Recent Advances in Zinc Complexes for Stereoselective Ring-Opening Polymerization and Copolymerization
Abstract
1. Introduction
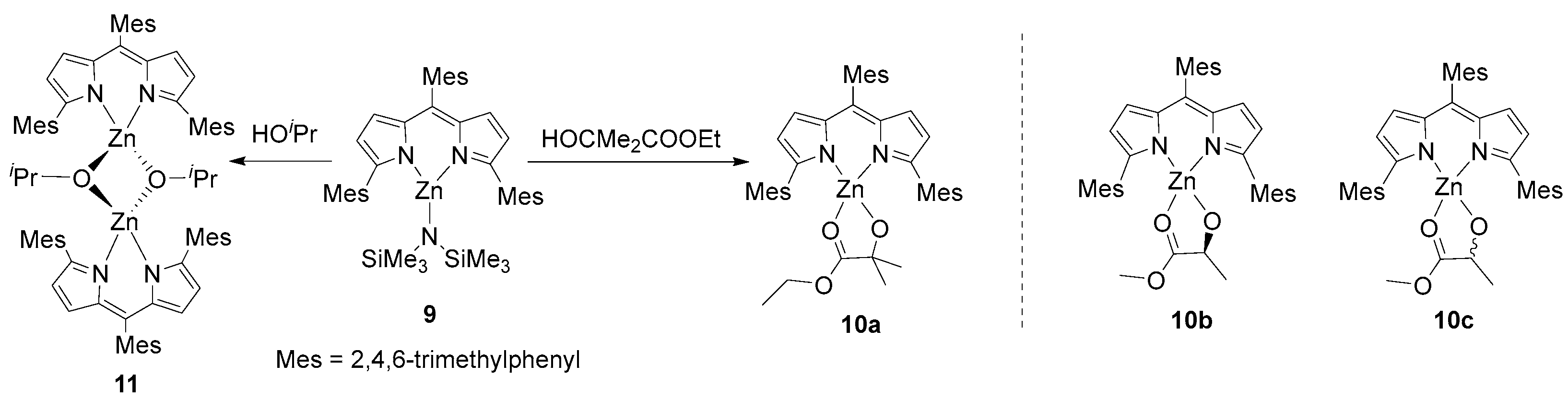
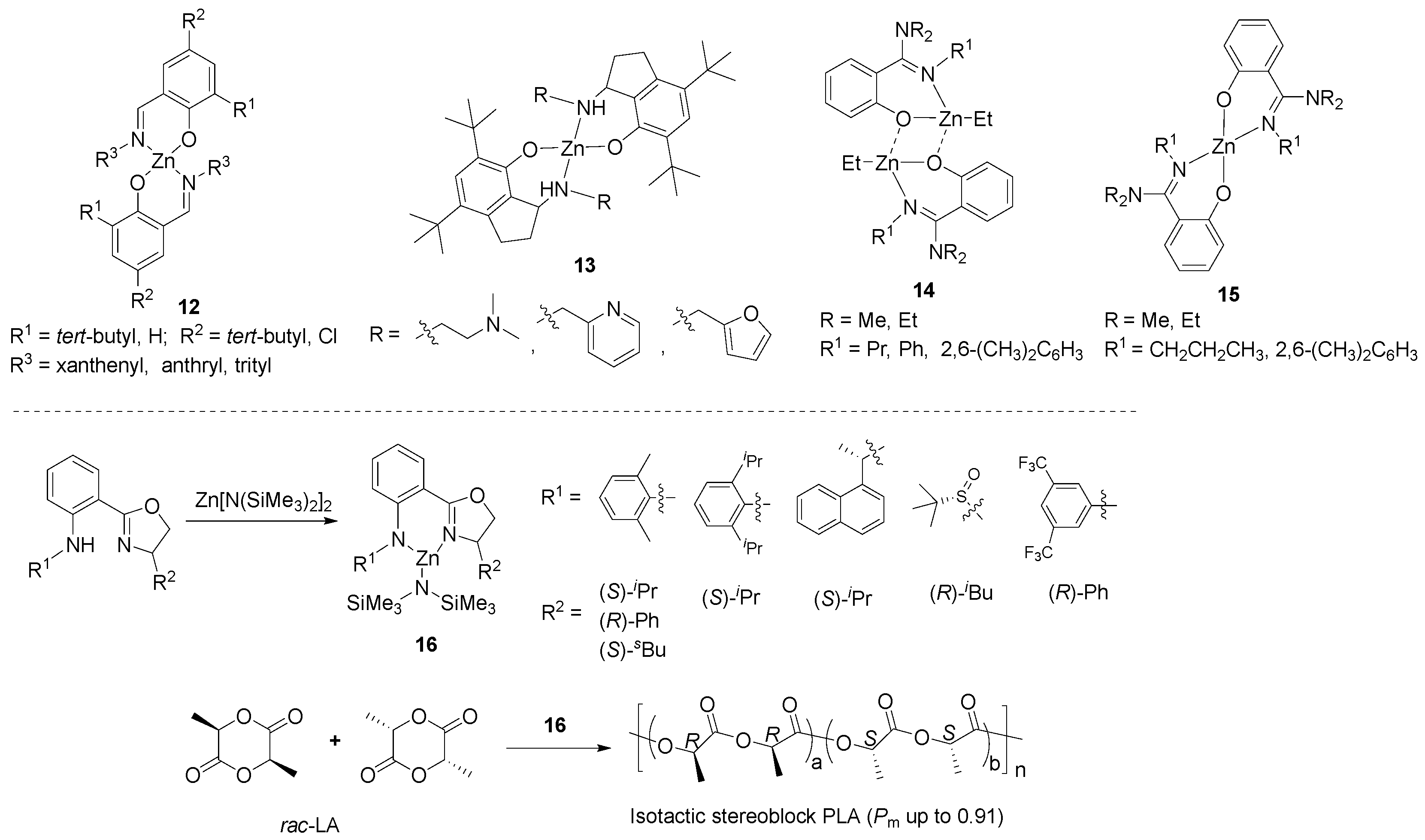
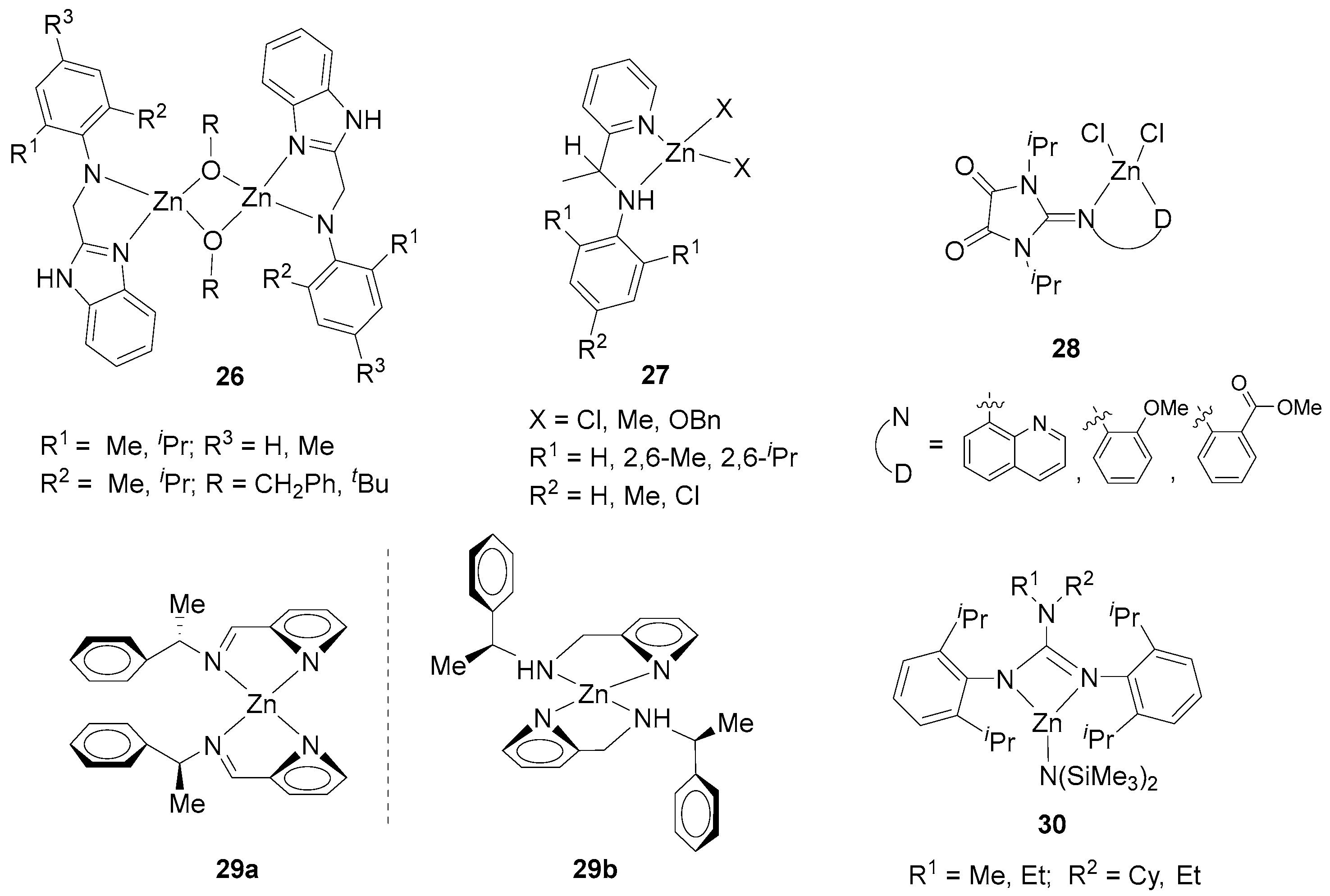
2. Zinc Complexes Containing Bidentate Ligands
| Run | Complex | M 1 | [M]:[C] 2 | T (°C) | Time (h) | Conv. (%) | Mn (kg/mol) | PDI | Tacticity | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
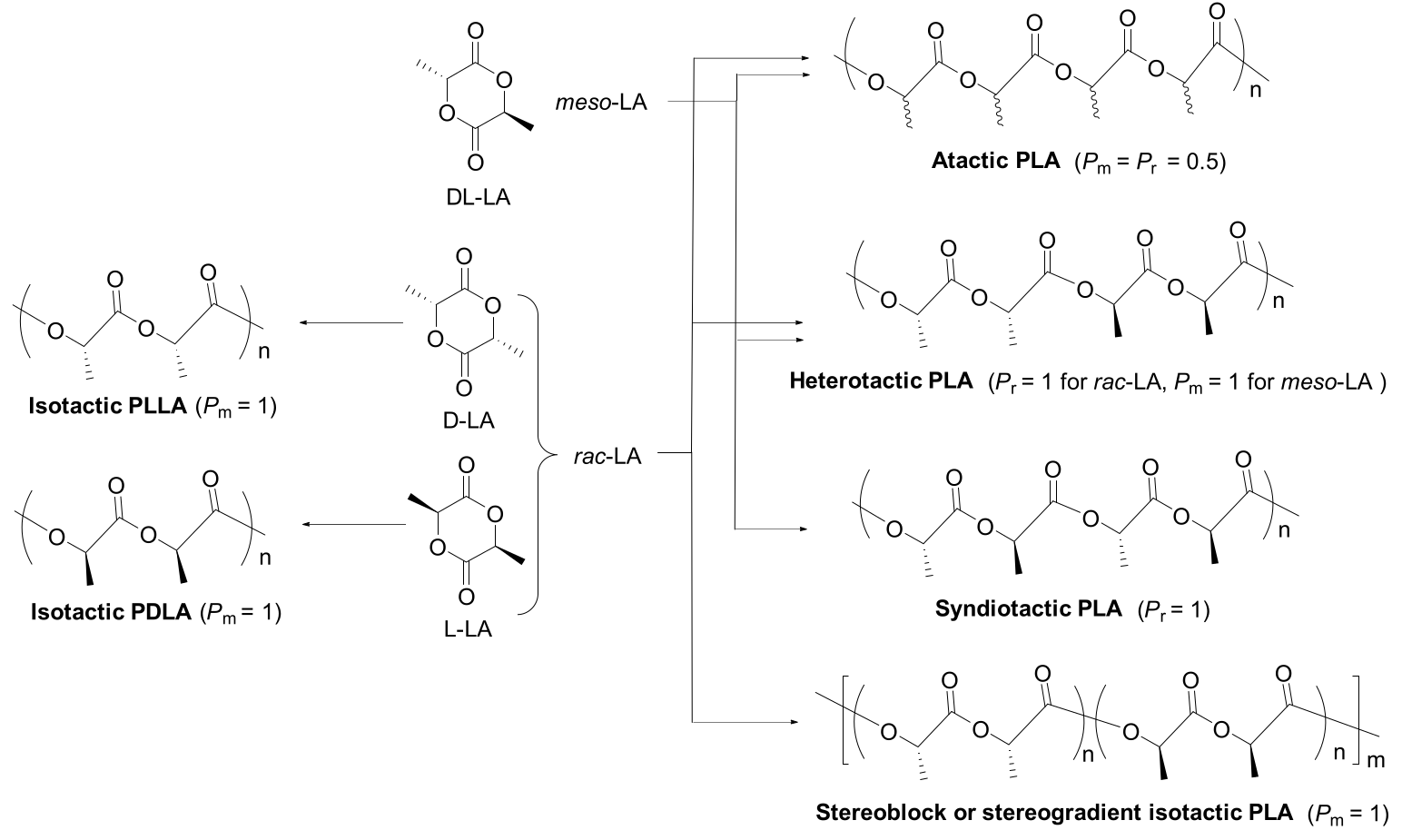
| 1 | 4b | rac-LA | 300:1 | 23 | 6 | 95 | 118 | 1.16 | Pr = 0.93 | [41] |
| 2 | 5 | rac-LA | 500:1 | 25 | 0.42 | 79 | 281 | 1.15 | Pr = 0.83 | [42] |
| 3 | 6 | rac-LA | 200:1 | 25 | 0.33 | 95 | 37.90 | 1.10 | Pr = 0.90 | [43] |
| 4 | 10c | rac-LacOCA | 100:1 | −70 | 10 | 90 | 6.60 | 1.25 | Pm = 0.97 | [48] |
| 5 | 12 | rac-LA | 100:1 | 130 | 1 | 92 | 10.80 | 1.16 | Pr = 0.62 | [49] |
| 6 | 13 | rac-LA | 100:1 | 30 | 0.017 | 98 | 13.20 | 1.05 | Pr = 0.68 | [51] |
| 7 | 15 | rac-LA | 100:1 | 25 | 2 | 72 | 10.40 | 1.09 | Pr = 0.75 | [52] |
| 8 | 16 | rac-LA | 100:1 | 23 | 44 | 96 | 52.20 | 1.32 | Pm = 0.91 | [53] |
| 9 | 17 | rac-LA | 50:1 | −25 | 0.5 | >99 | 5.54 | 1.31 | Pr = 0.80 | [54] |
| 10 | 18 | rac-LA | 100:1 | 25 | 0.033 | 100 | 8.01 | 1.36 | Pr = 0.77 | [55] |
| 11 | 19 | rac-LA | 100:1 | 0 | 0.33 | 96.6 | 6.273 | 1.25 | Pr = 0.74 | [56] |
| 12 | 20a | rac-LA | 100:1 | 25 | 0.033 | 94 | 7.01 | 1.33 | Pr = 0.90 | [57] |
| 13 | 21 | rac-LA | 100:1 | 25 | 0.083 | 100 | 6.57 | 1.31 | Pr = 0.77 | [58] |
| 14 | 22 | rac-LA | 100:1 | 25 | 0.33 | 100 | 8.42 | 1.26 | Pr = 0.78 | [59] |
| 15 | 23 | rac-LA | 100:1 | 25 | 0.042 | 100 | 7.67 | 1.26 | Pr = 0.80 | [60] |
| 16 | 24 | rac-LA | 100:1 | 25 | 0.05 | 100 | 8 | 1.21 | Pr = 0.81 | [61] |
| 17 | 25 | rac-LA | 100:1 | 70 | 0.33 | 78 | 6.792 | 1.09 | Pr = 0.54 | [62] |
| 18 | 26 | rac-LA | 200:1 | 110 | 18 | 98 | 8.675 | 1.34 | Pr = 0.78 | [63] |
| 19 | 28 | rac-LA | 100:1 | 135 | 24 | 100 | 2.10 | 1.10 | Pr = 0.60 | [65] |
| 20 | 29a | rac-LA | 100:1 | 70 | 3 | 90 | 9.19 | 1.49 | Pm = 0.71 | [66] |
| 21 | 31 | rac-LA | 100:1 | −50 | 12 | 90 | 16.20 | 1.22 | Pr = 0.95 | [69] |
| 22 | 32b | rac-LA | 100:1 | −25 | 2 | 97 | 7.56 | 1.16 | Pr = 0.94 | [70] |
| 23 | 33 | rac-LA | 100:1 | −25 | 2 | >99 | 13.93 | 1.29 | Pr = 0.93 | [71] |
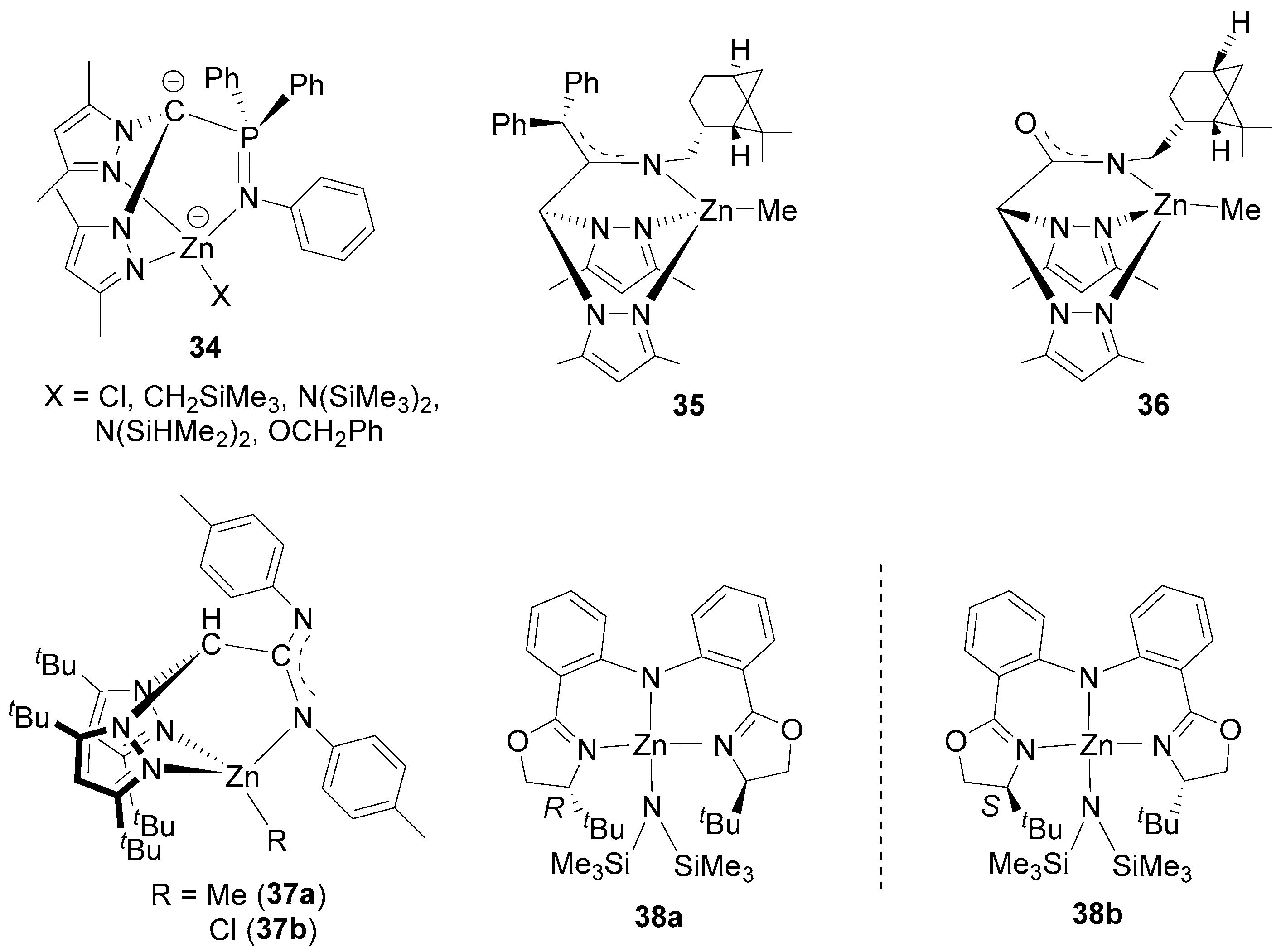
3. Zinc Complexes Containing Tridentate Ligands
| Run | Complex | M 1 | [M]:[C] 2 | T (°C) | Time (h) | Conv. (%) | Mn (kg/mol) | PDI | Tacticity | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | rac-LA | 200:1 | 30 | 8 | 96 | 5.10 | 1.37 | Pm = 0.85 | [75] |
| 2 | 35 | rac-LA | 100:1 | 50 | 2.5 | 37 | 4.90 | 1.07 | Pm = 0.88 | [76] |
| 3 | 37a | rac-LA | 100:1 | 50 | 3.75 | 95 | 13 | 1.13 | Pr = 0.74 | [78] |
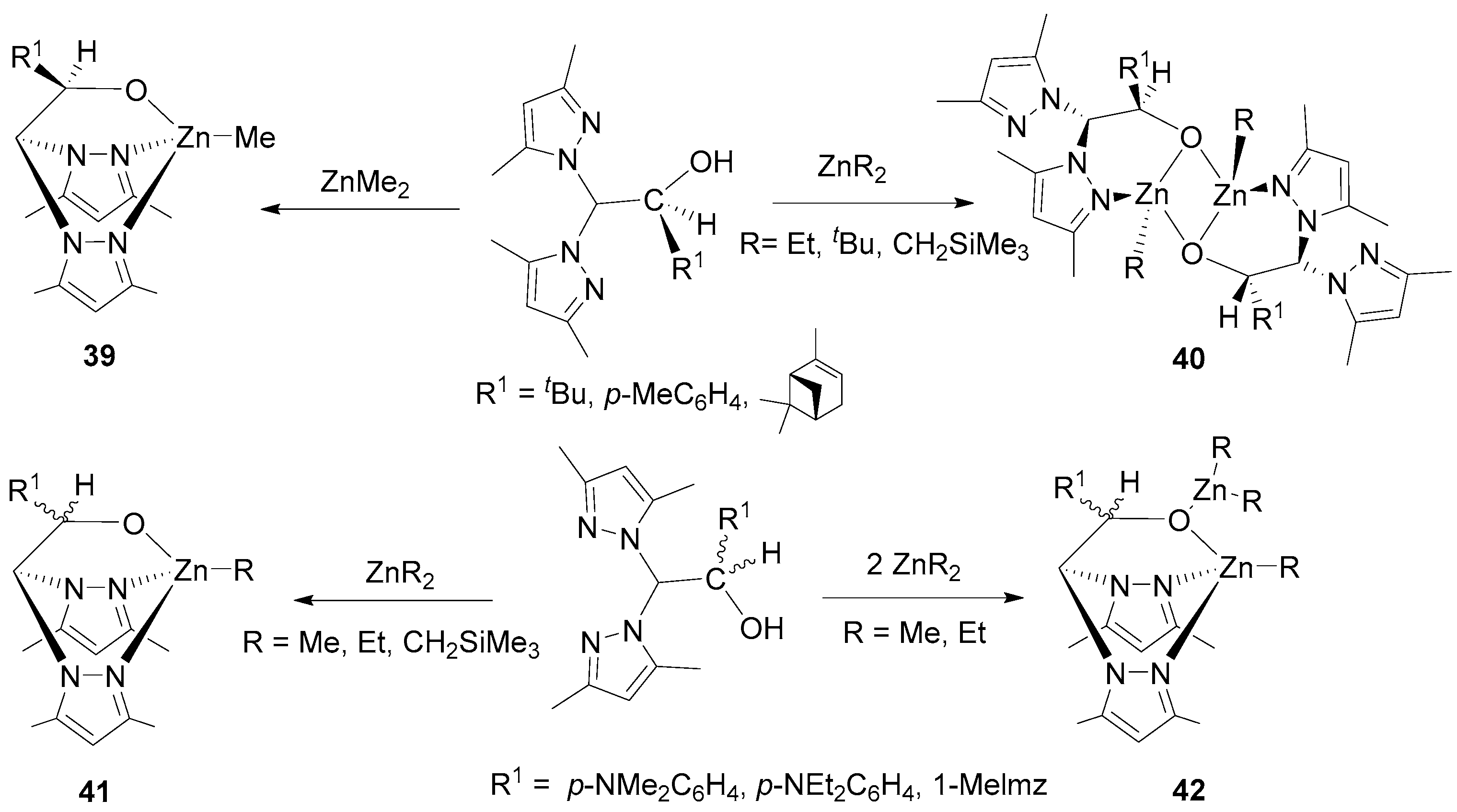
| 4 | 40 | rac-LA | 100:1 | 50 | 3.5 | 73 | 10.60 | 1.08 | Pr = 0.77 | [80] |
| 5 | 41 | rac-LA | 100:1 | 0 | 24 | 37 | 6.10 | 1.12 | Pr = 0.68 | [81] |
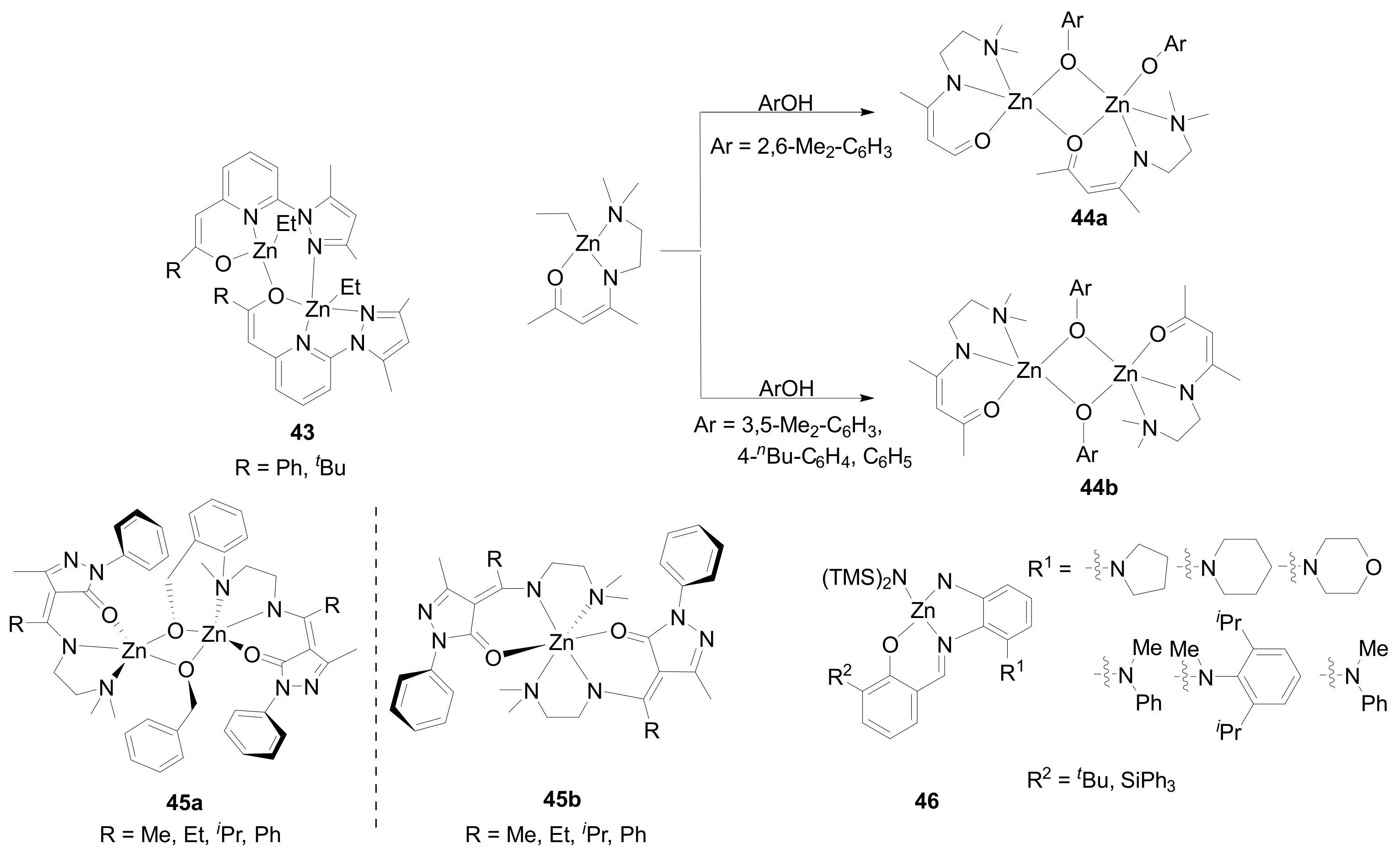
| 6 | 43 | rac-LA | 200:1 | 0 | 24 | 58 | 8.10 | 1.02 | Pr = 0.73 | [82] |
| 7 | 44a | rac-LA | 200:1 | 25 | 0.031 | 93 | 32 | 1.4 | Pm = 0.70 | [83] |
| 8 | 45a | rac-LA | 100:1 | 30 | 12 | 90 | 5.10 | 1.10 | Pr = 0.85 | [84] |
| 19 | 46 | rac-LA | 200:1 | −30 | 96 | 33 | 7.10 | 1.17 | Pr = 0.94 | [87] |
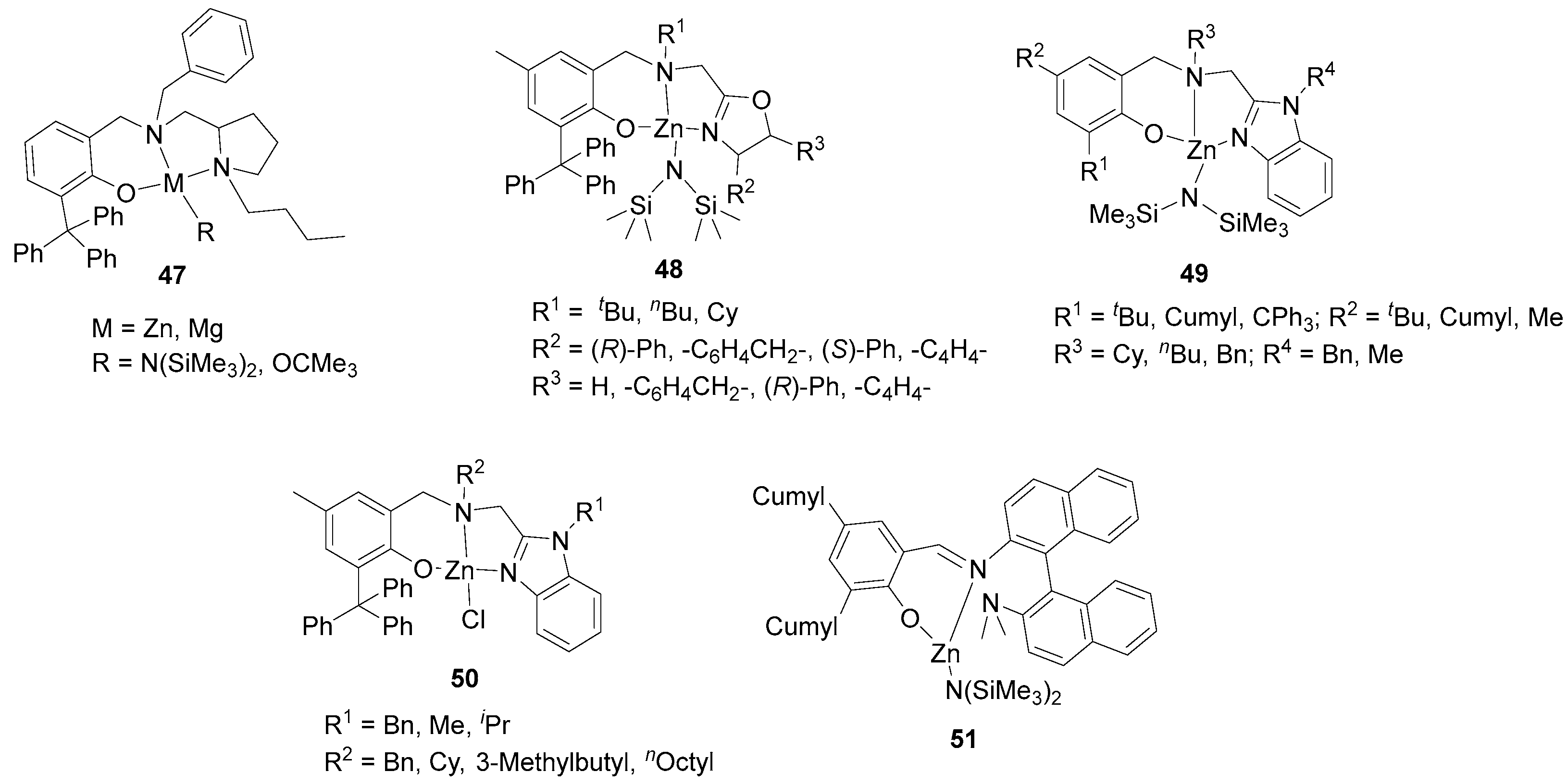
| 10 | 47 | rac-LA | 200:1 | 25 | 3 | 84 | 29.50 | 1.66 | Pm = 0.80 | [88] |
| 11 | 48 | rac-LA | 200:1 | −20 | 1.5 | 88 | 20.90 | 1.06 | Pm = 0.92 | [91] |
| 12 | 49 | rac-LA | 200:1 | 25 | 0.93 | 90 | 48.40 | 1.32 | Pm = 0.89 | [94] |
| 13 | 50 | rac-LA | 200:1 | −45 | 336 | 96 | 7.60 | 1.15 | Pm = 0.93 | [95] |
| 14 | 51 | rac-LA | 200:1 | 25 | 1.5 | 86 | 71.60 | 1.46 | Pr = 0.84 | [96] |
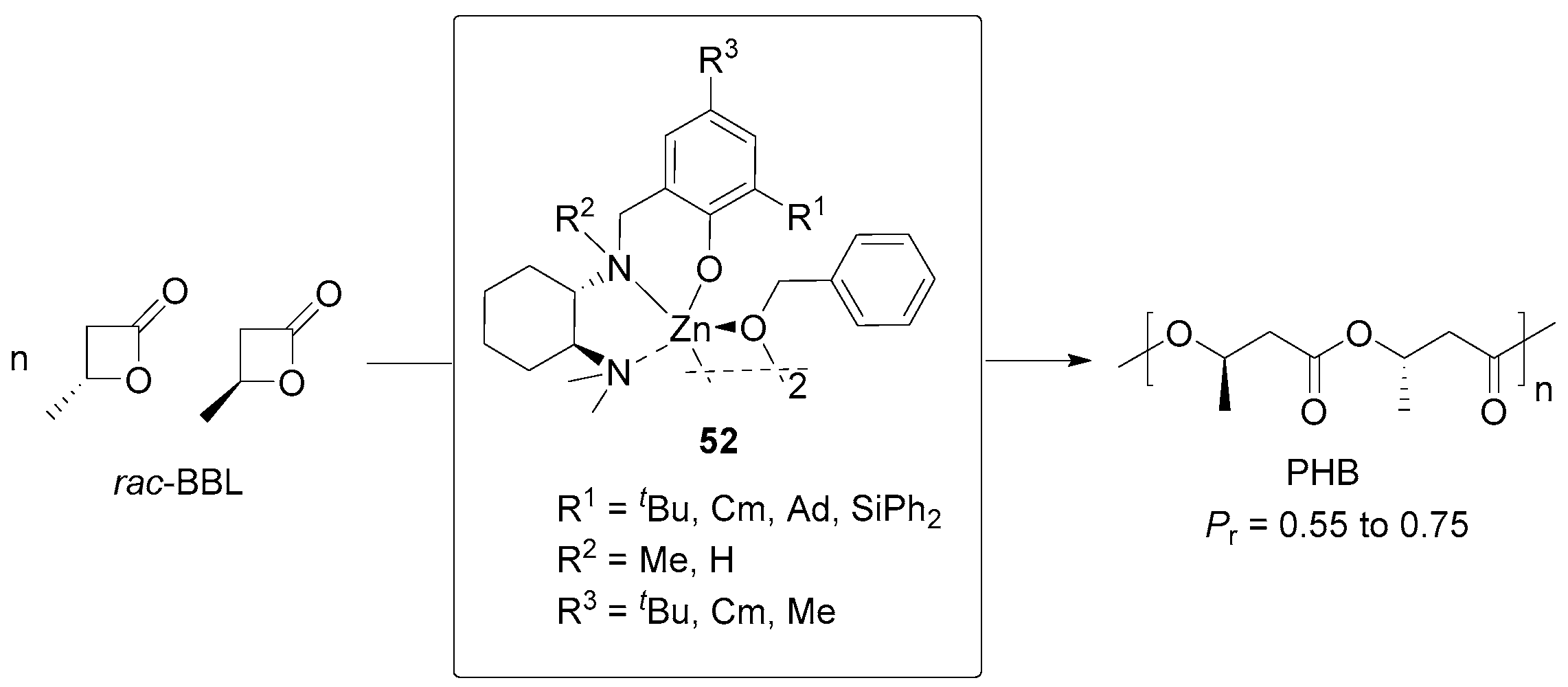
| 15 | 52 | rac-BBL | 400:1 | −30 | 16 | 75 | 21.20 | 1.34 | Pr = 0.75 | [98] |
| 16 | 53a | rac-CHO | 100:1 | 60 | 3 | 91 | 11.40 | 1.33 | Pm = ca.66% | [99] |
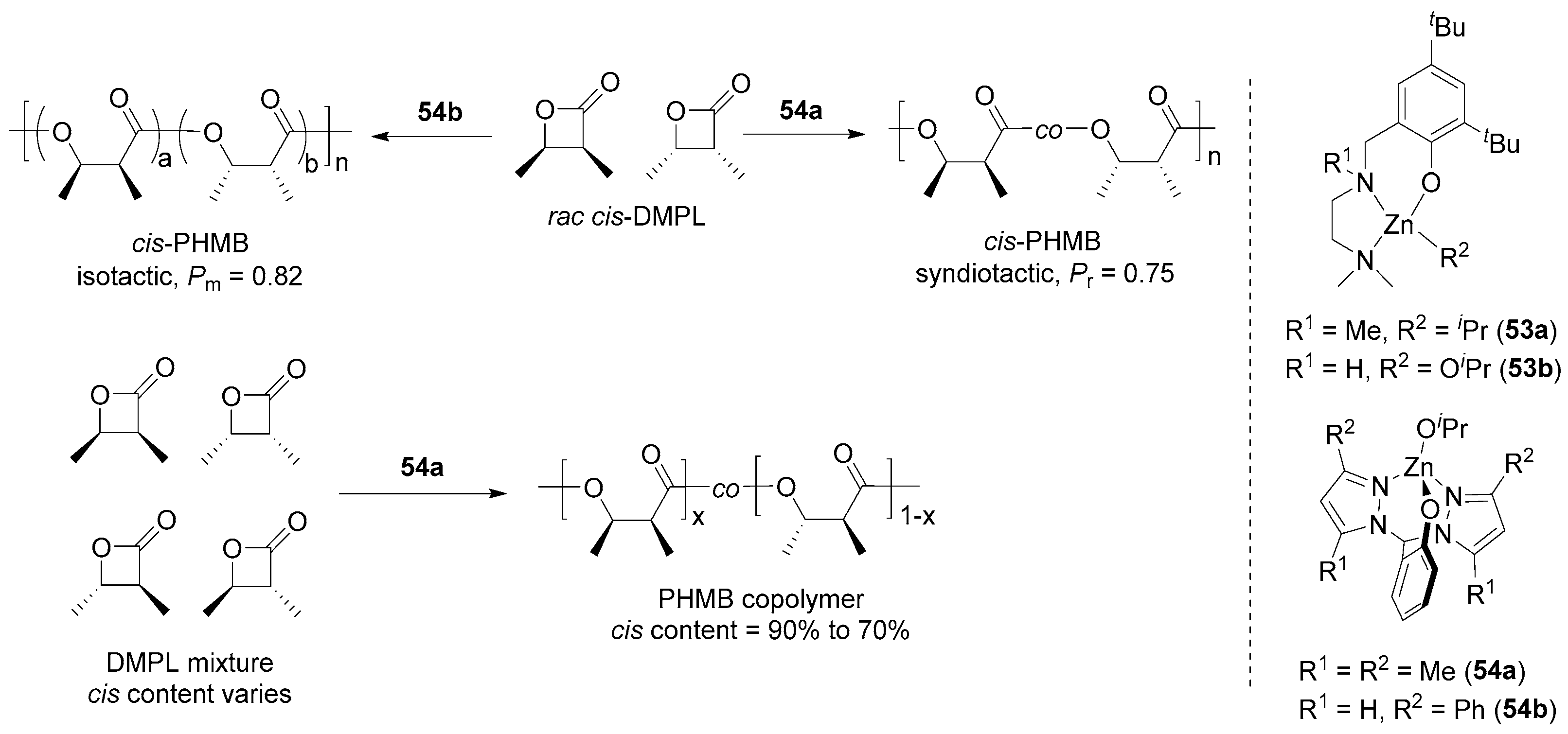
| 17 | 53b | cis-DMPL | 2650:1 | 25 | 24 | 90 | 202 | 1.03 | Pr = 0.63 | [100] |
| 18 | 54a | cis-DMPL | 2500:1 | 25 | 24 | 92 | 471 | 1.06 | Pr = 0.75 | [100] |
| 19 | 54b | cis-DMPL | 820:1 | 50 | 48 | 83 | 62.50 | 1.09 | Pm = 0.82 | [101] |
| 20 | 55a | rac-LA | 500:1 | 25 | 0.66 | 57 | 40.10 | 1.88 | Pr = 0.76 | [103] |
| 21 | 56 | rac-OCA | 50:1 | −50 | 9.5 | 79 | 5.30 | 1.12 | Pm = 0.92 | [104] |
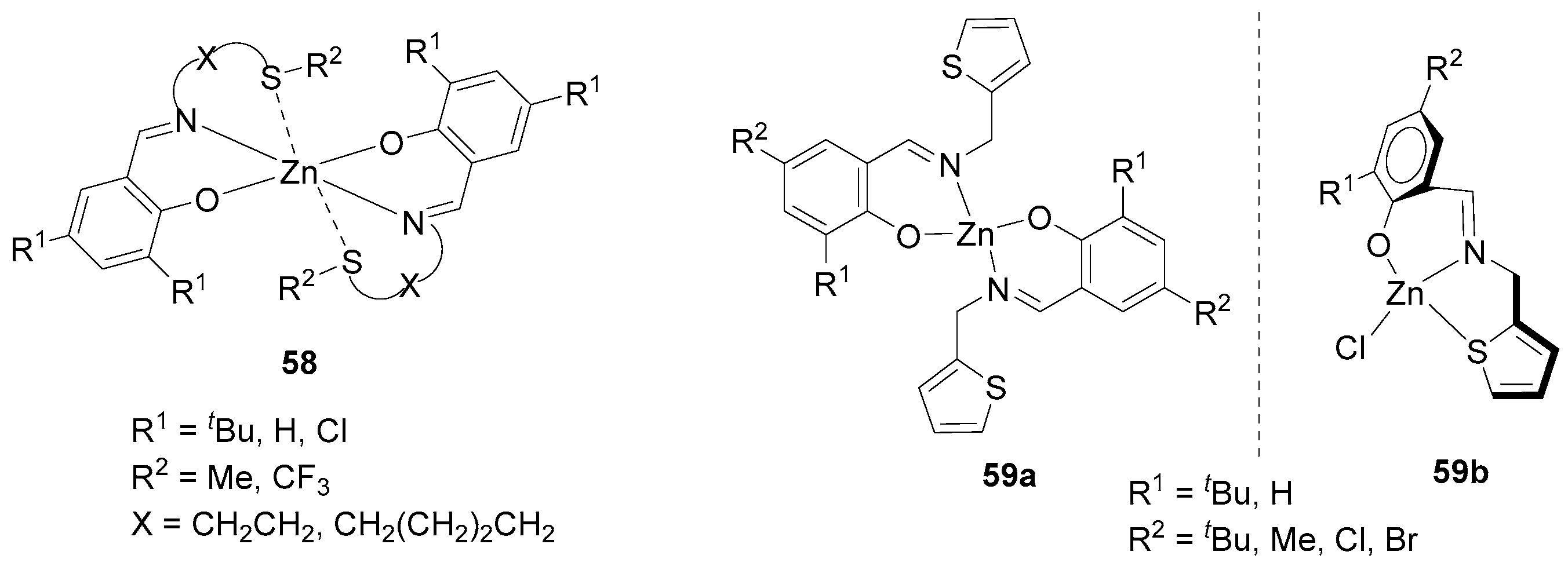
| 22 | 58 | rac-LA | 10,000:1 | 180 | 0.033 | 83 | 31.35 | 1.53 | Pm = 0.57 | [107] |
| 23 | 59a | rac-LA | 200:1 | 70 | 10 | 99 | 30.89 | 1.20 | Pr = 0.67 | [108] |
| 24 | 59b | rac-LA | 200:1 | 70 | 18 | 99 | 29.98 | 1.18 | Pm = 0.78 | [108] |
4. Zinc Complexes Containing Tetradentate Ligands and Others
| Run | Complex | M 1 | [M]:[C] 2 | T (°C) | Time (h) | Conv. (%) | Mn (kg/mol) | PDI | Tacticity | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
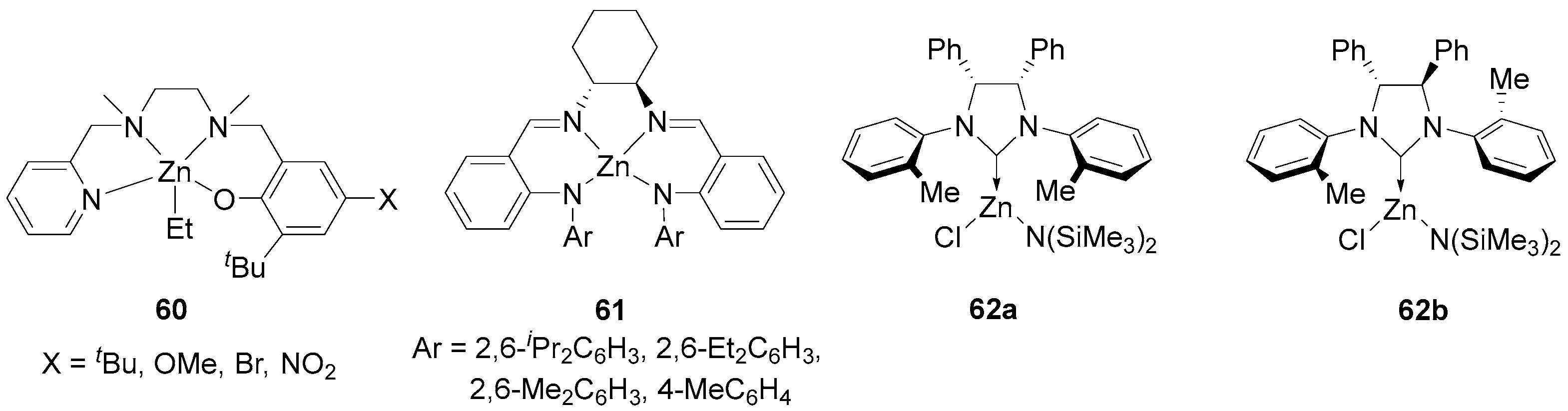
| 1 | 60 | rac-LA | 300:1 | 25 | 0.5 | >95 | 35.50 | 1.15 | Pm = 0.78 | [110] |
| 2 | 61 | rac-LA | 100:1 | 0 | 24 | 94 | 13.10 | 1.11 | Pr = 0.77 | [111] |
| 3 | 62b | rac-LA | 100:1 | 25 | 1.2 | 95 | 35.874 | 1.35 | Pr = 0.66 | [112] |
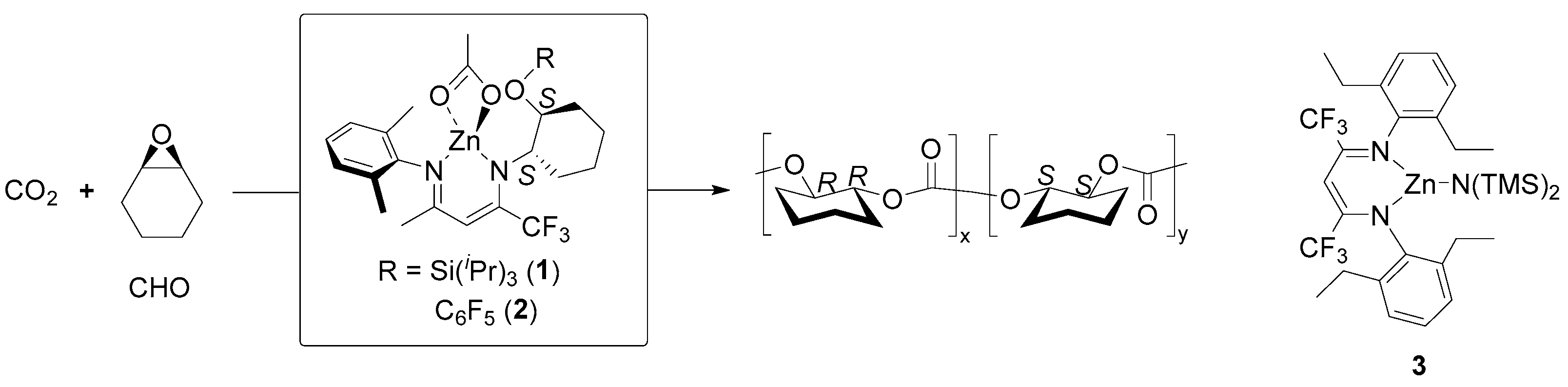
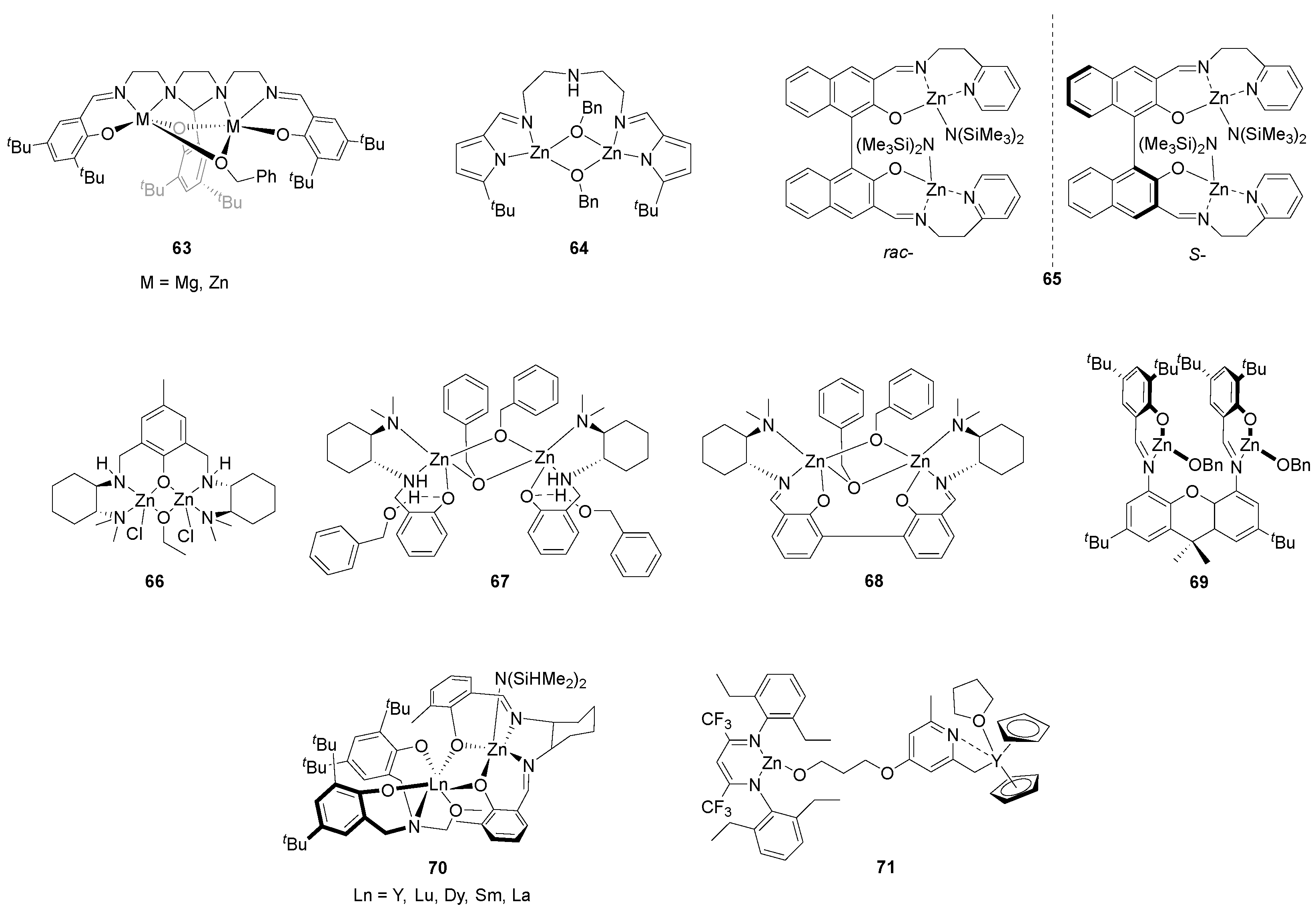
| 4 | 63 | rac-LA | 100:1 | 80 | 12 | 97 | 6.90 | 1.09 | Pm = 0.59 | [113] |
| 5 | 64 | rac-LA | 200:1 | 35 | 24 | 55 | 0.55 | 1.18 | Pr = 0.70 | [114] |
| 6 | 65 | rac-LA | 200:1 | 25 | 1.5 | 75 | 37.70 | 1.76 | Pm = 0.72 | [115] |
| 7 | 66 | rac-LA | 200:1 | 25 | 120 | 95 | 24.29 | 1.04 | Pr = 0.64 | [116] |
| 8 | 68 | rac-LA | 600:1 | 25 | 24 | 95 | 22.37 | 1.10 | Pr = 0.67 | [117] |
| 9 | 69 | rac-LA | 600:1 | 25 | 24 | 86 | 23.43 | 1.23 | Pr = 0.67 | [118] |
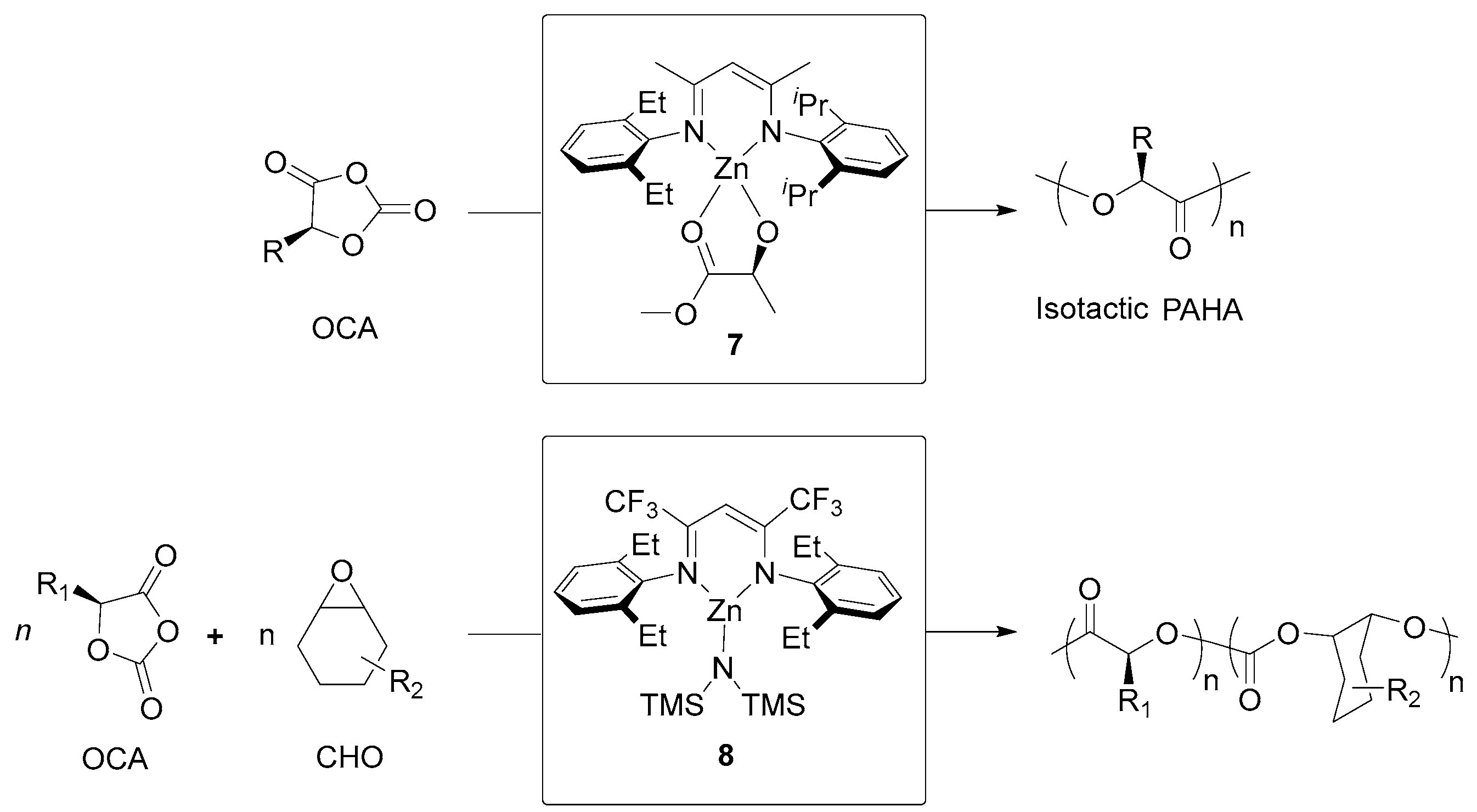
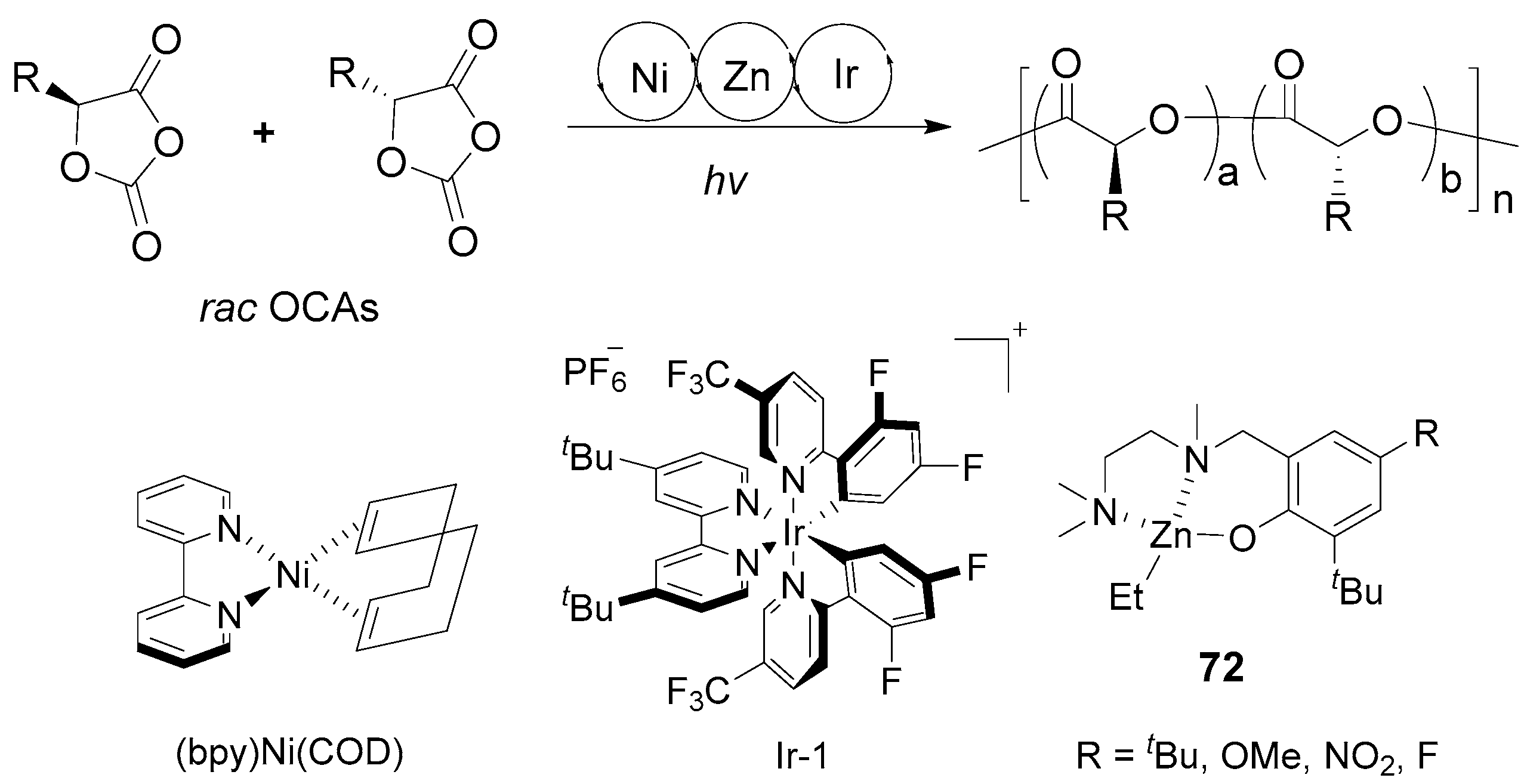
| 10 | 72 | rac-OCA | 150:1 | −15 | 4 | 100 | 45.70 | 1.06 | Pm = 0.97 | [126] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROP | Ring-opening polymerization |
| ROCOP | Ring-opening copolymerization |
| BDI | β-diketiminate |
| LA | Lactide |
| PLA | Polylactide |
| ε-CL | ε-caprolactone |
| PCL | Polycaprolactone |
| rac | Racemic |
| PHB | Poly(3-hydroxybutyrate) |
| CHO | Cyclohexene oxide |
| BBL | β-butyrolactone |
| OCA | O-carboxyanhydride |
| TMP | 1,5,9-trimesityldipyrromethene |
| CHC | Cyclohexene carbonate |
| PCHC | Poly(cyclohexene carbonate) |
| DMPL | 2,3-dimethyl-β-propiolactone |
| PHMB | Poly(3-hydroxy-2-methylbutyrate) |
| PET | Poly(ethylene terephthalate) |
| PO | Propylene oxide |
| TPPCl | Tetraphenylphosphonium chloride |
References
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Artz, J.; Muller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Huang, W.; Wen, X.; Tang, W. Are biodegradable plastics a promising solution to solve the global plastic pollution? Environ. Pollut. 2020, 263, 114469. [Google Scholar] [CrossRef]
- Millican, J.M.; Agarwal, S. Plastic Pollution: A Material Problem? Macromolecules 2021, 54, 4455–4469. [Google Scholar] [CrossRef]
- Pearson, P.N.; Palmer, M.R. Atmospheric carbon dioxide concentrations over the past 60 million years. Nature 2000, 406, 695–699. [Google Scholar] [CrossRef]
- Li, X.; Meng, L.; Zhang, Y.; Qin, Z.; Meng, L.; Li, C.; Liu, M. Research and Application of Polypropylene Carbonate Composite Materials: A Review. Polymers 2022, 14, 2159. [Google Scholar] [CrossRef]
- D’Alterio, M.C.; D’Auria, I.; Gaeta, L.; Tedesco, C.; Brenna, S.; Pellecchia, C. Are Well Performing Catalysts for the Ring Opening Polymerization of L-Lactide under Mild Laboratory Conditions Suitable for the Industrial Process? The Case of New Highly Active Zn (II) Catalysts. Macromolecules 2022, 55, 5115–5122. [Google Scholar] [CrossRef]
- Abel, B.A.; Coates, G.W. Introduction: The Future of Plastics Sustainability. Chem. Rev. 2025, 125, 1255–1256. [Google Scholar] [CrossRef]
- Brannigan, R.P.; Dove, A.P. Synthesis, properties and biomedical applications of hydrolytically degradable materials based on aliphatic polyesters and polycarbonates. Biomater. Sci. 2017, 5, 9–21. [Google Scholar] [CrossRef]
- Bolley, A.; Schnee, G.; Thévenin, L.; Jacques, B.; Dagorne, S. Sterically Bulky NHC Adducts of GaMe3 and InMe3 for H2 Activation and Lactide Polymerization. Inorganics 2018, 6, 23. [Google Scholar] [CrossRef]
- Feghali, E.; Tauk, L.; Ortiz, P.; Vanbroekhoven, K.; Eevers, W. Catalytic chemical recycling of biodegradable polyesters. Polym. Degrad. Stabil. 2020, 179, 109241. [Google Scholar] [CrossRef]
- Liao, X.; Su, Y.; Tang, X. Stereoselective synthesis of biodegradable polymers by salen-type metal catalysts. Sci. China Chem. 2022, 65, 2096–2121. [Google Scholar] [CrossRef]
- Li, Z.; Shen, Y.; Li, Z. Ring-Opening Polymerization of Lactones to Prepare Closed-Loop Recyclable Polyesters. Macromolecules 2024, 57, 1919–1940. [Google Scholar] [CrossRef]
- Paul, S.; Zhu, Y.; Romain, C.; Brooks, R.; Saini, P.K.; Williams, C.K. Ring-opening copolymerization (ROCOP): Synthesis and properties of polyesters and polycarbonates. Chem. Commun. 2015, 51, 6459–6479. [Google Scholar] [CrossRef]
- Karmel, I.S.R.; Batrice, R.J.; Eisen, M.S. Catalytic Organic Transformations Mediated by Actinide Complexes. Inorganics 2015, 3, 392–428. [Google Scholar] [CrossRef]
- Cybularczyk-Cecotka, M.; Dąbrowska, A.M.; Guńka, P.A.; Horeglad, P. Probing the Effect of Six-Membered N-Heterocyclic Carbene—6-Mes—On the Synthesis, Structure and Reactivity of Me2MOR(NHC) (M = Ga, In) Complexes. Inorganics 2018, 6, 28. [Google Scholar] [CrossRef]
- Pan, Y.; Li, W.; Wei, N.-N.; So, Y.-M.; Lai, X.; Li, Y.; Jiang, K.; He, G. Highly active rare-earth metal catalysts for heteroselective ring-opening polymerization of racemic lactide. Dalton Trans. 2019, 48, 9079–9088. [Google Scholar] [CrossRef]
- Lidston, C.A.L.; Severson, S.M.; Abel, B.A.; Coates, G.W. Multifunctional Catalysts for Ring-Opening Copolymerizations. ACS Catal. 2022, 12, 11037–11070. [Google Scholar] [CrossRef]
- Pan, Y.; Hao, M.; Li, X.; Meng, Y.; Kang, X.; Zhang, G.; Sun, X.; Song, X.-Z.; Zhang, L.; So, Y.-M. Anilido-Oxazoline-Ligated Iron Alkoxide Complexes for Living Ring-Opening Polymerization of Cyclic Esters with Controllability. Inorg. Chem. 2025, 64, 530–544. [Google Scholar] [CrossRef]
- Metz, A.; Heck, J.; Gohlke, C.M.; Kröckert, K.; Louven, Y.; McKeown, P.; Hoffmann, A.; Jones, M.D.; Herres-Pawlis, S. Reactivity of Zinc Halide Complexes Containing Camphor-Derived Guanidine Ligands with Technical rac-Lactide. Inorganics 2017, 5, 85. [Google Scholar] [CrossRef]
- Venezuela, J.; Dargusch, M.S. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: A comprehensive review. Acta Biomater. 2019, 87, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.M.D.; Omana, A.A.; Wilson, A.S.S.; Hill, M.S.; Aldridge, S.; Rivard, E. Molecular Main Group Metal Hydrides. Chem. Rev. 2021, 121, 12784–12965. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M. Stereocontrolled ring-opening polymerization of cyclic esters: Synthesis of new polyester microstructures. Chem. Soc. Rev. 2010, 39, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Stanford, M.J.; Dove, A.P. Stereocontrolled ring-opening polymerisation of lactide. Chem. Soc. Rev. 2010, 39, 486–494. [Google Scholar] [CrossRef]
- Dijkstra, P.J.; Du, H.; Feijen, J. Single site catalysts for stereoselective ring-opening polymerization of lactides. Polym. Chem. 2011, 2, 520–527. [Google Scholar] [CrossRef]
- Wang, Y.; Mehmood, A.; Zhao, Y.; Qu, J.; Luo, Y. Computational Studies on the Selective Polymerization of Lactide Catalyzed by Bifunctional Yttrium NHC Catalyst. Inorganics 2017, 5, 46. [Google Scholar] [CrossRef]
- Cheng, M.; Lobkovsky, E.B.; Coates, G.W. Catalytic Reactions Involving C1 Feedstocks: New High-Activity Zn(II)-Based Catalysts for the Alternating Copolymerization of Carbon Dioxide and Epoxides. J. Am. Chem. Soc. 1998, 120, 11018–11019. [Google Scholar] [CrossRef]
- Chamberlain, B.M.; Cheng, M.; Moore, D.R.; Ovitt, T.M.; Lobkovsky, E.B.; Coates, G.W. Polymerization of Lactide with Zinc and Magnesium β-Diiminate Complexes: Stereocontrol and Mechanism. J. Am. Chem. Soc. 2001, 123, 3229–3238. [Google Scholar] [CrossRef]
- Yadav, N.; Chundawat, T.S. Recent Trends on Zinc Complexes Bearing Bi-, Tri- and Tetra-Dentate Schiff Base Ligands for Catalytic Ring-Opening Polymerization of Lactides. ChemistrySelect 2024, 9, e202401619. [Google Scholar] [CrossRef]
- Sarazin, Y.; Carpentier, J.-F. Discrete cationic complexes for ring-opening polymerization catalysis of cyclic esters and epoxides. Chem. Rev. 2015, 115, 3564–3614. [Google Scholar] [CrossRef]
- Tschan, M.J.-L.; Gauvin, R.M.; Thomas, C.M. Controlling polymer stereochemistry in ring-opening polymerization: A decade of advances shaping the future of biodegradable polyesters. Chem. Soc. Rev. 2021, 50, 13587–13608. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-W.; Xie, R.; Zhang, Y.-Y.; Xu, C.-K.; Wu, G.-P. Evolution of Copolymers of Epoxides and CO2: Catalysts, Monomers, Architectures, and Applications. Chem. Rev. 2024, 124, 12305–12380. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Moore, D.R.; Reczek, J.J.; Chamberlain, B.M.; Lobkovsky, E.B.; Coates, G.W. Single-Site β-Diiminate Zinc Catalysts for the Alternating Copolymerization of CO2 and Epoxides: Catalyst Synthesis and Unprecedented Polymerization Activity. J. Am. Chem. Soc. 2001, 123, 8738–8749. [Google Scholar] [CrossRef]
- Dove, A.P.; Gibson, V.C.; Marshall, E.L.; Rzepa, H.S.; White, A.J.P.; Williams, D.J. Synthetic, Structural, Mechanistic, and Computational Studies on Single-Site β-Diketiminate Tin(II) Initiators for the Polymerization of rac-Lactide. J. Am. Chem. Soc. 2006, 128, 9834–9843. [Google Scholar] [CrossRef] [PubMed]
- Rosch, B.; Harder, S. New horizons in low oxidation state group 2 metal chemistry. Chem. Commun. 2021, 57, 9354–9365. [Google Scholar] [CrossRef]
- Balasanthiran, V.; Chisholm, M.H.; Choojun, K.; Durr, C.B. Ethyl 2-hydroxy-2-methylpropanoate derivatives of magnesium and zinc. The effect of chelation on the homo- and copolymerization of lactide and ε-caprolactone. Dalton Trans. 2014, 43, 2781–2788. [Google Scholar] [CrossRef]
- Shao, H.; Reddi, Y.; Cramer, C.J. Modeling the Mechanism of CO2/Cyclohexene Oxide Copolymerization Catalyzed by Chiral Zinc β-Diiminates: Factors Affecting Reactivity and Isotacticity. ACS Catal. 2020, 10, 8870–8879. [Google Scholar] [CrossRef]
- Ellis, W.C.; Jung, Y.; Mulzer, M.; Di Girolamo, R.; Lobkovsky, E.B.; Coates, G.W. Copolymerization of CO2and meso epoxides using enantioselective β-diiminate catalysts: A route to highly isotactic polycarbonates. Chem. Sci. 2014, 5, 4004–4011. [Google Scholar] [CrossRef]
- Kernbichl, S.; Reiter, M.; Adams, F.; Vagin, S.; Rieger, B. CO2-Controlled One-Pot Synthesis of AB, ABA Block, and Statistical Terpolymers from β-Butyrolactone, Epoxides, and CO2. J. Am. Chem. Soc. 2017, 139, 6787–6790. [Google Scholar] [CrossRef]
- Kernbichl, S.; Reiter, M.; Mock, J.; Rieger, B. Terpolymerization of β-Butyrolactone, Epoxides, and CO2: Chemoselective CO2-Switch and Its Impact on Kinetics and Material Properties. Macromolecules 2019, 52, 8476–8483. [Google Scholar] [CrossRef]
- Whitehorne, T.J.J.; Vabre, B.; Schaper, F. Lactide polymerization catalyzed by Mg and Zn diketiminate complexes with flexible ligand frameworks. Dalton Trans. 2014, 43, 6339–6352. [Google Scholar] [CrossRef] [PubMed]
- Keram, M.; Ma, H. Ring-opening polymerization of lactide, ε-caprolactone and their copolymerization catalyzed by β-diketiminate zinc complexes. Appl. Organomet. Chem. 2017, 31, e3893. [Google Scholar] [CrossRef]
- Chellali, J.E.; Alverson, A.K.; Robinson, J.R. Zinc Aryl/Alkyl β-diketiminates: Balancing Accessibility and Stability for High-Activity Ring-Opening Polymerization of rac-Lactide. ACS Catal. 2022, 12, 5585–5594. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, J.; Yin, Q.; Xu, Y.; Cheng, J.; Tong, R. Controlled Ring-Opening Polymerization of O-Carboxyanhydrides Using a β-Diiminate Zinc Catalyst. Angew. Chem. Int. Ed. 2016, 55, 13010–13014. [Google Scholar] [CrossRef]
- Wang, X.; Tong, R. Facile Tandem Copolymerization of O-Carboxyanhydrides and Epoxides to Synthesize Functionalized Poly(ester-b-carbonates). J. Am. Chem. Soc. 2022, 144, 20687–20698. [Google Scholar] [CrossRef]
- Chisholm, M.H.; Choojun, K.; Gallucci, J.C.; Wambua, P.M. Chemistry of magnesium alkyls supported by 1,5,9-trimesityldipyrromethene and 2-[(2,6-diisopropylphenyl)amino]-4-[(2,6-diisopropylphenyl)imino]pent-2-ene. A comparative study. Chem. Sci. 2012, 3, 3445–3457. [Google Scholar] [CrossRef]
- Balasanthiran, V.; Chisholm, M.H.; Choojun, K.; Durr, C.B.; Wambua, P.M. TMPZnN(SiMe3)2, [TMPZn(μ-OiPr)]2 and TMPZn[OCMe2C(O)OEt]. Their role in the ring-opening of rac-lactide and ε-caprolactone where TMP = 1,5,9-trimesityldipyrromethene. J. Organomet. Chem. 2016, 812, 56–65. [Google Scholar] [CrossRef]
- Cui, Y.; Jiang, J.; Pan, X.; Wu, J. Highly isoselective ring-opening polymerization of rac-O-carboxyanhydrides using a zinc alkoxide initiator. Chem. Commun. 2019, 55, 12948–12951. [Google Scholar] [CrossRef]
- Dai, Z.-R.; Yin, C.-F.; Wang, C.; Wu, J.-C. Zinc bis-Schiff base complexes: Synthesis, structure, and application in ring-opening polymerization of rac-lactide. Chin. Chem. Lett. 2016, 27, 1649–1654. [Google Scholar] [CrossRef]
- Ghosh, S.; Huse, K.; Wölper, C.; Tjaberings, A.; Gröschel, A.H.; Schulz, S. Fluorinated β-Ketoiminate Zinc Complexes: Synthesis, Structure and Catalytic Activity in Ring Opening Polymerization of Lactide. Z. Für Anorg. Allg. Chem. 2021, 647, 1744–1750. [Google Scholar] [CrossRef]
- Pongpanit, T.; Saeteaw, T.; Chumsaeng, P.; Chasing, P.; Phomphrai, K. Highly Active Homoleptic Zinc and Magnesium Complexes Supported by Constrained Reduced Schiff Base Ligands for the Ring-Opening Polymerization of Lactide. Inorg. Chem. 2021, 60, 17114–17122. [Google Scholar] [CrossRef] [PubMed]
- Vaillant-Coindard, V.; Théron, B.; Printz, G.; Chotard, F.; Balan, C.; Rousselin, Y.; Richard, P.; Tolbatov, I.; Fleurat-Lessard, P.; Bodio, E.; et al. Phenoxy-Amidine Ligands: Toward Lactic Acid-Tolerant Catalysts for Lactide Ring-Opening Polymerization. Organometallics 2022, 41, 2920–2932. [Google Scholar] [CrossRef]
- Abbina, S.; Du, G. Zinc-Catalyzed Highly Isoselective Ring Opening Polymerization of rac-Lactide. ACS Macro Lett. 2014, 3, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.S.; Nayab, S.; Lee, H.; Jeong, J.H. Synthesis and structural characterisation of zinc complexes bearing furanylmethyl and thiophenylmethyl derivatives of (R,R)-1,2-diaminocyclohexanes for stereoselective polymerisation of poly(rac-lactide). Polyhedron 2014, 77, 32–38. [Google Scholar] [CrossRef]
- Kang, M.-S.; Cho, J.; Nayab, S.; Jeong, J.H. Synthesis and characterization of Zn(II) and Cu(II) complexes bearing (chiral substituent)(diethyl)-ethanediamine derivatives as precatalysts for rac-lactide polymerisation. Polyhedron 2019, 158, 135–143. [Google Scholar] [CrossRef]
- Lee, J.; Melchakova, I.; Nayab, S.; Kim, K.; Ko, Y.H.; Yoon, M.; Avramov, P.; Lee, H. Synthesis and Characterization of Zinc(II), Cadmium(II), and Palladium(II) Complexes with the Thiophene-Derived Schiff Base Ligand. ACS Omega 2023, 8, 6016–6029. [Google Scholar] [CrossRef]
- Nayab, S.; Jeong, J.H. Facile separation of diastereo-chiral N,N′-diamine ligand via fractional crystallization and demetalation of corresponding Zn(II) complex. Inorg. Chem. Commun. 2016, 65, 35–38. [Google Scholar] [CrossRef]
- Kwon, K.S.; Nayab, S.; Jeong, J.H. Synthesis, characterisation and X-ray structures of zinc(II) complexes bearing camphor-based iminopyridines as pre-catalysts for heterotactic-enriched polylactide from rac-lactide. Polyhedron 2015, 85, 615–620. [Google Scholar] [CrossRef]
- Kwon, K.S.; Nayab, S.; Lee, H.-I.; Jeong, J.H. Synthesis, characterisation, and X-ray structures of Zn(II) complexes containing bis-camphoryldiimine ligands: Application to polymerisation of rac-lactide. Polyhedron 2017, 126, 127–133. [Google Scholar] [CrossRef]
- Kwon, K.S.; Nayab, S.; Jeong, J.H. Synthesis, characterisation and X-ray structure of Cu(II) and Zn(II) complexes bearing N, N -dimethylethylenamine-camphorylimine ligands: Application in the polymerisation of rac-lactide. Polyhedron 2017, 130, 23–29. [Google Scholar] [CrossRef]
- Cho, J.; Chun, M.K.; Nayab, S.; Jeong, J.H. Synthesis, characterisation, and X-ray structures of zinc(II) complexes bearing camphor-based ethyleneamineimines as pre-catalysts for heterotactic-enriched polylactide from rac-lactide. Transit. Met. Chem. 2019, 44, 175–185. [Google Scholar] [CrossRef]
- Bai, J.; Xiao, X.; Zhang, Y.; Chao, J.; Chen, X. β-Pyridylenolate zinc catalysts for the ring-opening homo- and copolymerization of ε-caprolactone and lactides. Dalton Trans. 2017, 46, 9846–9858. [Google Scholar] [CrossRef] [PubMed]
- Akpan, E.D.; Omondi, B.; Ojwach, S.O. Kinetics, mechanisms and polymer property studies of ring-opening polymerization of ɛ-caprolactone and lactides initiated by (benzimidazolylmethyl)amino Zn(II) alkoxides. Polym. Bull. 2018, 75, 5179–5195. [Google Scholar] [CrossRef]
- Munzeiwa, W.A.; Nyamori, V.O.; Omondi, B. Stereoselective homo- and co-polymerization of lactides and ε-caprolactone catalysed by highly active racemic zinc(II) pyridyl complexes. Transit. Met. Chem. 2022, 47, 93–111. [Google Scholar] [CrossRef]
- Khan, B.S.; Flores-Romero, V.; LeBlanc, J.; Lavoie, G.G. Lactide Polymerization Using Zinc Dichloride Complexes Containing a Neutral Bidentate Ligand with a Diacylated Cyclic Guanidine. Organometallics 2022, 41, 2668–2677. [Google Scholar] [CrossRef]
- Jia, B.; Hao, J.; Wei, X.; Tong, H.; Zhou, M.; Liu, D. Zinc and aluminum complexes of chiral ligands: Synthesis, characterization and application to rac-lactide polymerization. J. Organomet. Chem. 2017, 831, 11–17. [Google Scholar] [CrossRef]
- D’Auria, I.; Ferrara, V.; Tedesco, C.; Kretschmer, W.; Kempe, R.; Pellecchia, C. Guanidinate Zn(II) Complexes as Efficient Catalysts for Lactide Homo- and Copolymerization under Industrially Relevant Conditions. ACS Appl. Polym. Mater. 2021, 3, 4035–4043. [Google Scholar] [CrossRef]
- Akpan, E.D.; Ojwach, S.O.; Omondi, B.; Nyamori, V.O. Zn(ii) and Cu(ii) formamidine complexes: Structural, kinetics and polymer tacticity studies in the ring-opening polymerization of ε-caprolactone and lactides. New J. Chem. 2016, 40, 3499–3510. [Google Scholar] [CrossRef]
- Shin, S.; Cho, H.; Lee, H.; Nayab, S.; Kim, Y. Zinc(II) complexes containing N′-aromatic group substituted N,N′,N-bis((1H-pyrazol-1-yl)methyl)amines: Synthesis, characterization, and polymerizations of methyl methacrylate and rac-lactide. J. Coord. Chem. 2018, 71, 556–584. [Google Scholar] [CrossRef]
- Choe, S.; Lee, H.; Nayab, S. Synthesis, structures, and catalytic efficiency in ring opening polymerization of rac-lactide with tridentate vs. bidentate cobalt(ii), zinc(ii), and cadmium(ii) complexes containing N-substituted N,N-bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)amine ligands. RSC Adv. 2021, 11, 18840–18851. [Google Scholar] [CrossRef]
- Choe, S.; Lee, H.; Nayab, S. Diverse coordination geometry of cobalt (II), zinc (II), and cadmium (II) complexes comprising N,N-bis(1H-pyrazol-1-yl)methyl)amines derivatives: Synthesis, structures, and ring opening polymerization of rac-lactide. Appl. Organomet. Chem. 2021, 35, e6204. [Google Scholar] [CrossRef]
- Li, H.; Shakaroun, R.M.; Guillaume, S.M.; Carpentier, J.-F. Recent Advances in Metal-Mediated Stereoselective Ring-Opening Polymerization of Functional Cyclic Esters towards Well-Defined Poly(hydroxy acid)s: From Stereoselectivity to Sequence-Control. Chem. Eur. J. 2020, 26, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Barba, L.F.; Garces, A.; Lara-Sanchez, A.; Navarro, M.; Gonzalez-Lizana, D. Main advances in the application of scorpionate-based catalytic systems for the preparation of sustainable polymers. Chem. Commun. 2025, 61, 1087–1103. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.E.; Kleij, A.W. Recent progress in stereoselective synthesis of cyclic organic carbonates and beyond. Curr. Opin. Green Sustain. Chem. 2017, 3, 55–60. [Google Scholar] [CrossRef]
- Mou, Z.; Liu, B.; Wang, M.; Xie, H.; Li, P.; Li, L.; Li, S.; Cui, D. Isoselective ring-opening polymerization of rac-lactide initiated by achiral heteroscorpionate zwitterionic zinc complexes. Chem. Commun. 2014, 50, 11411–11414. [Google Scholar] [CrossRef]
- Honrado, M.; Sobrino, S.; Fernandez-Baeza, J.; Sanchez-Barba, L.F.; Garces, A.; Lara-Sanchez, A.; Rodriguez, A.M. Synthesis of an enantiopure scorpionate ligand by a nucleophilic addition to a ketenimine and a zinc initiator for the isoselective ROP of rac-lactide. Chem. Commun. 2019, 55, 8947–8950. [Google Scholar] [CrossRef]
- Navarro, M.; Sobrino, S.; Fernández, I.; Lara-Sánchez, A.; Garcés, A.; Sánchez-Barba, L.F. Exploring enantiopure zinc-scorpionates ascatalysts for the preparation of polylactides,cyclic carbonates, and polycarbonates. Dalton Trans. 2024, 53, 13933–13949. [Google Scholar] [CrossRef]
- Navarro, M.; Garces, A.; Sanchez-Barba, L.F.; de la Cruz-Martinez, F.; Fernandez-Baeza, J.; Lara-Sanchez, A. Efficient Bulky Organo-Zinc Scorpionates for the Stereoselective Production of Poly(rac-lactide)s. Polymers 2021, 13, 2356. [Google Scholar] [CrossRef]
- Peng, Z.; Xu, G.; Yang, R.; Guo, X.; Sun, H.; Wang, Q. Isoselective mechanism for asymmetric kinetic resolution polymerization of rac-lactide catalyzed by chiral tridentate bis(oxazolinylphenyl)amido ligand supported zinc complexes. Eur. Polym. J. 2022, 180, 111571. [Google Scholar] [CrossRef]
- Otero, A.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Tejeda, J.; Honrado, M.; Garcés, A.; Lara-Sánchez, A.; Rodríguez, A.M. Chiral N,N,O-Scorpionate Zinc Alkyls as Effective and Stereoselective Initiators for the Living ROP of Lactides. Organometallics 2012, 31, 4191–4202. [Google Scholar] [CrossRef]
- Otero, A.; Fernandez-Baeza, J.; Sanchez-Barba, L.F.; Sobrino, S.; Garces, A.; Lara-Sanchez, A.; Rodriguez, A.M. Mono- and binuclear chiral N,N,O-scorpionate zinc alkyls as efficient initiators for the ROP of rac-lactide. Dalton Trans. 2017, 46, 15107–15117. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.-L.; Chai, Z.-Y.; Wang, Z.-X. Synthesis of N,N,O-chelate zinc and aluminum complexes and their catalysis in the ring-opening polymerization of ε-caprolactone and rac-lactide. Dalton Trans. 2014, 43, 14470–14480. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Schafer, P.M.; Dittrich, D.; Scheiper, C.; Steiniger, P.; Fink, G.; Ksiazkiewicz, A.N.; Tjaberings, A.; Wolper, C.; Groschel, A.H.; et al. Heterolepic β-Ketoiminate Zinc Phenoxide Complexes as Efficient Catalysts for the Ring Opening Polymerization of Lactide. ChemistryOpen 2019, 8, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Kou, X.; Duan, Y.-L.; Ding, F.-F.; Yin, Y.-F.; Wang, W.; Yang, Y. Magnesium and zinc complexes bearing NNO-tridentate ketiminate ligands: Synthesis, structures and catalysis in the ring-opening polymerization of lactides. Dalton Trans. 2018, 47, 8121–8133. [Google Scholar] [CrossRef]
- Kirk, S.M.; McKeown, P.; Mahon, M.F.; Kociok-Köhn, G.; Woodman, T.J.; Jones, M.D. Synthesis of Zn(II) and Al(III) Complexes of Diaminocyclohexane-Derived Ligands and Their Exploitation for the Ring Opening Polymerisation of rac-Lactide. Eur. J. Inorg. Chem. 2017, 45, 5417–5426. [Google Scholar] [CrossRef]
- McKeown, P.; McCormick, S.N.; Mahon, M.F.; Jones, M.D. Highly active Mg(ii) and Zn(ii) complexes for the ring opening polymerisation of lactide. Polym. Chem. 2018, 9, 5339–5347. [Google Scholar] [CrossRef]
- Li, M.; Behzadi, S.; Chen, M.; Pang, W.; Wang, F.; Tan, C. Phenoxyimine Ligands Bearing Nitrogen-Containing Second Coordination Spheres for Zinc Catalyzed Stereoselective RingOpening Polymerization of rac-Lactide. Organometallics 2019, 38, 461–468. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Ma, H. Stereoselectivity Switch between Zinc and Magnesium Initiators in the Polymerization of rac-Lactide: Different Coordination Chemistry, Different Stereocontrol Mechanisms. Macromolecules 2014, 47, 7750–7764. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Ma, H. Stereoselective Polymerization of rac-Lactide Catalyzed by Zinc Complexes with Tetradentate Aminophenolate Ligands in Different Coordination Patterns: Kinetics and Mechanism. Inorg. Chem. 2015, 54, 5839–5854. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Ma, H. Exploring Steric Effects in Diastereoselective Synthesis of Chiral Aminophenolate Zinc Complexes and Stereoselective Ring-Opening Polymerization of rac-Lactide. Inorg. Chem. 2016, 55, 7356–7372. [Google Scholar] [CrossRef]
- Kan, C.; Hu, J.; Huang, Y.; Wang, H.; Ma, H. Highly Isoselective and Active Zinc Catalysts for rac-Lactide Polymerization: Effect of Pendant Groups of Aminophenolate Ligands. Macromolecules 2017, 50, 7911–7919. [Google Scholar] [CrossRef]
- Hu, J.; Kan, C.; Ma, H. Exploring Steric Effects of Zinc Complexes Bearing Achiral Benzoxazolyl Aminophenolate Ligands in Isoselective Polymerization of rac-Lactide. Inorg. Chem. 2018, 57, 11240–11251. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Ma, H. Ring-opening polymerization of rac-lactide, copolymerization of rac-lactide and ε-caprolactone by zinc complexes bearing pyridyl-based tridentate amino-phenolate ligands. Eur. Polym. J. 2019, 119, 289–297. [Google Scholar] [CrossRef]
- Gong, Y.; Ma, H. High performance benzoimidazolyl-based aminophenolate zinc complexes for isoselective polymerization of rac-lactide. Chem. Commun. 2019, 55, 10112–10115. [Google Scholar] [CrossRef]
- Wang, H.; Ma, H. Controlled Synthesis of High-Molecular-Weight and Isotactic Cyclic Polylactides from rac-Lactide Using Aminophenolate Zinc Chlorides. Macromolecules 2024, 57, 6156–6165. [Google Scholar] [CrossRef]
- Huang, M.; Pan, C.; Ma, H. Ring-opening polymerization of rac-lactide and α-methyltrimethylene carbonate catalyzed by magnesium and zinc complexes derived from binaphthyl-based iminophenolate ligands. Dalton Trans. 2015, 44, 12420–12431. [Google Scholar] [CrossRef]
- Huang, M.; Ma, H. Magnesium and Zinc Complexes Supported by N,N,O Tridentate Ligands: Synthesis and Catalysis in the Ring-Opening Polymerization of rac-Lactide and α-Methyltrimethylene Carbonate. Eur. J. Inorg. Chem. 2016, 2016, 3791–3803. [Google Scholar] [CrossRef]
- Ebrahimi, T.; Aluthge, D.C.; Hatzikiriakos, S.G.; Mehrkhodavandi, P. Highly Active Chiral Zinc Catalysts for Immortal Polymerization of β-Butyrolactone Form Melt Processable Syndio-Rich Poly(hydroxybutyrate). Macromolecules 2016, 49, 8812–8824. [Google Scholar] [CrossRef]
- Guerin, W.; Diallo, A.K.; Kirilov, E.; Helou, M.; Slawinski, M.; Brusson, J.-M.; Carpentier, J.-F.; Guillaume, S.M. Enantiopure Isotactic PCHC Synthesized by Ring-Opening Polymerization of Cyclohexene Carbonate. Macromolecules 2014, 47, 4230–4235. [Google Scholar] [CrossRef]
- Zhou, Z.; LaPointe, A.M.; Shaffer, T.D.; Coates, G.W. Nature-inspired methylated polyhydroxybutyrates from C1 and C4 feedstocks. Nat. Chem. 2023, 15, 856–861. [Google Scholar] [CrossRef]
- Zhou, Z.; LaPointe, A.M.; Coates, G.W. Atactic, Isotactic, and Syndiotactic Methylated Polyhydroxybutyrates: An Unexpected Series of Isomorphic Polymers. J. Am. Chem. Soc. 2023, 145, 25983–25988. [Google Scholar] [CrossRef] [PubMed]
- Di Iulio, C.; Middleton, M.; Kociok-Köhn, G.; Jones, M.D.; Johnson, A.L. Synthesis and Characterization of Zinc Ketoiminate and Zinc Alkoxide–/Phenoxide–Ketoiminate Complexes. Eur. J. Inorg. Chem. 2013, 2013, 1541–1554. [Google Scholar] [CrossRef]
- Chapurina, Y.; Roisnel, T.; Carpentier, J.-F.; Kirillov, E. Zinc, aluminum and group 3 metal complexes of sterically demanding naphthoxy-pyridine ligands: Synthesis, structure, and use in ROP of racemic lactide and β-butyrolactone. Inorg. Chim. Acta 2015, 431, 161–175. [Google Scholar] [CrossRef]
- Jiang, J.; Cui, Y.; Lu, Y.; Zhang, B.; Pan, X.; Wu, J. Weak Lewis Pairs as Catalysts for Highly Isoselective Ring-Opening Polymerization of Epimerically Labile rac-O-Carboxyanhydride of Mandelic Acid. Macromolecules 2020, 53, 946–955. [Google Scholar] [CrossRef]
- Jiang, J.; Cui, Y.; Jia, Z.; Pan, X.; Wu, J. Living Polymerization of Chiral O-Carboxyanhydride of Mandelic Acid and Precise Stereoblock Copolymer Syntheses Using Highly Active OOO-Tridentate Bis(phenolate) Zinc Complexes. Maromolecules 2021, 54, 2232–2241. [Google Scholar] [CrossRef]
- Zikode, M.; Fuchs, M.; Langletz, T.; Burkart, L.; Herres-Pawlis, S.; Ojwach, S.O. Mononuclear and Multinuclear O^N^O-donor Zn(II) Complexes as Robust Catalysts for the Production and Depolymerization of Poly(Lactide). ChemCatChem 2025, 17, e202400771. [Google Scholar] [CrossRef]
- Stewart, J.; Fuchs, M.; Payne, J.; Driscoll, O.; Kociok-Kohn, G.; Ward, B.D.; Herres-Pawlis, S.; Jones, M.D. Simple Zn(ii) complexes for the production and degradation of polyesters. RSC Adv. 2022, 12, 1416–1424. [Google Scholar] [CrossRef]
- Roy, S.S.; Sarkar, S.; Antharjanam, P.K.S.; Chakraborty, D. Mononuclear Zn(ii) compounds supported by iminophenolate proligands binding in the bidentate (N, O) and tridentate (N, O, S) coordination mode: Synthesis, characterization and polymerization studies. New J. Chem. 2023, 47, 635–652. [Google Scholar] [CrossRef]
- Williams, C.K.; Breyfogle, L.E.; Choi, S.K.; Nam, W.; Young, V.G., Jr.; Hillmyer, M.A.; Tolman, W.B. A Highly Active Zinc Catalyst for the Controlled Polymerization of Lactide. J. Am. Chem. Soc. 2003, 125, 11350–11359. [Google Scholar] [CrossRef]
- Stasiw, D.E.; Luke, A.M.; Rosen, T.; League, A.B.; Mandal, M.; Neisen, B.D.; Cramer, C.J.; Kol, M.; Tolman, W.B. Mechanism of the Polymerization of rac-Lactide by Fast Zinc Alkoxide Catalysts. Inorg. Chem. 2017, 56, 14366–14372. [Google Scholar] [CrossRef]
- Han, Y.; Feng, Q.; Zhang, Y.; Zhang, Y.; Yao, W. Ring-opening polymerization of rac-lactide by mononuclear zinc complexes that contain chiral tetra-azane ligands. Polyhedron 2017, 121, 206–210. [Google Scholar] [CrossRef]
- Tufano, F.; Santulli, F.; Grisi, F.; Lamberti, M. N-Heterocyclic Carbene-Based Zinc Complexes: Same Precursors for Different Lactide Ring-Opening Polymerization Mechanisms. ChemCatChem 2022, 14, e202200962. [Google Scholar] [CrossRef]
- Sun, Y.; Cui, Y.; Xiong, J.; Dai, Z.; Tang, N.; Wu, J. Different mechanisms at different temperatures for the ring-opening polymerization of lactide catalyzed by binuclear magnesium and zinc alkoxides. Dalton Trans. 2015, 44, 16383–16391. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.-L.; Wang, Z.-X. Dinuclear magnesium, zinc and aluminum complexes supported by bis(iminopyrrolide) ligands: Synthesis, structures, and catalysis toward the ring-opening polymerization of ε-caprolactone and rac-lactide. Dalton Trans. 2014, 43, 9126–9135. [Google Scholar] [CrossRef]
- Santulli, F.; Bruno, F.; Mazzeo, M.; Lamberti, M. Zinc Complexes Bearing Dinucleating Bis(imino-pyridine)binaphthol Ligands: Highly Active and Robust Catalysts for the Lactide Polymerization. ChemCatChem 2023, 15, e202300498. [Google Scholar] [CrossRef]
- Kremer, A.B.; Osten, K.M.; Yu, I.; Ebrahimi, T.; Aluthge, D.C.; Mehrkhodavandi, P. Dinucleating Ligand Platforms Supporting Indium and Zinc Catalysts for Cyclic Ester Polymerization. Inorg. Chem. 2016, 55, 5365–5374. [Google Scholar] [CrossRef]
- Knight, P.D.; White, A.J.P.; Williams, C.K. Dinuclear Zinc Complexes Using Pentadentate Phenolate Ligands. Inorg. Chem. 2008, 47, 11711–11719. [Google Scholar] [CrossRef]
- Soobrattee, S.; Zhai, X.; Nyamayaro, K.; Diaz, C.; Kelley, P.; Ebrahimi, T.; Mehrkhodavandi, P. Dinucleating Amino-Phenolate Platform for Zinc Catalysts: Impact on Lactide Polymerization. Inorg. Chem. 2020, 59, 5546–5557. [Google Scholar] [CrossRef]
- Hollingsworth, T.S.; Hollingsworth, R.L.; Rosen, T.; Groysman, S. Zinc bimetallics supported by a xanthene-bridged dinucleating ligand: Synthesis, characterization, and lactide polymerization studies. RSC Adv. 2017, 7, 41819–41829. [Google Scholar] [CrossRef]
- Xu, R.; Hua, L.; Li, X.; Yao, Y.; Leng, X.; Chen, Y. Rare-earth/zinc heterometallic complexes containing both alkoxy-amino-bis(phenolato) and chiral salen ligands: Synthesis and catalytic application for copolymerization of CO2 with cyclohexene oxide. Dalton Trans. 2019, 48, 10565–10573. [Google Scholar] [CrossRef]
- Denk, A.; Kernbichl, S.; Schaffer, A.; Kranzlein, M.; Pehl, T.; Rieger, B. Heteronuclear, Monomer-Selective Zn/Y Catalyst Combines Copolymerization of Epoxides and CO2 with Group-Transfer Polymerization of Michael-Type Monomers. ACS Macro Lett. 2020, 9, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Romain, C.; Williams, C.K. Chemoselective Polymerization Control: From Mixed-Monomer Feedstock to Copolymers. Angew. Chem. Int. Ed. 2014, 53, 1607–1610. [Google Scholar] [CrossRef] [PubMed]
- Diment, W.T.; Lindeboom, W.; Fiorentini, F.; Deacy, A.C.; Williams, C.K. Synergic Heterodinuclear Catalysts for the Ring-Opening Copolymerization (ROCOP) of Epoxides, Carbon Dioxide, and Anhydrides. Acc. Chem. Res. 2022, 55, 1997–2010. [Google Scholar] [CrossRef] [PubMed]
- Gruszka, W.; Garden, J.A. Advances in heterometallic ring-opening (co)polymerisation catalysis. Nat. Commun. 2021, 12, 3252. [Google Scholar] [CrossRef]
- Feng, Q.; Tong, R. Controlled Photoredox Ring-Opening Polymerization of O-Carboxyanhydrides. J. Am. Chem. Soc. 2017, 139, 6177–6182. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, L.; Zhong, Y.; Guo, D.; Liu, G.; Xie, L.; Huang, W.; Tong, R. Stereoselective photoredox ring-opening polymerization of O-carboxyanhydrides. Nat. Commun. 2018, 9, 1559. [Google Scholar] [CrossRef]
- Wang, X.; Chin, A.L.; Zhou, J.; Wang, H.; Tong, R. Resilient Poly(α-hydroxy acids) with Improved Strength and Ductility via Scalable Stereosequence-Controlled Polymerization. J. Am. Chem. Soc. 2021, 143, 16813–16823. [Google Scholar] [CrossRef]
- Zhong, Y.; Feng, Q.; Wang, X.; Chen, J.; Cai, W.; Tong, R. Functionalized Polyesters via Stereoselective Electrochemical Ring-Opening Polymerization of O-Carboxyanhydrides. ACS Macro Lett. 2020, 9, 1114–1118. [Google Scholar] [CrossRef]
- Zhong, Y.; Feng, Q.; Wang, X.; Yang, L.; Korovich, A.G.; Madsen, L.A.; Tong, R. Photocatalyst-independent photoredox ring-opening polymerization of O-carboxyanhydrides: Stereocontrol and mechanism. Chem. Sci. 2021, 12, 3702–3712. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Li, Y.; Zhang, G.; So, Y.-M.; Pan, Y. Recent Advances in Zinc Complexes for Stereoselective Ring-Opening Polymerization and Copolymerization. Inorganics 2025, 13, 185. https://doi.org/10.3390/inorganics13060185
Li X, Li Y, Zhang G, So Y-M, Pan Y. Recent Advances in Zinc Complexes for Stereoselective Ring-Opening Polymerization and Copolymerization. Inorganics. 2025; 13(6):185. https://doi.org/10.3390/inorganics13060185
Chicago/Turabian StyleLi, Xia, Yang Li, Gangqiang Zhang, Yat-Ming So, and Yu Pan. 2025. "Recent Advances in Zinc Complexes for Stereoselective Ring-Opening Polymerization and Copolymerization" Inorganics 13, no. 6: 185. https://doi.org/10.3390/inorganics13060185
APA StyleLi, X., Li, Y., Zhang, G., So, Y.-M., & Pan, Y. (2025). Recent Advances in Zinc Complexes for Stereoselective Ring-Opening Polymerization and Copolymerization. Inorganics, 13(6), 185. https://doi.org/10.3390/inorganics13060185