution of active sites, whether they are metal atoms, dopants, or defects, directly influence product selectivity [14]. Additionally, high electrical conductivity in the catalyst, typically associated with sp2-hybridized carbon frameworks or metal conductors, facilitates efficient charge transfer during the reaction [9].
Electrolyte composition also plays a vital role in modulating the reaction environment. The presence and type of cations in the electrolyte influence the local electric field and the stabilization of transition states. For example, larger alkali cations like Cs+ or K+ have been shown to enhance CO2 adsorption and suppress the HER by altering the interfacial electric double layer [5]. The pH of the electrolyte can shift the equilibrium between CO2, bicarbonate, and carbonate species, thereby influencing both the reaction pathway and the proton availability [7]. While alkaline conditions often help suppress the HER, they can also introduce additional transport and solubility challenges. Solvent choice further affects CO2 solubility, ionic mobility, and electrode wettability, all of which influence reaction kinetics [5].
Finally, operating conditions, such as applied potential, temperature, pressure, and cell design, strongly affect the overall performance of the eCO2RR system. The applied potential not only controls the thermodynamic driving force but also determines which reaction pathways are favored kinetically [7]. Higher temperatures generally enhance reaction rates but may compromise selectivity or catalyst stability [5]. Increasing CO2 partial pressure can improve reactant availability at the catalyst surface and shift product distributions [5]. Innovations in cell architecture, such as flow cells and gas diffusion electrodes, have been shown to significantly enhance mass transport and reduce concentration polarization, making them ideal for high-performance CO2 electrolysis [8].
Together, these mechanistic insights, performance metrics, and operating parameters form the foundation for rational catalyst design and system optimization in the field of electrochemical CO2 reduction. Understanding each component’s role in driving reaction pathways and determining selectivity is essential for guiding the development of next-generation CO2RR electrocatalysts.
2.4. In Situ Characterization Techniques
The eCO2RR performance is governed by multiple interrelated factors, including catalyst surface morphology (porosity, roughness, edge density), electronic structure (density of states, Fermi level alignment), and the nature and distribution of active sites, such as metal atoms, dopants, or defects. High electrical conductivity, often found in sp2-hybridized carbon frameworks or metallic conductors, further facilitates efficient charge transfer. Identifying the true active sites and reaction intermediates under operating conditions is essential for the rational design of catalysts with high activity and selectivity. In situ characterization techniques are powerful tools for this purpose, as they allow direct observation of structural and chemical changes during the reaction.
In situ Raman spectroscopy probes vibrational modes through inelastic light scattering and is well suited for aqueous systems due to the weak Raman signal of water. It provides valuable insights into active site structures and reaction pathways, though its limited spatial resolution and sensitivity require complementary methods.
In situ infrared spectroscopy (IR), particularly attenuated total reflection surface-enhanced infrared absorption spectroscopy (ATR-SEIRAS), enables real-time detection of CO2RR intermediates such as *COOH, *CO, and *OCHO with high sensitivity and time resolution. By depositing a thin metal layer (e.g., Au, Ag, Cu) on an IR-transparent prism, ATR-SEIRAS amplifies surface signals from species within 1–10 nm of the electrode while minimizing interference from electrolytes, making it a powerful tool for elucidating reaction mechanisms under operating conditions.
X-ray photoelectron spectroscopy (XPS) measures elemental composition and chemical states by detecting photoelectrons emitted under X-ray irradiation. While conventional XPS requires ultra-high vacuum, in situ or quasi in situ setups enable analysis of catalysts immediately after electrochemical operation without air exposure. XPS is often combined with X-ray absorption spectroscopy (XAS) for a more complete picture of surface and bulk properties.
In situ X-ray absorption spectroscopy (XAS), including X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS), provides detailed information on oxidation states, electronic structures, coordination environments, and atomic distributions. These methods are highly effective for tracking structural evolution and identifying the true active sites of electrocatalysts during the CO2RR.
3. C1-Producing Electrocatalysts
3.1. Metal-Based Electrocatalysts
Metal-based electrocatalysts constitute one of the most extensively investigated classes of materials in the field of the eCO2RR, owing to their unique electronic properties, surface reactivity, and tunable catalytic behavior [18]. These catalysts offer high electrical conductivity, accessible surface active sites, and the ability to lower the kinetic and thermodynamic barriers associated with the reduction of CO2 to a variety of value-added products, such as CO, formate, CH4, C2H4, ethanol (C2H5OH), and other multicarbon compounds [10]. These reaction products have important implications for renewable energy systems, including synthetic fuels, energy storage chemicals, and carbon-neutral industrial feedstocks [17]. This section will be focused on catalysts that mainly produce C1 products, namely, CO and formate.
3.1.1. CO-Producing Metals
Among the product-selective categories, CO-producing metal catalysts are particularly attractive due to the industrial importance of CO as a building block for syngas, Fischer–Tropsch synthesis, and methanol production [18,21]. CO production from the CO2RR proceeds through a two-electron transfer pathway involving the formation of a *COOH intermediate, which subsequently reduces to CO and desorbs from the surface. The effectiveness of CO-selective metals depends on their ability to stabilize *COOH while maintaining weak binding to *CO, thus preventing further reduction to hydrocarbons or alcohols.
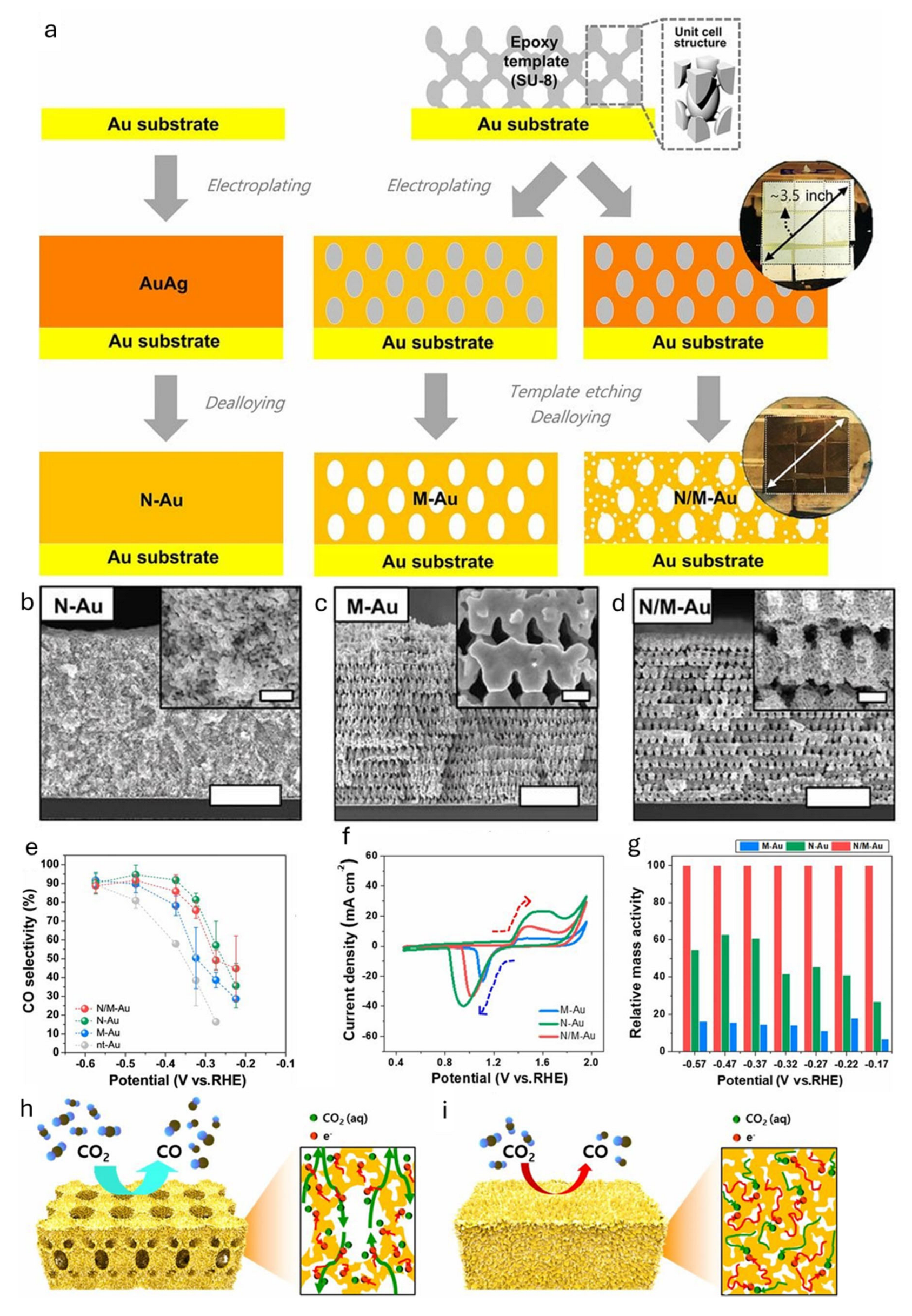
Au has emerged as a benchmark catalyst in this class due to its remarkable selectivity for CO production (>90% FE in many systems) and low overpotential, which can be further enhanced through nanostructuring [21,22]. For example, Kim and coworkers engineered Au nanostructures into pore-like and pillar-like forms by adjusting anodic potential and reduction current density during the electroreduction of Au(OH)3 [23]. These nanostructures showed improved CO selectivity compared to flat Au films, attributed to grain boundaries with rich surfaces that promote *CO2− intermediate stabilization. Pore-like structures exhibited better CO selectivity at low overpotentials, while pillar-like ones performed better at higher overpotentials. Hyun and coworkers reported 3D hierarchically porous gold (N/M-Au) nanostructures via Proximity-field nanoPatterning (PnP) lithography, creating networks of macropores (200–300 nm) and nanopores (~10 nm) [24]. The impact of electron and ion transport was systemically investigated by comparing different 3D Au nanostructures: nanoporous Au (N-Au), macroporous Au (M-Au), and N/M-Au (Figure 3a–d). Compared to nontemplated Au (nt-Au), these architectures significantly enhanced CO selectivity, with N/M-Au reaching 85.8% at an overpotential of 0.264 V (Figure 3e). Cyclic voltammetry (CV) showed a reduction peak shift to lower potentials in nanopore structures, indicating slower ion/electron transport (Figure 3f). Despite its larger surface area, N-Au suffered from mass transport limitations, while hierarchical N/M-Au exhibited up to 3.96 times higher mass activity than dealloyed nanoporous Au, due to its balance of high surface area and efficient electrolyte accessibility (Figure 3g–i).
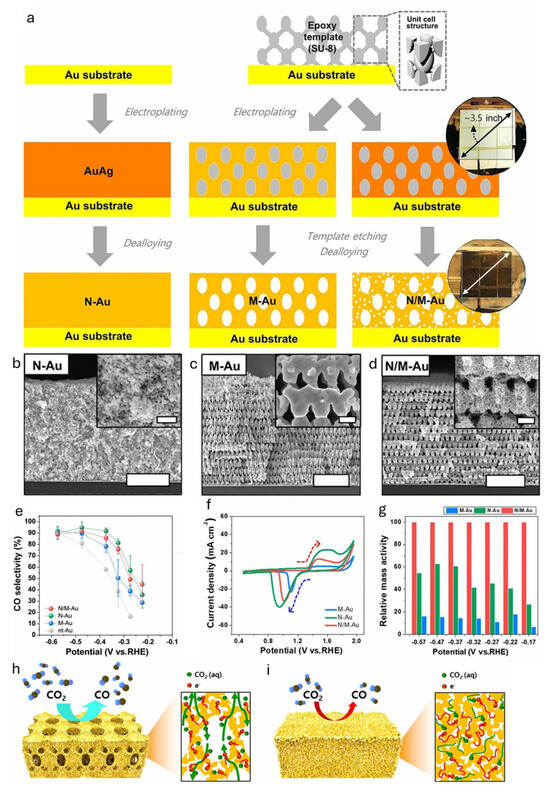
Figure 3.
(a) Schematic illustration of fabrication procedures for nanoporous gold (N-Au), macroporous gold (M-Au), and hierarchically porous gold (N/M-Au). Low- and high-magnification SEM images of (b) N-, (c) M-, and (d) N/M-Au (scale bars, 5 µm; inset scale bars, 500 nm). (e) CO selectivity of various gold nanostructures of N/M-, N-, M-, and nt-Au. (f) Cyclic voltammetry (CV) curves of various electrocatalysts in 0.5 M of H2SO4 with a scan rate of 25 mV s−1. (g) Relative mass activity comparing between M-, N-, and N/M-Au. Schematic illustration (left) and the cross-sectional view with the expected reaction pathway (right) for (h) N/M-Au and (i) N-Au electrodes [24].
Other metals in this category have also demonstrated promising CO2RR activity, especially when incorporated into nanostructured or heterostructured architectures. Zn, for instance, is considered a cost-effective and environmentally benign alternative to noble metals like Au and Ag. However, its widespread application has been limited by modest selectivity and activity [21]. Kang and coworkers reported porous, dendritic oxide-derived Zn (OD-Zn) catalysts capable of reducing CO2 to CO with a maximum FE of 86% at −0.95 V vs. RHE and a partial current density of −266 mA cm−2 at −1.00 V vs. RHE [25]. Compared to electrodeposited Zn, OD-Zn exhibits a greater density of undercoordinated sites, leading to enhanced CO2RR activity and selectivity. While oxygen vacancies were previously thought to be key active sites, combined experimental and density functional theory (DFT) studies reveal that Zn sites with high undercoordination offer even greater activity due to their near-optimal *COOH adsorption energies.
3.1.2. Formate-Producing Metals
Another valuable product of the CO2RR is formate (or formic acid), which serves as a hydrogen carrier, chemical feedstock, and potential fuel for direct formate fuel cells [26]. The formation of formate proceeds via a different two-electron reduction pathway, involving the formation and stabilization of the *OCOH intermediate [19]. Metals such as Sn, In, Bi, Cd, Hg, and Pb are known to selectively produce formate [19,26]. Their catalytic behavior is linked to their relatively low d-band centers, which result in weak binding to *CO intermediates and strong stabilization of *OCOH species. These materials generally exhibit low overpotentials and high selectivity toward formate but may suffer from structural instability or toxicity in some cases (e.g., Hg, Cd, Pb).
Sn-based electrocatalysts have been widely studied because of their low cost, earth abundance, and environmental benignity. Nanostructuring Sn or combining it with conductive supports enhances both catalytic activity and long-term durability. For example, high-surface-area tin oxide nanocrystals exhibit selective CO2 reduction to formate at overpotentials as low as ~340 mV [27]. In aqueous NaHCO3 solutions, formate FE exceeding 93% has been achieved on graphene supports with high stability and current densities above 10 mA cm−2. A Sn-Bi2O3 composite demonstrated an FE of 93.4% for formate at −0.97 V vs. RHE and a current density of 24.3 mA cm−2, although its performance declined after 8 h, highlighting the need for improved structural stability [28]. Another example utilized Sn supported on N/P-doped carbon derived from electroplating sludge, achieving a formate FE of 87.9% at −1.05 V vs. RHE and a stable current density of −8.05 mA cm−2 sustained over 105 h [29]. The heteroatom-doped carbon matrix promoted charge transfer and enhanced CO2 adsorption.
Similarly, In-based catalysts show excellent selectivity toward formate, particularly when nanostructured or supported on conductive substrates. A sulfur-doped indium catalyst, for example, delivered >85% FE across a wide current density range (25–100 mA cm−2), achieving a formate production rate of 1449 μmol h−1 cm−2 and a maximum FE of 93%, one of the highest reported to date [30]. Sulfur doping is believed to enhance the CO2RR by accelerating water activation to form hydrogen species that readily react with CO2 to produce formate. This chalcogen-modification strategy may be broadly applicable to other metal catalysts for enhanced activity.
Metal-based electrocatalysts offer a versatile and highly tunable platform for the electrochemical reduction of CO2 into a broad spectrum of value-added products, ranging from CO and formate to multicarbon hydrocarbons and alcohols. Their high electrical conductivity, adjustable surface binding characteristics, and abundance of active sites enable them to overcome the kinetic and thermodynamic barriers inherent in the CO2RR. Metals such as Au, Ag, Zn, and Sn have demonstrated high selectivity toward two-electron products like CO and formate, which can be integrated into renewable energy systems—CO as a syngas component for Fischer–Tropsch synthesis or methanol production, and formate/formic acid as hydrogen carriers in storage and direct formate fuel cells.
3.2. Metal-Free Electrocatalysts
In the pursuit of sustainable and cost-effective solutions for the eCO2RR, metal-free carbon-based electrocatalysts (C-MFECs) have garnered increasing attention as viable alternatives to traditional noble metal systems. These carbon-based materials benefit from natural abundance, structural tunability, and exceptional chemical stability under electrochemical conditions. Unlike noble metals, such as Au, Ag, and Pt, which are expensive and suffer from limited product selectivity, C-MFECs can be rationally engineered through defect creation and heteroatom doping to modulate their local electronic structures, thereby enhancing CO2 adsorption and catalytic activity [31]. This section critically examines two major subcategories of metal-free carbon catalysts: (i) defect-decorated carbon materials, where intrinsic structural irregularities, such as vacancies, edge sites, and topological distortions, act as active centers [31], and (ii) heteroatom-doped carbons, in which elements like nitrogen, boron, sulfur, or phosphorus are incorporated into the lattice to tailor reactivity and product selectivity. For each category, we highlight key advances in synthesis, structure–activity relationships, and product distribution, with emphasis on their mechanistic underpinnings and electrochemical performance. Together, these strategies represent a growing frontier in the rational design of low-cost, efficient, and selective catalysts for the CO2RR under ambient conditions.
3.2.1. Defect-Decorated Carbon Catalysts
Defect-engineered carbon materials represent a compelling category of metal-free electrocatalysts for the CO2RR, where intrinsic structural anomalies serve as catalytically active sites. Defects such as edge sites, vacancies, topological irregularities (e.g., pentagon–heptagon pairs, Stone–Wales defects), and non-hexagonal rings disrupt the sp2-hybridized carbon lattice, creating localized electronic states that facilitate CO2 adsorption and activation [31,32,33]. These perturbations redistribute the local electron density, effectively lowering the free energy barrier for CO2 chemisorption compared to pristine graphene [34]. Machine learning (ML) models are increasingly employed to screen large libraries of defective carbon configurations, enabling quantitative structure–activity correlations that inform defect design for tailored CO2RR selectivity (e.g., CO vs. formate) [33].
The controlled synthesis of defect-decorated carbon catalysts is essential for achieving a high-performance CO2RR. Strategies such as hard and soft templating are frequently employed to introduce hierarchical porosity and defect sites. For example, carbon precursors can be mixed with templating agents (e.g., silica), followed by carbonization and template removal to yield ordered mesoporous or macroporous architectures [35]. Alternative methods like ball milling and plasma etching offer precise control over the density and nature of defects, enabling the formation of edge-rich and topologically diverse active sites [31,36]. One chemical activation approach uses agents like KOH, ZnCl2, and H3PO4 to etch graphitic matrices, generating additional pore structures and exposing under-coordinated carbon atoms [31].
Hierarchical porosity plays a critical role in mass transport and reactant accessibility. Micropores (<2 nm) bring CO2 molecules in close contact with active sites, whereas mesopores (2–50 nm) and macropores enhance electrolyte diffusion and gas transport—factors particularly vital under high current densities for industrial CO2 electrolysis [35].
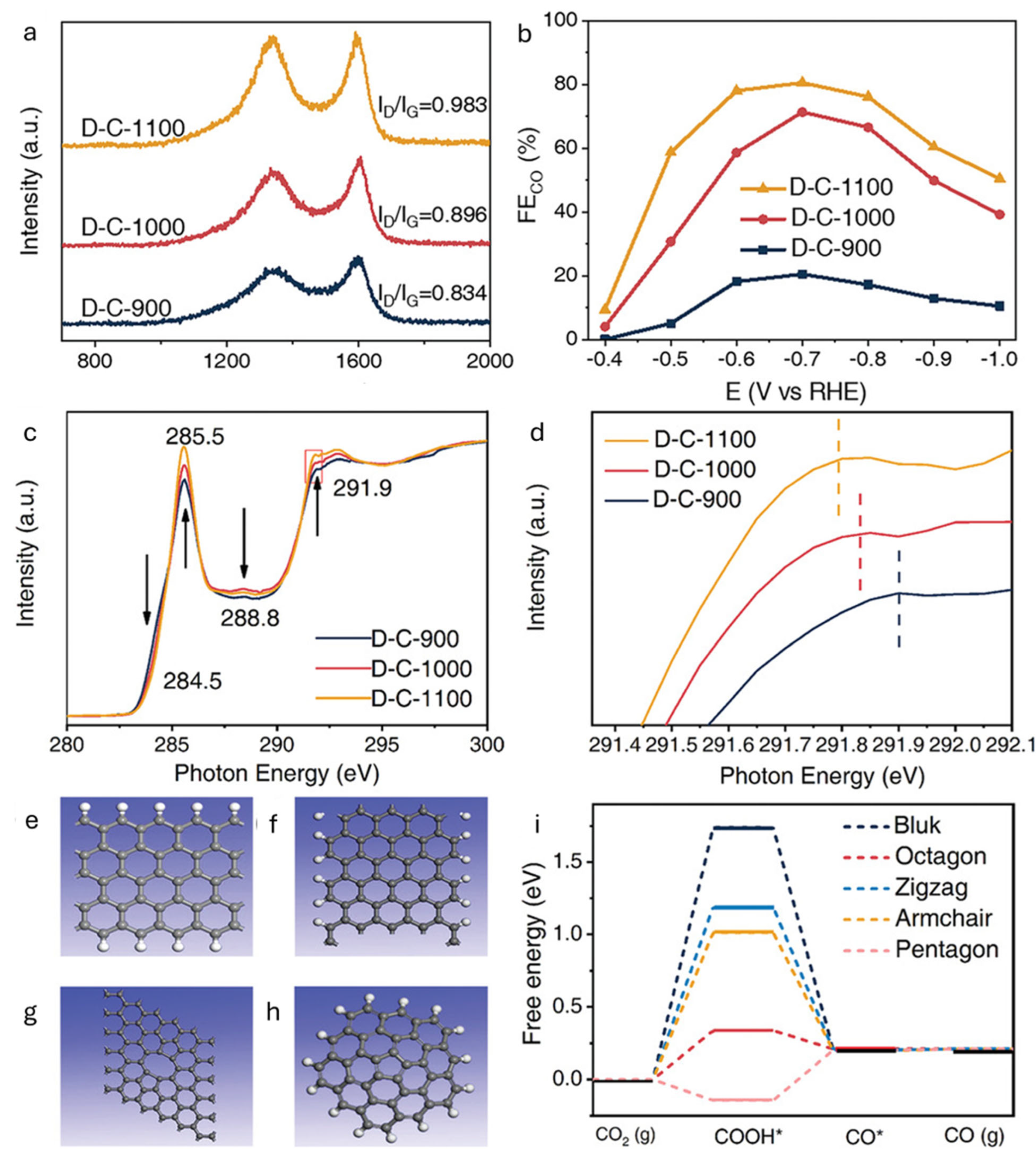
Defect structures such as edge sites and vacancies possess unique electronic properties. Edge atoms often carry unpaired π electrons, facilitating electron transfer to adsorbed CO2 molecules, while vacancies introduce localized charge redistribution, thereby lowering activation barriers [37]. Complex topological features, such as pentagon–octagon–pentagon arrangements, tailor local density of states and orbital overlap, thereby enhancing product specificity [34,38]. Electronic conductivity is also a vital consideration. sp2-hybridized carbons offer efficient charge transport, which can be further improved through partial doping or graphitization [39]. Defect-induced shifts in the Fermi level enhance the electronic coupling with reaction intermediates, promoting faster charge transfer. Importantly, the identity and distribution of defects directly influence product selectivity. Zigzag edges, for example, favor formate production, whereas pentagonal motifs tend to steer selectivity toward CO [33,38]. For example, Wang and coworkers synthesized defective carbon catalysts (D-C-X, where X refers to the pyrolysis temperature) by thermally treating MOF-5 (a metal–organic framework comprising Zn2+ and terephthalic acid) at 900–1100 °C under an Ar atmosphere (Figure 4) [38]. All materials exhibited a high degree of carbon disorder, with D-C-1100 showing the highest FE for CO (80.4% at −0.7 V vs. RHE) compared to D-C-1000 and D-C-900 (Figure 4b). C K-edge NEXAFS spectra revealed that the intensities of peaks associated with sp2-hybridized ring carbons (285.5 and 291.9 eV) increased with pyrolysis temperature, indicating a higher degree of graphitization (Figure 4c). Interestingly, the Raman ID/IG ratio also increased, suggesting that the enhanced D-band intensity originated from an increased concentration of ring defects rather than edge defects (Figure 4a). Moreover, the σ* peak near 291.9 eV shifted to higher energy from D-C-900 to D-C-1100, further confirming the rise in sp2 ring defects (Figure 4d). These findings imply that ring defects, rather than sp3 edge defects, are primarily responsible for the superior CO2RR activity of D-C-1100. DFT calculations further elucidated the role of various defect types, modeling zigzag and armchair graphene nanoribbons (sp3 edge defects) as well as octagonal and pentagonal rings (sp2 ring defects) (Figure 4e–h). The free energy diagrams for the CO2RR to CO showed that, for perfect sp2 carbon, zigzag and armchair edges, and octagonal defects, *COOH formation required additional free energy, whereas pentagonal defects lowered the barrier (Figure 4i). These computational results align with the NEXAFS data, indicating that sp2 ring defects, especially pentagonal motifs, are more active than sp3 edge defects in promoting CO2-to-CO conversion.
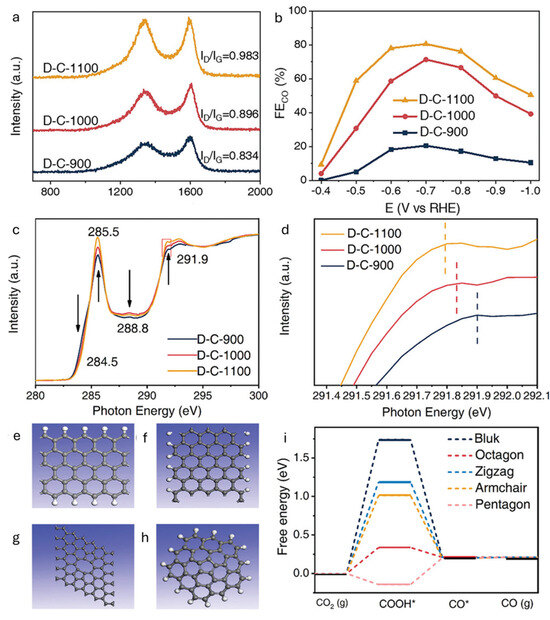
Figure 4.
(a) Raman spectra and ID/IG ratios for D-C-X synthesized at different temperatures. (b) FECO of D-C-X versus applied potential in a CO2-saturated aqueous 0.1 M KHCO3 solution. (c) C K-edge NEXAFS spectra for D-C-X and (d) its expanded view. (e–h) Defect models used for theoretical calculations: (e) zigzag edge, (f) armchair edge, (g) octagonal, and (h) pentagonal. (i) DFT calculations for eCO2RR activities of different defects [38].
By integrating advanced characterization methods with theoretical modeling and ML-guided screening, the rational design of defect-decorated carbon materials can continue to evolve, driving improvements in activity, selectivity, and durability for next-generation CO2 electroreduction.
3.2.2. Heteroatom-Doped Carbon Catalysts
Heteroatom doping represents a powerful strategy for tailoring the electronic structure and catalytic performance of carbon-based materials in the CO2RR. Introducing heteroatoms, such as nitrogen (N), boron (B), sulfur (S), phosphorus (P), or fluorine (F), into the carbon framework disrupts the uniform π-conjugated network and induces local charge polarization, thereby modifying adsorption energies and reaction pathways [31,40,41]. Among these dopants, nitrogen is the most extensively studied, owing to its comparable atomic size to carbon and multiple bonding configurations. Nitrogen doping generates positively charged adjacent carbon sites, with pyridinic-N species exhibiting the highest catalytic activity. For example, pyridinic-N functionalities have been shown to enhance the TOF for CO generation by a factor of 2.6 compared to graphitic-N at equivalent potentials [42]. Carbazole-based covalent triazine frameworks (CTFs) rich in pyridinic-N deliver an FE for CO up to 85% at −0.60 V vs. RHE, demonstrating the importance of site specificity [43]. Boron doping, on the other hand, creates electron-deficient sites that favor *OCHO intermediate binding, thus enhancing formate selectivity. Borocarbonitrides such as BC1.2N0.8 achieve FECO of 98% at −0.45 V with Tafel slopes as low as 87 mV dec−1 [40]. Sulfur and phosphorus dopants influence spin density and introduce geometric distortions, respectively, facilitating unique activation pathways. Co-doping strategies (e.g., N-B, N-S) exploit synergistic effects by establishing multiple active centers with diverse binding affinities. Notably, N,B-codoped nanodiamonds exhibit an FE for ethanol up to 12% at −1.0 V, among the highest reported for purely metal-free systems [44]. Similarly, N,S-codoped carbon spheres have demonstrated a record-low overpotential of 320 mV at 10 mA cm−2 in 0.5 M KHCO3, further highlighting the promise of co-doping strategies [34].
The synthesis of heteroatom-doped carbon materials involves balancing defect density, dopant incorporation, and hierarchical porosity to achieve optimal catalytic performance [35]. Pyrolysis of carbon-rich precursors, such as polymers or biomass, at 600–1000 °C yields partially graphitized structures with tunable nitrogen content [33]. Increasing the pyrolysis temperature from 700 to 900 °C enlarges graphitic domains but significantly reduces surface N content. Chemical activation using agents like KOH, ZnCl2, or H3PO4 etches the carbon matrix to enhance surface area and edge exposure. For example, a KOH:C ratio of 5:1 maximizes edge site accessibility, though excessive activation (e.g., >6:1) can collapse mesoporous structures. Alternatively, ionothermal synthesis in molten salts or ionic liquids serves as both solvent- and structure-directing templates, enabling the formation of N-rich frameworks, such as CTFs [43]. Beyond amorphous carbons, COFs synthesized through polycondensation reactions (e.g., Schiff-base) offer defined architectures with uniformly distributed heteroatoms. Incorporating B or N into the COF backbone yields thermally stable, metal-free catalysts with precisely engineered active sites [35].
The catalytic performance of heteroatom-doped carbon materials is governed by the interplay of structural defects, dopant type, and electronic band alignment. Dopants such as N and B modulate the Fermi level and local density of states, enhancing electronic coupling with CO2 intermediates [33,34]. The sp2-hybridized carbon network ensures high conductivity, which is further augmented by graphitization and partial heteroatom substitution [39].
Different dopants preferentially stabilize distinct intermediates: B dopants promote *OCHO formation and favor formate, whereas N dopants facilitate *COOH adsorption and CO evolution [40,43]. The initiation of the CO2RR typically involves the generation of a bent *CO2− radical or an adsorbed *CO2 species, which undergoes proton-coupled electron transfer toward either *COOH → CO or *OCHO → HCOO− pathways, depending on the active site’s electronic environment [33].
The role of dopant electronegativity and atomic size is crucial. For instance, S and P, having larger atomic radii than carbon, introduce significant local distortions, creating highly reactive sites [34]. These structural distortions, combined with charge redistribution, contribute to the suppression of the competing HER by lowering proton adsorption affinity. DFT calculations reveal that zigzag edges lower the Gibbs free energy (ΔG) for *COOH formation to ~0.56 eV, close to the Sabatier optimum, while B-doped centers stabilize *OCHO intermediates at −0.22 eV [40]. These insights underscore how precise dopant control can dictate reaction pathways and product distribution.
Metal-free carbon-based materials offer a promising avenue for efficient and sustainable CO2 electroreduction, circumventing the cost and scarcity issues associated with traditional metal catalysts. This section reviewed two main classes of carbon-based metal-free electrocatalysts: defect-decorated and heteroatom-doped carbon materials. In both systems, local structural and electronic modifications introduced by defects or dopants significantly enhance CO2 activation and product selectivity. Defect-decorated carbons primarily rely on intrinsic irregularities, such as edge sites, vacancies, and non-hexagonal rings, that disrupt the π-conjugated network to create active sites. These defects lower energy barriers for CO2 adsorption and promote electron transfer, resulting in high selectivity toward CO or formate, depending on the defect type. On the other hand, heteroatom-doped carbons benefit from electronic perturbations introduced by dopants such as N, B, S, and P. These dopants tune local reactivity, shift intermediate binding energies, and enable product steering via single-site or cooperative mechanisms. Synthetic strategies, including templating, activation, and framework polymerization, allow precise control over morphology, porosity, and chemical composition. Both catalysts exhibit high activity and stability under mild conditions, with CO and formate being the predominant products. Future efforts should focus on improving multielectron product formation, mitigating the HER, and integrating operando studies with computational screening to guide the rational design of next-generation metal-free CO2RR catalysts.
4. C2+-Producing Electrocatalysts
Among the diverse range of electrocatalysts explored for the eCO2RR, Cu has emerged as the only metal capable of catalyzing the formation of significant quantities of multicarbon products, such as ethylene, ethanol, n-propanol, and other hydrocarbons and oxygenates [45,46,47,48]. This exceptional behavior has attracted extensive research interest, positioning Cu at the forefront of CO2RR catalyst design. Unlike other metals that predominantly produce CO (e.g., Au, Ag) or formate/formic acid (e.g., In, Sn, Bi), Cu exhibits a unique balance in its binding strength to key intermediates, particularly *CO and *H, enabling complex proton-coupled electron transfer (PCET) pathways and C-C coupling reactions that lead to the generation of C2 and C3 species [49].
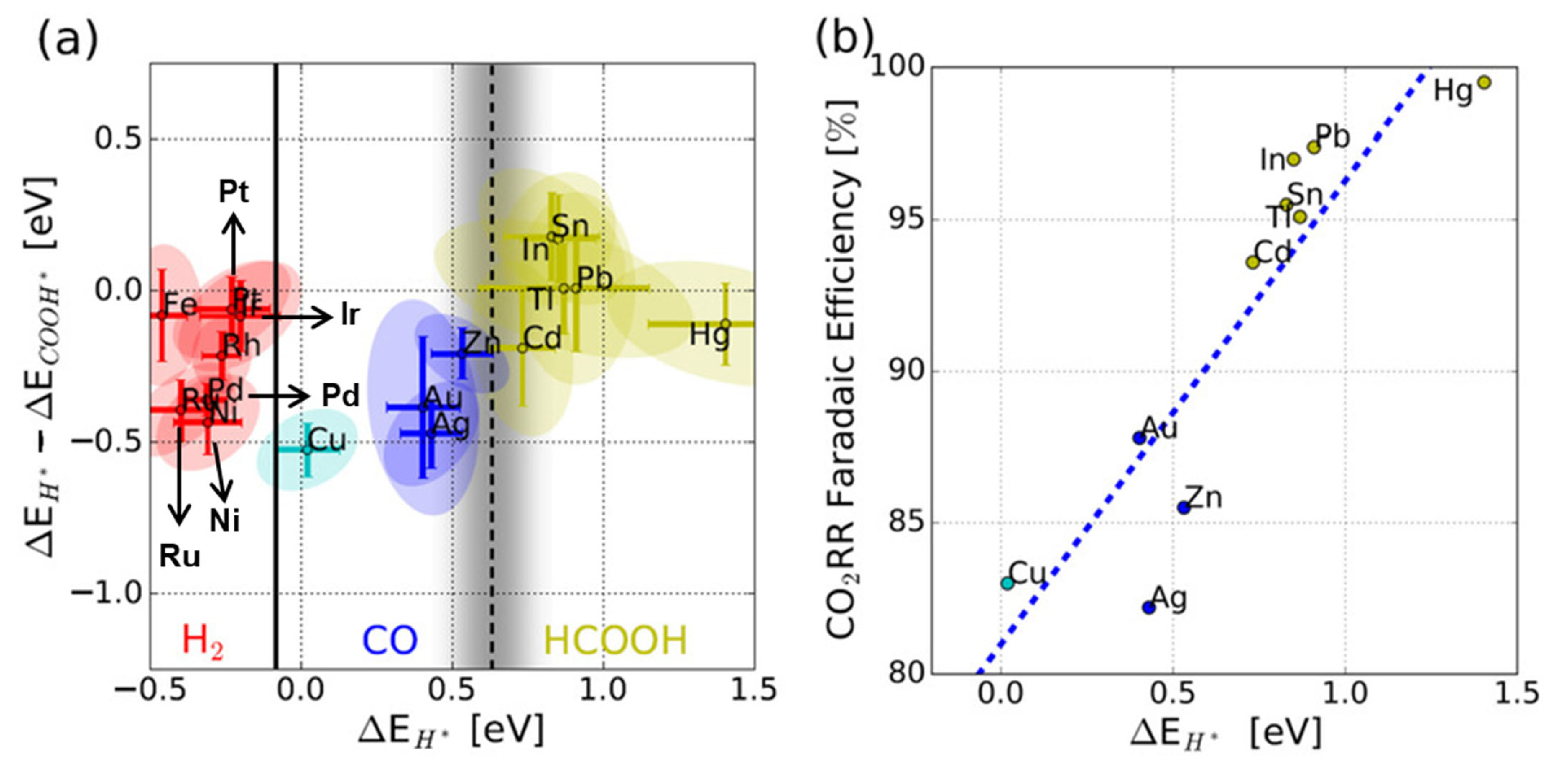
This intermediate binding behavior is further illustrated in studies analyzing the relationship between hydrogen (*H) and *COOH adsorption energies and catalytic product distributions. Bagger and coworkers demonstrated that catalysts with weaker H adsorption (higher ΔEH) tend to suppress the competing HER, thereby promoting selectivity toward carbon-based products (Figure 5) [2]. However, on Cu surfaces, *CO is not tightly bound, leading to a wide distribution of reaction pathways and products. For example, Kuhl and coworkers reported that the electrochemical CO2RR on polycrystalline Cu yields over 16 different products, including 12 distinct C2 and C3 compounds [50]. This reflects both the opportunity and challenge in utilizing Cu for selective CO2 electroreduction.
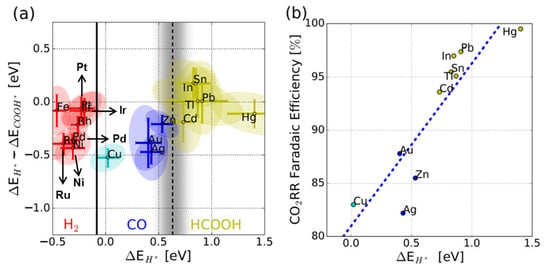
Figure 5.
(a) The experimental product classification of H2, CO, and HCOOH by the ΔEH* − ΔECOOH* and ΔEH* descriptors. (b) The CO2RR FE as a function of ΔEH* [2].
To overcome issues such as poor product selectivity and competition with the HER, researchers have developed a wide variety of Cu-based catalyst architectures and compositions. These include metallic Cu, Cu oxides, Cu-based alloys, and even single-atom Cu catalysts, each tailored to promote specific reaction pathways and enhance overall performance.
4.1. Metallic Cu and Cu-Derived Catalysts
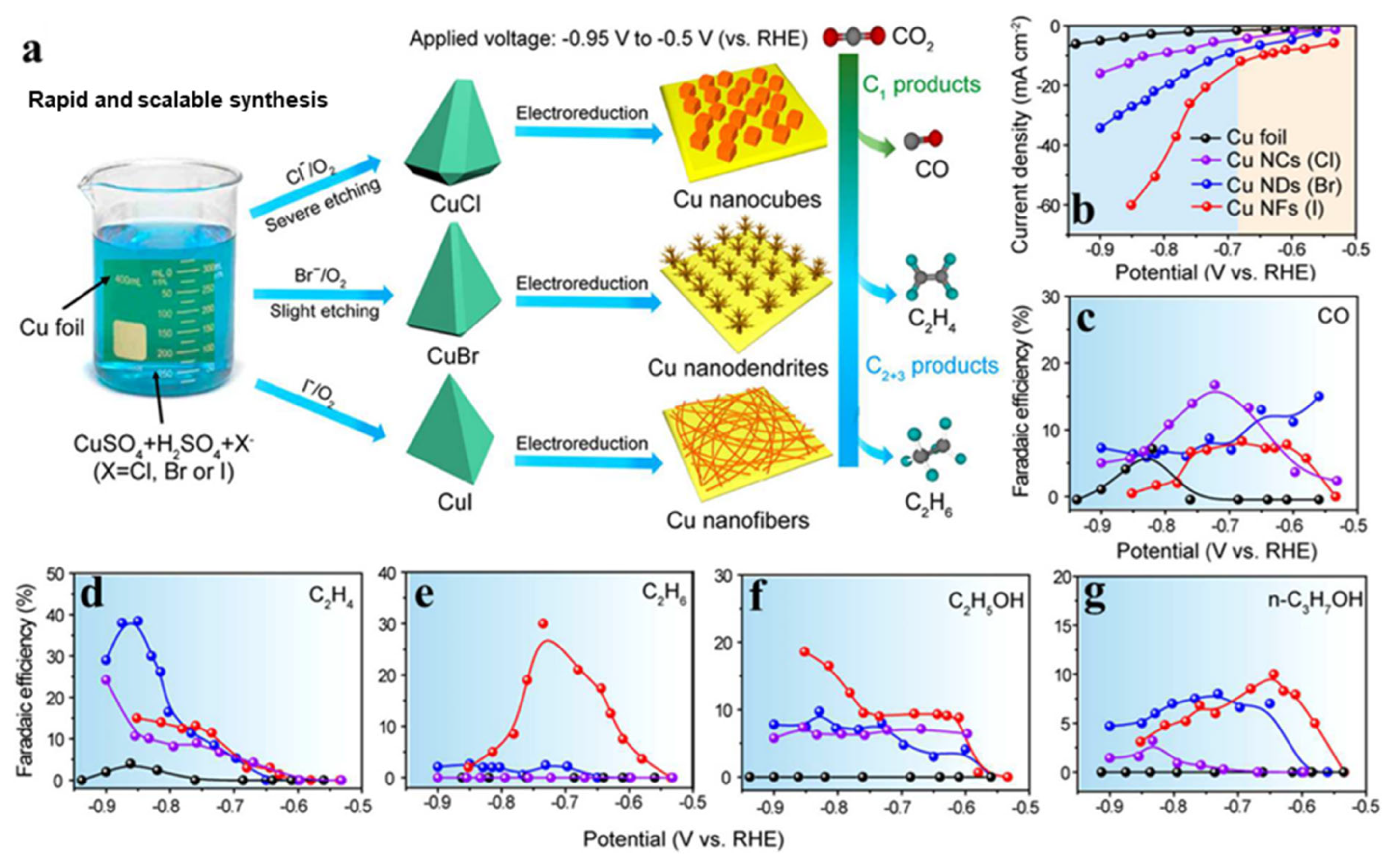
The electrochemical reduction behavior of metallic Cu is highly sensitive to its surface structure, facet exposure, and oxidation state. Engineering these parameters through pre-treatment, oxidation/reduction cycling, and potential-induced restructuring significantly affects product distributions. Notably, the electrochemical reduction of Cu halides (CuCl, CuBr, CuI) into metallic Cu has been shown to produce distinct nanostructures (Figure 6a), such as nanofibers (from CuI), nanodendrites (from CuBr), and nanocubes (from CuCl), with dramatically different activity (Figure 6b) and selectivity (Figure 6c–g) profiles during the CO2RR [51]. For instance, CuBr-derived Cu nanodendrites favor the formation of C1 products at low overpotentials but gradually shift toward C2+ production (particularly ethylene) at more negative potentials. These transformations are closely linked to changes in local coordination environments and roughness of the reconstructed Cu surface.
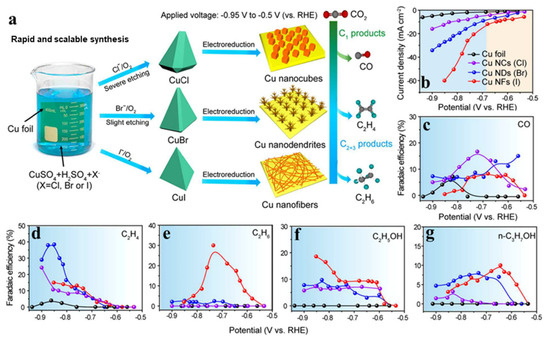
Figure 6.
(a) Scheme illustrating the synthesis of CuCl, CuBr, and CuI electrodes, corresponding Cu nanostructures, and product alterations of the CO2RR due to the distinct Cu morphologies after electrochemical reduction. Electrocatalytic CO2 reduction performance on Cu foil and halide-derived Cu NCs, (b) current densities, and Faradaic efficiencies of (c) CO, (d) C2H4, (e) C2H6, (f), C2H5OH, and (g) n-C3H7OH at a range of −0.5 to −0.95 V applied potential in CO2-saturated 0.1 M KHCO3. Adapted with permission from [51]. Copyright 2019 American Chemical Society.
Furthermore, crystal phase control offers another lever for tuning Cu catalyst performance. Specific crystallographic orientations, such as Cu(100), Cu(111), and Cu(110), exhibit distinct adsorption behaviors and reaction energetics [52,53]. For instance, Cu(100) surfaces are more favorable for *CO dimerization and ethylene formation, while Cu(111) promotes methane production. Engineering the predominance of high-index facets or twin boundaries can thus serve as an effective method for guiding selectivity.
4.2. Cu-Based Alloys and Composition-Tuned Catalysts
Alloying Cu with secondary metal elements is a powerful strategy for tuning both electronic and geometric properties. Common alloying elements include Au, Ag, Sn, Zn, Pb, Ga, and Bi [7,10]. These modifications influence the binding strength of *CO, stabilize C-C coupling intermediates, and adjust the catalyst’s ability to suppress the HER. The resulting improvements in selectivity are attributed to synergistic effects, alloying effects, chemical state changes, and microstructure modulation.
4.3. Cu Oxide-Derived Catalysts
Cu oxides (Cu2O, CuO, CuOH) serve as effective precursors for active Cu sites during in situ reduction under CO2RR conditions. The dynamic transformation from Cu oxides to metallic Cu during the reaction generates high-density grain boundaries, defects, and undercoordinated sites, which are believed to play a crucial role in facilitating C-C bond formation [11,13]. These oxide-derived Cu catalysts often exhibit superior selectivity for ethylene and ethanol compared to pure Cu foil.
4.4. Single-Atom Cu Catalysts
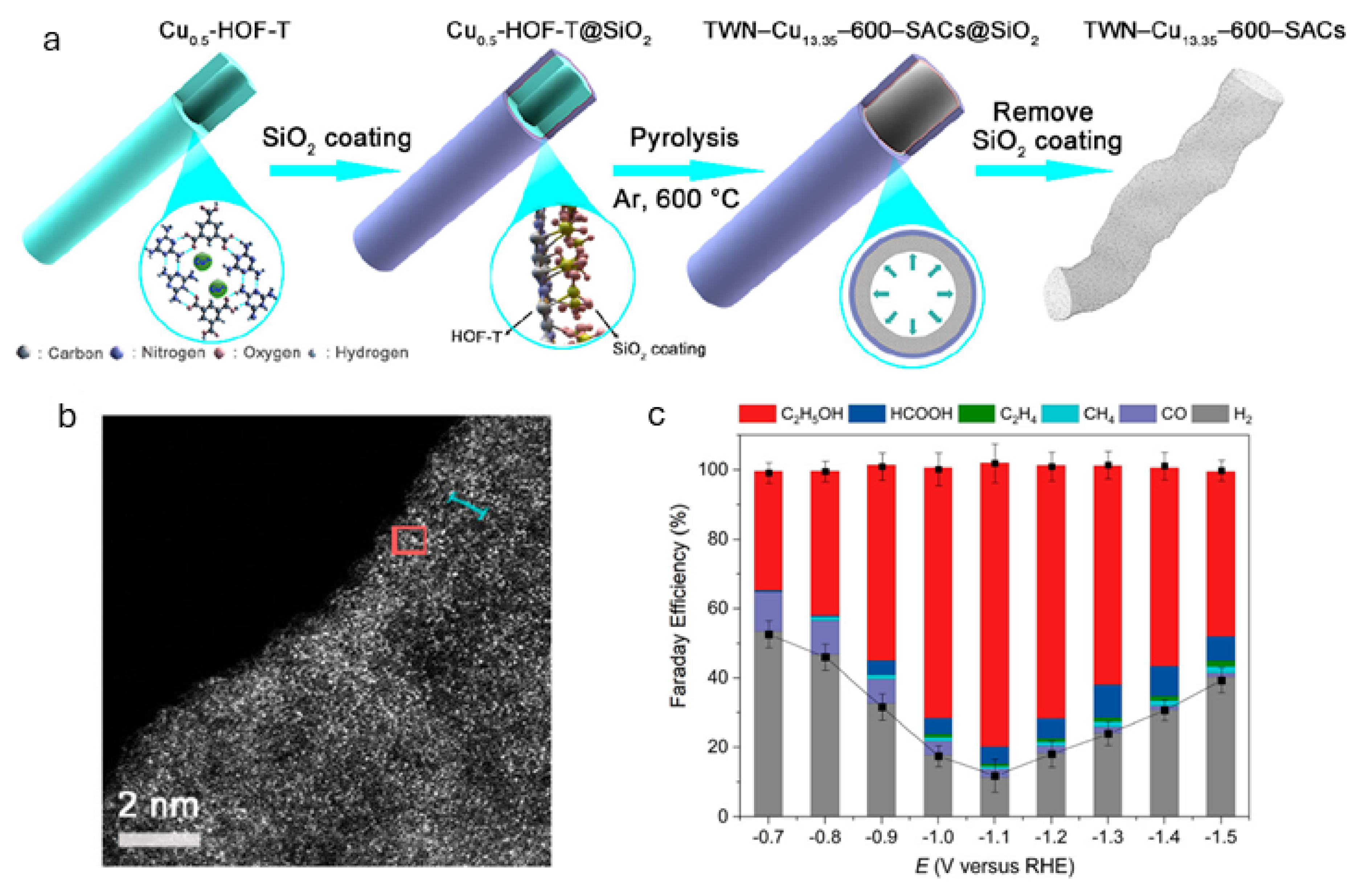
The development of single-atom Cu catalysts represents an exciting frontier in the eCO2RR. These catalysts feature atomically dispersed Cu atoms coordinated with heteroatoms (e.g., N, O, S) on carbon supports or within porous frameworks, such as metal–organic frameworks (MOFs) [7]. For example, Xia and coworkers developed a silica-mediated, hydrogen-bonded organic framework (HOF)-templated method to fabricate ultrahigh-density Cu single-atom catalysts (SACs) supported by thin-walled N-doped carbon nanotubes (TWNs) (Figure 7a,b) [54]. This approach enabled a high Cu loading of up to 13.35 wt. %, with the resulting catalysts achieving an FE of up to 81.9% for ethanol production and a partial current density of 35.6 mA cm−2 in an H-type cell, one of the highest reported performances to date (Figure 7c). The catalyst also demonstrated stable operation over 25 h. Experimental data and DFT calculations identified adjacent Cu-N3 moieties as the active sites responsible for ethanol formation. These closely spaced Cu-N3 units facilitate synergistic C-C coupling, highlighting the importance of high active site density. The remaining challenges for single-atom Cu catalysts include ensuring atomic stability under reductive potentials and enabling efficient multielectron and proton transfer [55]. Continued progress in computational modeling is critical for the rational design of active motifs and for improving selectivity toward multicarbon products.
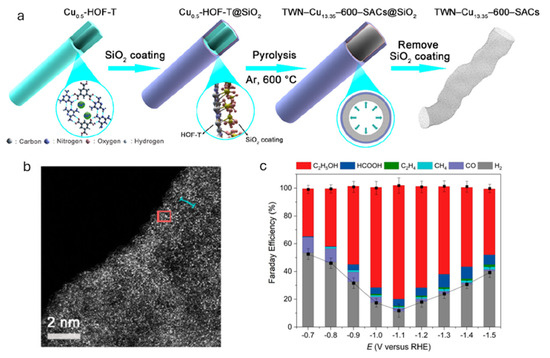
Figure 7.
(a) Schematic illustration of the fabrication processes of the thin-walled nanotubes shaped TWN-Cu13.35-600-SACs catalyst and (b) its representative HAADF-STEM image showing the presence of ultrahigh-density isolated Cu SAs. (c) FE and the product distribution of TWN-Cu13.35-600-SACs at different potentials. The data were averaged over three repeated measurements, with the standard deviations marked by black error bars for the total products. Reprinted with permission from [54]. Copyright 2023 American Chemical Society.
Generally, Cu-based electrocatalysts stand out for their unparalleled ability to produce multicarbon products, such as ethylene, ethanol, and propanol, making them uniquely promising for carbon-neutral petrochemical substitutes. A wide range of Cu catalyst architectures, metallic, oxide-derived, alloyed, and even single-atom Cu, have been explored to tune selectivity and improve performance. Despite significant progress, the field still faces key challenges, including low long-term stability, limited product selectivity control, and the scalable synthesis of catalysts with well-defined surface structures. Looking forward, continued advances in catalyst design, durability enhancement, and system integration are essential to bring metal-based CO2RR technologies closer to commercial viability. Cu-based systems must meet industrial standards in terms of cost, current density, and longevity. The integration of in situ/operando characterization, DFT modeling, and machine learning-guided screening is expected to accelerate the rational development of next-generation electrocatalysts capable of efficiently converting CO2 into fuels and chemicals at scale.
5. Summary and Outlook
Electrochemical CO2 reduction has emerged as a promising strategy to address the dual challenges of mitigating carbon emissions and enabling sustainable chemical production. This review presented a comprehensive overview of the current landscape of the eCO2RR, encompassing fundamental mechanisms, key performance indicators, and the structural and electronic factors that govern catalytic activity. Emphasis was placed on the classification and design of electrocatalysts, metal-based and metal-free, with in-depth discussion on the role of surface properties, local electronic environments, and material composition in determining product selectivity and efficiency.
Metal-based catalysts, including noble metals like Ag and Au, as well as p-block elements, such as Sn and Bi, demonstrate high selectivity toward two-electron products like CO and formate at relatively low overpotentials. In contrast, metal-free catalysts, such as defect-rich or heteroatom-doped carbons, offer a sustainable alternative with considerable advantages in terms of cost, abundance, and tunability. Although metal-free systems currently lag in producing multielectron products, they exhibit high Faradaic efficiency for CO and formate, and co-doping strategies have begun to bridge the gap toward deeper reduction products. Furthermore, Cu-based catalysts, uniquely capable of producing multicarbon products, remain at the forefront of hydrocarbon and oxygenate generation research. Recent advances in Cu nanostructuring and surface modification have shown promise in steering product selectivity toward higher-value compounds.
Despite significant progress, several challenges remain. The intrinsic complexity of the eCO2RR, involving multiple competing pathways and intermediates, makes it difficult to achieve both high activity and selectivity across a wide range of products. Many systems still suffer from high overpotentials, limited current densities, and short operational stability, especially under industrially relevant conditions. Furthermore, the dominant competing reaction, the hydrogen evolution reaction (HER), poses a substantial challenge, particularly in aqueous electrolytes.
Looking forward, further progress in this field will require a multifaceted approach that integrates materials design, mechanistic understanding, and systems engineering. Operando spectroscopy and computational modeling are expected to play increasingly vital roles in elucidating the nature of active sites and the evolution of reaction intermediates. In parallel, machine learning and high-throughput screening offer promising tools for accelerating catalyst discovery and optimization. For practical applications, the development of scalable electrode architectures and efficient cell designs, such as membrane–electrode assemblies, gas diffusion electrodes, and flow cells, will be crucial to enable high-performance CO2 conversion with long-term durability.
Ultimately, realizing the full potential of the eCO2RR will depend on our ability to design robust, selective, and scalable electrocatalyst systems while simultaneously integrating renewable electricity sources. Interdisciplinary collaboration among materials scientists, chemists, engineers, and computational theorists will be essential for translating laboratory breakthroughs into industrial solutions. With continued innovation, electrochemical CO2 reduction stands poised to become a cornerstone of a circular carbon economy and a key technology in the transition toward carbon-neutral energy systems.
Author Contributions
Writing—original draft preparation, L.J., D.A., S.R. and Q.S.; writing—review and editing, visualization, Q.S. and Y.F.; supervision, project administration, and guidance and support, M.Z. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
All the authors were enrolled in the Electrochemistry class (CHME 478/578) in Spring 2025. Yuhuan Fei (co-instructor) made major contributions by organizing the team, guiding the students, and writing and modifying this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wu, J.; Huang, Y.; Ye, W.; Li, Y. CO2 Reduction: From the Electrochemical to Photochemical Approach. Adv. Sci. 2017, 4, 1700194. [Google Scholar] [CrossRef] [PubMed]
- Bagger, A.; Ju, W.; Varela, A.S.; Strasser, P.; Rossmeisl, J. Electrochemical CO2 Reduction: A Classification Problem. ChemPhysChem 2017, 18, 3266–3273. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Jaroniec, M.; Qiao, S.-Z. Cocatalysts in Semiconductor-based Photocatalytic CO2 Reduction: Achievements, Challenges, and Opportunities. Adv. Mater. 2018, 30, 1704649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fan, Q.; Xia, R.; Meyer, T.J. CO2 Reduction: From Homogeneous to Heterogeneous Electrocatalysis. Acc. Chem. Res. 2020, 53, 255–264. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.-J.; Gong, J. Nanostructured Materials for Heterogeneous Electrocatalytic CO2 Reduction and their Related Reaction Mechanisms. Angew. Chem. Int. Ed. 2017, 56, 11326–11353. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Sethuraman, V.; Michalsky, R.; Peterson, A.A. Competition between CO2 Reduction and H2 Evolution on Transition-Metal Electrocatalysts. ACS Catal. 2014, 4, 3742–3748. [Google Scholar] [CrossRef]
- Lu, Q.; Jiao, F. Electrochemical CO2 reduction: Electrocatalyst, reaction mechanism, and process engineering. Nano Energy 2016, 29, 439–456. [Google Scholar] [CrossRef]
- Weekes, D.M.; Salvatore, D.A.; Reyes, A.; Huang, A.; Berlinguette, C.P. Electrolytic CO2 Reduction in a Flow Cell. Acc. Chem. Res. 2018, 51, 910–918. [Google Scholar] [CrossRef]
- Nam, D.-H.; De Luna, P.; Rosas-Hernández, A.; Thevenon, A.; Li, F.; Agapie, T.; Peters, J.C.; Shekhah, O.; Eddaoudi, M.; Sargent, E.H. Molecular enhancement of heterogeneous CO2 reduction. Nat. Mater. 2020, 19, 266–276. [Google Scholar] [CrossRef]
- He, J.; Johnson, N.J.J.; Huang, A.; Berlinguette, C.P. Electrocatalytic Alloys for CO2 Reduction. ChemSusChem 2018, 11, 48–57. [Google Scholar] [CrossRef]
- Raciti, D.; Wang, C. Recent Advances in CO2 Reduction Electrocatalysis on Copper. ACS Energy Lett. 2018, 3, 1545–1556. [Google Scholar] [CrossRef]
- Garza, A.J.; Bell, A.T.; Head-Gordon, M. Mechanism of CO2 Reduction at Copper Surfaces: Pathways to C2 Products. ACS Catal. 2018, 8, 1490–1499. [Google Scholar] [CrossRef]
- Todorova, T.K.; Schreiber, M.W.; Fontecave, M. Mechanistic Understanding of CO2 Reduction Reaction (CO2RR) Toward Multicarbon Products by Heterogeneous Copper-Based Catalysts. ACS Catal. 2020, 10, 1754–1768. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Li, L.; Sun, Y.; Xie, Y. Progress and Perspective for In Situ Studies of CO2 Reduction. J. Am. Chem. Soc. 2020, 142, 9567–9581. [Google Scholar] [CrossRef]
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim. Acta 1994, 39, 1833–1839. [Google Scholar] [CrossRef]
- Hori, Y. Electrochemical CO2 Reduction on Metal Electrodes. In Modern Aspects of Electrochemistry; Vayenas, C.G., White, R.E., Gamboa-Aldeco, M.E., Eds.; Springer: New York, NY, USA, 2008; pp. 89–189. [Google Scholar]
- Yu, M.; Sui, P.-F.; Fu, X.-Z.; Luo, J.-L.; Liu, S. Specific Metal Nanostructures toward Electrochemical CO2 Reduction: Recent Advances and Perspectives. Adv. Energy Mater. 2023, 13, 2203191. [Google Scholar] [CrossRef]
- Vijay, S.; Ju, W.; Brückner, S.; Tsang, S.-C.; Strasser, P.; Chan, K. Unified mechanistic understanding of CO2 reduction to CO on transition metal and single atom catalysts. Nat. Catal. 2021, 4, 1024–1031. [Google Scholar] [CrossRef]
- Han, N.; Ding, P.; He, L.; Li, Y.; Li, Y. Promises of Main Group Metal-Based Nanostructured Materials for Electrochemical CO2 Reduction to Formate. Adv. Energy Mater. 2020, 10, 1902338. [Google Scholar] [CrossRef]
- Dutta, N.; Bagchi, D.; Chawla, G.; Peter, S.C. A Guideline to Determine Faradaic Efficiency in Electrochemical CO2 Reduction. ACS Energy Lett. 2024, 9, 323–328. [Google Scholar] [CrossRef]
- Zheng, T.; Jiang, K.; Wang, H. Recent Advances in Electrochemical CO2-to-CO Conversion on Heterogeneous Catalysts. Adv. Mater. 2018, 30, 1802066. [Google Scholar] [CrossRef]
- Tao, Z.; Pearce, A.J.; Mayer, J.M.; Wang, H. Bridge Sites of Au Surfaces Are Active for Electrocatalytic CO2 Reduction. J. Am. Chem. Soc. 2022, 144, 8641–8648. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Song, J.T.; Ryoo, H.; Kim, J.-G.; Chung, S.-Y.; Oh, J. Morphology-controlled Au nanostructures for efficient and selective electrochemical CO2 reduction. J. Mater. Chem. A 2018, 6, 5119–5128. [Google Scholar] [CrossRef]
- Hyun, G.; Song, J.T.; Ahn, C.; Ham, Y.; Cho, D.; Oh, J.; Jeon, S. Hierarchically porous Au nanostructures with interconnected channels for efficient mass transport in electrocatalytic CO2 reduction. Proc. Natl. Acad. Sci. USA 2020, 117, 5680–5685. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.P.L.; Kolb, M.J.; Calle-Vallejo, F.; Yeo, B.S. The Role of Undercoordinated Sites on Zinc Electrodes for CO2 Reduction to CO. Adv. Funct. Mater. 2022, 32, 2111597. [Google Scholar] [CrossRef]
- Wang, W.-H.; Himeda, Y.; Muckerman, J.T.; Manbeck, G.F.; Fujita, E. CO2 Hydrogenation to Formate and Methanol as an Alternative to Photo- and Electrochemical CO2 Reduction. Chem. Rev. 2015, 115, 12936–12973. [Google Scholar] [CrossRef]
- Zhang, S.; Kang, P.; Meyer, T.J. Nanostructured Tin Catalysts for Selective Electrochemical Reduction of Carbon Dioxide to Formate. J. Am. Chem. Soc. 2014, 136, 1734–1737. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Li, J.; Huang, J.; Ji, L.; Leng, Z.; Qian, N.; Yang, D.; Zhang, H. Sn-Doped Bi2O3 nanosheets for highly efficient electrochemical CO2 reduction toward formate production. Nanoscale 2021, 13, 19610–19616. [Google Scholar] [CrossRef]
- Zhong, X.; Zhong, Z.; Liang, S.; Zeng, G.; Cheng, S.; Deng, H.; Lin, Z. Towards a broad-operation window for stable CO2 electroreduction to HCOOH by a design involving upcycling electroplating sludge-derived Sn@N/P-doped carbon. Environ. Sci. Nano 2022, 9, 511–522. [Google Scholar] [CrossRef]
- Ma, W.; Xie, S.; Zhang, X.-G.; Sun, F.; Kang, J.; Jiang, Z.; Zhang, Q.; Wu, D.-Y.; Wang, Y. Promoting electrocatalytic CO2 reduction to formate via sulfur-boosting water activation on indium surfaces. Nat. Commun. 2019, 10, 892. [Google Scholar] [CrossRef]
- Xue, D.; Xia, H.; Yan, W.; Zhang, J.; Mu, S. Defect Engineering on Carbon-Based Catalysts for Electrocatalytic CO2 Reduction. Nano-Micro Lett. 2020, 13, 5. [Google Scholar] [CrossRef]
- Zhu, J.; Mu, S. Defect Engineering in Carbon-Based Electrocatalysts: Insight into Intrinsic Carbon Defects. Adv. Funct. Mater. 2020, 30, 2001097. [Google Scholar] [CrossRef]
- Kim, C.; Talapaneni, S.N.; Dai, L. Porous carbon materials for CO2 capture, storage and electrochemical conversion. Mater. Rep. Energy 2023, 3, 100199. [Google Scholar] [CrossRef]
- Zhai, Q.; Huang, H.; Lawson, T.; Xia, Z.; Giusto, P.; Antonietti, M.; Jaroniec, M.; Chhowalla, M.; Baek, J.-B.; Liu, Y.; et al. Recent Advances on Carbon-Based Metal-Free Electrocatalysts for Energy and Chemical Conversions. Adv. Mater. 2024, 36, 2405664. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Mohanty, B.; Nurhuda, M.; Dalapati, S.; Jana, R.; Addicoat, M.; Datta, A.; Jena, B.K.; Bhaumik, A. A Thiadiazole-Based Covalent Organic Framework: A Metal-Free Electrocatalyst toward Oxygen Evolution Reaction. ACS Catal. 2020, 10, 5623–5630. [Google Scholar] [CrossRef]
- Yuan, R.; Dong, Y.; Hou, R.; Shang, L.; Zhang, J.; Zhang, S.; Chen, X.; Song, H. Structural transformation of porous and disordered carbon during ball-milling. Chem. Eng. J. 2023, 454, 140418. [Google Scholar] [CrossRef]
- Argirusis, C.; Alizadeh, N.; Katsanou, M.-Ε.; Argirusis, N.; Sourkouni, G. Advances in Metal-Organic Frameworks (MOFs) for Rechargeable Batteries and Fuel Cells. Batteries 2025, 11, 192. [Google Scholar] [CrossRef]
- Wang, W.; Shang, L.; Chang, G.; Yan, C.; Shi, R.; Zhao, Y.; Waterhouse, G.I.N.; Yang, D.; Zhang, T. Intrinsic Carbon-Defect-Driven Electrocatalytic Reduction of Carbon Dioxide. Adv. Mater. 2019, 31, 1808276. [Google Scholar] [CrossRef]
- Rasool, F.; Pirzada, B.M.; Talib, S.H.; Alkhidir, T.; Anjum, D.H.; Mohamed, S.; Qurashi, A. In Situ Growth of Interfacially Nanoengineered 2D-2D WS2/Ti3C2Tx MXene for the Enhanced Performance of Hydrogen Evolution Reactions. ACS Appl. Mater. Interfaces 2024, 16, 14229–14242. [Google Scholar] [CrossRef]
- Ayyub, M.M.; Rao, C.N.R. Borocarbonitrides As Metal-Free Electrocatalysts for the Electrochemical Reduction of CO2. Chem. Mater. 2022, 34, 6626–6635. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, W.; Ma, C.; Zhang, C.; Li, C.; Hong, Y.; Sun, Y.; Niu, J.; Guo, S.; Yao, S. Nitrogen/Phosphorus/Fluorine Heteroatoms Codoped Carbon Nanotube Networks as Free-Standing Cathode for Rechargeable Li-CO2 Batteries. ACS Appl. Nano Mater. 2025, 8, 1499–1507. [Google Scholar] [CrossRef]
- Jiang, M.; Yu, X.; Yang, H.; Chen, S. Optimization strategies of preparation of biomass-derived carbon electrocatalyst for boosting oxygen reduction reaction: A minireview. Catalysts 2020, 10, 1472. [Google Scholar] [CrossRef]
- Yu, W.; Gu, S.; Fu, Y.; Xiong, S.; Pan, C.; Liu, Y.; Yu, G. Carbazole-decorated covalent triazine frameworks: Novel nonmetal catalysts for carbon dioxide fixation and oxygen reduction reaction. J. Catal. 2018, 362, 1–9. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Z.; Suo, J.; Liao, L.; Li, L.; Yu, Z.; Zhang, H.; Valtchev, V.; Qiu, S.; Fang, Q. Exploring metal-free ionic covalent organic framework nanosheets as efficient OER electrocatalysts via cationic-π interactions. Chem. Eng. J. 2023, 478, 147403. [Google Scholar] [CrossRef]
- Tan, D.; Zhang, J.; Yao, L.; Tan, X.; Cheng, X.; Wan, Q.; Han, B.; Zheng, L.; Zhang, J. Multi-shelled CuO microboxes for carbon dioxide reduction to ethylene. Nano Res. 2020, 13, 768–774. [Google Scholar] [CrossRef]
- Altaf, N.; Liang, S.; Huang, L.; Wang, Q. Electro-derived Cu-Cu2O nanocluster from LDH for stable and selective C2 hydrocarbons production from CO2 electrochemical reduction. J. Energy Chem. 2020, 48, 169–180. [Google Scholar] [CrossRef]
- Rayer, A.V.; Reid, E.; Kataria, A.; Luz, I.; Thompson, S.J.; Lail, M.; Zhou, J.; Soukri, M. Electrochemical carbon dioxide reduction to isopropanol using novel carbonized copper metal organic framework derived electrodes. J. CO2 Util. 2020, 39, 101159. [Google Scholar] [CrossRef]
- Ebaid, M.; Jiang, K.; Zhang, Z.; Drisdell, W.S.; Bell, A.T.; Cooper, J.K. Production of C2/C3 Oxygenates from Planar Copper Nitride-Derived Mesoporous Copper via Electrochemical Reduction of CO2. Chem. Mater. 2020, 32, 3304–3311. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, J. Architectural Design for Enhanced C2 Product Selectivity in Electrochemical CO2 Reduction Using Cu-Based Catalysts: A Review. ACS Nano 2021, 15, 7975–8000. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059. [Google Scholar] [CrossRef]
- Wang, H.; Matios, E.; Wang, C.; Luo, J.; Lu, X.; Hu, X.; Li, W. Rapid and Scalable Synthesis of Cuprous Halide-Derived Copper Nano-Architectures for Selective Electrochemical Reduction of Carbon Dioxide. Nano Lett. 2019, 19, 3925–3932. [Google Scholar] [CrossRef]
- Quan, Z.; Wang, Y.; Fang, J. High-Index Faceted Noble Metal Nanocrystals. Acc. Chem. Res. 2013, 46, 191–202. [Google Scholar] [CrossRef]
- Woldu, A.R. From low to high-index facets of noble metal nanocrystals: A way forward to enhance the performance of electrochemical CO2 reduction. Nanoscale 2020, 12, 8626–8635. [Google Scholar] [CrossRef]
- Xia, W.; Xie, Y.; Jia, S.; Han, S.; Qi, R.; Chen, T.; Xing, X.; Yao, T.; Zhou, D.; Dong, X.; et al. Adjacent Copper Single Atoms Promote C–C Coupling in Electrochemical CO2 Reduction for the Efficient Conversion of Ethanol. J. Am. Chem. Soc. 2023, 145, 17253–17264. [Google Scholar] [CrossRef]
- Yang, C.-H.; Nosheen, F.; Zhang, Z.-C. Recent progress in structural modulation of metal nanomaterials for electrocatalytic CO2 reduction. Rare Met. 2021, 40, 1412–1430. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).