Catalytic Activity of Rhenium(I) Tricarbonyl Complexes Containing Polypyridine and Phosphorus–Nitrogen Ligands in the Hydrogen Transfer of Acetophenone
Abstract
1. Introduction
2. Experimental Section
2.1. General Procedure
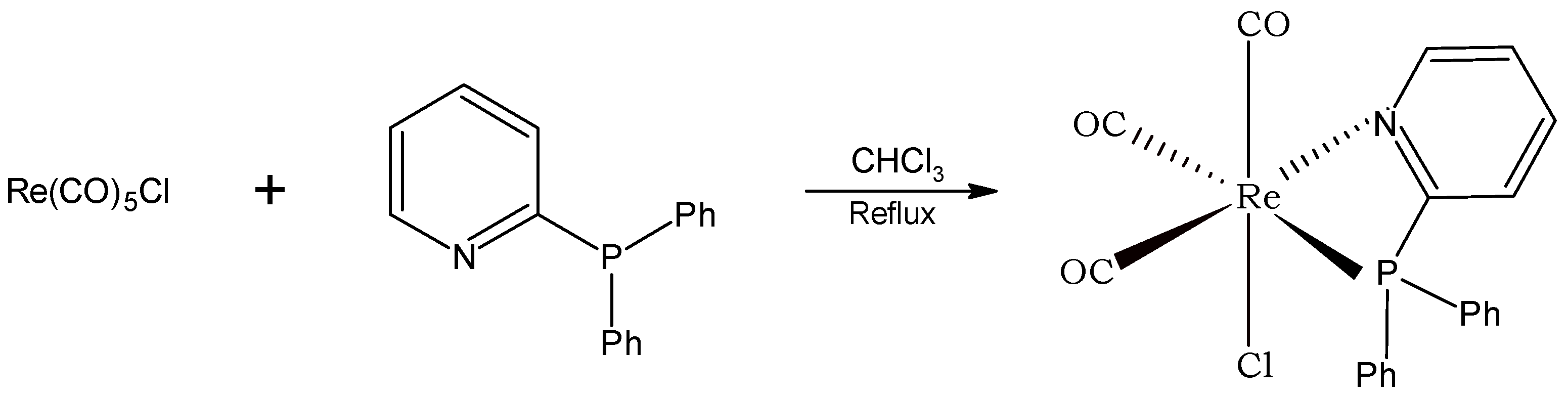
2.2. Synthesis of Fac-[Re(CO)3(PN)Cl] Complex
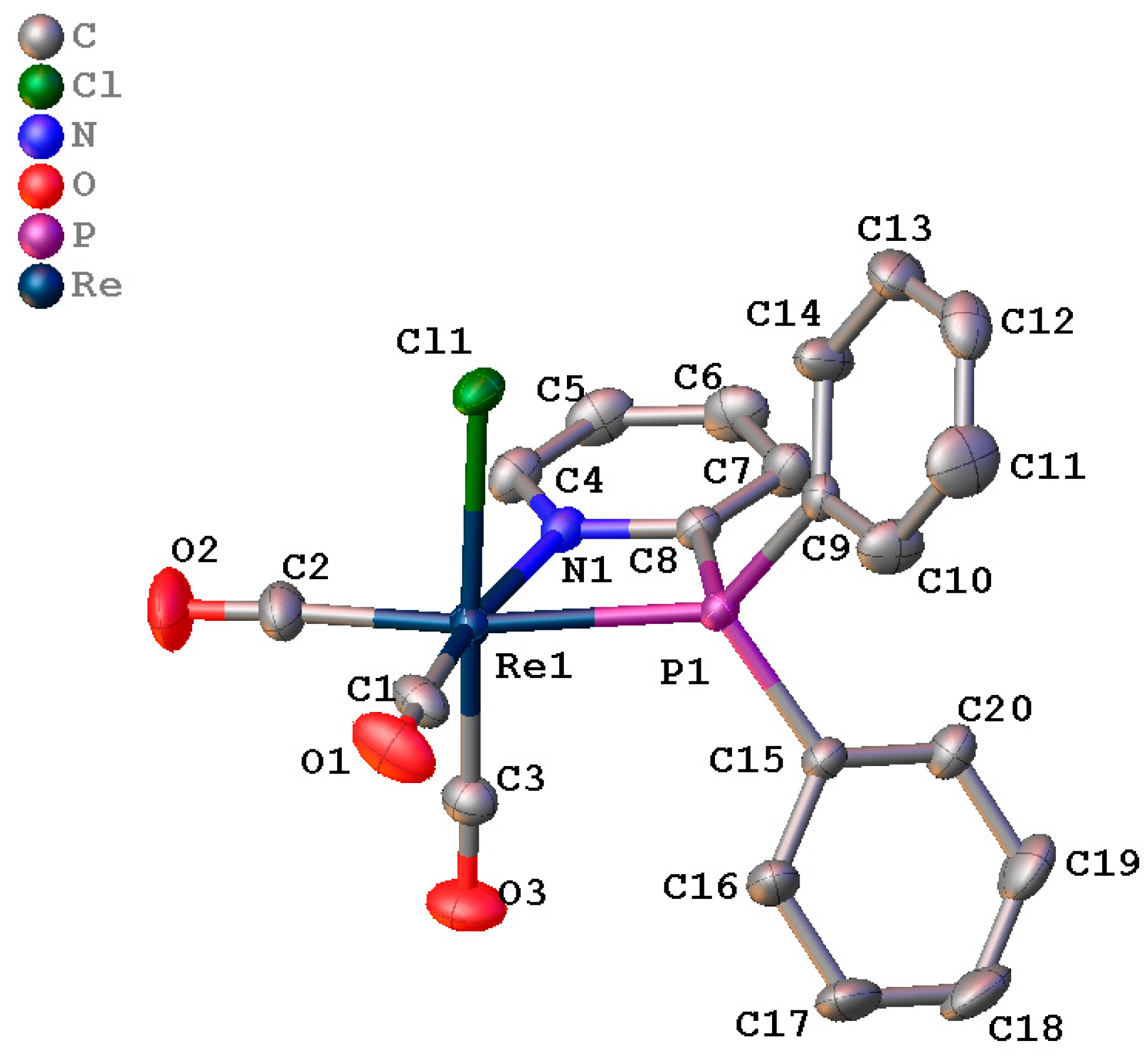
2.3. X-Ray Crystal Structure Determination for Fac-[Re(CO)3(PN)Cl] Complex
3. Results and Discussion
3.1. Synthesis and Characterization of Fac-[Re(CO)3(PN)Cl] Complex
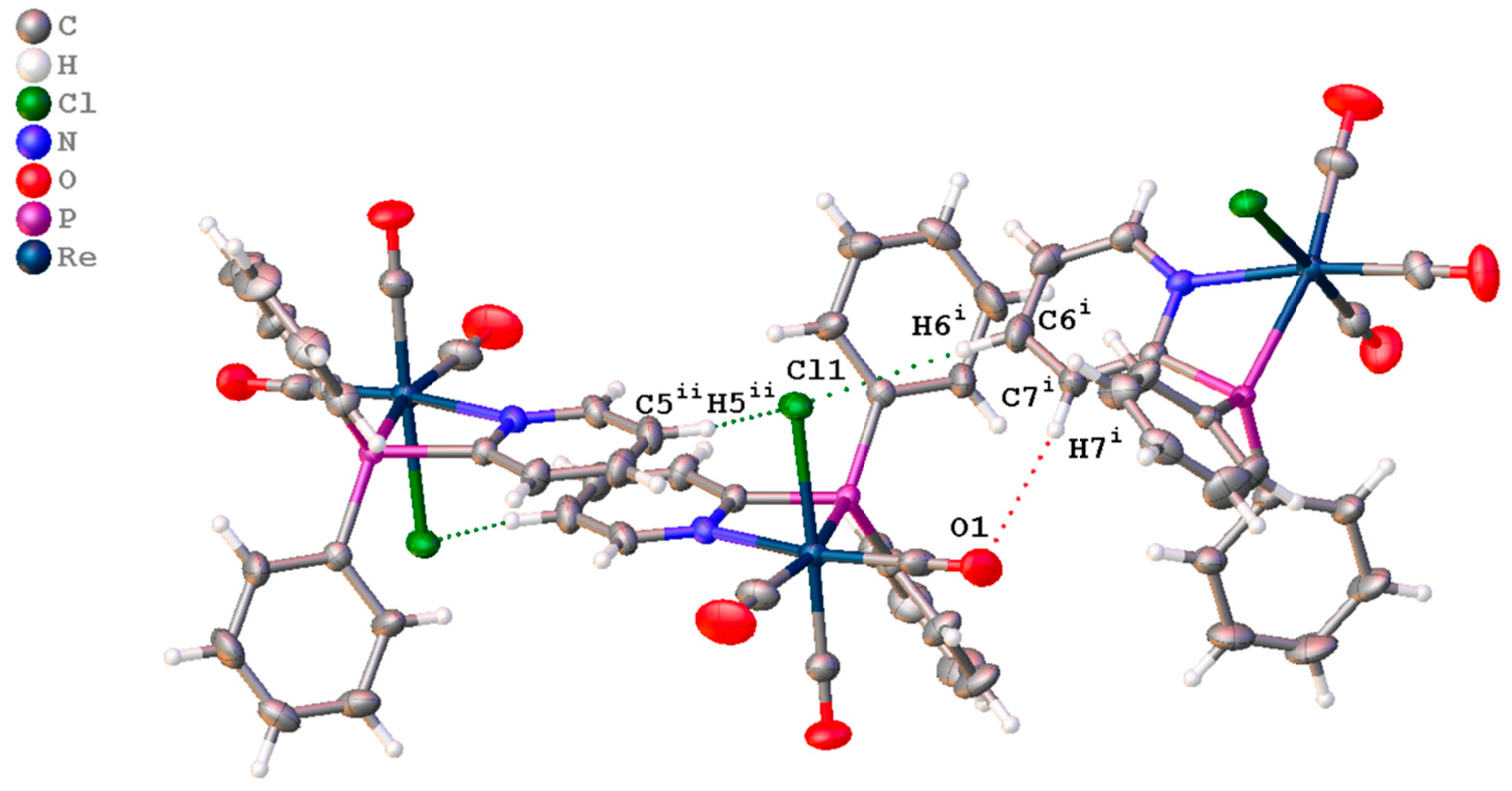
3.2. X-Ray Crystallographic Study
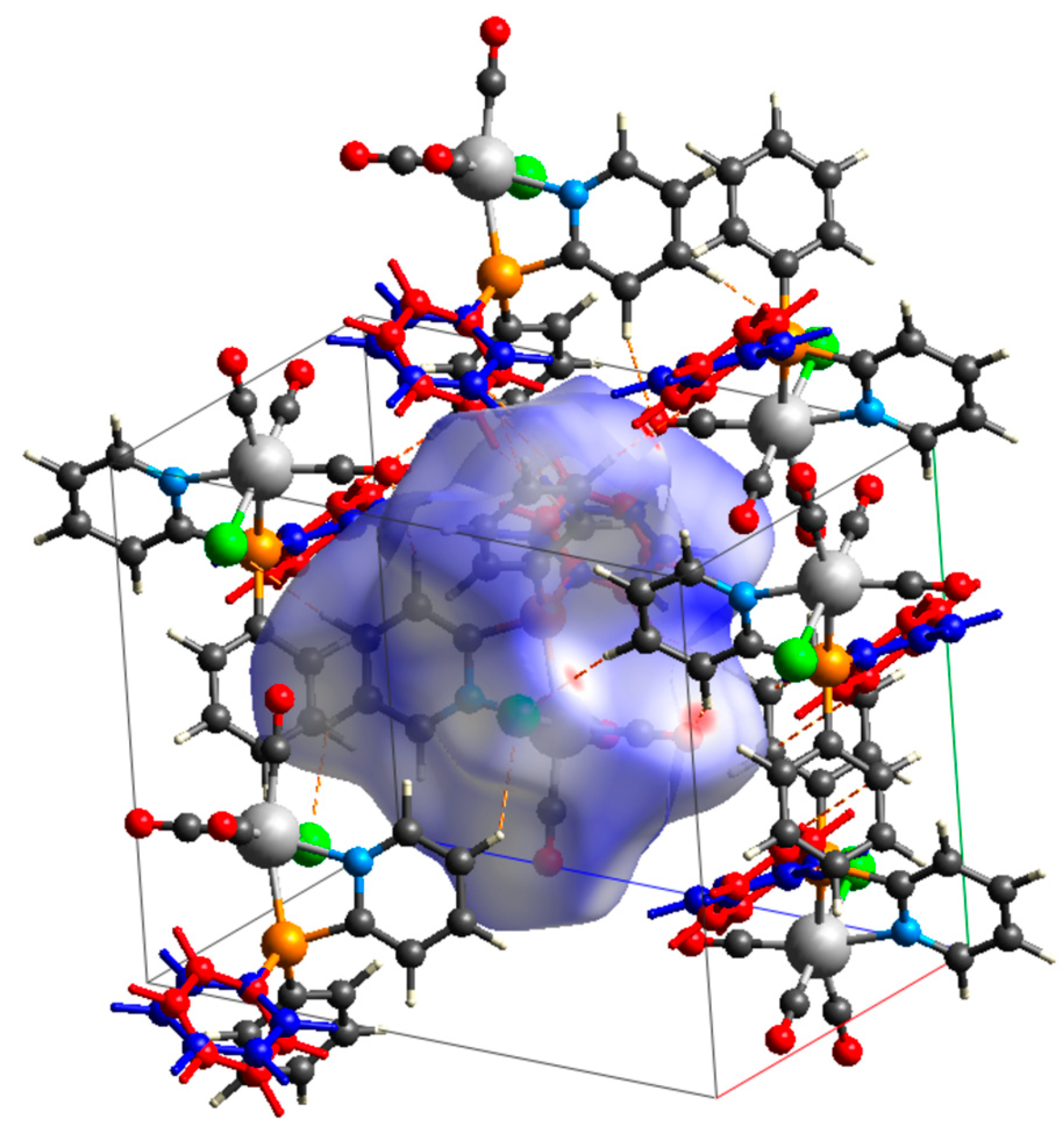
Hirshfeld Surface Analysis
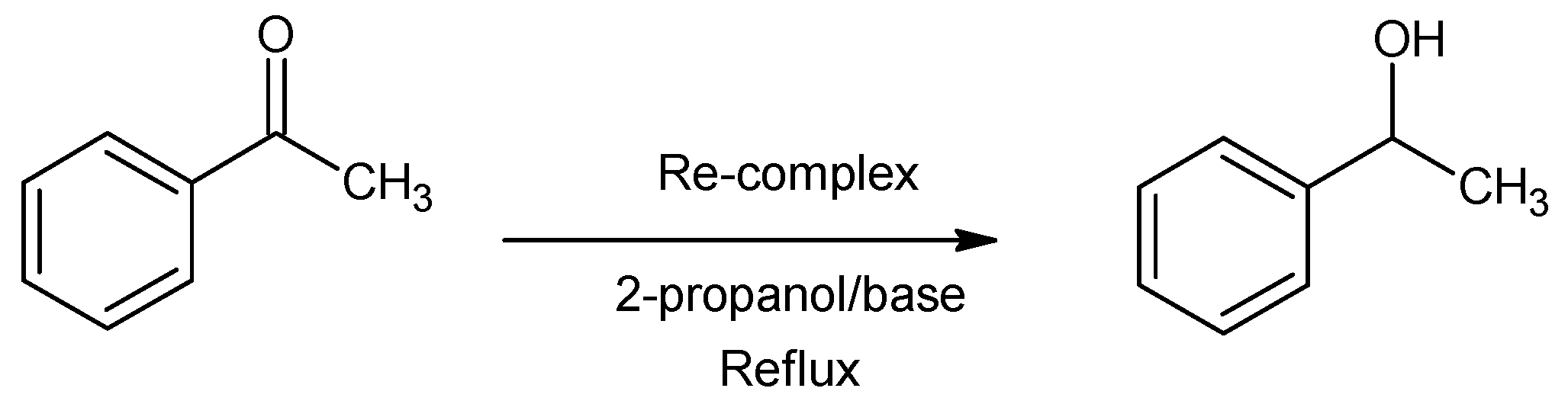
3.3. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graham, T.W.; Cariou, R.P.-Y.; Sánchez-Nieves, J.; Allen, A.E.; Udachin, K.A.; Regragui, R.; Carty, A.J. Reactivity of Terminal Electrophilic Phosphinidene Complexes: Synthesis of the First Rhenium Phosphinidene, [Re(CO)5(η1-PNiPr2)][AlCl4], and Novel Reactions with Azobenzene. Organometallics 2005, 24, 2023–2026. [Google Scholar] [CrossRef]
- Cowley, A.R.; Dilworth, J.R.; Nairn, A.K.; Robbie, A.J. Preparation and Characterization of Diarylphosphazene and Diarylphosphinohydrazide Complexes of Titanium, Tungsten and Ruthenium and Phosphorylketimido Complexes of Rhenium. Dalton Trans. 2005, 4, 680. [Google Scholar] [CrossRef] [PubMed]
- Saucedo Anaya, S.A.; Hagenbach, A.; Abram, U. Tricarbonylrhenium(I) and -Technetium(I) Complexes with Bis(2-Pyridyl)Phenylphosphine and Tris(2-Pyridyl)Phosphine. Polyhedron 2008, 27, 3587–3592. [Google Scholar] [CrossRef]
- Villafañe, F. Re I (CO)3 Complexes with Diimine Ligands Synthesized in Situ. Coord. Chem. Rev. 2017, 339, 128–137. [Google Scholar] [CrossRef]
- Edwards, P.G.; Newman, P.D.; Stasch, A. Coordination Chemistry of an Asymmetric P,N,O Tridentate Ligand Containing Primary Phosphine, Amine and Alcohol Donors. J. Organomet. Chem. 2011, 696, 1652–1658. [Google Scholar] [CrossRef]
- Correia, J.D.G.; Domingos, Â.; Santos, I.; Alberto, R.; Ortner, K. Re Tricarbonyl Complexes with Ligands Containing P,N,N and P,N,O Donor Atom Sets: Synthesis and Structural Characterization. Inorg. Chem. 2001, 40, 5147–5151. [Google Scholar] [CrossRef]
- Ranjan, S.; Lin, S.-Y.; Hwang, K.-C.; Chi, Y.; Ching, W.-L.; Liu, C.-S.; Tao, Y.-T.; Chien, C.-H.; Peng, S.-M.; Lee, G.-H. Realizing Green Phosphorescent Light-Emitting Materials from Rhenium(I) Pyrazolato Diimine Complexes. Inorg. Chem. 2003, 42, 1248–1255. [Google Scholar] [CrossRef]
- Yıldız, C.A.; Güney, E.; Nasif, V.; Karakaş, D.; Erkan, S. Investigation of Substituent Effect on Rhenium Complexes by DFT Methods: Structural Analysis, IR Spectrum, Quantum Chemical Parameter, NLO and OLED Properties, Molecular Docking. J. Mol. Struct. 2023, 1278, 134835. [Google Scholar] [CrossRef]
- Hu, Y.-X.; Zhao, G.-W.; Dong, Y.; Lü, Y.-L.; Li, X.; Zhang, D.-Y. New Rhenium(I) Complex with Thiadiazole-Annelated 1,10-Phenanthroline for Highly Efficient Phosphorescent OLEDs. Dyes Pigments 2017, 137, 569–575. [Google Scholar] [CrossRef]
- Gupta, D.; Sathiyendiran, M. Rhenium-Carbonyl-Based Supramolecular Coordination Complexes: Synthesis, Structure and Properties. ChemistrySelect 2018, 3, 7439–7458. [Google Scholar] [CrossRef]
- Luengo, A.; Fernández-Moreira, V.; Marzo, I.; Gimeno, M.C. Trackable Metallodrugs Combining Luminescent Re(I) and Bioactive Au(I) Fragments. Inorg. Chem. 2017, 56, 15159–15170. [Google Scholar] [CrossRef] [PubMed]
- Kamecka, A.; Prachnio, K.; Kapturkiewicz, A. The Luminescence Properties of [Re(CO)2(P^P)(N^N)]+ Complexes: Comparison with Their [Re(CO)3(N^N)(Cl)] Analogues. J. Lumin. 2018, 203, 409–419. [Google Scholar] [CrossRef]
- Ray, S.; Rajak, K.K. Rhenium(I) Complexes Incorporating Pyrene Bearing N, N Ligand: Luminescent Based Sensors for DNA. J. Organomet. Chem. 2024, 1009, 123077. [Google Scholar] [CrossRef]
- Veronese, L.; Quartapelle Procopio, E.; Moehl, T.; Panigati, M.; Nonomura, K.; Hagfeldt, A. Triarylamine-Based Hydrido-Carboxylate Rhenium(I) Complexes as Photosensitizers for Dye-Sensitized Solar Cells. Phys. Chem. Chem. Phys. 2019, 21, 7534–7543. [Google Scholar] [CrossRef]
- Takeda, H.; Koike, K.; Morimoto, T.; Inumaru, H.; Ishitani, O. Photochemistry and Photocatalysis of Rhenium(I) Diimine Complexes. In Advances in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 2011; Volume 63, pp. 137–186. ISBN 978-0-12-385904-4. [Google Scholar]
- Vlček, A.; Busby, M. Ultrafast Ligand-to-Ligand Electron and Energy Transfer in the Complexes Fac-[ReI(L)(CO)3(Bpy)]n+. Coord. Chem. Rev. 2006, 250, 1755–1762. [Google Scholar] [CrossRef]
- Kurtz, D.A.; Brereton, K.R.; Ruoff, K.P.; Tang, H.M.; Felton, G.A.N.; Miller, A.J.M.; Dempsey, J.L. Bathochromic Shifts in Rhenium Carbonyl Dyes Induced through Destabilization of Occupied Orbitals. Inorg. Chem. 2018, 57, 5389–5399. [Google Scholar] [CrossRef]
- Acosta, A.; Antipán, J.; Fernández, M.; Prado, G.; Sandoval-Altamirano, C.; Günther, G.; Gutiérrez-Urrutia, I.; Poblete-Castro, I.; Vega, A.; Pizarro, N. Photochemistry of P,N-Bidentate Rhenium(I) Tricarbonyl Complexes: Reactive Species Generation and Potential Application for Antibacterial Photodynamic Therapy. RSC Adv. 2021, 11, 31959–31966. [Google Scholar] [CrossRef]
- Pizarro, N.; Duque, M.; Chamorro, E.; Nonell, S.; Manzur, J.; De La Fuente, J.R.; Günther, G.; Cepeda-Plaza, M.; Vega, A. Dual Emission of a Novel (P,N) ReI Complex: A Computational and Experimental Study on [P,N-{(C6H5)2(C5H4N)P}Re(CO)3Br]. J. Phys. Chem. A 2015, 119, 3929–3935. [Google Scholar] [CrossRef]
- Nguyen, P.N.; Dao, T.-B.-N.; Tran, T.T.; Tran, N.-A.T.; Nguyen, T.A.; Phan, T.-D.L.; Nguyen, L.P.; Dang, V.Q.; Nguyen, T.M.; Dang, N.N. Electrocatalytic CO2 Reduction by [Re(CO)3Cl(3-(Pyridin-2-Yl)-5-Phenyl-1,2,4-Triazole)] and [Re(CO)3Cl(3-(2-Pyridyl)-1,2,4-Triazole)]. ACS Omega 2022, 7, 34089–34097. [Google Scholar] [CrossRef]
- Clark, M.L.; Cheung, P.L.; Lessio, M.; Carter, E.A.; Kubiak, C.P. Kinetic and Mechanistic Effects of Bipyridine (Bpy) Substituent, Labile Ligand, and Brønsted Acid on Electrocatalytic CO2 Reduction by Re(Bpy) Complexes. ACS Catal. 2018, 8, 2021–2029. [Google Scholar] [CrossRef]
- Willkomm, J.; Bouzidi, S.; Bertin, E.; Birss, V.I.; Piers, W.E. Aqueous CO2 Reduction by a Re(Bipyridine)-Polypyrrole Film Deposited on Colloid-Imprinted Carbon. ACS Catal. 2021, 11, 1096–1105. [Google Scholar] [CrossRef]
- Murata, K.; Tanaka, H.; Ishii, K. Electrochemical Reduction of CO2 by a Gas-Diffusion Electrode Composed of Fac-Re(Diimine)(CO)3Cl and Carbon Nanotubes. J. Phys. Chem. C 2019, 123, 12073–12080. [Google Scholar] [CrossRef]
- Nguyen, P.N.; Pham, H.P.; Dang, Q.V.; Pham, K.D.; Doan, G.N.; Ho, T.H.; Nguyen, T.M.; Dang, N.N. Low-Intensity-Visible-Light-Driven Photocatalytic CO2 Reduction by Rhenium Tricarbonyl Complexes Based on Pyridyl-Triazole Ligands. J. Organomet. Chem. 2025, 1023, 123438. [Google Scholar] [CrossRef]
- Baráth, E. Hydrogen Transfer Reactions of Carbonyls, Alkynes, and Alkenes with Noble Metals in the Presence of Alcohols/Ethers and Amines as Hydrogen Donors. Catalysts 2018, 8, 671. [Google Scholar] [CrossRef]
- Santana, C.G.; Krische, M.J. From Hydrogenation to Transfer Hydrogenation to Hydrogen Auto-Transfer in Enantioselective Metal-Catalyzed Carbonyl Reductive Coupling: Past, Present, and Future. ACS Catal. 2021, 11, 5572–5585. [Google Scholar] [CrossRef]
- Popovici, I.; Tannoux, T.; Bourcier, S.; Casaretto, N.; Auffrant, A. Coordination of NNN Iminophosphorane Ligands to MnII, CoII, and FeII and Application in the Transfer Hydrogenation of Ketones. Inorg. Chem. Commun. 2025, 180, 114917. [Google Scholar] [CrossRef]
- Namlı, M.; Işık, U.; Kantar, C.; Aydemir, M. Application of Phthalocyanine Complexes of Cu(II), Co(II), Ni(II), and Zn(II) as Catalysts in the Transfer Hydrogenation of Acetophenone and Its Derivatives. J. Mol. Struct. 2024, 1312, 138447. [Google Scholar] [CrossRef]
- Vermaak, V.; Vosloo, H.C.M.; Swarts, A.J. The Development and Application of Homogeneous Nickel Catalysts for Transfer Hydrogenation and Related Reactions. Coord. Chem. Rev. 2024, 507, 215716. [Google Scholar] [CrossRef]
- Venkatachalam, G.; Ramesh, R. Ruthenium(III) Bis-Bidentate Schiff Base Complexes Mediated Transfer Hydrogenation of Imines. Inorg. Chem. Commun. 2006, 9, 703–707. [Google Scholar] [CrossRef]
- Dayan, S.; Ozpozan, N.K.; Özdemir, N.; Dayan, O. Synthesis of Some Ruthenium(II)–Schiff Base Complexes Bearing Sulfonamide Fragment: New Catalysts for Transfer Hydrogenation of Ketones. J. Organomet. Chem. 2014, 770, 21–28. [Google Scholar] [CrossRef]
- Prakash, G.; Viswanathamurthi, P. New Ruthenium(II) Carbonyl Complexes Bearing Disulfide Schiff Base Ligands and Their Applications as Catalyst for Some Organic Transformations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 129, 352–358. [Google Scholar] [CrossRef]
- Singh, P.; Singh, A.K. Transfer Hydrogenation of Ketones and Catalytic Oxidation of Alcohols with Half-Sandwich Complexes of Ruthenium(II) Designed Using Benzene and Tridentate (S, N, E) Type Ligands (E = S, Se, Te). Organometallics 2010, 29, 6433–6442. [Google Scholar] [CrossRef]
- Moya, S.A.; Vidal, M.; Brown, K.; Negrete-Vergara, C.; Abarca, G.; Aguirre, P. Ruthenium Carbonyl Compounds Containing Polypyridine Ligands as Catalysts in the Reaction of N-Benzylideneaniline Hydrogenation. Inorg. Chem. Commun. 2012, 22, 146–148. [Google Scholar] [CrossRef]
- Moya, S.A.; Araya, J.C.; Gajardo, J.; Guerchais, V.; Le Bozec, H.; Toupet, L.; Aguirre, P. Synthesis and Characterization of Ruthenium (II) Carbonyl Complexes Containing Naphthyridine and Acetylacetonate Ligands and Their Catalytic Activity in the Hydrogen Transfer Reaction. Inorg. Chem. Commun. 2013, 27, 108–110. [Google Scholar] [CrossRef]
- Revathi, S.; Ghatak, T. Ethanol as Hydrogen Donor: An Efficient Transfer Hydrogenation of Aldehydes, Ketones, and Nitroarenes with H-Bonded Ru(II)-N-Heterocyclic Iminium Complex. J. Catal. 2024, 429, 115207. [Google Scholar] [CrossRef]
- Subaramanian, M.; Sivakumar, G.; Landge, V.G.; Kumar, R.; Natte, K.; Jagadeesh, R.V.; Balaraman, E. General and Selective Homogeneous Ru-Catalyzed Transfer Hydrogenation, Deuteration, and Methylation of Functional Compounds Using Methanol. J. Catal. 2023, 425, 386–405. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Wu, K.; Wang, Q.; Yu, Z. Mono- and Multinuclear Pincer-Type Ru(II) Complex Catalysts and Their Catalytic Applications. Inorg. Chim. Acta 2023, 551, 121458. [Google Scholar] [CrossRef]
- Prakash, O.; Singh, P.; Mukherjee, G.; Singh, A.K. Efficient Catalysis of Transfer Hydrogenation of Ketones and Oxidation of Alcohols with Newly Designed Half-Sandwich Rhodium(III) and Iridium(III) Complexes of Half-Pincer Chalcogenated Pyridines. Organometallics 2012, 31, 3379–3388. [Google Scholar] [CrossRef]
- Prakash, O.; Joshi, H.; Sharma, K.N.; Gupta, P.L.; Singh, A.K. Transfer Hydrogenation (pH Independent) of Ketones and Aldehydes in Water with Glycerol: Ru, Rh, and Ir Catalysts with a COOH Group near the Metal on a (Phenylthio)Methyl-2-Pyridine Scaffold. Organometallics 2014, 33, 3804–3812. [Google Scholar] [CrossRef]
- Ak, B.; Aydemir, M.; Durap, F.; Meriç, N.; Baysal, A. The First Application of C2-Symmetric Ferrocenyl Phosphinite Ligands for Rhodium-Catalyzed Asymmetric Transfer Hydrogenation of Various Ketones. Inorganica Chim. Acta 2015, 438, 42–51. [Google Scholar] [CrossRef]
- Archana, B.; Sinha, A. Unprecedented Homogeneous Transfer Hydrogenation Reaction of Aldehydes and Ketones by Μ-Mono Oxo Bridged Iron (III) Complex. Inorg. Chem. Commun. 2025, 180, 114891. [Google Scholar] [CrossRef]
- Işık, U.; Meriç, N.; Kayan, C.; Kılıç, A.; Belyankova, Y.; Zazybin, A.; Aydemir, M. Synthesis of Half-Sandwich Ruthenium(II) and Iridium(III) Complexes Containing Imidazole-Based Phosphinite Ligands and Their Use in Catalytic Transfer Hydrogenation of Acetophenone with Isopropanol. J. Organomet. Chem. 2023, 998, 122800. [Google Scholar] [CrossRef]
- Zhu, X.-H.; Cai, L.-H.; Wang, C.-X.; Wang, Y.-N.; Guo, X.-Q.; Hou, X.-F. Efficient and Versatile Transfer Hydrogenation Catalysts: Iridium (III) and Ruthenium (II) Complexes with 4-Acetylbenzyl-N-Heterocyclic Carbenes. J. Mol. Catal. Chem. 2014, 393, 134–141. [Google Scholar] [CrossRef]
- Smith, I.G.; Zgrabik, J.C.; Gutauskas, A.C.; Gray, D.L.; Domski, G.J. Synthesis, Characterization, and Catalytic Behavior of Mono- and Bimetallic Ruthenium(II) and Iridium(III) Complexes Supported by Pyridine-Functionalized N -Heterocyclic Carbene Ligands. Inorg. Chem. Commun. 2017, 81, 27–32. [Google Scholar] [CrossRef]
- Ak, B.; Aydemir, M.; Durap, F.; Meriç, N.; Elma, D.; Baysal, A. Highly Efficient Iridium Catalysts Based on C2-Symmetric Ferrocenyl Phosphinite Ligands for Asymmetric Transfer Hydrogenations of Aromatic Ketones. Tetrahedron Asymmetry 2015, 26, 1307–1313. [Google Scholar] [CrossRef]
- Landwehr, A.; Dudle, B.; Fox, T.; Blacque, O.; Berke, H. Bifunctional Rhenium Complexes for the Catalytic Transfer-Hydrogenation Reactions of Ketones and Imines. Chem.—Eur. J. 2012, 18, 5701–5714. [Google Scholar] [CrossRef]
- Zúñiga, C.; Oyarzún, D.P.; Martin-Transaco, R.; Yáñez-S, M.; Tello, A.; Fuentealba, M.; Cantero-López, P.; Arratia-Pérez, R. Synthesis, Characterization and Relativistic DFT Studies of fac-Re(CO)3(Isonicotinic Acid)2Cl Complex. Chem. Phys. Lett. 2017, 688, 66–73. [Google Scholar] [CrossRef]
- Portenkirchner, E.; Oppelt, K.; Ulbricht, C.; Egbe, D.A.M.; Neugebauer, H.; Knör, G.; Sariciftci, N.S. Electrocatalytic and Photocatalytic Reduction of Carbon Dioxide to Carbon Monoxide Using the Alkynyl-Substituted Rhenium(I) Complex (5,5′-Bisphenylethynyl-2,2′-Bipyridyl)Re(CO)3Cl. J. Organomet. Chem. 2012, 716, 19–25. [Google Scholar] [CrossRef]
- Frin, K.P.M.; Murakami Iha, N.Y. Modulation of Trans-to-Cis Photoisomerization and Photoluminescence of 1,2-Bis(4-Pyridyl)Ethylene or 4-Styrylpyridine Coordinated to fac-Tricarbonyl(5-Chloro-1,10-Phenathroline)Rhenium(I). Inorg. Chim. Acta 2011, 376, 531–537. [Google Scholar] [CrossRef]
- Sanhueza, L.; Barrera, M.; Crivelli, I. Experimental and Theoretical Studies of a New Donor–Acceptor Re(I) Complexes Using Nitropolypyridil Ligand. Analysis of the NLO Potential Response. Polyhedron 2013, 57, 94–104. [Google Scholar] [CrossRef]
- Lundin, N.J.; Walsh, P.J.; Howell, S.L.; Blackman, A.G.; Gordon, K.C. A Synthetic, Structural, Spectroscopic and DFT Study of ReI, CuI, RuII and IrIII Complexes Containing Functionalised Dipyrido [3,2-a:2′,3′-c]Phenazine (Dppz). Chem. Eur. J. 2008, 14, 11573–11583. [Google Scholar] [CrossRef]
- Zúñiga, C.; Crivelli, I.; Loeb, B. Synthesis, Characterization, Spectroscopic and Electrochemical Studies of Donor–Acceptor Ruthenium(II) Polypyridine Ligand Derivatives with Potential NLO Applications. Polyhedron 2015, 85, 511–518. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Mayberry, D.D.; Nesterov, V.N.; Richmond, M.G. Ambidentate Ligand Reactivity with the Rhenium(I) Compounds [BrRe(CO)4]2 and Cis-BrRe(CO)4 L: A Kinetic and Mechanistic Study. Eur. J. Inorg. Chem. 2017, 2017, 3990–3998. [Google Scholar] [CrossRef]
- McCusker, J.K.; Rheingold, A.L.; Hendrickson, D.N. Variable-Temperature Studies of Laser-Initiated 5T2→1A1 Intersystem Crossing in Spin-Crossover Complexes: Empirical Correlations between Activation Parameters and Ligand Structure in a Series of Polypyridyl Ferrous Complexes. Inorg. Chem. 1996, 35, 2100–2112. [Google Scholar]
- Pritchard, R.; Barrett, S.A.; Kilner, C.A.; Halcrow, M.A. The Influence of Ligand Conformation on the Thermal Spin Transitions in Iron(III) Saltrien Complexes. Dalton Trans. 2008, 24, 3159. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Mitchell, A.S.; Spackman, M.A. Hirshfeld Surfaces: A New Tool for Visualising and Exploring Molecular Crystals. Chem.—Eur. J. 1998, 4, 2136–2141. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel Tools for Visualizing and Exploring Intermolecular Interactions in Molecular Crystals. Acta Crystallogr. B 2004, 60, 627–668. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting Intermolecular Interactions in Molecular Crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Parra-Melipán, S.; López, V.; Moya, S.A.; Valdebenito, G.; Aranda, B.; Aguirre, P. Valorization of Furfural Using Ruthenium (II) Complexes Containing Phosphorus-Nitrogen Ligands under Homogeneous Transfer Hydrogen Condition. Mol. Catal. 2021, 513, 111729. [Google Scholar] [CrossRef]
| Compound | Fac-[Re(CO)3(PN)Cl] |
|---|---|
| Empirical formula | C20H14ClNO3PRe |
| Formula weight | 568.94 |
| Temperature/K | 298 |
| Crystal system | monoclinic |
| Space group | P21/c |
| a/Å | 10.3912 (14) |
| b/Å | 12.4141 (16) |
| c/Å | 16.222 (2) |
| α/° | 90 |
| β/° | 94.930 (5) |
| γ/° | 90 |
| Volume/Å3 | 2084.9 (5) |
| Z | 4 |
| ρcalcg/cm3 | 1.813 |
| μ/mm−1 | 6.052 |
| F (000) | 1088.0 |
| Crystal size/mm3 | 0.3 × 0.256 × 0.152 |
| Radiation | MoKα (λ = 0.71073) |
| 2Θ range for data collection/° | 5.04 to 52.864 |
| Index ranges | −13 ≤ h ≤ 12, −15 ≤ k ≤ 15, −20 ≤ l ≤ 20 |
| Reflections collected | 37,744 |
| Independent reflections | 4274 [Rint = 0.0272, Rsigma = 0.0134] |
| Data/restraints/parameters | 4274/15/239 |
| Goodness-of-fit on F2 | 1.085 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.0165, wR2 = 0.0338 |
| Final R indexes [all data] | R1 = 0.0213, wR2 = 0.0354 |
| Largest diff. peak/hole/e Å−3 | 0.58/−0.67 |
| Bond Distances (Å) | |
|---|---|
| Re1-Cl1 | 2.4817 (7) |
| Re1-P1 | 2.4800 (7) |
| Re1-N1 | 2.201 (2) |
| Re1-C1 | 1.911 (3) |
| Re1-C2 | 1.930 (3) |
| Re1-C3 | 1.907 (3) |
| Bond Angles (°) | |
| P1-Re1-Cl1 | 84.43 (2) |
| N1-Re1-Cl1 | 83.72 (5) |
| N1-Re1-P1 | 65.13 (5) |
| C1-Re1-Cl1 | 92.82 (9) |
| C1-Re1-P1 | 104.54 (9) |
| C1-Re1-N1 | 169.32 (10) |
| C1-Re1-C2 | 90.16 (15) |
| C2-Re1-Cl1 | 93.82 (10) |
| C2-Re1-P1 | 165.25 (12) |
| C2-Re1-N1 | 100.13 (13) |
| C3-Re1-Cl1 | 176.93 (9) |
| C3-Re1-P1 | 93.52 (9) |
| C3-Re1-N1 | 93.35 (10) |
| C3-Re1-C1 | 89.91 (12) |
| C3-Re1-C2 | 87.59 (13) |
| D-H⋯A | d(D-H)/Å | d(H-A)/Å | d(D-A)/Å | D-H-A/° |
|---|---|---|---|---|
| C7 i-H7 i⋯O1 | 0.93 | 2.55 | 3.347 (4) | 143.7 |
| C6 ii-H6 ii⋯Cl1 | 0.93 | 2.87 | 3.794 (3) | 170.4 |
| C5 ii-H5 ii⋯Cl1 | 0.93 | 2.87 | 3.528 (4) | 128.6 |
| % Conversion (TON, TOF h−1) | ||||||
|---|---|---|---|---|---|---|
| Entry | Complex | 1 h | 2 h | 3 h | 4 h | 7 h |
| 1 | [Re(CO)5Cl] | 3 (30, 30) | 7 (70, 35) | 8 (80, 27) | 8 (80, 27) | 8 (80, 27) |
| 2 | [Re(CO)3(PN)Cl] | 15 (150,150) | 37 (370, 185) | 65 (650, 217) | 80 (800, 200) | 99 (990, 141) |
| 3 | [Re(CO)3(PyCOOH)2Cl] | 24 (240,240) | 44 (440, 220) | 56 (560, 187) | 65 (650, 163) | 70 (700, 100) |
| 4 | [Re(CO)3(bpy)Cl] | 33 (330, 330) | 53 (530, 265) | 70 (700, 233) | 88 (880, 220) | 90 (900, 129) |
| 5 | [Re(CO)3(Phen)Cl] | 30 (300, 300) | 47 (470, 235) | 55 (550, 183) | 68 (680, 170) | 71 (710, 101) |
| 6 | [Re(CO)3(Phen-NO2)Cl] | 36 (360, 360) | 52 (520, 260) | 63(630, 210) | 66 (660, 165) | 68 (680, 97) |
| 7 | [Re(CO)3(dppz-NO2)Cl] | 34 (340, 340) | 65 (650, 325) | 71(710, 240) | 68 (680, 170) | 69 (690, 98) |
| 8 | [Re(CO)3(dppz-CN)Cl] | 29 (290, 290) | 42 (420, 210) | 56 (560, 187) | 62 (620, 155) | 68 (680, 97) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zúñiga, C.; Fuentealba, M.; Olave, E.; Oyarzún, D.P.; Aracena, A.; Yañez-S, M.; Cantero-López, P.; Aguirre, P.A. Catalytic Activity of Rhenium(I) Tricarbonyl Complexes Containing Polypyridine and Phosphorus–Nitrogen Ligands in the Hydrogen Transfer of Acetophenone. Inorganics 2025, 13, 338. https://doi.org/10.3390/inorganics13100338
Zúñiga C, Fuentealba M, Olave E, Oyarzún DP, Aracena A, Yañez-S M, Cantero-López P, Aguirre PA. Catalytic Activity of Rhenium(I) Tricarbonyl Complexes Containing Polypyridine and Phosphorus–Nitrogen Ligands in the Hydrogen Transfer of Acetophenone. Inorganics. 2025; 13(10):338. https://doi.org/10.3390/inorganics13100338
Chicago/Turabian StyleZúñiga, César, Mauricio Fuentealba, Elizabeth Olave, Diego P. Oyarzún, Andrés Aracena, Mauricio Yañez-S, Plinio Cantero-López, and Pedro A. Aguirre. 2025. "Catalytic Activity of Rhenium(I) Tricarbonyl Complexes Containing Polypyridine and Phosphorus–Nitrogen Ligands in the Hydrogen Transfer of Acetophenone" Inorganics 13, no. 10: 338. https://doi.org/10.3390/inorganics13100338
APA StyleZúñiga, C., Fuentealba, M., Olave, E., Oyarzún, D. P., Aracena, A., Yañez-S, M., Cantero-López, P., & Aguirre, P. A. (2025). Catalytic Activity of Rhenium(I) Tricarbonyl Complexes Containing Polypyridine and Phosphorus–Nitrogen Ligands in the Hydrogen Transfer of Acetophenone. Inorganics, 13(10), 338. https://doi.org/10.3390/inorganics13100338








