Synthesis of Some Novel Cr(III), Mn(II), and Pd(II) Complexes via the Sono-Chemical Route with a Chlorinated Quinolinyl-Imine Ligand: Structural Elucidation, Bioactivity Analysis, and Docking Simulations
Abstract
1. Introduction
2. Result and Discussion
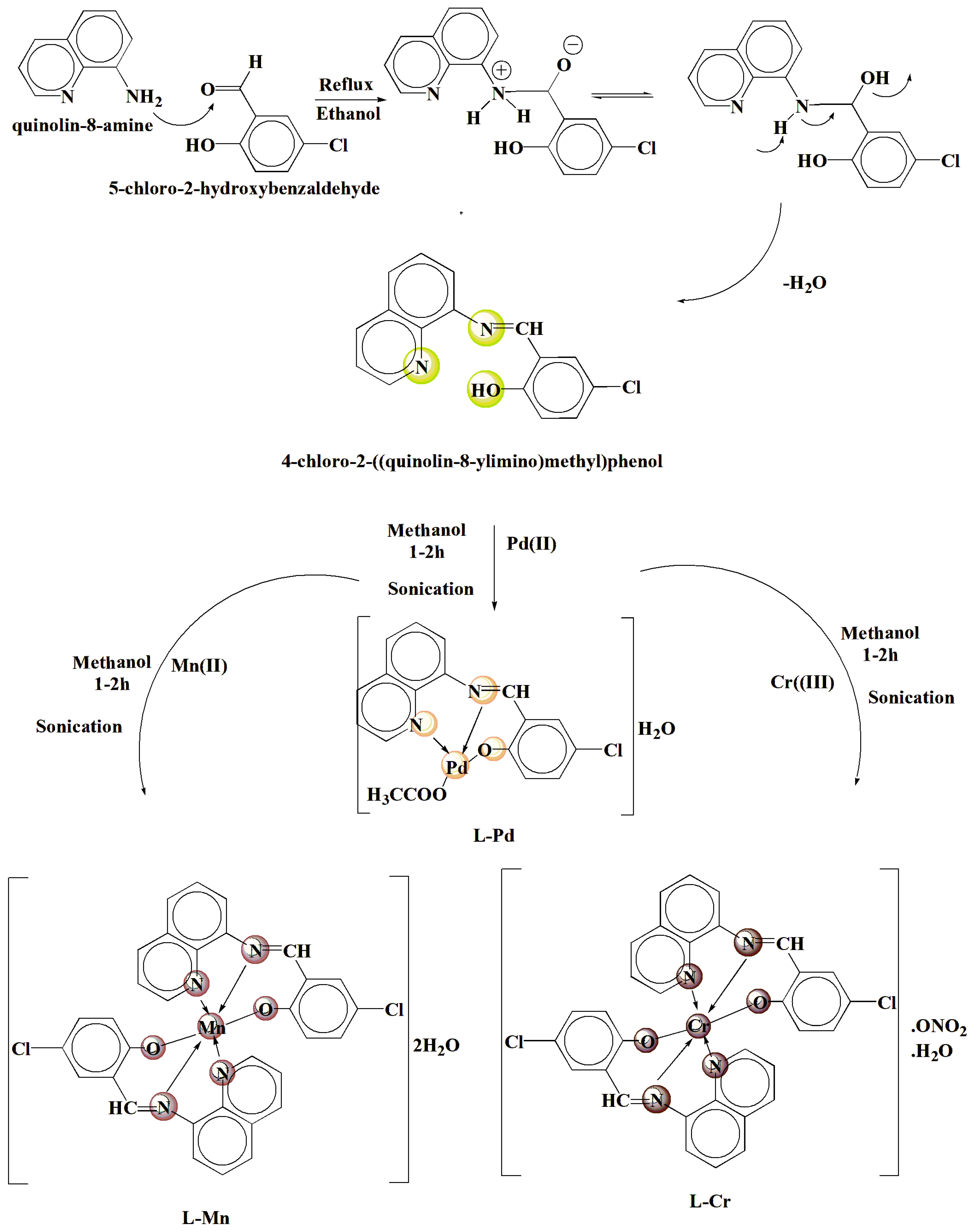
2.1. Characterization of 4-Chloro-2-(Quinolin-8-Yliminomethyl)-Phenol Ligand and Its Complexes
2.2. FTIR Spectrum
2.3. 1H-NMR and 13C-NMR Spectral Evaluations
2.4. Elemental Analysis and Molar Conductance
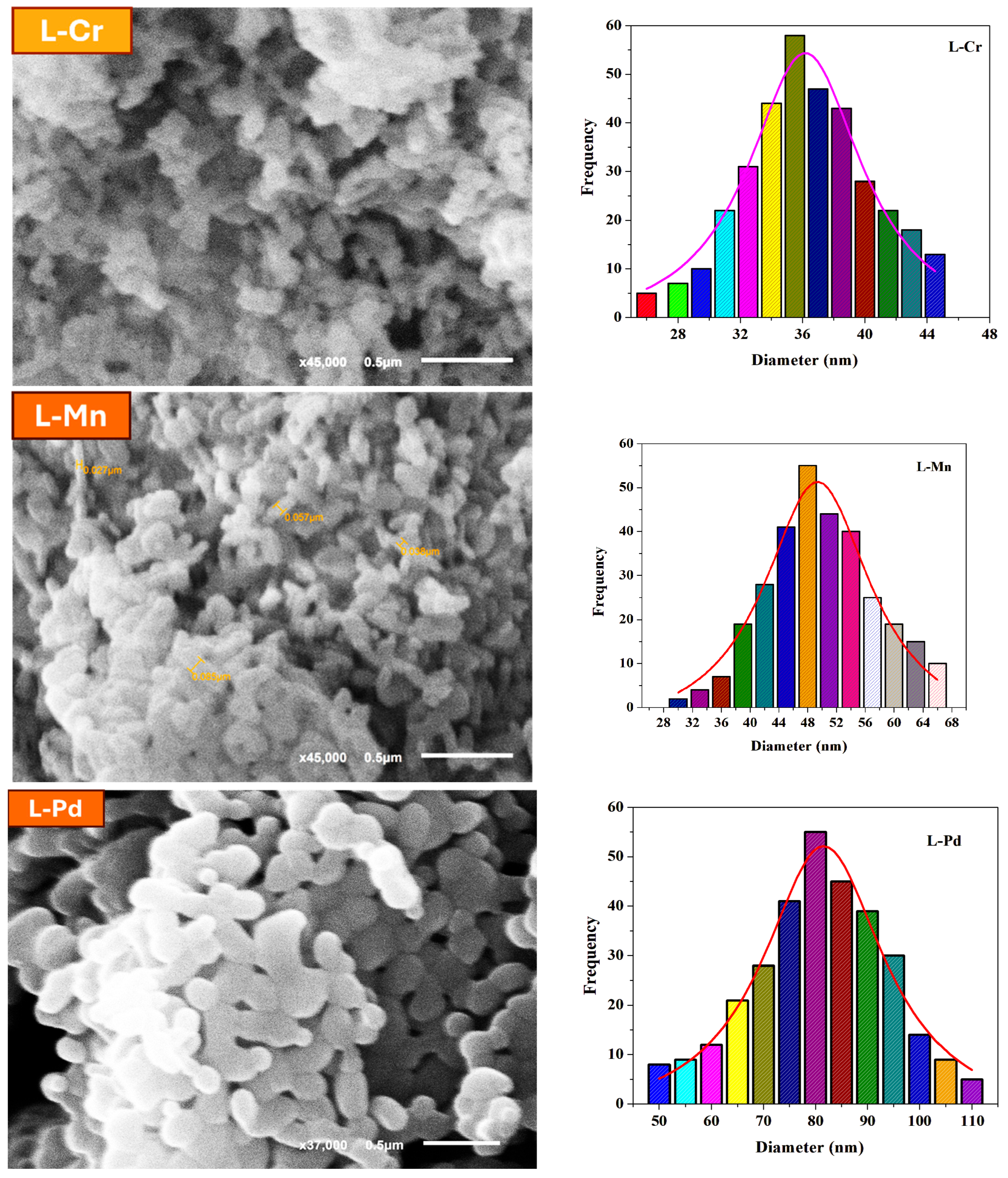
2.5. SEM Analysis of the Prepared Nanosized Cr(III), Mn(II), and Pd(II) Metal Complexes
- The SEM images of the Cr(III) complex showed an aggregated but uniformly distributed morphology with spherical-like nanosized particles. The observed structures suggest a tendency for slight agglomeration due to intermolecular interactions.
- The Mn(II) complex displayed rod-like or irregular morphology, indicating variations in nucleation and growth mechanisms during sono-chemical synthesis.
- The Pd(II) complex exhibited a well-dispersed, granular, and slightly crystalline nanostructure. The smaller particle size observed for Pd(II) could be attributed to the strong coordination interactions between the Schiff base ligand and the palladium ion, stabilizing the nanostructures effectively.
2.6. Electronic Absorption Spectra (EAS)
2.7. Magnetic Moment
2.8. Thermal Analysis
Kinetic Parameter
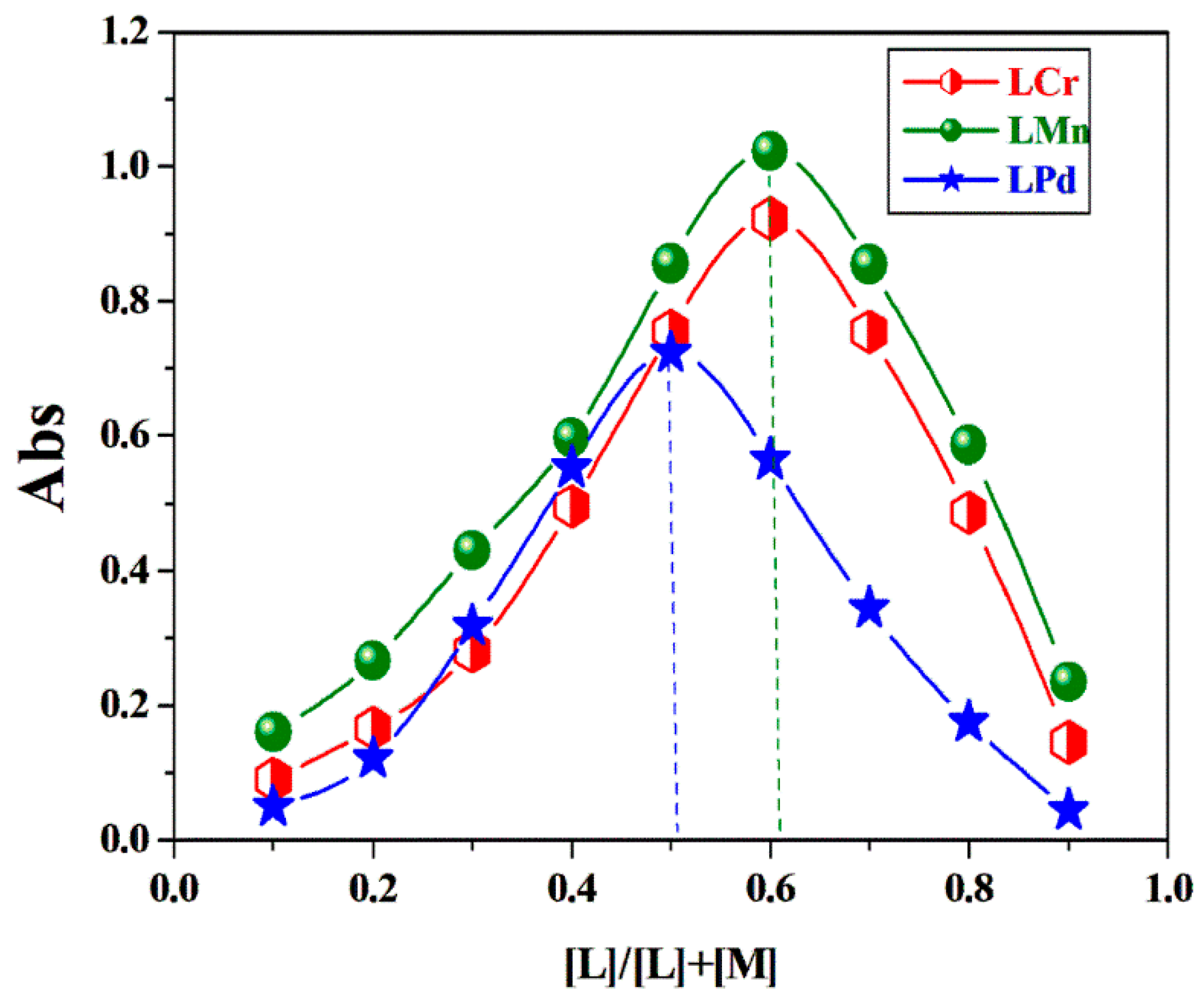
2.9. Stoichiometry of Complexes in Solution
2.10. The Apparent Formation Constants of the Synthesized Complexes
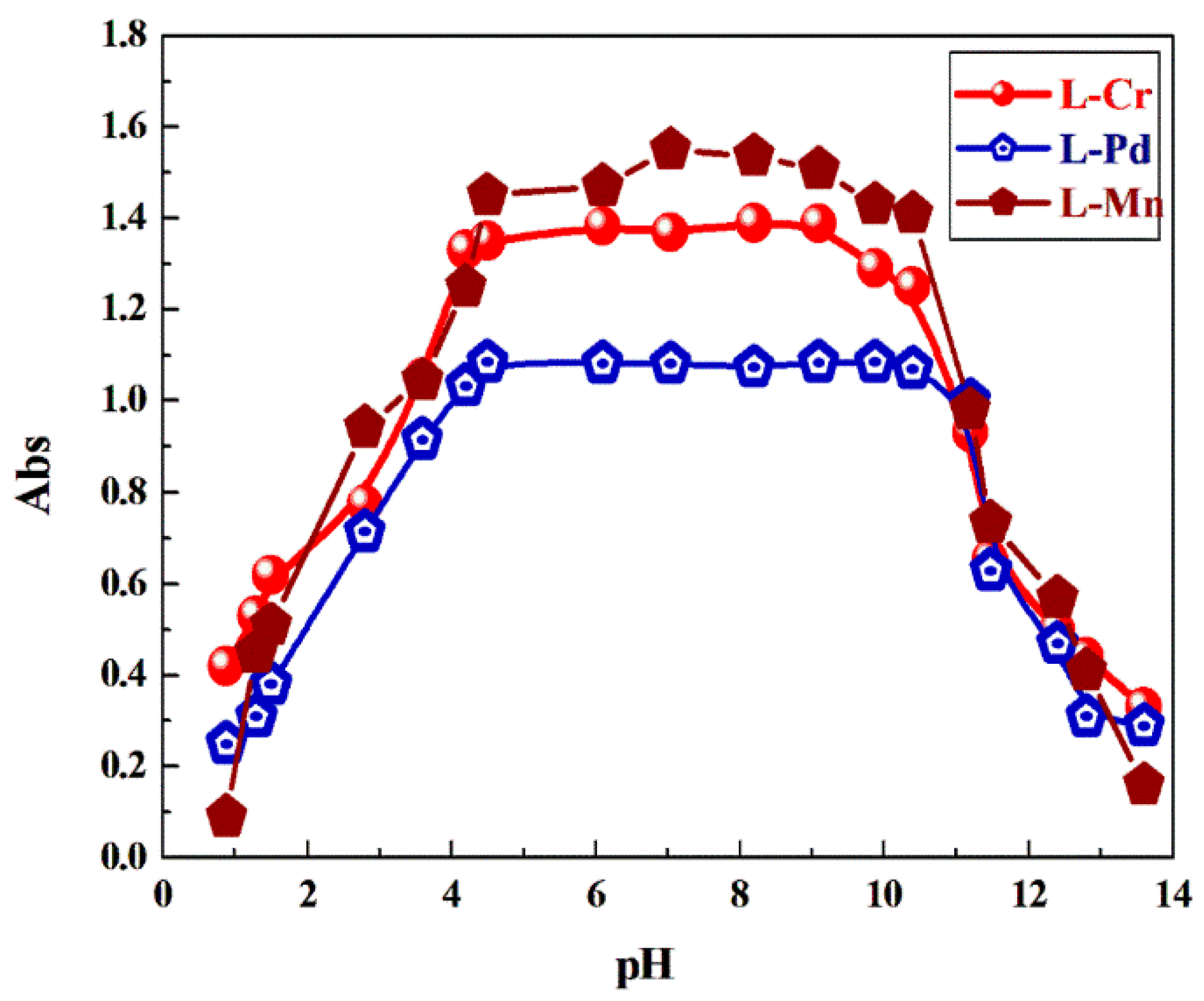
2.11. pH Profile of the Investigated Complexes
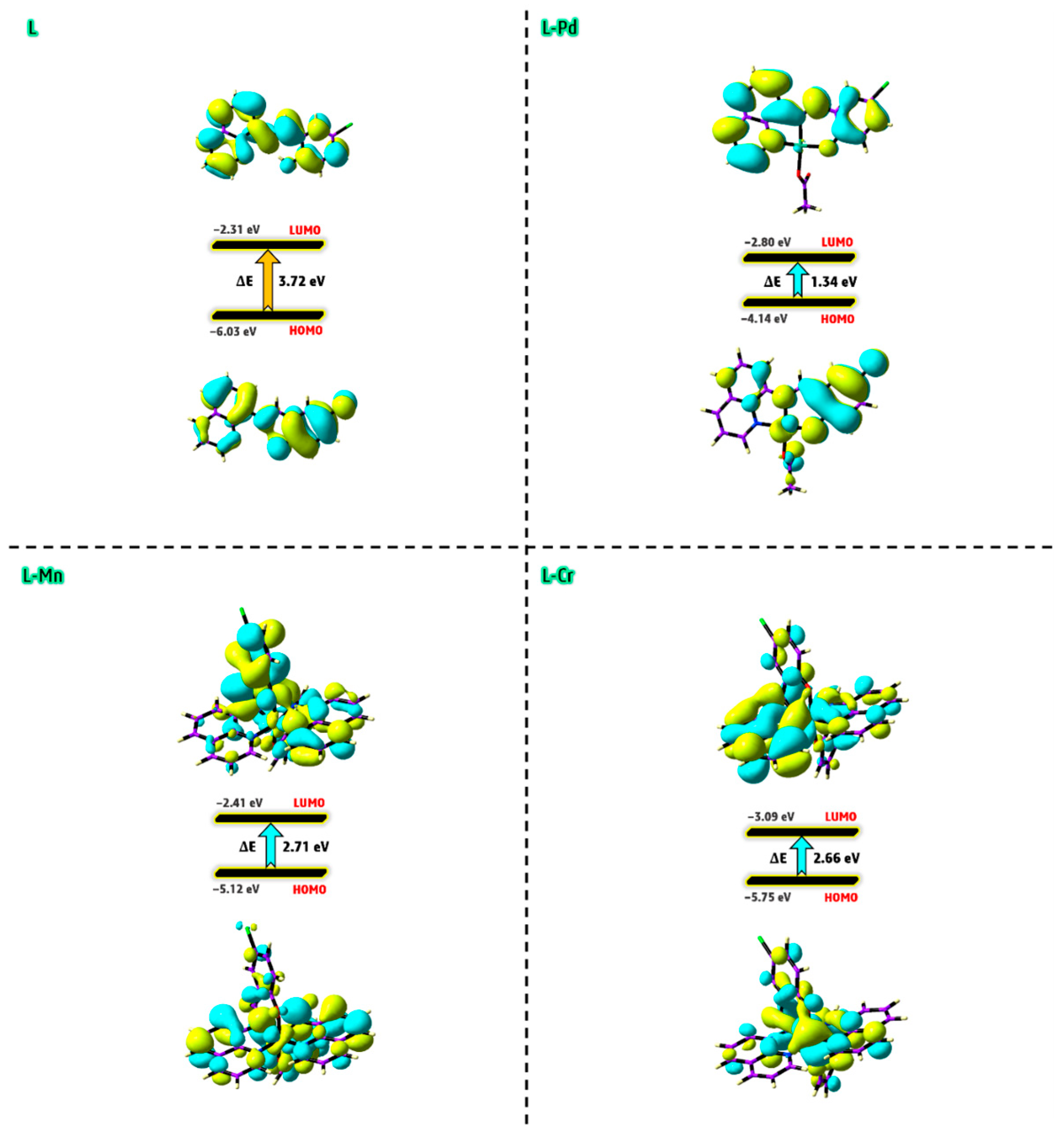
2.12. DFT Details
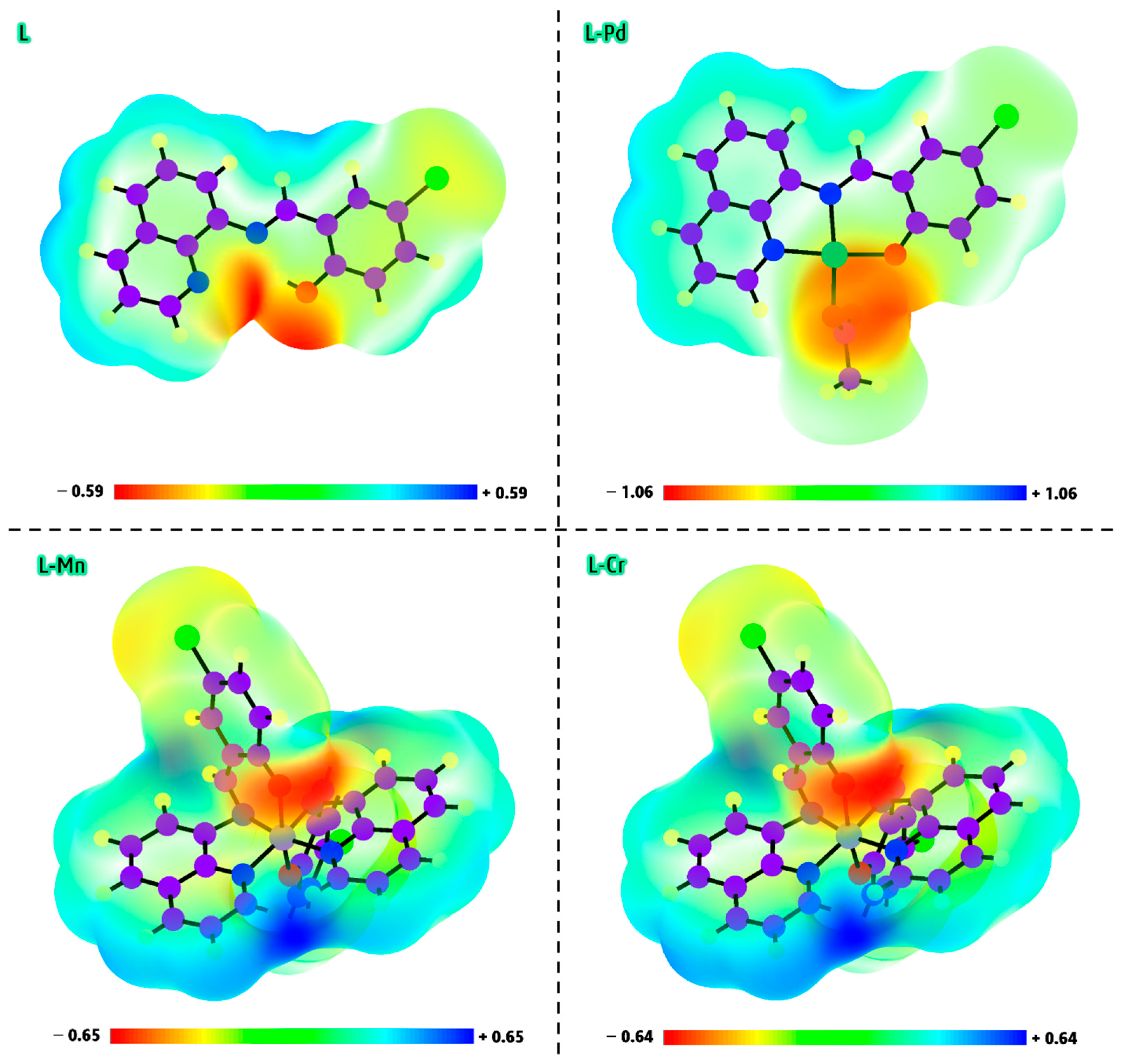
2.12.1. Geometry Optimization and Mulliken Charge
2.12.2. Electrophilic and Nucleophilic Reaction Sites
2.12.3. Molecular Orbital Analysis
2.12.4. Physicochemical Parameters
- (i)
- The degree of electron transfer within a compound can be assessed using the additional electronic charge parameter (ΔNmax), which measures a molecule’s tendency to accept electrons from another species. Based on this parameter, the results suggest that the L-Pd, L-Mn, and L-Cr complexes possess enhanced electron transfer capabilities compared to the free ligand (L). Among these, the Pd(II) complex exhibits the highest electron-accepting ability, highlighting its superior electronic properties.
- (ii)
- Balancing a compound’s chemical reactivity with its hardness or softness is essential in determining its interaction potential. The Hard-Soft Acid-Base (HSAB) principle offers valuable insights into molecular reactivity, suggesting that hard acids preferentially bind to hard bases, while soft acids form more stable interactions with soft bases. In biological systems, key components such as cells and proteins are classified as soft molecules, making them more likely to interact with other soft molecules rather than hard ones. This explains why softer chemical environments generally enhance biological activity, whereas harder environments tend to suppress it [48]. Based on chemical hardness and softness parameters, the predicted reactivity trend for the studied compounds follows the order of L-Pd > L-Cr > L-Mn > L (Table 4), indicating that L-Pd exhibits the highest reactivity among them.
- (iii)
- The global electrophilicity index (ω) quantifies a molecule’s ability to accept electrons, classifying it as strong (ω > 1.5 eV), moderate (0.9 eV < ω < 1.4 eV), or marginal (ω < 0.8 eV). The electrophilicity index values for the studied metal complexes range from 5.23 to 8.98 eV, confirming their strong electrophilic nature. This result suggests that these complexes possess significant reactivity, which may contribute to their potential biological activity [49].
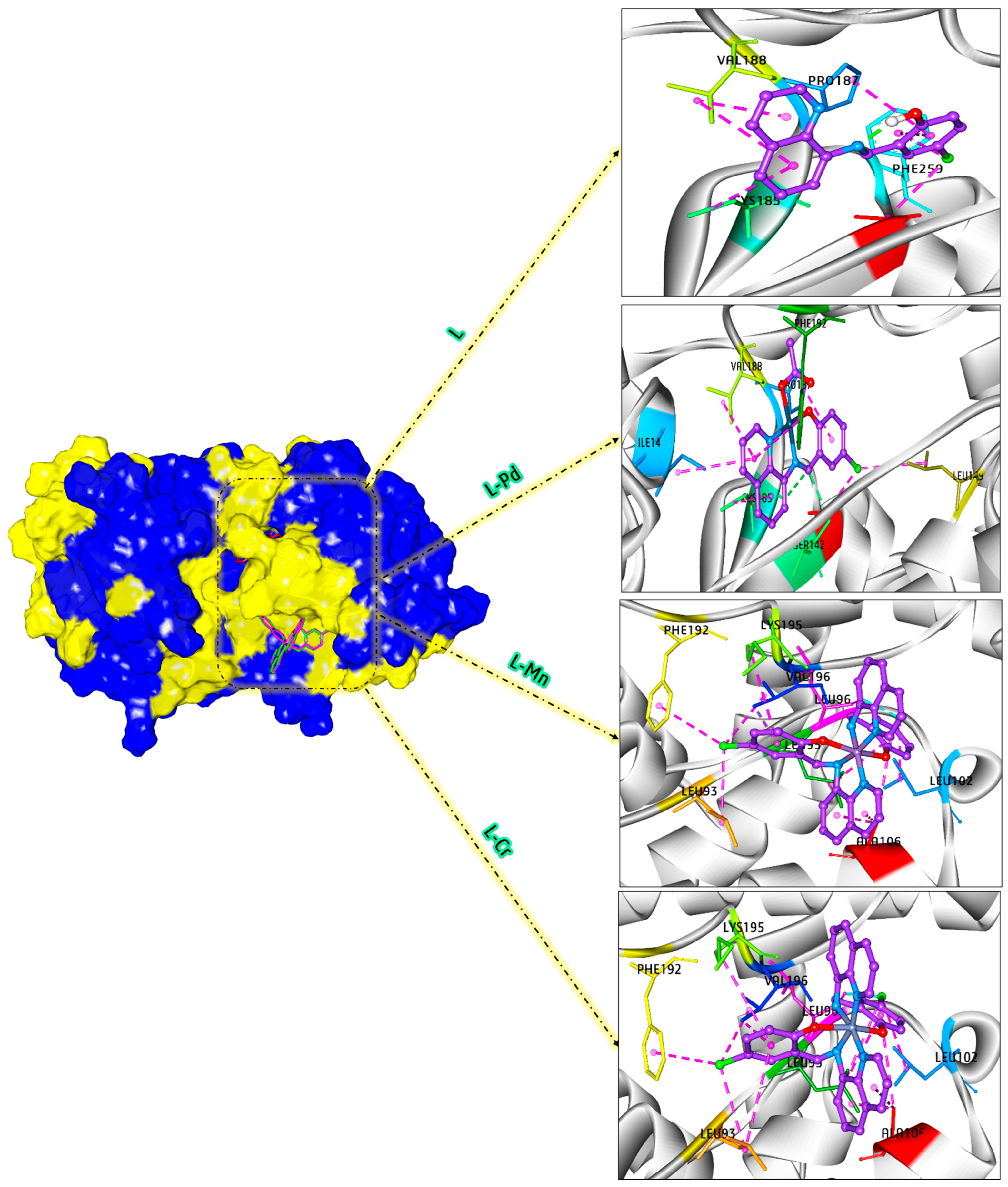
2.13. Molecular Modeling
2.14. Biological Activity
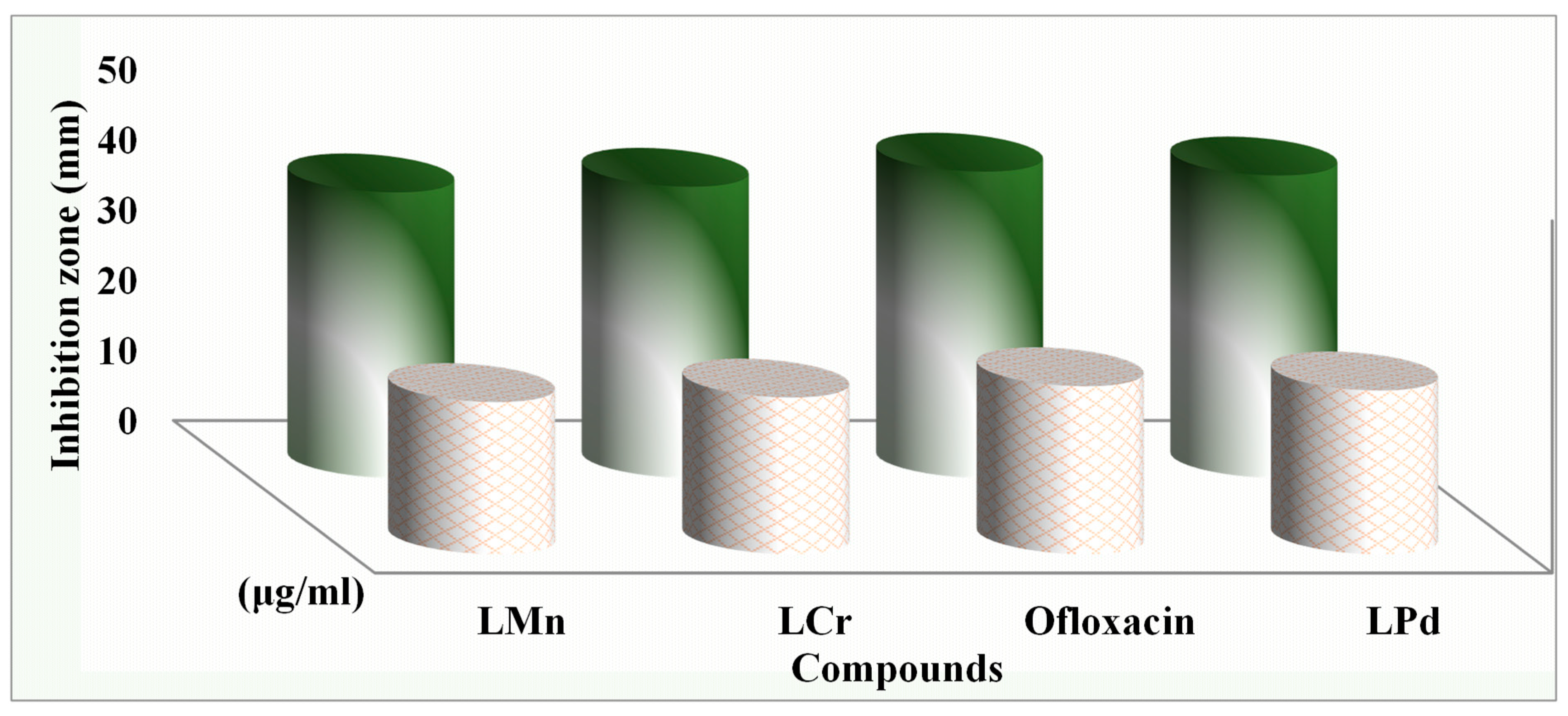
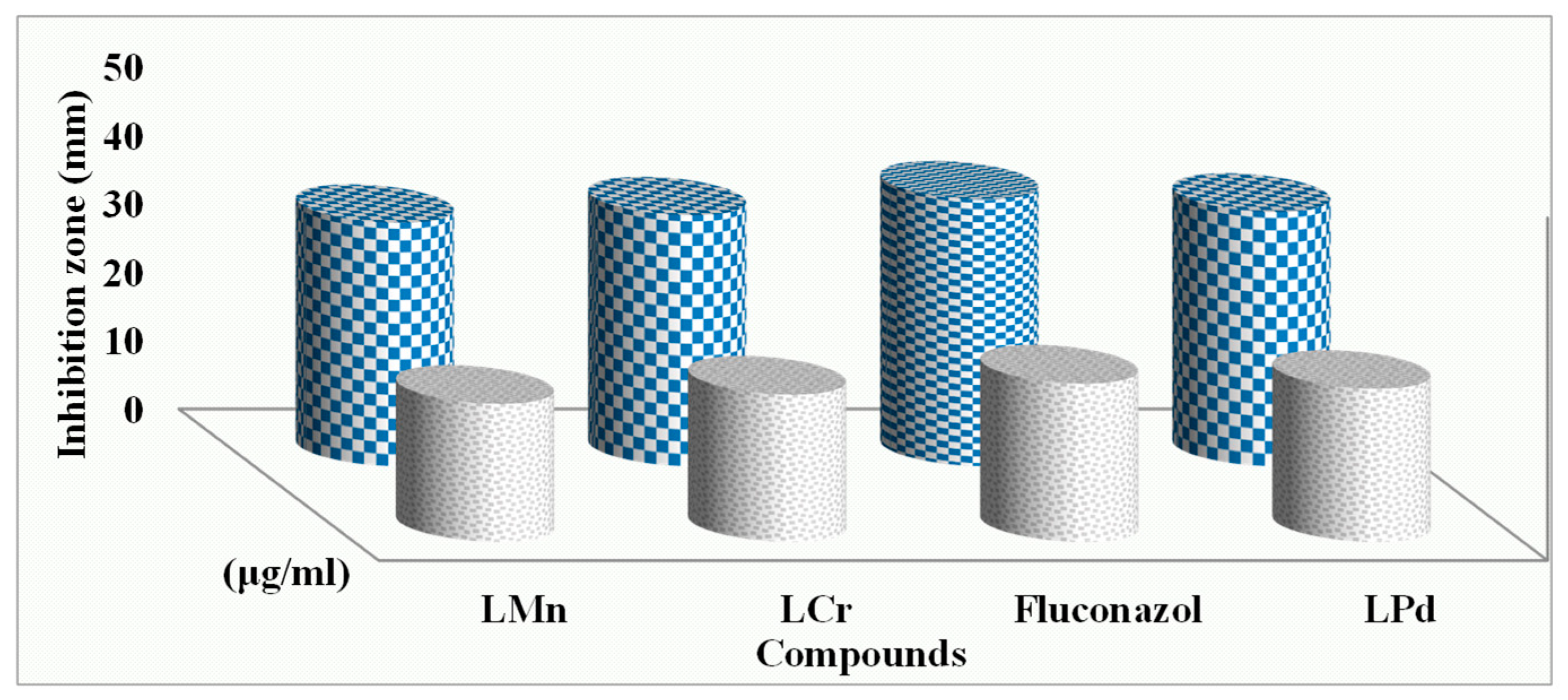
2.14.1. Antimicrobial Activity
2.14.2. Determination of Minimum Inhibition Concentration
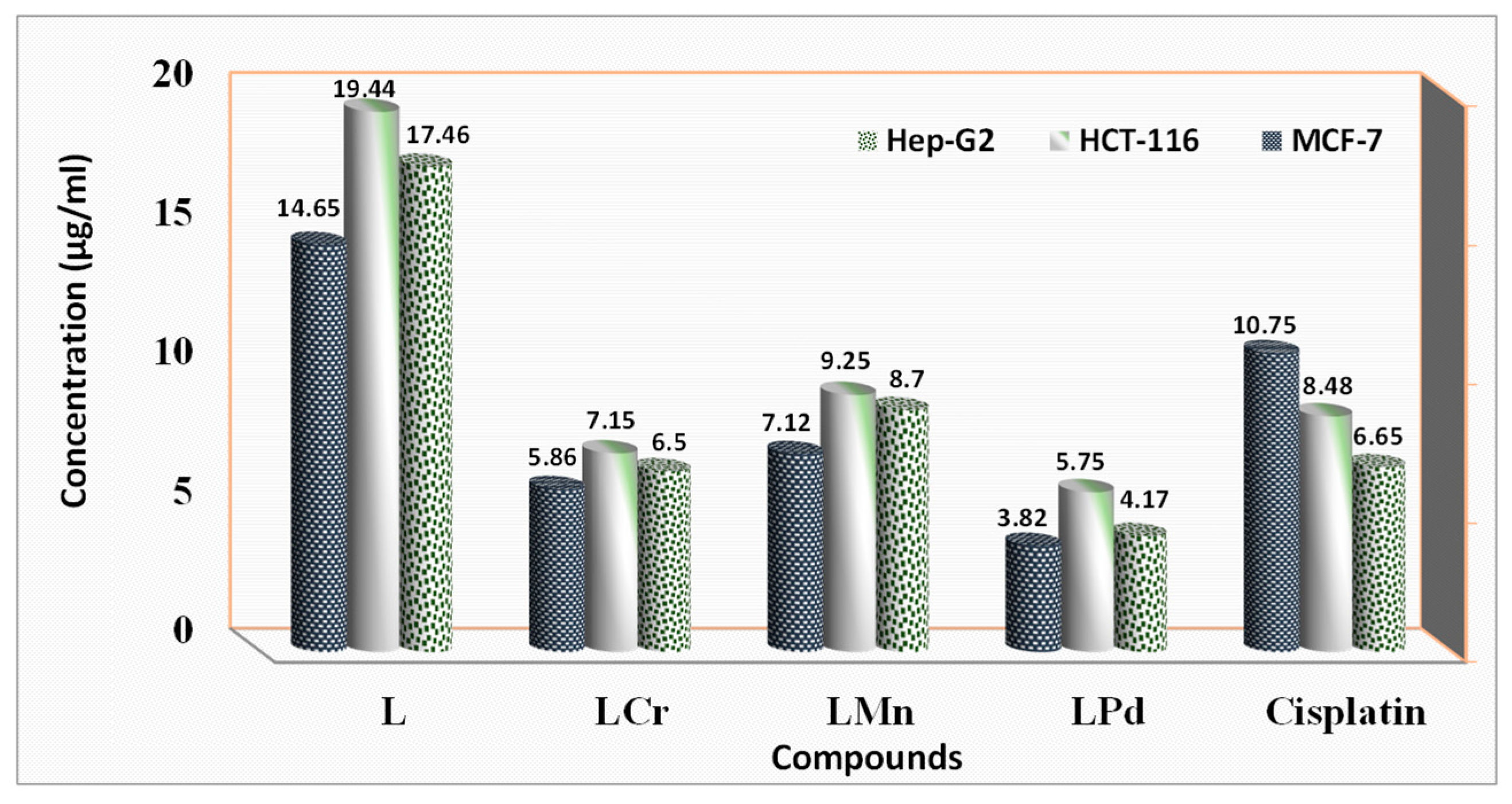
2.15. Anti-Cancer
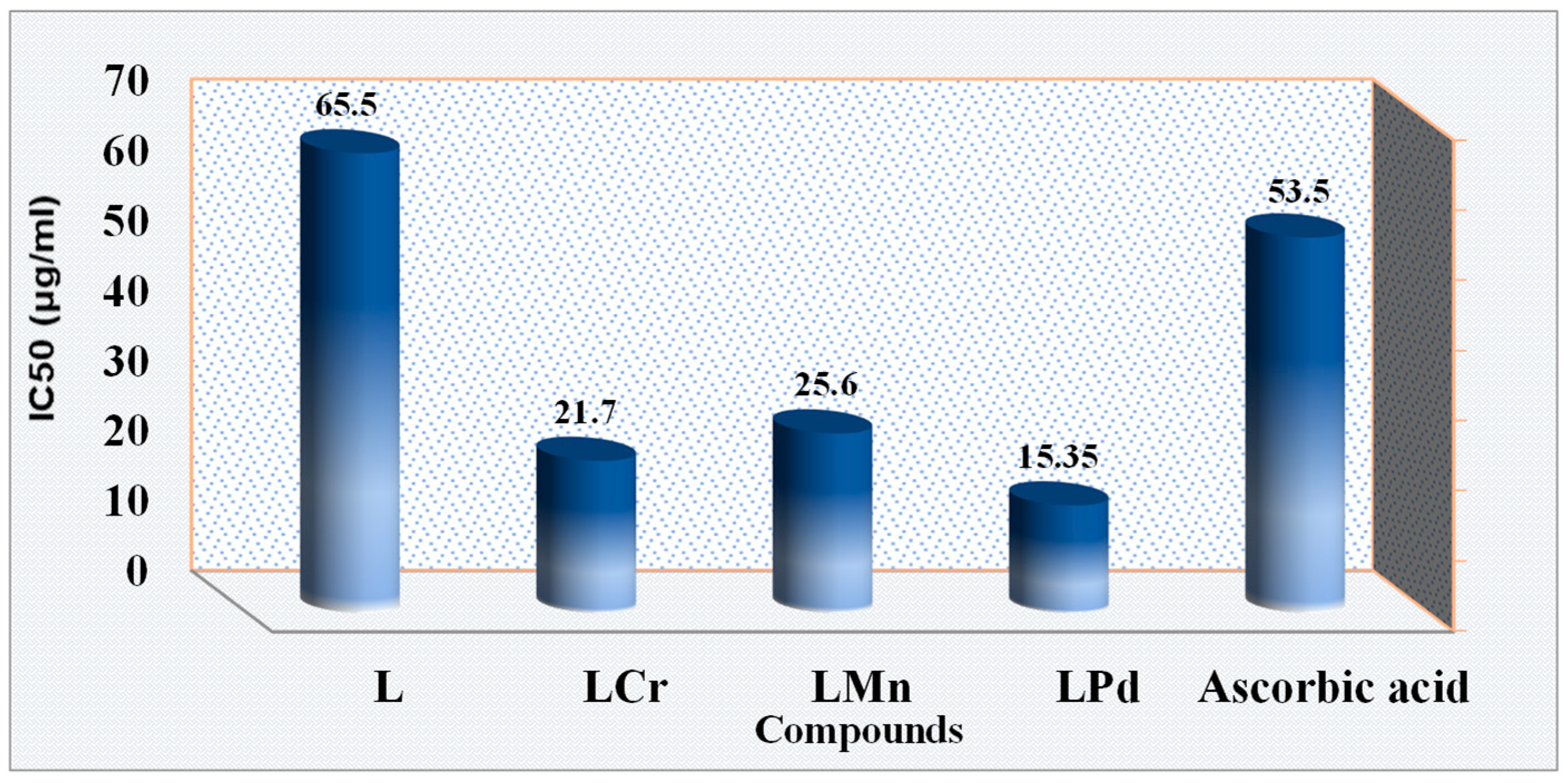
2.16. Examination of DPPH Radicals Scavenging Efficiency
3. Experimental
3.1. Reagents
3.2. Instrumentation
3.3. Synthesis of Tri-Dentate L Imine Ligand
3.4. Sono-Chemical Synthesis of Pd(II), Mn(II), and Cr(III) Complexes with 4-Chloro-2-(Quinolin-8-Yliminomethyl)-Phenol Ligand
3.5. Estimating the Stoichiometry of Chelates Using Job’s and Molar Ratio Methods
3.6. Assessment of the Apparent Constant of Complexes
3.7. Magnetic Moment Measurements
3.8. Spectrophotometric Studies
3.9. Thermogravimetric Analysis and Kinetic Studies
3.10. DFT and Docking Studies
3.10.1. DFT Calculations
3.10.2. Molecular Docking Approaches
3.11. Biological Evaluate
3.11.1. Antibacterial Activity
3.11.2. Anticancer Evaluation of Ligand and Its Metal Complexes
3.11.3. Assessment of Antioxidant Activity Using DPPH Radical Scavenging Assay
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Gogoi, H.P.; Barman, P. Salophen type ONNO donor Schiff base complexes: Synthesis, characterization, bioactivity, computational, and molecular docking investigation. Inorg. Chim. Acta 2023, 556, 121668. [Google Scholar] [CrossRef]
- Al-Farraj, E.S.; Alharbi, S.K.; Feizi-Dehnayebi, M.; Asghar, B.H.; Alahmadi, N.; Eskander, T.N.A.; Alghamdi, M.A.; Abu-Dief, A.M. Molecular, Stochiometric, Stability and Biological Investigations of Novel Multifunctional Salen Metal Chelates: From Synthesis to Therapeutic Potential Supported by Theoretical Approaches. Appl. Organomet. Chem. 2025, 39, e70273. [Google Scholar] [CrossRef]
- El-Kasaby, R.A.; Al-Farraj, E.S.; Abdou, A.; Abu-Dief, A.M. Synthesis, spectral analysis, physicochemical investigation and biomedical potential of some novel Cu(II), Ru(III) and VO(II) complexes with anthraquinone-based Schiff base supported by DFT and molecular docking insights. J. Mol. Struct. 2025, 1345, 143010. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Abdel-Mawgoud Azza, A.H. Novel Di-and Tri-azomethine compounds as chemo sensors for the detection of various metal ions. Int. J. Nano Chem. 2019, 5, 1–17. [Google Scholar]
- Vernekar, B.K.; Sawant, P.S. Interaction of metal ions with Schiff bases having N2O2 donor sites: Perspectives on synthesis, structural features, and applications. Results Chem. 2023, 6, 101039. [Google Scholar] [CrossRef]
- Bulatov, E.; Sayarova, R.; Mingaleeva, R.; Miftakhova, R.; Gomzikova, M.; Ignatyev, Y.; Petukhov, A.; Davidovich, P.; Rizvanov, A.; Barlev, N.A. Isatin-Schiff base-copper (II) complex induces cell death in p53-positive tumors. Cell Death Discov. 2018, 4, 103. [Google Scholar] [CrossRef]
- Hosny, S.; Shehata, M.R.; Aly, S.A.; Alsehli, A.H.; Salaheldeen, M.; Abu-Dief, A.M.; Abu-El-Wafa, S.M. Synergistic broad-spectrum bioactivity of some multifunctional novel Anil metal chelates: Design, synthesise, nonlinear optical properties, and biomedical applications supported by DFT and molecular docking insights. J. Mol. Struct. 2025, 1339, 142390. [Google Scholar] [CrossRef]
- Hosny, S.; Shehata, M.R.; Aly, S.A.; Alsehli, A.H.; Salaheldeen, M.; Abu-Dief, A.M.; Abu-El-Wafa, S.M. Designing of novel nano-sized coordination compounds based on Spinacia oleracea extract: Synthesis, structural characterization, molecular docking, computational calculations, and biomedical applications. Inorg. Chem. Commun. 2024, 160, 111994. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Khalaf, M.M.; Shehata, M.R.; Abu-Dief, A.M. Fabrication, DFT Calculation, and Molecular Docking of Two Fe(III) Imine Chelates as Anti-COVID-19 and Pharmaceutical Drug Candidate. Int. J. Mol. Sci. 2022, 23, 3994. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Said, M.A.; Elhady, O.; Al-Abdulkarim, H.A.; Alzahrani, S.; Eskander, T.N.A.; El-Remaily, M.A.E.A.A.A. Innovation of Fe(III), Ni(II), and Pd(II) complexes derived from benzothiazole imidazolidin-4-ol ligand: Geometrical elucidation, theoretical calculation, and pharmaceutical studies. Appl. Organomet. Chem. 2023, 37, e7162. [Google Scholar] [CrossRef]
- Gupta, K.C.; Sutar, A.K. Catalytic activities of Schiff base transition metal complexes. Coord. Chem. Rev. 2008, 252, 1420–1450. [Google Scholar] [CrossRef]
- Shaaban, S.; Yousef, T.A.; Al-Janabi, A.S.; Alammar, T.; Alaasar, M.; Shalabi, K.; Al-Karmalawy, A.A.; Ferjani, H.; Al-Dakhil, A.; Abu-Dief, A.M. Zn(II), Cu(II), and Fe(III) complexes of 2-(((4-(Methylselanyl) phenyl)imino)methyl)phenol: Synthesis, characterization, and multidisciplinary investigations. Polyhedron 2025, 279, 117652. [Google Scholar] [CrossRef]
- Al-Ghamdi, K.; Alharas, M.M.; Abdel-Latif, S.A.; Alhashmialameer, D.; Al-Farraj, E.S.; Almalki, M.A.; El-Khatib, R.M.; Abu-Dief, A.M. Selective Novel Metal-Coordinated Biomedical Agents Encompassing Tetradentate Salen Ligand: Structural Elucidation, DFT Calculation, Cytotoxic, and Antioxidant Activities Supported by Molecular Docking Approach. Appl. Organomet. Chem. 2025, 39, e7991. [Google Scholar] [CrossRef]
- Al-Abdulkarim, H.A.; El-khatib, R.M.; Aljohani, F.S.; Mahran, A.; Alharbi, A.; Mersal, G.A.; El-Metwaly, N.M.; Abu-Dief, A.M. Optimization for synthesized quinoline-based Cr3+, VO2+, Zn2+ and Pd2+ complexes: DNA interaction, biological assay and in-silico treatments for verification. J. Mol. Liq. 2021, 339, 116797. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Shehata, M.R.; Hassan, A.E.; Altayeb, B.M.; Aljohani, F.S.; Barnawi, I.O.; Abo-Dief, H.M.; Ragab, M.S. Tailoring of novel water soluble Pd(II), Cu(II), Fe(III) and VO(II) chelates based on 4-[(5-bromo-2-hydroxy-benzylidene)-amino]-benzenesulfonate ligand: Synthesis, spectral investigations, DNA interaction and pharmaceutical applications supported by molecular docking approach. J. Mol. Struct. 2025, 1334, 141780. [Google Scholar]
- Al-Fakeh, M.S.; Alsikhan, M.A.; Alnawmasi, J.S. Physico-chemical study of Mn(II), Co(II), Cu(II), Cr(III), and Pd(II) complexes with schiff-base and aminopyrimidyl derivatives and anti-cancer, antioxidant, antimicrobial applications. Molecules 2023, 28, 2555. [Google Scholar] [CrossRef]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.J.; Peters, D. Practical Sonochemistry: Power Ultrasound Uses and Applications; Woodhead Publishing: Cambridge, UK, 2002. [Google Scholar]
- Abu-Dief, A.M.; Said, M.A.; Elhady, O.; Alahmadi, N.; Alzahrani, S.; Eskander, T.N.A.; Ali, M.A.E.A.A. Designing of some novel Pd(II), Ni(II) and Fe(III) complexes: Synthesis, structural elucidation, biomedical applications, DFT and docking approaches against COVID-19. Inorg. Chem. Commun. 2023, 155, 110955. [Google Scholar] [CrossRef]
- Gozdas, S.; Kose, M.; Mckee, V.; Elmastas, M.; Demirtas, I.; Kurtoglu, M. Crystal structures, electronic spectra and anticancer properties of new azo-azomethines and their nickel(II) and copper(II) chelates. J. Mol. Struct. 2024, 1304, 137691. [Google Scholar] [CrossRef]
- Shaaban, S.; Abdullah, K.T.; Shalabi, K.; Yousef, T.A.; Al Duaij, O.K.; Alsulaim, G.M.; Althikrallah, H.A.; Alaasar, M.; Al-Janabi, A.S.; Abu-Dief, A.M. Synthesis, structural characterization, anticancer, antimicrobial, antioxidant, and computational assessments of zinc(II), iron(II), and copper(II) chelates derived from selenated Schiff base. Appl. Organomet. Chem. 2024, 38, e7712. [Google Scholar] [CrossRef]
- Khalaf, M.M.; Abd El-Lateef, H.M.; Taha, A.A.; Abdou, A. Binuclear Fe(II) and Cu(II) complexes with 2-(pyridin-2-yl)-1H-benzimidazole and 4,4′-bipyridine: Synthesis, characterization, DFT insights, broad-spectrum bioactivity and docking study. Inorganica Chim. Acta 2025, 577, 122505. [Google Scholar] [CrossRef]
- Abdel-Rhman, M.H.; Motawea, R.; Belal, A.; Hosny, N.M. Spectral, structural and cytotoxicity studies on the newly synthesized n′1, n′3-diisonicotinoylmalonohydrazide and some of its bivalent metal complexes. J. Mol. Struct. 2022, 1251, 131960. [Google Scholar] [CrossRef]
- Hosny, N.M.; Ibrahim, O.A.; Belal, A.; Hussien, M.A.; Abdel-Rhman, M.H. Synthesis, characterization, DFT, cytotoxicity evaluation and molecular docking of a new carbothioamide ligand and its coordination compounds. Results Chem. 2023, 5, 100776. [Google Scholar] [CrossRef]
- Ali, I.; Wani, W.A.; Saleem, K. Empirical formulae to molecular structures of metal complexes by molar conductance. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2013, 43, 1162–1170. [Google Scholar] [CrossRef]
- Khalaf, M.M.; Abd El-Lateef, H.M.; Gouda, M.; Sayed, F.N.; Mohamed, G.G.; Abu-Dief, A.M. Design, structural inspection and bio-medicinal applications of some novel imine metal complexes based on acetylferrocene. Materials 2022, 15, 4842. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.A. Characterization, thermal and fluorescence study of Mn(II) and Pd(II) Schiff base complexes. J. Therm. Anal. Calorim. 2017, 128, 1579–1590. [Google Scholar] [CrossRef]
- Soliman, M.H.; Mohamed, G.G. Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II) and Zn(II) new complexes of 5-aminosalicylic acid: Spectroscopic, thermal characterization and biological activity studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 107, 8–15. [Google Scholar] [CrossRef]
- Zare, N.; Zabardasti, A. A new nano-sized mononuclear Cu (II) complex with N,N-donor Schiff base ligands: Sonochemical synthesis, characterization, molecular modeling and biological activity. Appl. Organomet. Chem. 2019, 33, e4687. [Google Scholar] [CrossRef]
- Al-Hazmi, G.A.; Abou-Melha, K.S.; Althagafi, I.; El-Metwaly, N.; Shaaban, F.; Abdul Galil, M.S.; El-Bindary, A.A. Synthesis and structural characterization of oxovanadium(IV) complexes of dimedone derivatives. Appl. Organomet. Chem. 2020, 34, e5672. [Google Scholar] [CrossRef]
- Refat, M.S.; Altalhi, T.; Bakare, S.B.; Al-Hazmi, G.H.; Alam, K. New Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Zn(II), Cd(II), and Hg(II) gibberellate complexes: Synthesis, structure, and inhibitory activity against COVID-19 protease. Russ. J. Gen. Chem. 2021, 91, 890–896. [Google Scholar] [CrossRef] [PubMed]
- El-Remaily, M.A.E.A.A.A.; Eskander, T.N.A.; Elhady, O.; Alhashmialameer, D.; Alsehli, M.; Kamel, M.S.; Feizi-Dehnayebi, M.; Abu-Dief, A.M. A comparative study for the efficiency of Pd(II) and Fe(III) complexes as efficient catalysts for synthesis of dihydro-7H-5-thia-hexaaza-s-indacen-6-one derivatives supported with DFT approach. Appl. Organomet. Chem. 2024, 38, e7653. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Aboelez, M.O.; Abdel-Mawgoud, A.A.H. DNA interaction, antimicrobial, anticancer activities and molecular docking study of some new VO(II), Cr(III), Mn(II) and Ni(II) mononuclear chelates encompassing quaridentate imine ligand. J. Photochem. Photobiol. B Biol. 2017, 170, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Yousef, T.A.; El-Reash, G.A.; El-Gammal, O.A.; Bedier, R.A. Co(II), Cu(II), Cd(II), Fe(III) and U(VI) complexes containing a NSNO donor ligand: Synthesis, characterization, optical band gap, in vitro antimicrobial and DNA cleavage studies. J. Mol. Struct. 2012, 1029, 149–160. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Moustafa, H.; Hamdan, S.K. Ni(II) and Cu(II) complexes with ONNO asymmetric tetradentate Schiff base ligand: Synthesis, spectroscopic characterization, theoretical calculations, DNA interaction and antimicrobial studies. Appl. Organomet. Chem. 2017, 31, e3555. [Google Scholar] [CrossRef]
- Aljohani, E.T.; Shehata, M.R.; Alkhatib, F.; Alzahrani, S.O.; Abu-Dief, A.M. Development and structure elucidation of new VO2+, Mn2+, Zn2+, and Pd2+ complexes based on azomethine ferrocenyl ligand: DNA interaction, antimicrobial, antioxidant, anticancer activities, and molecular docking. Appl. Organomet. Chem. 2021, 35, e6154. [Google Scholar] [CrossRef]
- Munde, A.S.; Jagdale, A.N.; Jadhav, S.M.; Chondhekar, T.K. Synthesis, characterization and thermal study of some transition metal complexes of an asymmetrical tetradentate Schiff base ligand. J. Serbian Chem. Soc. 2010, 75, 349–359. [Google Scholar] [CrossRef]
- El-Remaily, M.A.E.A.A.A.; Elhady, O.; Eskander, T.N.A.; Mohamed, S.K.; Abu-Dief, A.M. Development of novel guanidine iron (III) complexes as a powerful catalyst for the synthesis of tetrazolo [1,5-a] pyrimidine by green protocol. Sohag J. Sci. 2024, 9, 7–15. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Said, M.A.; Elhady, O.; Alzahrani, S.; Aljohani, F.S.; Eskander, T.N.A.; Ali, M.A.E.A.A. Design, structural inspection of some new metal chelates based on benzothiazol-pyrimidin-2-ylidene ligand: Biomedical studies and molecular docking approach. Inorg. Chem. Commun. 2023, 158, 111587. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Feizi-Dehnayebi, M.; Nafady, A.; Almalki, M.A.; Kassem, A.M.; Abu Al-Ola, K.A.; Altayeb, B.M.; Abdel-Rahman, L.H. Fabrication, structural inspection, stability studies in solution and DFT calculations of some novel complexes drived from 4-(Benzothiazol-2-yliminomethyl)-phenol ligand: Pharmaceutical applications supported by molecular docking approach. J. Mol. Struct. 2025, 1328, 141284. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Issaoui, N.; Holikulov, U.; Al-Dossary, O.M.; Ponomarev, I.S.; Kazachenko, A.S.; Akman, F.; Bousiakou, L.G. Noncovalent interactions in N-methylurea crystalline hydrates. Z. Phys. Chem. 2024, 238, 89–114. [Google Scholar] [CrossRef]
- Akman, F.; Demirpolat, A.; Kazachenko, A.S.; Kazachenko, A.S.; Issaoui, N.; Al-Dossary, O. Molecular structure, electronic properties, reactivity (ELF, LOL, and Fukui), and NCI-RDG studies of the binary mixture of water and essential oil of Phlomis bruguieri. Molecules 2023, 28, 2684. [Google Scholar] [CrossRef]
- Dehghani, N.; Ziarani, G.M.; Feizi-Dehnayebi, M.; Mirhosseyni, M.; Badiei, A. Synthesis and theoretical investigation of a new Hg2+ chemosensor based nanomagnetic particle (Fe3O4@SiO2@ Pr-NCIM). Mater. Res. Bull. 2025, 183, 113200. [Google Scholar] [CrossRef]
- Mohammadi Ziarani, G.; Rezakhani, M.; Feizi-Dehnayebi, M.; Nikolova, S. Fumed-Si-Pr-Ald-Barb as a fluorescent chemosensor for the Hg2+ detection and Cr2O72− ions: A combined experimental and computational perspective. Molecules 2024, 29, 4825. [Google Scholar] [CrossRef]
- Mohammadi Ziarani, G.; Ebrahimi, D.; Feizi-Dehnayebi, M.; Badiei, A.; Abu-Dief, A.M. Tailored Silica-Based Sensors (SBA-Pr-Ald-MA) for Efficient Detection of Iron (III) Ions: A Comprehensive Theoretical and Experimental Viewpoint. Appl. Organomet. Chem. 2025, 39, e7917. [Google Scholar] [CrossRef]
- Zamiran, F.; Mohammadi Ziarani, G.; Feizi-Dehnayebi, M.; Mirhosseyni, M.; Badiei, A.; Abu-Dief, A.M. Design, Preparation, Characterization, Density Functional Theory, and HOMO-LUMO Perspective of Fe3O4@SiO2-Pr-NH-IC as a New Nanomagnetic Chemosensor. Appl. Organomet. Chem. 2025, 39, e7998. [Google Scholar] [CrossRef]
- Gungor, O.; Gul, A.; Comertpay, S.; McKee, V.; Yalcinkaya, O.B.; Kose, M. Copper(II) and Zinc(II) complexes of new water-soluble Schiff base ligands and their antiproliferative properties towards mesothelioma cell line. J. Photochem. Photobiol. A Chem. 2025, 459, 116049. [Google Scholar] [CrossRef]
- Canakdag, M.; Feizi-Dehnayebi, M.; Kundu, S.; Sahin, D.; İlhan, İ.Ö.; Alhag, S.K.; Al-Shuraym, L.A.; Akkoc, S. Comprehensive evaluation of purine analogues: Cytotoxic and antioxidant activities, enzyme inhibition, DFT insights, and molecular docking analysis. J. Mol. Struct. 2025, 1323, 140798. [Google Scholar] [CrossRef]
- El-Remaily, M.A.E.A.A.A.; El-Dabea, T.; El-Khatib, R.M.; Abdou, A.; El Hamd, M.A.; Abu-Dief, A.M. Efficiency and development of guanidine chelate catalysts for rapid and green synthesis of 7-amino-4,5-dihydro-tetrazolo [1,5-a] pyrimidine-6-carbonitrile derivatives supported by density functional theory (DFT) studies. Appl. Organomet. Chem. 2023, 37, e7262. [Google Scholar] [CrossRef]
- Pérez, P.; Domingo, L.R.; Aizman, A.; Contreras, R. The electrophilicity index in organic chemistry. Theor. Comput. Chem. 2007, 19, 139–201. [Google Scholar]
- Raman, N.; Raja, J.D. Synthesis, structural characterization and antibacterial studies of some biosensitive mixed ligand copper (II) complexes. Indian J. Chem. Sect. A 2007, 46, 1611–1614. [Google Scholar]
- Abdel-Rahman, L.H.; Abdelhamid, A.A.; Abu-Dief, A.M.; Shehata, M.R.; Bakheet, M.A. Facile synthesis, X-ray structure of new multi-substituted aryl imidazole ligand, biological screening and DNA binding of its Cr(III), Fe(III) and Cu(II) coordination compounds as potential antibiotic and anticancer drugs. J. Mol. Struct. 2020, 1200, 127034. [Google Scholar] [CrossRef]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular mechanisms of membrane targeting antibiotics. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 980–987. [Google Scholar] [CrossRef]
- Ansel, H.C.; Norred, W.P.; Roth, I.L. Antimicrobial activity of dimethyl sulfoxide against Escherichia coli, Pseudomonas aeruginosa, and Bacillus megaterium. J. Pharm. Sci. 1969, 58, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Tweedy, B.G. Possible mechanism for reduction of elemental sulfur by Monilinia fructicola. Phytopathology 1964, 54, 910. [Google Scholar]
- Abu-Dief, A.M.; El-Metwaly, N.M.; Alzahrani, S.O.; Alkhatib, F.; Abualnaja, M.M.; El-Dabea, T.; Ali, M.A.E.A.A. Synthesis and characterization of Fe(III), Pd(II) and Cu(II)-thiazole complexes; DFT, pharmacophore modeling, in-vitro assay and DNA binding studies. J. Mol. Liq. 2021, 326, 115277. [Google Scholar] [CrossRef]
- Sindhu, Y.; Athira, C.J.; Sujamol, M.S.; Joseyphus, R.S.; Mohanan, K. Synthesis, characterization, DNA cleavage, and antimicrobial studies of some transition metal complexes with a novel Schiff base derived from 2-aminopyrimidine. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2013, 43, 226–236. [Google Scholar] [CrossRef]
- Mohamed, G.G.; Soliman, M.H. Synthesis, spectroscopic and thermal characterization of sulpiride complexes of iron, manganese, copper, cobalt, nickel, and zinc salts. Antibacterial and antifungal activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 76, 341–347. [Google Scholar] [CrossRef]
- Aljohani, F.S.; Abu-Dief, A.M.; El-Khatib, R.M.; Al-Abdulkarim, H.A.; Alharbi, A.; Mahran, A.; Khalifa, M.E.; El-Metwaly, N.M. Structural inspection for novel Pd(II), VO(II), Zn(II) and Cr(III)-azomethine metal chelates: DNA interaction, biological screening and theoretical treatments. J. Mol. Struct. 2021, 1246, 131139. [Google Scholar] [CrossRef]
- El-Wakiel, N.A. Synthesis and characterization of azo sulfaguanidine complexes and their application for corrosion inhibition of silicate glass. Appl. Organomet. Chem. 2016, 30, 664–673. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Adam, M.S.S.; Abu-Dief, A.M.; Moustafa, H.; Basha, M.T.; Aboraia, A.S.; Al-Farhan, B.S.; Ahmed, H.E.S. Synthesis, theoretical investigations, biocidal screening, DNA binding, in vitro cytotoxicity and molecular docking of novel Cu(II), Pd(II) and Ag(I) complexes of chlorobenzylidene Schiff base: Promising antibiotic and anticancer agents. Appl. Organomet. Chem. 2018, 32, e4527. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Shehata, M.R.; Atlam, F.M.; Abdel-Mawgoud, A.A.H. Some new Ag(I), VO(II) and Pd(II) chelates incorporating tridentate imine ligand: Design, synthesis, structure elucidation, density functional theory calculations for DNA interaction, antimicrobial and anticancer activities and molecular docking studies. Appl. Organomet. Chem. 2019, 33, e4699. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- El-Remaily, M.A.E.A.A.A.; Elhady, O.; Alzubi, M.S.H.; Eskander, T.N.A.; El Hamd, M.A.; Al-Ghamdi, K.; Abu-Dief, A.M. Development of new thiazole-guanidine complexes as rapid and recoverable catalysts for the synthesis of 6-piperidin-dihydro-thia-hexaaza-s-indacene derivatives supported by DFT studies. Appl. Organomet. Chem. 2024, 38, e7454. [Google Scholar] [CrossRef]
- El-Remaily, M.A.E.A.A.A.; Eskander, T.N.A.; Alzahrani, A.Y.A.; Alsehli, M.; Al-Ghamdi, K.; ALMughram, M.H.; El-Hady, O.M.; Feizi-Dehnayebi, M.; Abu-Dief, A.M. Tailoring of novel Ru(III) and Cr(III) Salen complexes as catalysts for a sustainable and green synthesis of dihydro-tetrazolo [1,5-a] Thiazolo [4,5-d] pyrimidin-6-yl morpholine: Experimental and theoretical approaches. Appl. Organomet. Chem. 2025, 39, e7879. [Google Scholar] [CrossRef]
- Ismael, M.; Abdel-Mawgoud, A.M.M.; Rabia, M.K.; Abdou, A. Design and synthesis of three Fe(III) mixed-ligand complexes: Exploration of their biological and phenoxazinone synthase-like activities. Inorganica Chim. Acta 2020, 505, 119443. [Google Scholar] [CrossRef]
- Al-Shamry, A.A.; Khalaf, M.M.; El-Lateef, H.M.A.; Yousef, T.A.; Mohamed, G.G.; El-Deen, K.M.K.; Gouda, M.; Abu-Dief, A.M. Development of new azomethine metal chelates derived from isatin: DFT and pharmaceutical studies. Materials 2022, 16, 83. [Google Scholar] [CrossRef]
- Alkhatib, F.; Hameed, A.; Sayqal, A.; Bayazeed, A.A.; Alzahrani, S.; Al-Ahmed, Z.A.; Zaky, R.; El-Metwaly, N.M. Green-synthesis and characterization for new Schiff-base complexes; spectroscopy, conductometry, Hirshfeld properties and biological assay enhanced by in-silico study. Arab. J. Chem. 2020, 13, 6327–6340. [Google Scholar] [CrossRef]
- Al-Farraj, E.S.; Qasem, H.A.; Aouad, M.R.; Al-Abdulkarim, H.A.; Alsaedi, W.H.; Khushaim, M.S.; Feizi-Dehnayebi, M.; Al-Ghamdi, K.; Abu-Dief, A.M. Synthesis, Structural Determination, DFT Calculation and Biological Evaluation Supported by Molecular Docking Approach of Some New Complexes Incorporating (E)-N′-(3,5-di-Tert-Butyl-2-Hydroxybenzylidene) Isonicotino Hydrazide Ligand. Appl. Organomet. Chem. 2025, 39, e7768. [Google Scholar] [CrossRef]
- Ali, M.A.E.A.A.; Elhady, O.; Abdou, A.; Alhashmialameer, D.; Eskander, T.N.A.; Abu-Dief, A.M. Development of new 2-(Benzothiazol-2-ylimino)-2,3-dihydro-1H-imidazol-4-ol complexes as a robust catalysts for synthesis of thiazole 6-carbonitrile derivatives supported by DFT studies. J. Mol. Struct. 2023, 1292, 136188. [Google Scholar]
- Frisch, M.; Trucks, G.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009; 201p. [Google Scholar]
- Mazumdar, M.; Fournier, D.; Zhu, D.W.; Cadot, C.; Poirier, D.; Lin, S.X. Binary and ternary crystal structure analyses of a novel inhibitor with 17β-HSD type 1: A lead compound for breast cancer therapy. Biochem. J. 2009, 424, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.H.; Goh, E.B.; Keasling, J.D.; Beller, H.R.; Adams, P.D. Structure of FabH and factors affecting the distribution of branched fatty acids in Micrococcus luteus. Biol. Crystallogr. 2012, 68, 1320–1328. [Google Scholar] [CrossRef]
- Keniya, M.V.; Sabherwal, M.; Wilson, R.K.; Woods, M.A.; Sagatova, A.A.; Tyndall, J.D.; Monk, B.C. Crystal structures of full-length lanosterol 14α-demethylases of prominent fungal pathogens Candida albicans and Candida glabrata provide tools for antifungal discovery. Antimicrob. Agents Chemother. 2018, 62, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Feizi-Dehnayebi, M.; Ziarani, G.M.; Lohith, T.N.; Ghareghomi, S.; Panahande, Z.; Farsadrooh, M.; Majidinia, M. Probing the biological activity of isatin derivatives against human lung cancer A549 cells: Cytotoxicity, CT-DNA/BSA binding, DFT/TD-DFT, topology, ADME-Tox, docking and dynamic simulations. J. Mol. Liq. 2025, 428, 127475. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Todorova, M.; Bakalska, R.; Feizi-Dehnayebi, M.; Ziarani, G.M.; Pencheva, M.; Stojnova, K.; Milusheva, M.; Nedialkov, P.; Cherneva, E.; Kolev, T.; et al. Synthesis, Anti-Inflammatory Activity, and Docking Simulation of a Novel Styryl Quinolinium Derivative. Appl. Sci. 2024, 15, 284. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; El-Khatib, R.M.; Nassr, L.A.; Abu-Dief, A.M.; Ismael, M.; Seleem, A.A. Metal based pharmacologically active agents: Synthesis, structural characterization, molecular modeling, CT-DNA binding studies and in vitro antimicrobial screening of iron (II) bromosalicylidene amino acid chelates. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 366–378. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Nassr, L.A. Tailoring, physicochemical characterization, antibacterial and DNA binding mode studies of Cu(II) Schiff bases amino acid bioactive agents incorporating 5-bromo-2-hydroxybenzaldehyde. J. Iran. Chem. Soc. 2015, 12, 943–955. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; El-Khatib, R.M.; Abdel-Fatah, S.M. Sonochemical synthesis, DNA binding, antimicrobial evaluation and in vitro anticancer activity of three new nano-sized Cu(II), Co(II) and Ni(II) chelates based on tri-dentate NOO imine ligands as precursors for metal oxides. J. Photochem. Photobiol. B Biol. 2016, 162, 298–308. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Newair, E.F.; Hamdan, S.K. Some new nano-sized Cr(III), Fe(II), Co(II), and Ni(II) complexes incorporating 2-((E)-(pyridine-2-ylimino) methyl) napthalen-1-ol ligand: Structural characterization, electrochemical, antioxidant, antimicrobial, antiviral assessment and DNA interaction. J. Photochem. Photobiol. B Biol. 2016, 160, 18–31. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Ismail, N.M.; Ismael, M.; Abu-Dief, A.M.; Ahmed, E.A.H. Synthesis, characterization, DFT calculations and biological studies of Mn(II), Fe(II), Co(II) and Cd(II) complexes based on a tetradentate ONNO donor Schiff base ligand. J. Mol. Struct. 2017, 1134, 851–862. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Nassar, I.F.; Elsayed, W.H. Magnetic NiFe2O4 nanoparticles: Efficient, heterogeneous and reusable catalyst for synthesis of acetylferrocene chalcones and their anti-tumour activity. Appl. Organomet. Chem. 2016, 30, 917–923. [Google Scholar] [CrossRef]
- Ferrari, E.; Asti, M.; Benassi, R.; Pignedoli, F.; Saladini, M. Metal binding ability of curcumin derivatives: A theoretical vs. experimental approach. Dalton Trans. 2013, 42, 5304–5313. [Google Scholar] [CrossRef] [PubMed]




| Compounds | Empirical Formula (Formula Weight) | Color | (M. p.) and Decomp. Temp. (°C) | Λm (Ω−1 cm2 mol−1) | µeff (B.M.) | Analysis (%) Found (Calc.) | IR, Cm−1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | (ʋOH)/H2O | ʋ(CH=N)υph | (C=N)py | ʋ(C-O) | ʋ(M-N) | ʋ(M-O) | ||||||
| L | C16H11ClON2 (282.72) | Canary yellow | 180 | - | - | 67.89 (67.96) | 3.88 (3.92) | 9.97 (9.91) | 3428 | 1618 | 1552 | 1269 | - | - |
| L-Cr | C32H22Cl2N5O6 Cr (695.45) | Dark brown | >300 | 60.50 | 3.65 | 55.33 (55.27) | 3.14 (3.19) | 10.14 (10.07) | 3359 | 1607 | 1526 | 1249 | 513 | 457 |
| L-Mn | C32H24Cl2N4O4 Mn (654.40) | brown | >300 | 8.50 | 5.32 | 58.79 (58.73) | 3.76 (3.70) | 8.51 (8.56) | 3345 | 1610 | 1533 | 1258 | 517 | 445 |
| L-Pd | C18H15ClN2O4 Pd (465.20) | Orange | >300 | 10.21 | - | 46.54 (46.47) | 3.19 (3.25) | 6.10 (6.02) | 3365 | 1602 | 1517 | 1242 | 478 | 524 |
| Complexes | Temp (°C) | Fragment Loss % | Weight Loss % | E* (KJmol−1) | A (S−1) | ∆H* (KJmol−1) | ∆G* (KJmol−1) | ∆S* (Jmol−1K−1) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| M. Formula | M. Wt. | Found | Calc | |||||||
| L-Cr 695.45 Residue | 38–121 | H2O | 18 | 2.62 | 2.58 | 30.25 | 0.011 | 30.06 | 51.73 | −270.79 |
| 122–225 | NO3 | 62 | 8.96 | 8.92 | 29.08 | 79.35 | −275.25 | |||
| 230–305 | C9H5NCl2 | 198.05 | 28.40 | 28.48 | 28.20 | 104.85 | −277.84 | |||
| 310–415 | C13H8N | 178 | 25.66 | 25.59 | 27.71 | 130.57 | −283.36 | |||
| 420–595 | C10H7N2O | 171 | 24.50 | 24.58 | 26.51 | 171.88 | −286.16 | |||
| >600 | CrO | 68 | 9.85 | 9.77 | - | - | - | |||
| L-Mn 654.40 Residue | 34–118 | 2H2O | 36 | 5.42 | 5.50 | 49.28 | 0.008 | 48.65 | 69.46 | −273.73 |
| 120–210 | C7H5Cl2 | 160 | 24.55 | 24.46 | 47.91 | 94.14 | −280.18 | |||
| 215–365 | C7H4NO | 118 | 17.95 | 18.04 | 46.87 | 129.48 | −284.87 | |||
| 370–460 | C10H6N2 | 154 | 23.60 | 23.54 | 45.83 | 165.29 | −287.85 | |||
| 465–545 | C8H5N | 115 | 17.55 | 17.58 | 45.08 | 191.27 | −289.48 | |||
| >550 | MnO | 71 | 10.82 | 10.85 | - | - | - | |||
| L-Pd 465.20 Residue | 38–125 | H2O | 18 | 3.80 | 3.86 | 26.35 | 0.01 | 25.66 | 48.015 | −272.51 |
| 125–232 | C2H3O2 | 59 | 12.75 | 12.68 | 24.86 | 74.80 | −279.01 | |||
| 235–410 | C7H4NCl | 137.5 | 29.51 | 29.55 | 23.66 | 115.36 | −283.91 | |||
| 415–685 | C9H6N | 128 | 27.55 | 27.51 | 21.77 | 180.36 | −288.33 | |||
| >690 | PdO | 122.5 | 26.29 | 26.33 | - | - | - | |||
| Complexes | Kf | pK | ΔG≠ kJ mol−1 |
|---|---|---|---|
| L-Cr | 4.75 × 107 | 7.67 | −43.99 |
| L-Mn | 6.18 × 107 | 7.79 | −44.45 |
| L-Pd | 4.87 × 104 | 4.69 | −26.67 |
| Parameter | L | L-Pd | L-Mn | L-Cr |
|---|---|---|---|---|
| EHOMO | −6.03 | −4.14 | −5.12 | −5.75 |
| ELUMO | −2.31 | −2.80 | −2.41 | −3.09 |
| ΔE(LUMO–HOMO) | 3.72 | 1.34 | 2.71 | 2.66 |
| χ | 4.17 | 3.47 | 3.76 | 4.42 |
| η | 1.86 | 0.67 | 1.35 | 1.33 |
| σ | 0.53 | 1.49 | 0.74 | 0.75 |
| Pi | −4.17 | −3.47 | −3.76 | −4.42 |
| ω | 4.67 | 8.98 | 5.23 | 7.34 |
| ΔNmax | 2.24 | 5.18 | 2.78 | 3.32 |
| Compound | (MIC) Minimum Inhibition Concentration µg/mL | |||||
|---|---|---|---|---|---|---|
| Bacteria | Fungi | |||||
| S. marcescence | E. coli | M. luteus | A. flavus | C. albicans | F. oxysporum | |
| L | 7.5 | 8.25 | 6.5 | 8.75 | 6.25 | 7 |
| L-Cr | 2.75 | 3.5 | 2 | 3.75 | 2.25 | 3.25 |
| L-Mn | 3 | 3.75 | 2.5 | 4 | 3 | 3.75 |
| L-Pd | 2.25 | 3 | 1.75 | 3 | 2.25 | 2.75 |
| Ofloxacin | 2 | 2.75 | 1.5 | |||
| Fluconazole | 2.25 | 1.75 | 2.5 | |||
| Compound | Activity Index (%) | |||||
|---|---|---|---|---|---|---|
| Bacteria | Fungi | |||||
| S. marcescence | E. coli | M. luteus | A. flavus | C. albicans | F. oxysporum | |
| L | 44.05 | 38.48 | 37.7 | 39.41 | 34.96 | 33.65 |
| L-Cr | 94.82 | 91.58 | 95.06 | 92.87 | 94.09 | 91.23 |
| L-Mn | 89.94 | 86.97 | 93.22 | 88.26 | 91.26 | 86.28 |
| L-Pd | 97.71 | 98.2 | 98.74 | 96.23 | 95.37 | 95.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhashmialameer, D. Synthesis of Some Novel Cr(III), Mn(II), and Pd(II) Complexes via the Sono-Chemical Route with a Chlorinated Quinolinyl-Imine Ligand: Structural Elucidation, Bioactivity Analysis, and Docking Simulations. Inorganics 2025, 13, 271. https://doi.org/10.3390/inorganics13080271
Alhashmialameer D. Synthesis of Some Novel Cr(III), Mn(II), and Pd(II) Complexes via the Sono-Chemical Route with a Chlorinated Quinolinyl-Imine Ligand: Structural Elucidation, Bioactivity Analysis, and Docking Simulations. Inorganics. 2025; 13(8):271. https://doi.org/10.3390/inorganics13080271
Chicago/Turabian StyleAlhashmialameer, Dalal. 2025. "Synthesis of Some Novel Cr(III), Mn(II), and Pd(II) Complexes via the Sono-Chemical Route with a Chlorinated Quinolinyl-Imine Ligand: Structural Elucidation, Bioactivity Analysis, and Docking Simulations" Inorganics 13, no. 8: 271. https://doi.org/10.3390/inorganics13080271
APA StyleAlhashmialameer, D. (2025). Synthesis of Some Novel Cr(III), Mn(II), and Pd(II) Complexes via the Sono-Chemical Route with a Chlorinated Quinolinyl-Imine Ligand: Structural Elucidation, Bioactivity Analysis, and Docking Simulations. Inorganics, 13(8), 271. https://doi.org/10.3390/inorganics13080271






