Abstract
In the present work, we describe the use of the potentially tridentate ligand pyridine-2-amidoxime (NH2paoH) in Fe-Co chemistry. The 1:1:3 FeIII(NO3)3·9H2O/CoII(ClO4)2·6H2O/NH2paoH reaction mixture in MeOH gave complex [CoIII2FeIII(NH2pao)6](ClO4)2(NO3) (1) in ca. 55% yield, the cobalt(II) being oxidized to cobalt(III) under the aerobic conditions. The same complex was isolated using cobalt(II) and iron(II) sources, the oxidation now taking place at both metal sites. The structure of 1 contains two structurally similar, crystallographically independent cations [CoIII2FeIII(NH2pao)6]3+ which are strictly linear by symmetry. The central high-spin FeIII ion is connected to each of the terminal low-spin CoIII ions through the oximato groups of three 2.1110 (Harris notation) NH2pao− ligands, in such a way that the six O atoms are bonded to the octahedral FeIII center ({FeIIIO6} coordination sphere). Each terminal octahedral CoIII ions is bonded to six N atoms (three oximato, three 2-pyridyl) from three NH2pao− groups ({CoIIIN6} coordination sphere). The IR and Raman spectra of the complex are discussed in terms of the coordination mode of the organic ligand, and the non-coordinating nature of the inorganic ClO4− and NO3− counterions. The UV/VIS spectrum of the complex in EtOH shows the two spin-allowed d-d transitions of the low-spin 3d6 cobalt(III) and a charge-transfer NH2pao− → FeIII band. The δ and ΔΕQ 57Fe-Mössbauer parameter of 1 at 80 K show the presence of an isolated high-spin FeIII center. Variable-temperature (1.8 K–300 K) and variable-field (0–7 T) magnetic studies confirm the isolated character of FeIII. A critical discussion of the importance of NH2paoH and its anionic forms (NH2pao−, NHpao2−) in homo- and heterometallic chemistry is also attempted.
1. Introduction
Classical inorganic materials based on mixed-metal atoms or ions are at the forefront of several fields, including condensed-matter physics, solid-state chemistry, and quantum chemistry, primarily due to their unique properties and wide-ranging applications [1]. Most of them contain monoatomic bridges (e.g., O2−, S2− or X−, where X = F, Cl, Br, I) [2,3]; a characteristic example is BiMnO3, which combines large magnetization and electric polarization [2]. Few of them contain no bridge and are named intermetallics [4,5,6]; examples are materials with the compositions FeGa3, Sm2NiGa12, YNiGe2, SmNiS3, Pt3Co, Pd31Bi12, and crystalline MnNi2Ga. The latter [5] has been shown to produce ~10% magnetic field-induced strain. Mixed-metal molecular materials (i.e., materials based on molecules) are also of great interest because they exploit the advances of molecular chemistry, e.g., synthesis under mild conditions, reproducibility, better control of changes in composition, high solubility in many organic solvents and sometimes in water, etc. Such molecule-based materials are related to several, currently “hot” scientific areas, e.g., bioinorganic chemistry [7,8], catalysis [9], metallosupramolecular chemistry [10], quantum technology [11], molecular nanomagnetism [12], preparation of porous compounds [13], and development of precursor compounds for multifunctional materials [14]. Mixed-metal molecular materials consist mainly of heterometallic dinuclear, polynuclear, or polymeric complexes often deposited on surfaces, e.g., graphene, MoS2, Au, etc. In these complexes, the metal ions are bridged exclusively by inorganic groups (OH−, O2−, S2−, halido or pseudohalido, CN−, …), organic groups (e.g., the dianionic oxalate ligand, pyrazine, 4,4′-bipyridine, …) or a combination of them. This work is related to the second category, i.e., in cases where the different metal ions are bridged solely by organic ligands.
The synthesis of heterometallic complexes is not an easy task. The main strategies involve the use of appropriate inorganic ligands (e.g., CN−, SCN−, NCO−, …) [15,16] or/and organic ligands (e.g., polytopic Schiff bases) [17,18,19]. The former have two different donor atoms, each of which can bind a different metal ion. The latter contain compartments (sometimes called “pockets”) capable of binding different metal ions. In both cases, the ligands introduce preprogrammed coordination information that is “stored” in the donor atoms (diatomic or oligoatomic inorganic ligands) or in the compartments (polytopic organic ligands). When the ligands combine with different metal ions, the latter interpret this information according to their own “coordination algorithms”, i.e., according to their coordination geometry preference. The Hard–Soft Acid Base (HSAB) model [20] is sometimes helpful for successful synthetic processes. When the two different metal ions belong to two different types according to this model (i.e., soft, hard, or borderline acids), the presence of complementary atoms or sites (e.g., soft, hard, or borderline bases, respectively) in the bridging ligand is highly desirable, because it favors selectivity and strong binding. The nuclearity of the products and metal topology depend on the ligand chosen, the favorable coordination geometries of the metal centers, and the reaction and crystallization conditions. Thus, it is apparent that the choice of the bridging ligand is of paramount importance in heterometallic synthesis.
Despite the tremendous growth of complexes possessing two different first-row transition metal ions (3d-3d’) [21], their number remains significantly smaller compared to the thousands of 3d-4f coordination species [17,18,19,22,23,24].
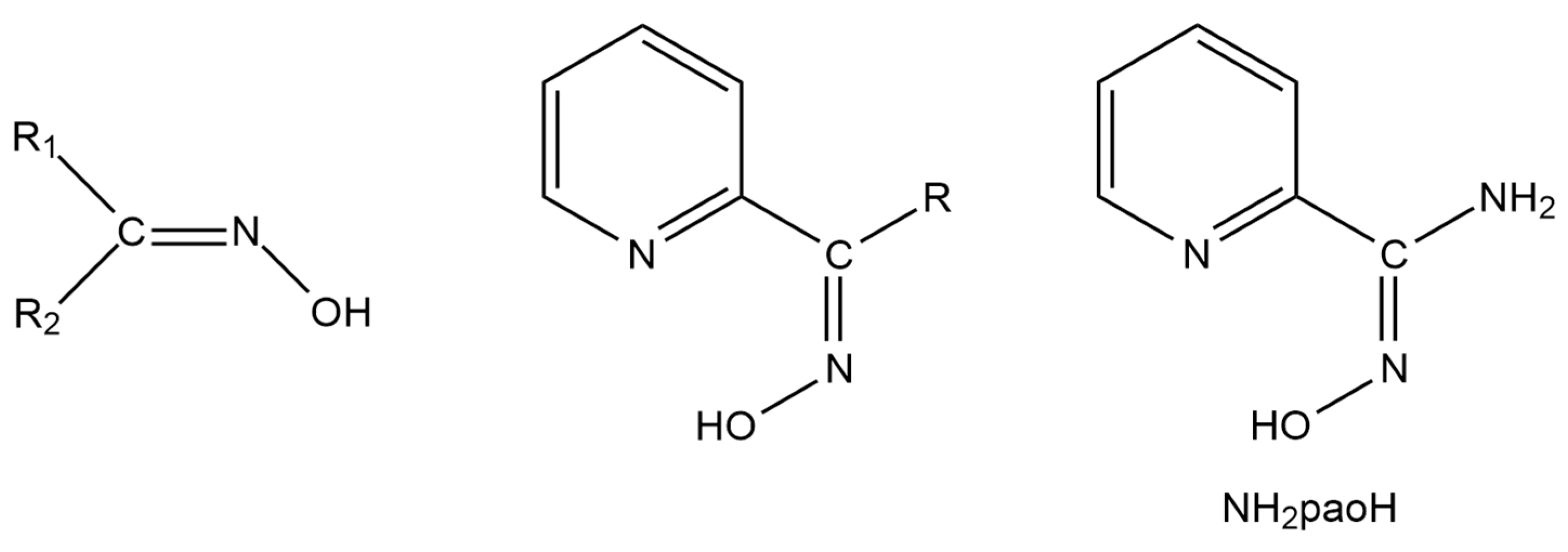
Restricting further discussion to heterometallic complexes containing organic bridging ligands, most of them are based on polytopic Schiff bases [21]. Another, much less studied, class of compounds suitable for this purpose are oximes (Figure 1, left) [25]. The deprotonated oxime group (oximate) is an ideal candidate for the synthesis of 3d-4f (the 3d-metal ion should be a soft or borderline acid according to the HSAB criteria) and 3d-3d’ (the 3d-metal ion should be a soft or borderline acid, whereas the 3d’ one should be a hard acid) compounds. The deprotonated oxygen atom behaves as a hard base and has a great affinity to form a coordination bond (even two bonds) with 4f- or 3d’- metal ions, which are hard acids. In an analogous manner, the oximate nitrogen atom (borderline base) has a higher affinity for borderline, 3d-metal ion, acid. In practically all cases, the oxime group is a part of an organic ligand that contains one or more other donor sites. Typical examples are the various 2-pyridyl oximes (Figure 1, middle), where is R is a non-donor group. The anionic 2-pyridyl oximes possess a 2-pyridyl nitrogen atom in a position that offers the possibility of the formation of a stable 5-membered chelating ring with the participation of the oximate nitrogen atom, ensuring strong binding (due to the chelate effect) to the 3d-metal ion and leaving the oximate oxygen atom available for binding to the oxophilic (hard acid) 4f- or 3d’-metal ion. Our group has exploited these features of 2-pyridyloximates for the synthesis of a plethora of 3d-4f compounds [19], the 3d-3d’ chemistry with such ligands being still at its infancy [26].
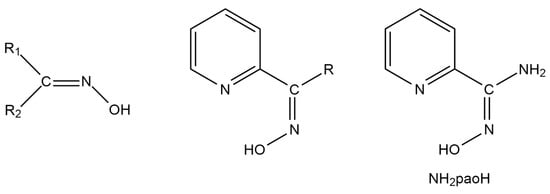
Figure 1.
The general structural formulae of simple oximes (left; R1, R2 = various non-donor groups including H atoms) and 2-pyridyl oximes (middle; R = various non-donor groups, e.g., H, Me, Ph…), and pyridine-2-amidoxime (NH2paoH; right).
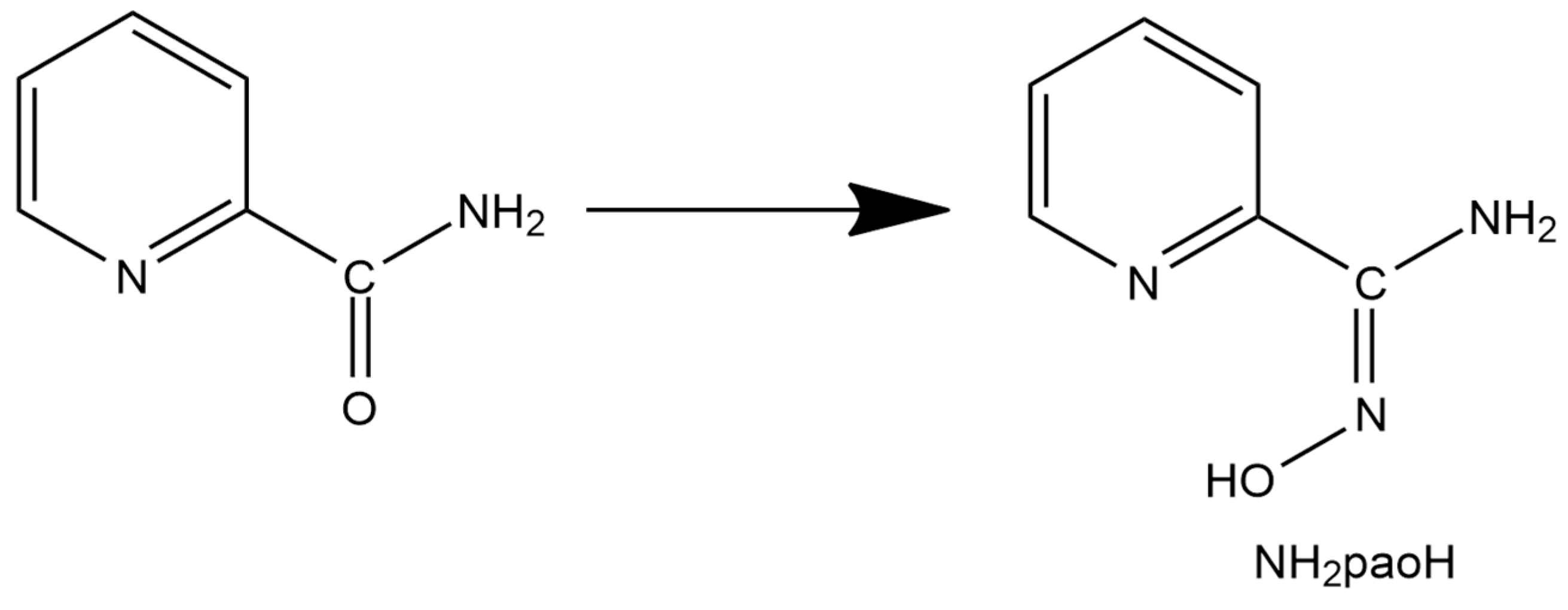
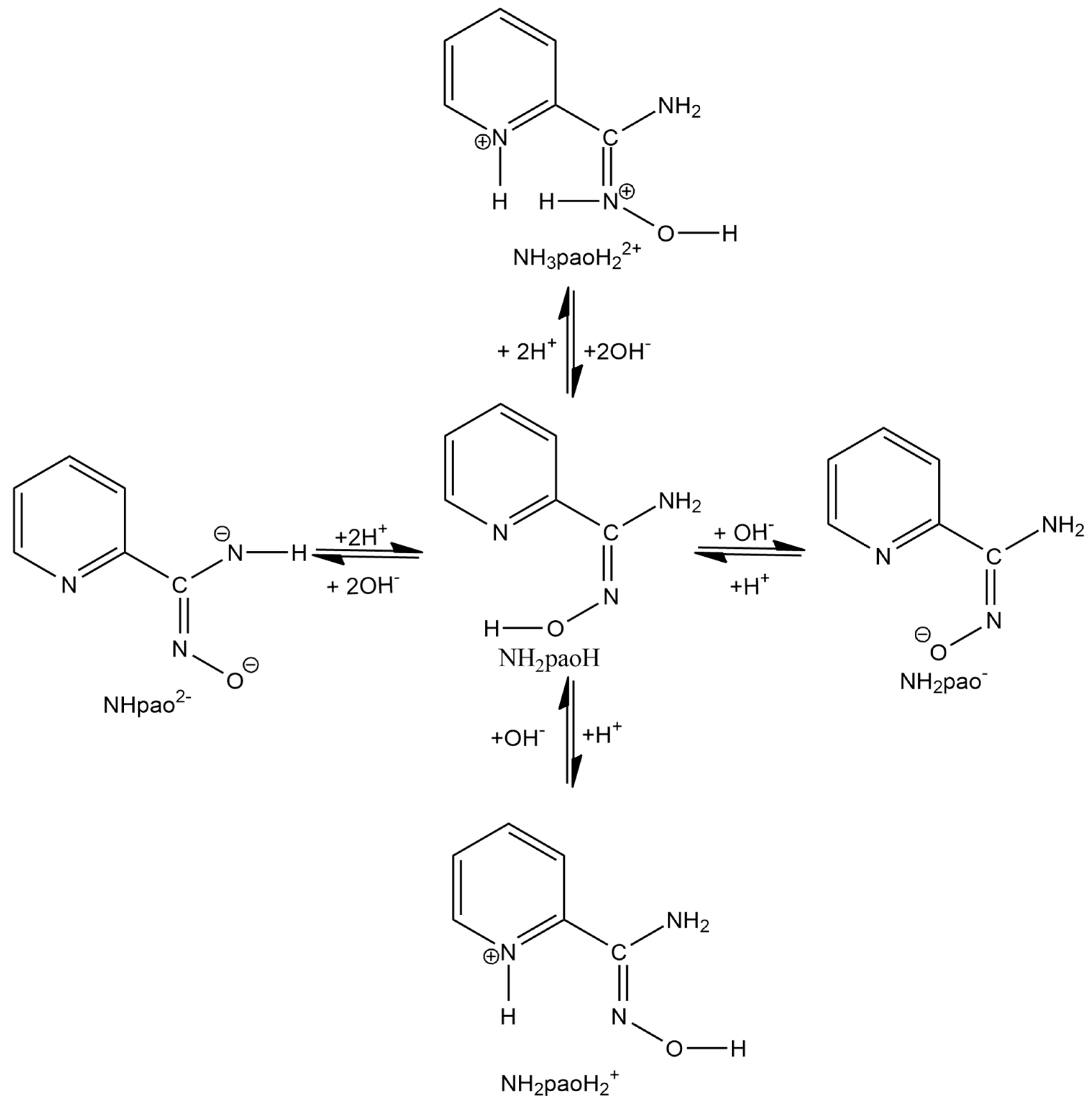
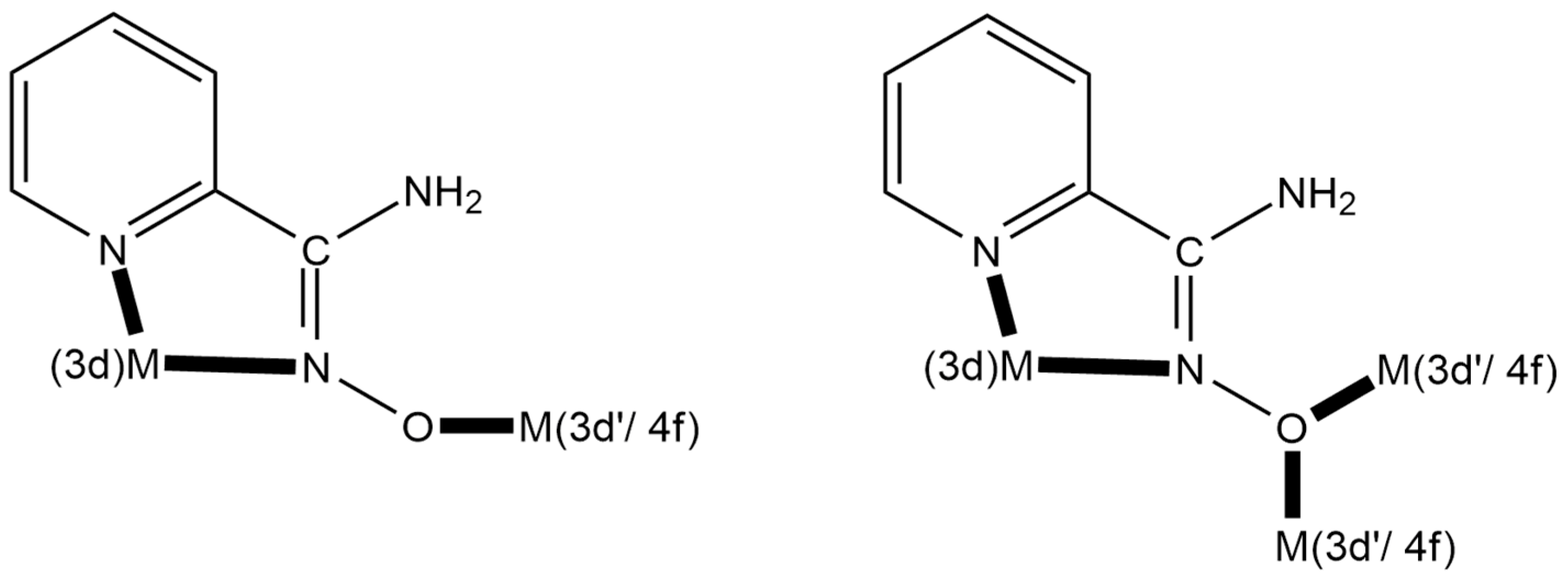
A unique “member” of 2-pyridyl oximes is pyridine-2-amidoxime, abbreviated as NH2paoH (Figure 1, right). Other names of this compound (less commonly used) are pyridine-2-carboxamide oxime and {[amino(pyridine-2-yl)methylidene]amino}oxidanide. This is a derivative of pyridine-2-carboxamide or picolinamide (Figure 2). The presence of the -NH2 group results in a significant mesomeric effect, and this creates a difference in the chemistry of NH2paoH and that of simple 2-pyridyl oximes. The amidoxime group is important in organic, pharmaceutical, theoretical, coordination, bioinorganic, supramolecular, and material chemistry [27,28,29,30,31,32,33,34,35], and in the area of the reactivity of coordinated ligands [36]. The 2-pyridyl and oxime N atoms of NH2paoH are basic, whereas the -OH group is acidic. The simplified acid–base chemistry of NH2paoH is illustrated in Figure 3. The N atom of the -NH2 group is sp2 hybridized and it thus has a negligible basicity. Therefore, the neutral amino group is uncoordinated in metal complexes; it rarely can be acidic under basic conditions in the presence of metal ions, and in this case, the amidoxime group has an overall charge of -2 (NHpao2−), favoring coordination of its N atom [37]. The singly deprotonated ligand is ideal (like the 2-pyridyloximates) for the preparation of 3d-4f and 3d-3d’ species (Figure 4), based on the concept mentioned above for the anionic 2-pyridyl oximes. Somewhat to our surprise, the number of heterometallic complexes (both 3d-4f and 3d-3d’) based on NH2paoH is very limited [38,39,40,41].
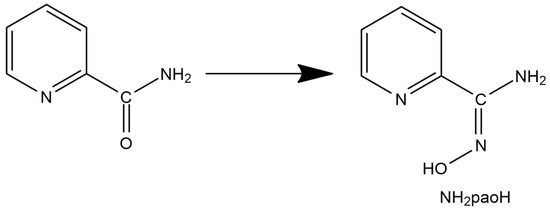
Figure 2.
The relation between picolinamide and pyridine-2-amidoxime.
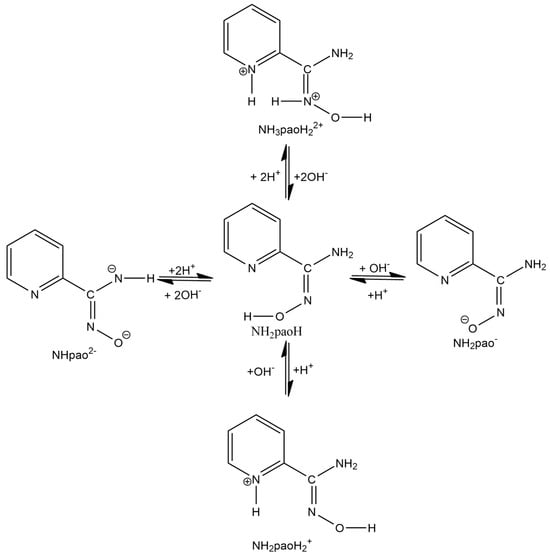
Figure 3.
Simplified picture of the acid–base chemistry of NH2paoH.
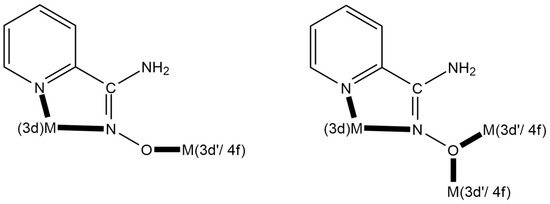
Figure 4.
Strategic approach and expected coordination modes for the preparation of 3d-3d’ and 3d-4f metal complexes using the singly deprotonated NH2pao− ligand; 3d’- and 4f-metal ions are hard acids (HSAB), whereas the 3d-metal ion is a soft or borderline acid (HSAB); see text for details. The coordination bonds are drawn with bold lines.
We have recently embarked on a new program aiming to synthesize 3d-3d’ complexes [21]. In this work, we describe efforts to prepare Co-Fe complexes using the NH2pao− ligand. Heterometallic CoII-FeIII complexes attract the intense interest of the materials’ scientific community as molecular analogues of the mixed-metal oxide CoIIFeIII2O4, which has an inverse spinel structure [42,43,44]; CoIII-FeIII compounds are also exciting in the field of molecular magnetism because they are capable of undergoing proton-induced reversible conversion between a low- and a high-spin CoIII center within the heterometallic core [45]; in addition, discrete cyanido-bridged FeII-CoIII and FeIII-CoII complexes are excellent models for the study of switchable Fe-Co Prussian blue networks [46]. This work can also be considered as a continuation of the activity of our groups in several aspects of the coordination chemistry of NH2paoH [37,41].
2. Results and Discussion
2.1. Synthetic Comments
As mentioned in the Introduction, our goal was to isolate Co-Fe compounds with the anionic form of pyridine-2-amidoxime as primary bridging ligands. Many synthetic parameters were studied, including the source of the two metal ions, the solvent, the temperature, the pressure (solvothermal reactions), the presence or absence of external bases, the reaction time, and the crystallization procedures before arriving at the optimized preparation described in Section 3 (vide infra). For the isolation of single crystals, the ideal choices of metal-containing starting materials were CoII(ClO4)2·6H2O and FeIII(NO3)3·9H2O. The 1:1:3 reaction between CoII(ClO4)2·6H2O, FeIII(NO3)3·9H2O, and NH2paoH in MeOH at room temperature gave a dark red solution, from which were subsequently isolated X-ray quality crystals of [CoIII2FeIII(NH2pao)6](ClO4)2(NO3) (1) as the 5MeOH/1.5H2O solvate in ca. 55% yield. It is easily seen that oxidation of CoII has taken place, the atmospheric oxygen being the oxidant. Assuming that the structurally characterized complex is the only product in solution, the formation of the compound is summarized in Equation (1).
Some synthetic points deserve discussion: (i) The use of Et3N as external base also gives 1 in microcrystalline form (IR evidence, microanalytical data), Equation (2), but—somewhat to our surprise—in comparable yields (45–55%). (ii) The excess of FeIII(NO3)3·9H2O (the stoichiometric Co: Fe ratio of the product is 2:1, but the experimental one is 2:2) appears necessary for the isolation of pure 1; when the stoichiometric ratio is used, 1 is contaminated with dark brown crystals of [CoII2CoIII(NH2pao)6](NO3) [38], as proven by unit-cell determination. Presumably, reaction (1) is an equilibrium, and the excess of FeIII(NO3)3·9H2O moves it to the right. (iii) The 2:1:3 CoII(ClO4)2·6H2O/FeIII(ClO4)3·6H2O/NH2paoH and CoII(NO3)2·6H2O/FeIII(NO3)3·9H2O/NH2paoH in MeOH gives the powders analyzed as [Co2Fe(NH2pao)6](ClO4)3 and [Co2Fe(NH2pao)6](NO3)3, respectively; thus, using the same inorganic anion (ClO4− vs. NO3−) in separate reactions leads to the same {CoIII2FeIII} cation. Since we could not crystallize these powders, we did not pursue their characterization further. (iv) Small increases in the NH2paoH ratio, i.e., CoII(ClO4)2·6H2O/FeIII(NO3)3·9H2O/NH2paoH = 2:2:4, results in 1 (IR evidence). (v) Changes in the oxidation state in the iron reactant do not affect the identity of the products; the 1:1:3 CoII(NO3)2·6H2O/FeII(ClO4)2·6H2O/NH2paoH reaction mixture in MeOH leads again to 1 in rather moderate yields (30–40%) (Equation (3)), as evidenced using IR spectroscopy. All the above five synthetic comments show that the cation of 1 is the thermodynamically stable species from the 2:2:3 CoII/FeIII or FeII/NH2paoH reaction systems in the simultaneous presence of ClO4− and/or NO3− ions.
It should be noted that the complex (after pulverizing and drying it) was analyzed as 1·H2O; this sample was used for the spectroscopic (IR, Raman, UV/VIS, Mössbauer) studies. The purity of the samples was ensured because only single crystals of the product were pulverized and dried before the spectroscopic measurements. On the contrary, the magnetic measurements were performed on freshly isolated crystals in order to detect intermolecular exchange interactions (if any) between the trinuclear cations, mediated by the large number of lattice solvent molecules and counterions.
2.2. Description of Structure
The structure of 1·5MeOH·1.5H2O was determined using single-crystal X-ray crystallography. The crystallographic data are gathered in Table 1. Selected interatomic distances and angles are listed in Table 2. Structural plots are shown in Figure 5, Figure 6, and Figures S1–S3.

Table 1.
Crystallographic data and structural refinement parameters for complex 1·5MeOH·1.5H2O.

Table 2.
Selected interatomic distances (Å) and bond angles (°) for one of the two crystallographically independent trinuclear cations of complex [CoIII2FeIII(NH2pao)6](ClO4)2(NO3)·5MeOH·1.5H2O (1·5MeOH·1.5H2O) a,b.
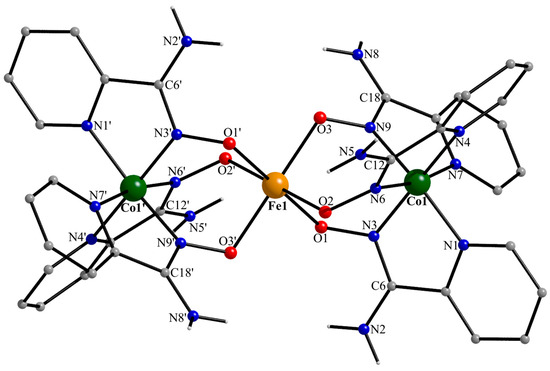
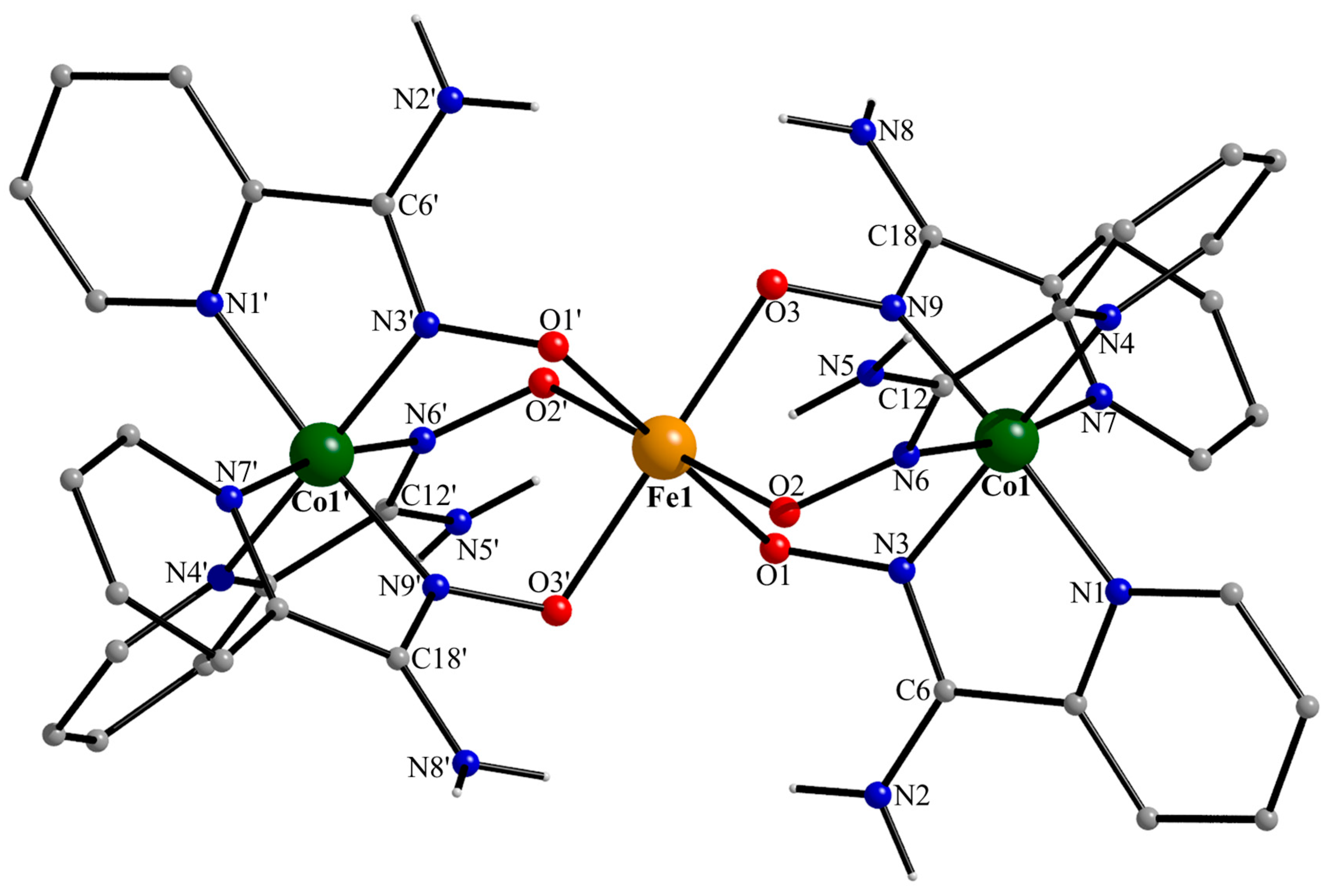
Figure 5.
Partially labeled structural plot for one (of the two) crystallographically independent trinuclear cation [CoIII2FeIII(NH2pao)6]3+ of 1·5MeOH·1.5H2O. Symmetry code: (‘) −x + 1, −y + 1, −z + 1.
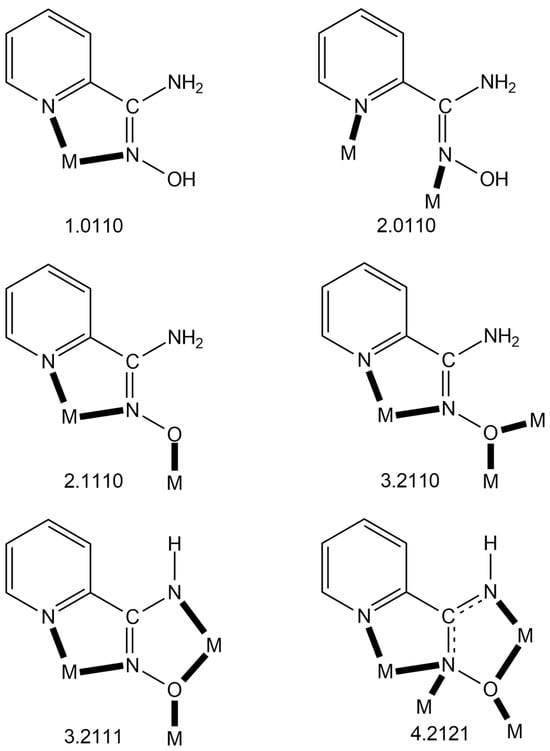
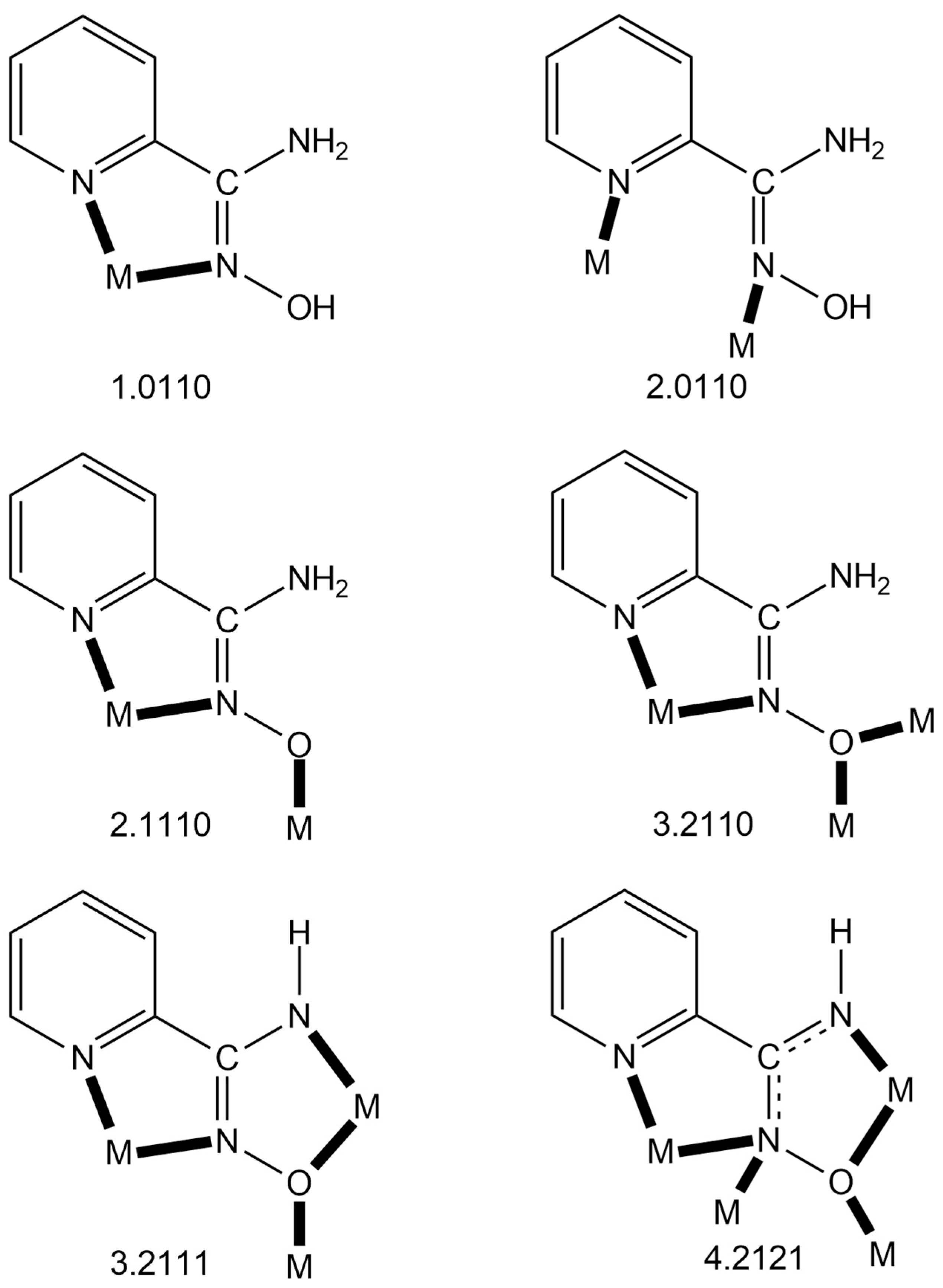
Figure 6.
The to-date crystallographically established ligation modes of NH2paoH, NH2pao− and NHpao2−, and the Harris notation that describes these modes. The 4.2121 mode of NHpao2− has been observed only in Ni(II) chemistry, while the 3.2111 one exists only in Ni(II) and mixed-valence Mn chemistry. The coordination bonds are drawn with bold lines.
Compound 1·5MeOH·1.5H2O crystallizes in the centrosymmetric space group P. There are two, structurally very similar, crystallographically independent [CoIII2FeIII(NH2pao)6]3+ cations in the crystal structure; thus, the complete formula of the complex is {[CoIII2FeIII(NH2pao)6](ClO4)2(NO3)}2·10MeOH·3H2O. A remarkable feature of the crystal structure is the fact that the positive charge is balanced by a combination of two inorganic anions in a 2:1 ClO4−:NO3− ratio. Due to the structural similarity of the cations (Figure 5 and Figure S1), only the structure of the cation possessing Fe1, Co1, and Co1′ (−x + 1, −y + 1, −z + 1) will be described. The central iron(III) center (Fe1 in Figure 5) is located on a crystallographically imposed inversion center resulting in a strictly linear cation. Fe1 is surrounded by six deprotonated oxygen atoms from the six 2.1110 (Figure 6) NH2pao− ligands. Each cobalt(III) ion is bonded to six nitrogen atoms that belong to three “chelating” parts of three ligands, resulting in a Co1/1′ (N2-pyridyl)3(Noximato)3 facial coordination sphere. The Fe1…Co1/1′ distance is 3.488(2) Å. The Fe1-O bond lengths are in the range of 2.005(2)–2.053(2) Å, suggesting a high-spin 3d5 FeIII center [21]. The Co1/1′-N bond distances [1.884(3)–1.961(3) Å] are typical of a low-spin 3d6 CoIII ion [41], the cobalt(III)-N(2-pyridyl) lengths being slightly larger than the cobal(III)-N(oximato) ones. The C-N“amino” distances [1.340(4)–1.348(4) Å] are larger than the C-Noximato ones [1.302(5)–1.304(4) Å], as expected. However, their difference (~0.45 Å) is not as large as expected for single and double carbon-nitrogen bonds; this indicates a partial delocalization in the N“amino”-C-Noximato moiety.
The Fe1O6 coordination polyhedron is almost a perfect octahedron, the cis angles being in the narrow range of 88.2(1)–91.8(1)°. The octahedral coordination geometry of Co1/1′ is more distorted, and the three trans Noximato-CoIII-N2-pyridyl bond angles are ~171°. Angular distortions from the perfect octahedral geometry are primarily a consequence of the chelating rings and their restricted (~82°) bite angles.
At the supramolecular level, each of the two crystallographically different cations is H-bonded with two “bridging” MeOH molecules through weak N-H…O and O-H…O interactions (dN…o = 2.90–2.94 Å; do…o = 2.71–2.78 Å). These units are connected by direct weak N-H…O (dN…o = 2.89–3.11 Å) and N-H…N (dN…N = 3.11–3.17 Å) H bonds along the b crystallographic axis, and by a “bridging” MeOH (dN…o = 2.76 Å) and O-H…O interactions (dO…o = 2.80 Å; see Figure S2). In the crystal, these 1D H-bonded supramolecular networks are separated by ClO4− and NO3− ions and H2O and MeOH molecules (Figure S3).
Complex 1 joins a handful of structurally characterized heterometallic complexes based on pyridine-2-amidoxime (Table 3). With the exception of the neutral dimer [CoIIIDy(NH2pao)3(NO3)3] [41], all the other complexes consist of strictly (by symmetry) trinuclear cations [38,39,40] and various counterions, and they possess six 2.1110 NH2pao− ligands. The central metal ion (CrIII, MnIV, FeIII) is oxophilic, bonded to six oxygen atoms, while the terminal metal ions are intermediate (borderline) acids (HSAB), and each is bonded to six nitrogen atoms that belong to three ligands. Complex 1 is the first trinuclear complex of this type, containing three trivalent metals.

Table 3.
Structurally characterized heterometallic complexes based on the singly deprotonated form (NH2pao−) of pyridine-2-amidoxime and the Harris notation that describes the coordination mode of the anionic ligand.
Table 3.
Structurally characterized heterometallic complexes based on the singly deprotonated form (NH2pao−) of pyridine-2-amidoxime and the Harris notation that describes the coordination mode of the anionic ligand.
| Complex a | Coordination Mode b,c | Reference |
|---|---|---|
| [Ni2CrIII(NH2pao)6](OH) | 2.1112120 | [38] |
| [Ni2CrIII(NH2pao)6](ClO4) | 2.1112120 | [39] |
| [Ni2MnIV(NH2pao)6](ClO4)Cl | 2.1112120 | [39] |
| [Ni2MnIV(NH2pao)6](ClO4)2 | 2.1112120 | [39] |
| [Ni2FeIII(NH2pao)6](NO3) | 2.1112120 | [40] |
| [Ni2FeIII(NH2pao)6]Cl | 2.1112120 | [38] |
| [Ni2FeIII(NH2pao)6](ClO4) | 2.1112120 | [39] |
| [CoIIIDy(NH2pao)3(NO3)3] | 2.1112120 | [41] |
| [CoIII2FeIII(NH2pao)6](ClO4)2 (NO3) | This work |
a Lattice solvent molecules have been omitted from the formulae. b Using the Harris notation. c The subscript 1 refers to CrIII, MnIV, FeIII, and DyIII, while the subscript 2 refers to NiII and CoIII.

Table 4.
Structurally characterized metal complexes possessing the neutral form (NH2paoH) of pyridine-2-amidoxime, and a compound containing both the neutral and singly deprotonated (NH2pao−) forms of the ligand, along with their coordination modes.
Table 4.
Structurally characterized metal complexes possessing the neutral form (NH2paoH) of pyridine-2-amidoxime, and a compound containing both the neutral and singly deprotonated (NH2pao−) forms of the ligand, along with their coordination modes.
| Complex a | Coordination Mode f | References |
|---|---|---|
| [MnIICl2(NH2paoH)2] | 1.0110 | [47] |
| [MnIICl(N3)(NH2paoH)2] | 1.0110 | [47] |
| [MnII(O2CMe)2(NH2paoH)2] | 1.0110 | [47,48] |
| {[Mn(bdc)(NH2paoH)2]}n b | 1.0110 | [49] |
| {[MnII(SO4)(NH2paoH)2]}n | 1.0110 | [50] |
| [Ni(NO3)2(NH2paoH)2] | 1.0110 | [51] |
| [Ni(NH2paoH)3](NO3)2 | 1.0110 | [51] |
| [Ni(O2CMe)2(NH2paoH)2] | 1.0110 | [52] |
| {[Ni(SO4)(NH2paoH)2(H2O)]}n | 1.0110 | [50] |
| {[Ni(bdc)(NH2paoH)2]}n b | 1.0110 | [49] |
| {[Ni(Hbtc)(NH2paoH)2}n c | 1.0110 | [49] |
| [Ni(H2btc)2(NH2paoH)2] d | 1.0110 | [49] |
| [Cu(NH2paoH)2(H2O)]Cl2 | 1.0110 | [53,54] |
| [Zn(O2CMe)2(NH2paoH)2] | 1.0110 | [48,55] |
| [Zn(O2CPh)2(NH2paoH)2] | 1.0110 | [55] |
| [Zn(NO3)(NH2paoH)2](NO3) | 1.0110 | [55] |
| [Zn2(O2CMe)3(NH2paoH)4](OH) | 1.0110 | [50] |
| {[Zn(bdc)(NH2paoH)(DMF)]}n b | 1.0110 | [49] |
| [Cd(O2CMe)2(NH2paoH)2] | 1.0110 | [56] |
| [Hg2(SCN)4(NH2paoH)2] | 1.0110 | [57] |
| [ReIBr(CO)3(NH2paoH)] | 1.0110 | [58] |
| [RhIIICl(Cp*)(NH2paoH)](PF6) e | 1.0110 | [59] |
| [IrIIICl(Cp*)(NH2paoH)](PF6) e | 1.0110 | [59] |
| {[Ag(NO3)(NH2paoH)]}n | 2.0110 | [60] |
| [Dy2(NO3)6(NH2paoH)2] | 1.0110 | [61] |
| [Zn2(NH2pao)2(NH2paoH)2](NO3)2 | 2.1110 g, 1.0110 h | [62] |
a Lattice solvent molecules are not written in the formulae. b The ligand bdc2− is the dianion of 1,4-benzenedicarboxylic acid. c The ligand Hbtc2− is the dianion of 1,3,5-benzenetricarboxylic acid. d The ligand H2btc− is the monoanion of 1,3,5-dicarboxylic acid. e Cp*− is the pentamethylcyclopentadienido ligand. f Using the Harris notation. g For the singly deprotonated ligand. h For the neutral ligand.
Since the area of the homometallic complexes based on neutral or/and deprotonated pyridine-2-amidoxime is now quite mature, we felt it timely to collect and briefly discuss them. Table 4, Table 5 and Table 6 list all the to-date structurally characterized metal complexes, classified according to the charge (0, −1, −2) of the ligand. The following points deserve comments: (a) With the exception of {[Ag(NO3)(NH2paoH)]}n, the neutral NH2paoH ligand is bidentate chelating (1.0110) (Figure 6). In the Ag(I) coordination polymer, NH2paoH adopts the bridging 2.0110 mode (Figure 6), which partially contributes to polymerization (the nitrato ligand is also bidentate bridging) [60]. In the other NH2paoH-containing metal polymers [49,50], polymerization is achieved through ancillary di- or tricarboxylato ligands. (b) The only complex that contains both the neutral and singly deprotonated ligand is [Zn2(NH2pao)2(NH2paoH)2](NO3)2 [62], and the 2.1110 mode (Figure 6) of the NH2pao− ions is responsible for the dimerization. (c) There are only five complexes (Table 5) containing exclusively the singly deprotonated ligand (NH2pao−) [38,50,55,63,64]; all contain the NH2pao− ligand in its 2.1110 coordination mode. The mixed-valence complex [CoII2CoIII(NH2pao)6](NO3)] [38] is analogous to those listed in Table 3, the CoIII ion being the central site with a {CoIIIO6} coordination sphere. Finally, (d) more exciting from the structural and magnetic viewpoints are the complexes listed in Table 6 containing both NHpao2− and NH2pao− ligands. With the exception of the mixed-valence compound [MnII2MnIII10MnIV2O8(N3)2(NHpao)2(NH2pao)14](ClO4)2(OH)2 [65], the metal center in the other complexes is Ni(II). The capability of the NHpao2− ligand to adopt the 4.2121 and 3.2111 modes, combined with the 3.2110 and 2.1110 ligation modes of NH2pao−, create a “blend”, which gives rise to Ni4, Ni5, Ni8, Ni12, and Ni16 complexes with aesthetically beautiful structures [37,65,66,67,68], such as single- (Ni4), double- (Ni8), triple- (Ni12), and quadruple (Ni16)-decker exhibiting intramolecular ferromagnetic interactions.

Table 5.
Structurally characterized homometallic complexes based on the singly deprotonated form (NH2pao−) of pyridine-2-amidoxime and the Harris notation that describes the coordination mode of the anionic ligand.

Table 6.
Structurally characterized metal complexes containing both the doubly deprotonated (NHpao2−) and the singly deprotonated (NH2pao−) forms of pyridine-2-amidoxime and the Harris notation that describes the coordination modes of the anionic ligands.
2.3. Spectroscopic and Physical Studies
Complex 1 was characterized using several spectroscopic and physical techniques in the solid state and in solution. The data are presented in Figure 7, Figure 8, Figure 9 and Figure 10 and Figures S4–S8. For the spectroscopic characterization, analytically pure samples (1·H2O) of the complex were used, whereas for the magnetic measurements, the sample was in the form of crystals (1·5MeOH·1.5H2O) collected directly from the mother liquor. The strong/medium bands at 3465 and 3349 cm−1 in the IR spectrum of free NH2paoH (Figure S4) are assigned to the νas(NH2) and νs(NH2) modes, respectively [57,61]. The absence of large systematic shifts of these bands in the spectrum (Figure S5) [at 3467 and 3330/3306 cm−1, respectively] implies the non-involvement of the -NH2 group in coordination. The relatively broad band centered at ~3125 cm−1 in the latter can be attributed to the ν(OH) vibration of the lattice H2O contained in the sample 1·H2O. The in-plane deformation of the 2-pyridyl group of NH2paoH at 594 cm−1 [57] shifts upwards (at 628 cm−1), confirming the coordination of the ring-N atom [57,61]. The ν(C = N)oxime band of free NH2paoH at 1650 cm−1 [likely also having δ(ΝH2) and δ(OH) character] shifts slightly to a lower wavenumber (at 1643 cm−1) in the spectrum of the complex, suggesting the ligation of the imino nitrogen. The ν(NO)oximate band of the complex is located at 1145 cm−1 [41], while the corresponding band in the free ligand is at a lower wavenumber (1098 cm−1). The shift to a higher wavenumber in the complex has been previously discussed [41]. This result is in accordance with the concept that upon deprotonation and oximato O-coordination, there is a higher contribution of the double bond character (N = O) to the electronic structure of the oximato group; thus, the ν(NO) vibration shifts to a higher wavenumber in complexes containing the 2.1110 NH2pao− ligand, relative to the free ligand. The presence of the NO3− and ClO4− ions in the complex is clearly evident in its IR spectrum. The band at 1384 cm−1 is assigned to the IR-active ν3(Ε’)[νd(NO)] vibration of the planar D3h nitrate ion [70]. The bands at 1082 and 628 cm−1 can be safely attributed to the IR-active ν3(F2)[νd(ClO)] and ν4(F2)[δd(OClO)] modes of the tetrahedral (Td) perchlorate ion [70], the former possibly overlapping with a ligand vibration.
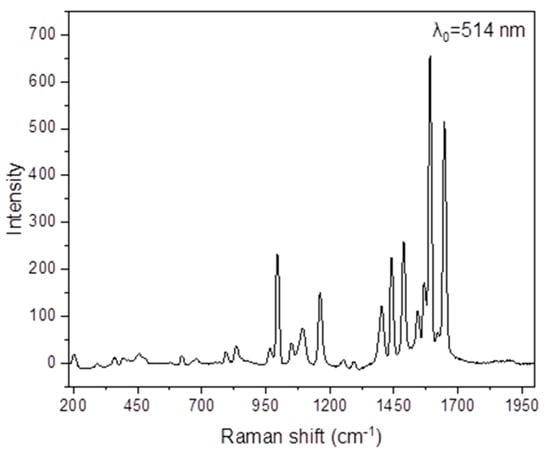
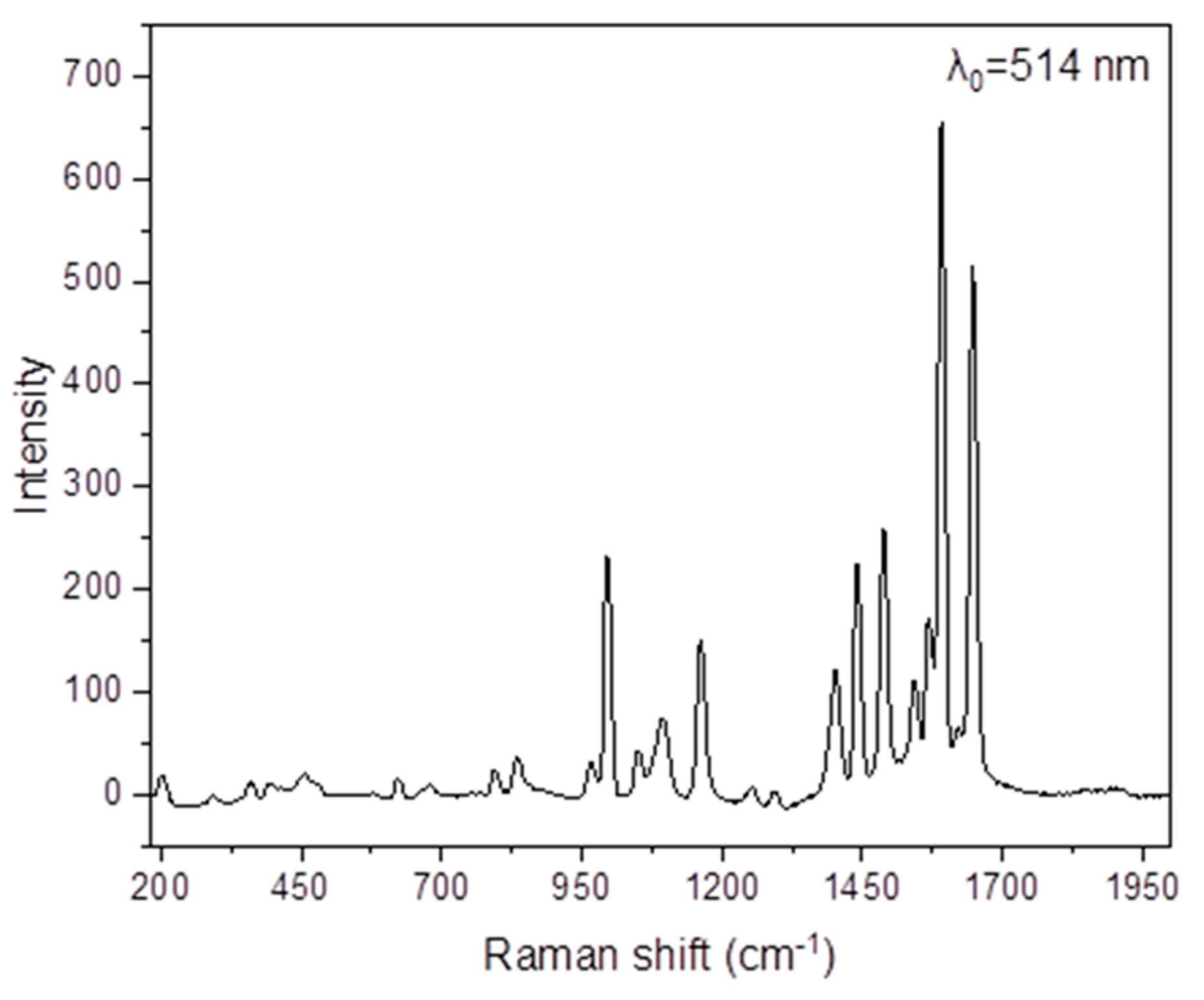
Figure 7.
The Raman spectrum of NH2paoH in the 2000–190 cm−1 region (excitation wavelength: 514 nm).
The νas(NH2), νs(NH2), and ν(OH) modes are not practically visible in the Raman spectra of NH2paoH (Figure 7) and the complex (Figure S6), as expected. The peaks at 1648 and 1093 cm−1 in the spectrum of NH2paoH are assigned to the ν(C = N) and ν(NO) modes, respectively. The corresponding peaks in the spectrum of 1·H2O appear at 1634 and 1162 cm−1, respectively [71], the latter overlapping with a NH2pao− vibration. The shoulder at 1405 cm−1 and the peak at 705 cm−1 are assigned [70] to the Raman-active vibrations ν3(E’)[νd(NO)] and ν4(Ε’)[δd(ONO)], respectively. The Raman-active modes ν4(F2)[δd(OClO), ν1(A1)[νs(ClO)], and ν3(F2)[νd(ClO)] of the tetrahedral (Td) perchlorate ion are located at 642, 936, and 1095 cm−1, respectively [70].
The molar conductivity of the 1·H2O in EtOH (ΛM/10−3 M, 25 °C) is 135 S cm2 mol−1, suggesting a 1:3 electrolyte [72]. This might indicate that the solid-state structure of the trinuclear cation [CoIII2FeIII(NH2pao)]3+ is retained in EtOH.
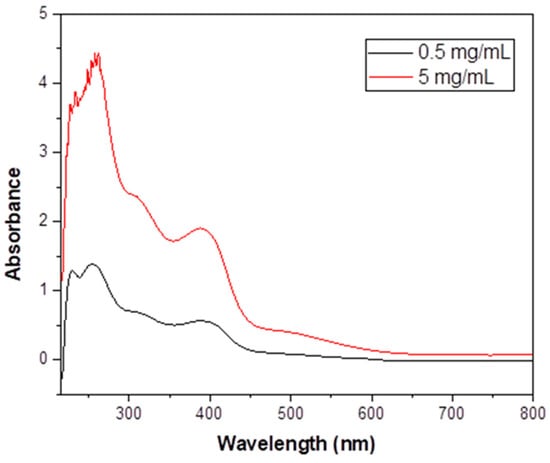
Figure 8.
The UV/VIS spectrum (nm) of 1·H2O in EtOH at two different concentrations.
Figure 8.
The UV/VIS spectrum (nm) of 1·H2O in EtOH at two different concentrations.
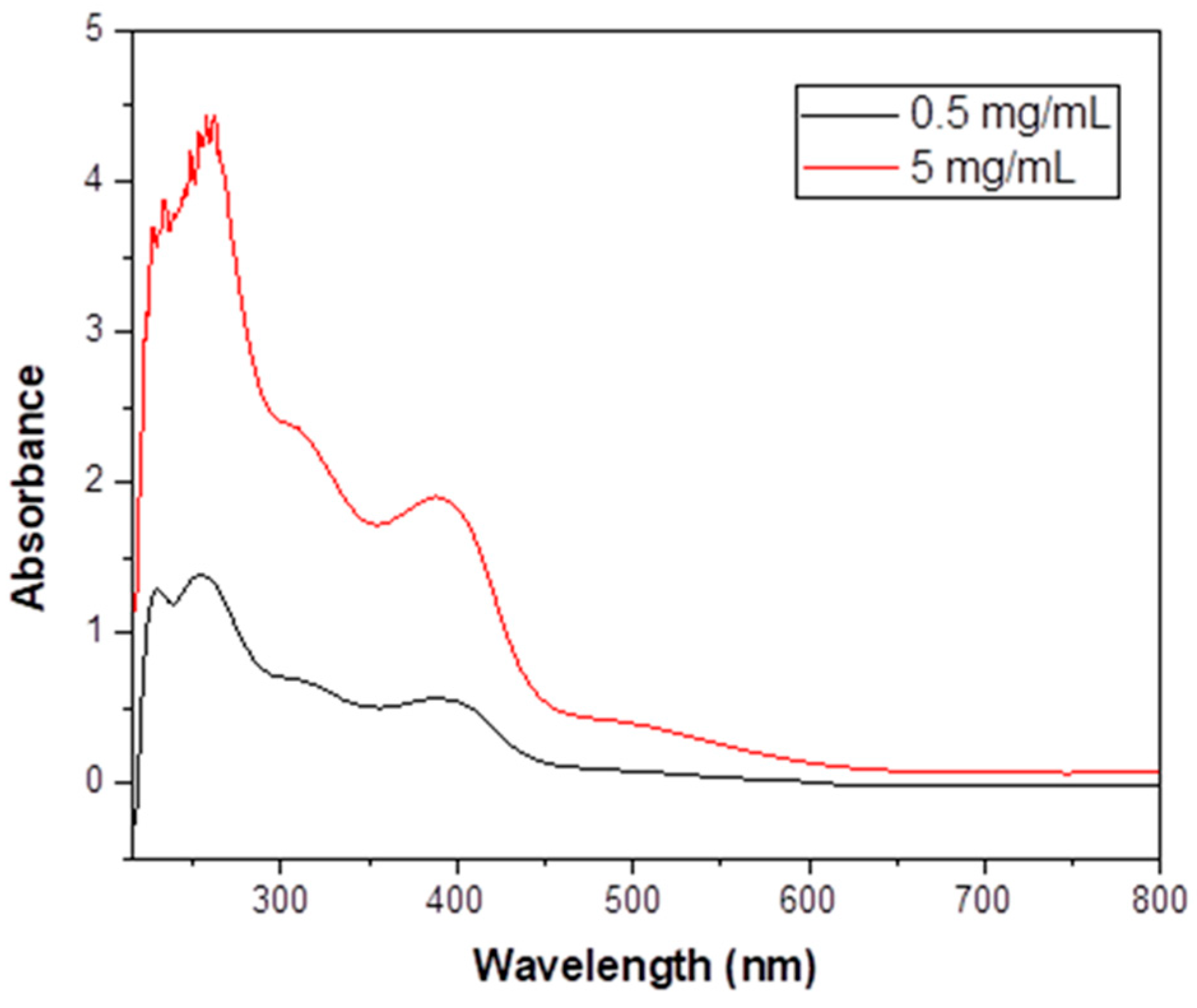
The UV/VIS spectrum of free NH2paoH in EtOH (Figure S7) exhibits a high-intensity band at 283 nm, assigned to a transition having a -C(NH2) = N- character [73], and two shoulders below 250 nm. These bands also exist in the spectrum of 1·H2O in the same solvent, albeit slightly shifted due to deprotonation and coordination. The spectrum of the complex additionally shows three bands at 312, 390, and ~ 505 nm. The low-spin octahedral CoIII (t2g6eg0) ground term is 1A1g, and three are two spin-allowed transitions, with lower lying spin triplet partners, all derived from (t2g)5(eg)1. The spin-allowed transitions 1A1g → 1T2g and 1A1g → 1T1g give absorption maxima at 312 and 505 nm, respectively; the wavelengths are characteristic of low-spin {CoIIIN6} chromophores [41,74]. The spin-forbidden 1A1g → 3T1g and 1A1g → 3T2g transitions above 600 nm are too weak to be observed. The band at 390 nm is assigned to a NH2pao− -to-FeIII charge-transfer transition [74]. The charge-transfer transition is from a π orbital of NH2pao− to a t2g orbital of FeIII. Since iron(III) is an oxidizing agent, the ligand-to-metal charge-transfer transition obscures any very weak, spin-forbidden transition of the high-spin 3d5 FeIII center. The possibility that the 505 nm band also has a charge-transfer character, overlapping with a d-d transition of CoIII, cannot be ruled out [74].
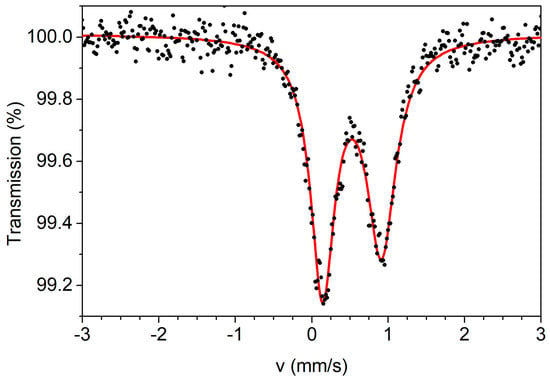
Figure 9.
The 57Fe-Mössbauer spectrum of an analytically pure sample of complex (1·H2O) recorded at 80 K in zero applied field. The solid line is the theoretical spectrum assuming an asymmetric doublet.
Figure 9.
The 57Fe-Mössbauer spectrum of an analytically pure sample of complex (1·H2O) recorded at 80 K in zero applied field. The solid line is the theoretical spectrum assuming an asymmetric doublet.
The zero-field 57Fe-Mössbauer spectrum of a powdered, analytically pure sample of the complex was recorded at 80 K. The spectrum is shown in Figure 9. The spectrum consists of an asymmetric doublet. The doublet can be simulated with an isomer shift (δ) value of 0.53 mm s−1 (relative to metallic iron at room temperature) and a quadrupole splitting value (ΔEQ) of 0.79 mm s−1. These values are consistent with a high-spin iron(III) center in an O-rich coordination environment [21,75]. The neighboring CoIII ions may contribute to the relatively large ΔEQ value. The asymmetry and broad character of the peaks (width at half maximum 0.39 mm s−1 for the left peak and 0.47 mm s−1 for the right one) is attributed to magnetic relaxation effects, as it is often observed in isolated high-spin FeIII (3d5) ions in the solid state (the closest intermolecular FeIII…FeIII distance is ~7.7Å).
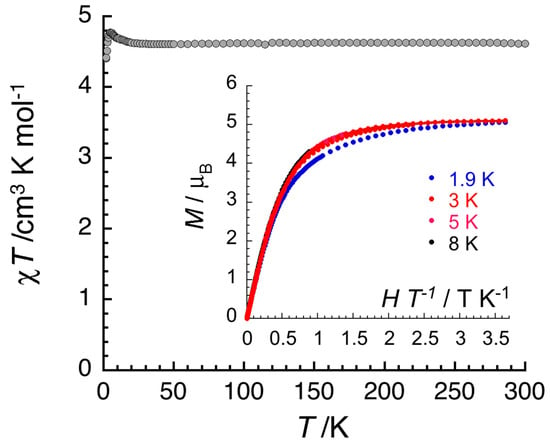
Figure 10.
Temperature dependence of the χT product (where χ is the molar magnetic susceptibility that equals M/H per complex, and T is the absolute temperature) in an applied dc magnetic field (H) of 0.1 T for 1·5MeOH·1.5H2O. The solid line is a guide for the eyes. Inset: Field dependence of the magnetization (M) for 1·5MeOH·1.5H2O in the form of M vs. H/T below 8 K. The solid line is the best fit of the experimental data to a S = 5/2 Brillouin function.
Figure 10.
Temperature dependence of the χT product (where χ is the molar magnetic susceptibility that equals M/H per complex, and T is the absolute temperature) in an applied dc magnetic field (H) of 0.1 T for 1·5MeOH·1.5H2O. The solid line is a guide for the eyes. Inset: Field dependence of the magnetization (M) for 1·5MeOH·1.5H2O in the form of M vs. H/T below 8 K. The solid line is the best fit of the experimental data to a S = 5/2 Brillouin function.
Variable-temperature (1.8–300 K) dc magnetic susceptibility studies were performed on a crystalline sample of 1·5MeOH·1.5H2O at 0.1 and 1 T. The results are shown in Figure 10. The crystals were filtered directly from the mother liquor and immediately prepared for measurements. The χT value at room temperature is 4.6 cm3 K mol−1, identical with the expected value (g = 2.05) for one isolated, i.e., non-interacting, high-spin FeIII center (χ is the molar magnetic susceptibility). The value of the χT product remains constant in the 300–9 K range, suggesting the practical absence of interactions between the trinuclear cations; the slight variation of the product at lower temperatures might indicate tiny ferromagnetic interactions, likely coexisting with even smaller antiferromagnetic interactions, although the FeIII…FeIII distances are long (vide supra). Magnetization (M) data (Figure 10, inset) at 1.9 K are approaching saturation at 7 T with a value of 5.1 μΒ for one independent S = 5/2 ion and g = 2.05. Overall, the complex is characterized by S = 5/2 Curie and Brillouin paramagnetic behavior. The isolated character of the central, high-spin FeIII atom would be expected because of the presence of two diamagnetic, i.e., low-spin CoIII, sites at the terminal positions within each molecule. On the contrary, in the structurally related cation [Ni2FeIII(NH2pao)6]+, the paramagnetic nature of the terminal NiII sites (each having S = 1) results in an antiferromagnetic exchange interaction between the central FeIII atom and the terminal NiII atoms.
3. Materials and Methods
3.1. Materials and Instrumentation
All manipulations took place under aerobic conditions in a normal laboratory atmosphere. Reagents and solvents were purchased from Alfa Aesar (Karlsruhe, Germany) and Sigma-Aldrich (Tanfrichen, Germany) and used as received. The free pyridine-2-amidoxime (NH2paoH) was synthesized as reported in the literature [76]. The yield was 79%. Its purity was assessed using 1H NMR spectroscopy (Figure S8), and its melting point was determined (reported 114–116 °C, found 113 °C). Deionized water was obtained from the in-house facility.
C, H, and N microanalyses were conducted at the University of Patras Instrumental Analysis Laboratory. The conductivity experiment was carried out at 25 ± 2 °C with a Metrohm-Herisau E-527 bridge and a cell of standard design (Metrohm AG, Herisau, Switzerland); the concentration was ~10−3 M (assuming the formula 1·H2O), and the solvent (EtOH) was used as received. The 1H NMR spectrum of free NH2paoH in d6-DMSO was run on a 600.13 MHz Bruker Avance DPX spectrometer (Bruker Avance, Billerica, MA, USA). FT-IR spectra (4000–400 cm−1) were recorded using a Perkin-Elmer 16PC spectrometer (Waltham, MA, USA); the samples were in the form of KBr pellets obtained using pressure. Backscattering Raman spectra were obtained using the T-64000 model of Jobin Yvon (Horiba group), excited with either a He-Ne (Optronics Technologies SA, Moschato, Greece, Model HLA-20P, 20 mV) or a Cobolt Fandango TM ISO laser (Hübner Photonics Gmbh, Solna, Sweden), operating at 632.8 and 514 nm, respectively; the resolution was ± 4 cm−1. The calibration of the instrument was achieved via the standard Raman peak position of Si at 520.5 cm−1. The rather poor quality of the spectrum of the complex was due to its dark color. UV/VIS spectra in EtOH were recorded on a Hitachi U-3000 spectrometer (Slough, UK). The 57Fe-Mössbauer spectrum from a powdered sample of 1·H2O at 80 K with a Janis cryostat (Cryogenic Technology, Woburn, MA, USA) was recorded using a constant-acceleration conventional spectrometer with a source of 57Co (Rh matrix). Magnetic susceptibility and magnetization measurements were performed on a Quantum Design SQUID magnetometer MPMS-XL (San Diego, CA, USA) in the ranges of 1.8–300 K and 1.9–8 K, respectively; for the magnetization measurement, the dc magnetic fields ranged from −7 to +7 T. The data were collected on a polycrystalline sample suspended in mineral oil and introduced in a sealed polyethylene bag. Prior to the experiment, the field-dependent magnetization was measured at 100 K in order to detect the presence of any bulk ferromagnetic impurities; paramagnetic materials should exhibit a strictly linear dependence of magnetization that extrapolates to zero dc field. No ferromagnetic impurities were observed. The magnetic susceptibility was corrected for the oil, the sample holder, and the intrinsic diamagnetic contributions.
3.2. Preparation of the Complex
Method (a): Solid NH2paoH (0.041g, 0.30 mmol), CoII(ClO4)2·6H2O (0.037g, 0.10 mmol), and FeIII(NO3)3·9H2O (0.040g, 0.10 mmol) were added to MeOH (10 mL). The resulting dark red solution was stirred for 10 min and stored in a closed flask. X-ray quality, red-brown crystals of the product were precipitated within 6 days. The crystals were collected via filtration, washed with Et2O (3 × 2 mL), and dried in a vacuum desiccator over silica gel. The yield was ~ 55% (based on the CoII available). The sample was analyzed satisfactorily as 1·H2O. Anal. Calcd. (%) for C36H38N19O18Cl2Co2Fe: C, 34.07; H, 3.02; N, 20.98. Found (%): C, 33.79; H, 3.06; N, 20.74. ΛM (EtOH, 25 °C, 10−3 M) = 135 S cm2 mol−1. IR (KBr, cm−1): 3467wb, 3330w, 3306wb, 3124wb, 1643s, 1609m, 1564w, 1494m, 1423m, 1384s, 1301w, 1268w, 1183m, 1145w, 1095sh, 1082s, 1027w, 866w, 783m, 751w, 702m, 628m, 500w, 470m, 420s. Selected Raman peaks (cm−1): 1634m, 1599s, 1538m, 1493s, 1421m, 1405sh, 1267w, 1162m, 1095m, 936w, 705w, 642w. UV/VIS (nm, EtOH): 225, 267, 312, 390, ~ 505. 57Fe-Mössbauer (mm s−1, 80 K): δ = 0.53, ΔEQ = 0.79.
Method (b): Solid NH2paoH (0.041g, 0.30 mmol), CoII(ClO4)2·6H2O (0.037g, 0.10 mmol), FeIII(NO3)3·9H2O (0.040g, 0.10 mmol) and Et3N (28 μL, 0.20 mmol) were added to MeOH (10 mL). The resulting dark-red brown solution was stirred for 10 min and stored in a closed flask. Red-brown crystals of the product were precipitated within 2–3 days. When precipitation was judged to be complete, the crystals were collected via filtration, washed with Et2O (3 × 2 mL), and dried in a vacuum desiccator over silica gel. The yield was 49% (based on the CoII available). Th IR and Raman spectra of the powdered crystals were identical to the corresponding spectra of authentic 1·H2O prepared using method (a).
Method (c): To a stirred, almost colorless solution of NH2paoH (0.041g, 0.30 mmol), solid CoII(NO3)2·6H2O (0.029g, 0.10 mmol) and FeII(ClO4)2·6H2O (0.036 g, 0.10 mmol) were added. The resulting yellow solution was stirred for 5 h in an open beaker, during which time the color turned to dark red. The solution was stored in a closed flask. Red-brown crystals of the product were precipitated within 4 weeks. The crystals were collected via filtration, washed with Et2O (4 × 2 mL), and dried in a vacuum desiccator over anhydrous CaCl2. The yield was 36% (based on the CoII available). The sample was analyzed as 1·H2O. Anal. Calcd. (%) for C36H38N19O18Cl2Co2Fe: C, 34.07; H, 3.02; N, 20.98. Found (%): C, 34.23; H, 2.99; N, 20.77. The IR spectrum of the powdered crystals was identical to that of the authentic complex prepared using method (a).
3.3. Single-Crystal X-Ray Crystallography
A brown crystal (0.55 × 0.10 × 0.02 mm) was taken directly from the mother liquor. Crystallographic data were collected with a Brucker APEX II Quasar diffractometer, equipped with a graphite monochromator centered on the path of Mo Kα radiation. The crystal was coated with Cargille TM immersion oil and mounted on a fiber loop, followed by data collection at 120 K. The program SAINT was used to integrate the data, which was thereafter corrected using SADABS [77]. The structure was solved using SHELXT [78] and refined using a full matrix least-squares method on F2 using SHELXL-2019 [79]. The structure was hard to solve, but thanks to the very good quality of the crystal and the dataset, our efforts were successful. It has been rather hard to distinguish Co from Fe with single-crystal X-ray crystallography, but the confidence factors are slightly better if there is Fe in the middle and Co at both ends, thus forming a CoIII…FeIII…CoIII complex. If so, BVS calculations suggest formal III oxidation states. The CoIIIFeIIICoIII formulation is also consistent with the spectroscopic and magnetic data (vide supra). One of the ClO4− ions was found to be disordered over two positions with 0.75:0.25 relatice occupancies. The two parts were refined using EADP constraints. Two NO3− ions were present in the asymmetric unit, with 0.5 occupancies, residing close to hydrogen-bonded H2O molecules (also at 0.5 occupancies). The NO3− ions were refined using SADI, FLAT, SIMU, and DFIX restraints and constraints. All non-H atoms were refined anisotropically. H atoms were assigned to ideal positions and refined isotropically using a riding model, except those of the −NH2 groups, which were introduced from the difference Fourier map and refined using DFIX constraints. The H atoms of the lattice H2O molecules were not introduced, but they are taken into account in the compound formula. The residual electronic density of 2.51 electron/Å3 is located at 0.71 Å from the N20 atom of the lattice (counterion) nitrate and at equidistance of O23 and O25 from the same nitrate, too close to be a relevant missing atom. It likely results from the disorder of the nitrate and H2O molecules, for which the elaboration of a more complex model would be too artificial.
Crystallographic data were submitted to the Cambridge Crystallographic Data Center, No. 2440064. Copies of the data can be obtained free of charge upon application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK. Telephone: +(44)-1223-336033; E-mail: deposit@ccdc.uk, or via https://www.ccdc.cam.ac.uk/structures/.
4. Conclusions and Perspectives
The important message of this work is the confirmation of the ability of NH2paoH to form 3d-3d’ complexes. Compound 1 is the first such heterometallic species containing two trivalent metals, see Table 3. The N2-pyridyl, Noximato, O donor-atom system of NH2pao− is ideal for linking one borderline (HSAB) metal ion and one oxophilic (i.e., hard acid) metal ion (Figure 4). All to-date structurally characterized metal complexes are cationic linear of the general type 3d…3d’…3d. The N6 donor-atom set of three NH2pao− ligands is suitable for octahedral coordination of the borderline 3d-metal ion site, while their deprotonated oxygen atoms are ideal for fac coordination at three corners of the central 3d’-metal ion octahedron. An exception in nuclearity has been observed only in the 3d-4f complex [CoIIIDy(NH2pao)6(NO3)3] [41].
Current efforts in our groups are directed, among others, towards the following: (a) preparation of CuII-M (M = CrIII, MnIII, MnIV, FeIII) complexes based on NH2pao−; and (b) study of the ZnII/MIII/NH2pao− reaction systems (M = Eu, Tb) to isolate compounds with photoluminescence in the visible region of the electromagnetic spectrum.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics13050171/s1, Figure S1: Partially labeled plot of the structure of the second, crystallographically independent, trinuclear cation [CoIII2FeIII(NH2pao)6]3+ of compound 1·5MeOH·1.5H2O; Figure S2: Stick representation of the packing of 1·5MeOH·1.5H2O along the b crystallographic axis; Figure S3: Projection of the crystal structure of 1·5MeOH·1.5H2O along the (ac) plane; Figure S4: The IR spectrum (KBr, cm−1) of free NH2paoH; Figure S5: The IR spectrum (KBr, cm−1) of the sample 1·H2O; Figure S6: The Raman spectrum of the sample 1·H2O in the 1750–190 cm−1 region (excitation wavelength: 632.8 nm); Figure S7: The UV/VIS spectrum (nm) of NH2paoH in EtOH; Figure S8: The 1H NMR spectrum (δ/ppm) of NH2paoH in d6-DMSO.
Author Contributions
S.G.S. and C.D.P. synthesized and proved the purity of the known free ligand NH2paoH, prepared and crystallized the complex and contributed to its IR and UV/VIS characterization. Z.G.L. recorded the Raman spectra of the compounds, interpreted the results, and performed a comprehensive literature search. R.C. recorded the variable-temperature and variable-field magnetic data and interpreted the results. Y.S. recorded the 80 K 57Fe-Mössbauer spectrum, interpreted the data, and wrote the relevant part of the paper. P.D. collected single-crystal crystallographic data, solved the structure, and performed its refinement; he also studied the supramolecular features of the crystal structures and wrote the relevant part of the article. S.P.P. coordinated the research, contributed to the interpretation of the results, and wrote the paper based on the reports of his collaborators. All the authors exchanged opinions concerning the interpretation and study of the results and commented on the various drafts of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Sotiris G. Skiadas was financially supported by the “Andreas Mentzelopoulos Foundation”. R.C. and P.D. thank the University of Bordeaux, the Region Nouvelle Aquitaine, Quantum Matter Bordeaux, the Association Française de Magnétisme Moléculaire, and the Centre National de la Recherche Scientifique (CNRS) for support. Y.S. acknowledges support by the Special Account of NCSR “Demokritos” through the internal program ELKE #12611.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data presented in this study are included in the article and Supplementary Materials, and further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank George A. Voyiatzis (ICE-HT/FORTH), for the access to the Raman Facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, N.; Homann, C.; Morfin, S.; Kesanakurti, M.S.; Calvert, N.D.; Shukendler, A.J.; Al, T.; Hemmer, E. Core-multi-shell design: Unlocking multimodal capabilities in lanthanide-based nanoparticles as upconverting, T2-weighted MRI and CT probes. Nanoscale 2013, 15, 19546–19556. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.-W.; Mostovoy, M. Multiferroics: A magnetic twist for ferroelectricity. Nat. Mater. 2007, 6, 13–20. [Google Scholar] [CrossRef]
- Seo, D.; Somjit, V.; Wi, D.H.; Galli, G.; Choi, K.-S. p-Type BiVO4 for Solar O2 Reduction to H2O2. J. Am. Chem. Soc. 2025, 147, 3261–3273. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhang, W.; Zhu, C.; Guo, W.; He, M.; Zhao, H.; Chen, R. Platinum group metal-based intermetallic compounds: Syntheses and application in electrocatalysis. Coord. Chem. Rev. 2025, 530, 216473. [Google Scholar] [CrossRef]
- Kanatzidis, M.G.; Pöttgen, R.; Jeitschko, W. The Metal Flux: A Preparative Tool for the Exploration of Intermetallic Compounds. Angew. Chem. Int. Ed. 2005, 44, 6996–7023. [Google Scholar] [CrossRef] [PubMed]
- Sagawa, M.; Fujimura, S.; Togawa, N.; Yamamoto, H.; Matsura, Y. New material for permanent magnets on a base of Nd and Fe. J. Appl. Phys. 1984, 55, 2083–2087. [Google Scholar] [CrossRef]
- Mukherjee, S.; Stull, J.A.; Yano, J.; Stamatatos, T.C.; Pringouri, K.; Stich, T.A.; Abboud, K.A.; Britt, R.D.; Yachandra, V.K.; Christou, G. Synthetic model of the asymmetric [Mn3CaO4] subane core of the oxygen-evolving complex of photosystem II. Proc. Natl. Acad. Sci. USA 2012, 109, 2257–2262. [Google Scholar] [CrossRef]
- Kanady, J.S.; Tsui, E.Y.; Day, M.W.; Agapie, T. A Synthetic Model of the Mn3Ca Subsite of the Oxygen-Evolving Complex in Photosystem II. Science 2011, 333, 733–736. [Google Scholar] [CrossRef]
- Blakemore, J.D.; Crabtree, R.H.; Brudvig, G.W. Molecular Catalysts for Water Oxidation. Chem. Rev. 2015, 115, 12794–13005. [Google Scholar] [CrossRef]
- Atwood, J.L.; Lehn, J.M. Comprehensive Supramolecular Chemistry; Pergamon: Oxford, UK, 1996. [Google Scholar]
- Aguilà, D.; Barrios, L.; Belasco, V.; Roubeau, O.; Repollés, A.; Alonso, P.J.; Sesé, J.; Teat, S.J.; Luis, F.; Aromi, G. Heterodimetallic [LnLn’] Lanthanide Complexes: Toward a Chemical Design of Two-Qubit Molecular Spin Quantum Gates. J. Am. Chem. Soc. 2014, 136, 14215–14222. [Google Scholar] [CrossRef]
- Miyasaka, H.; Julve, M.; Yamashita, M.; Clérac, R. Slow Dynamics of the Magnetization in One-Dimensional Coordination Polymers: Single-Chain Magnets. Inorg. Chem. 2009, 48, 3420–3437. [Google Scholar] [CrossRef]
- O’ Keeffe, M.; Yaghi, O.M. Deconstructing the Crystal Structures of Metal-Organic Frameworks and Related Materials into their Underlying Nets. Chem. Rev. 2012, 112, 675–702. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadis, N.C.; Polyzou, C.D.; Kostakis, G.E.; Bekiari, V.; Lan, Y.; Perlepes, S.P.; Konidaris, K.F.; Powell, A.K. Dinuclear lanthanide(III)/zinc(II) complexes with methyl 2-pyridyl ketone oxime. Dalton Trans. 2015, 44, 19791–19795. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Li, Z.-Y.; Yamashita, M.; Bu, X.-H. Recent progress on cyano-bridged transition-metal-based single-molecule magnets and single-chain magnets. Coord. Chem. Rev. 2021, 428, 213617. [Google Scholar] [CrossRef]
- Chorazy, S.; Rams, M.; Nakabayashi, K.; Sieklucka, B.; Ohkoshi, S.-i. White Light Emissive DyIII Single-Molecule Magnets Sensitized by Diamagnetic [CoIII(CN)6]3- Linkers. Chem. Eur. J. 2016, 22, 7371–7375. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Shi, W.; Cheng, P. Toward heterometallic single-molecule magnets: Synthetic strategy, structures and properties of 3d-4f discrete complexes. Coord. Chem. Rev. 2015, 289-290, 74–122. [Google Scholar] [CrossRef]
- Sessoli, R.; Powell, A.K. Strategies towards single-molecule magnets based on lanthanide ions. Coord. Chem. Rev. 2009, 253, 2328–2341. [Google Scholar] [CrossRef]
- Lada, Z.G.; Polyzou, C.D.; Nika, V.; Stamatatos, T.C.; Konidaris, K.F.; Perlepes, S.P. Adventures in the coordination chemistry of 2-pyridyl oximes: On the way to 3d/4f-metal coordination clusters. Inorg. Chim. Acta 2022, 539, 120954. [Google Scholar] [CrossRef]
- Ribas Gispert, J. Coordination Chemistry; Wiley-VCH: Weinheim, Germany, 2008; pp. 205–208. [Google Scholar]
- Pantelis, K.N.; Skiadas, S.G.; Lada, Z.G.; Clérac, R.; Sanakis, Y.; Dechambenoit, P.; Perlepes, S.P. Hexanuclear {ZnII4FeIII2} and {ZnII4CrIII2} complexes from the use of potentially tetradentate NOO′O″ Schiff-based ligands. New J. Chem. 2024, 48, 11221–11232. [Google Scholar] [CrossRef]
- Rosado Piquer, L.; Sañudo, E.C. Heterometallic 3d-4f single-molecule magnets. Dalton Trans. 2015, 44, 8771–8780. [Google Scholar] [CrossRef]
- Wang, H.-S.; Zhang, K.; Song, Y.; Pan, Z.-Q. Recent advances in 3d-4f magnetic complexes with several types of non-carboxylate ligands. Inorg. Chim. Acta 2021, 521, 120318. [Google Scholar] [CrossRef]
- Dey, A.; Acharya, J.; Chandrasekhar, V. Heterometallic 3d-4f Complexes as Single-Molecule Magnets. Chem. Asian J. 2019, 14, 4433–4453. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, P. Homo- and hetero-polymetallic exchange coupled metal-oximates. Coord. Chem. Rev. 2003, 243, 143–190. [Google Scholar] [CrossRef]
- Ross, S.; Weyhermüller, T.; Bill, E.; Wieghardt, K.; Chaudhuri, P. Tris(pyridinealdoximato)metal Complexes as Ligands for the Synthesis of Asymmetric Heterodinuclear CrIIIM Species [M = Zn(II), Cu(II), Ni(II), Fe(II), Mn(II), Cr(II), Co(III)]: A Magneto-Structural Study. Inorg. Chem. 2001, 40, 6656–6665. [Google Scholar] [CrossRef] [PubMed]
- Sahyoun, T.; Arrault, A.; Schneider, R. Amidoximes and Oximes: Synthesis, Structure, and their Key Role as NO Donors. Molecules 2019, 24, 2470. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Sinha, A.S.; Epa, K.N.; Chopade, P.D.; Smith, M.M.; Desper, J. Structural Chemistry of Oximes. Cryst. Growth Des. 2013, 13, 2687–2695. [Google Scholar] [CrossRef]
- Novikov, A.S.; Bolotin, D.S. Tautomerism of Amidoxime and Other Oxime Species. J. Phys. Org. Chem. 2018, 31, e3772. [Google Scholar] [CrossRef]
- Tavakol, H.; Arshadi, S. Theoretical Investigation of N-Hydroxy Amidines. J. Mol. Model. 2009, 15, 807–816. [Google Scholar] [CrossRef]
- Abney, C.W.; Mayers, R.T.; Saito, T.; Dai, S. Materials for the Recovery of Uranium Seawater. Chem. Rev. 2017, 117, 13935–14013. [Google Scholar] [CrossRef]
- Abney, C.W.; Liu, S.; Lin, W. Tuning Amidoximate to Enhance Uranyl Binding: A Density Functional Theory. J. Phys. Chem. A 2013, 117, 11558–11565. [Google Scholar] [CrossRef]
- Vukovich, S.; Hay, B.P. De Novo Structure-Based Design of Bis-amidoxime Uranophiles. Inorg. Chem. 2013, 52, 7805–7810. [Google Scholar] [CrossRef]
- Wei, Y.; Salih, K.A.M.; Lu, S.; Hamza, M.F.; Fujita, T.; Vincent, T.; Guibal, E. Amidoxime Functionalization of Algal/Polyethyleneimine Beads for the Sorption of Sr(II) from Aqueous Solutions. Molecules 2019, 24, 3893. [Google Scholar] [CrossRef]
- Kelley, S.P.; Rogers, R.D. Lanthanide complexes with zwitterionic amidoximes stabilized by noncoordinating water molecules. Supramol. Chem. 2018, 30, 411–417. [Google Scholar] [CrossRef]
- Bolotin, D.S.; Bokach, N.A.; Kukushkin, V.Y. Coordination chemistry and metal-involving reactions of amidoximes: Relevance to the chemistry of oximes and oxime ligands. Coord. Chem. Rev. 2016, 313, 62–93. [Google Scholar] [CrossRef]
- Efthymiou, C.G.; Cunha-Silva, L.; Perlepes, S.P.; Brechin, E.K.; Inglis, R.; Evangelisti, M.; Papatriantafyllopoulou, C. In search of molecules displaying ferromagnetic exchange; multiple-decker Ni12 and Ni16 complexes from the use of pyridine-2-amidoxime. Dalton Trans. 2016, 45, 17409–17419. [Google Scholar] [CrossRef]
- Ran, J.-W.; Tong, Y.-P. Crystal Structures, Magnetic Properties, and Theoretical Investigations of three Linear Cluster Complexes with Pyridine-2-Amidoxime. J. Struct. Chem. 2018, 59, 1433–1439. [Google Scholar] [CrossRef]
- An, G.-Y.; Yuan, B.; Tao, J.; Cui, A.-L.; Kou, H.-Z. Synthesis, structure and magnetic properties of trinuclear transition metal complexes based on pyridine-2-amidoxime. Inorg. Chim. Acta 2012, 387, 401–406. [Google Scholar] [CrossRef]
- Ran, J.; Li, X.; Zhao, Q.; Hou, Y.; Tang, W.; Chen, G. High-efficient photooxidative degradation of dyes catalyzed by heteronuclear complex under light irradiation. Inorg. Chem. Commun. 2010, 13, 1527–1529. [Google Scholar] [CrossRef]
- Polyzou, C.D.; Koumousi, E.S.; Lada, Z.G.; Raptopoulou, C.P.; Psycharis, V.; Rouzières, M.; Tsipis, A.C.; Mathonière, C.; Clèrac, R.; Perlepes, S.P. “Switching on” the single-molecule magnet properties within a series of dinuclear cobalt(III)-dysprosium(III) 2-pyridyloximate complexes. Dalton Trans. 2017, 46, 14812–14825. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry, 5th ed.; Pearson: Harlow, UK, 2018; p. 415. [Google Scholar]
- Hou, Y.H.; Zhao, Y.J.; Liu, Z.W.; Yu, H.Y.; Zhong, X.C.; Qiu, W.Q.; Zeng, D.C.; Wen, L.S. Structural, electronic and magnetic properties of partially inverse spinel CoFe2O4: A first principles study. J. Phys. D Appl. Phys. 2010, 43, 445003. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, J.; Zhu, S.; Deng, X.; Ma, H.; Zhang, J.; Zhang, Q.; Li, P.; Xue, D.; Mellors, N.J.; et al. Direct observation of cation distributions of ideal inverse spinel CoFe2O4 nanofibres and correlated magnetic properties. Nanoscale 2017, 9, 7493–7500. [Google Scholar] [CrossRef] [PubMed]
- Phu, P.N.; Lee, J.L.; Biswas, S.; Ziller, J.W.; Bominaar, E.L.; Hendrich, M.P.; Borovic, A.S. Proton-Induced Switching of Paramagnetism: Reversible Conversion between a Low and High Spin CoIII Center within a Heterobimetallic Core. J. Am. Chem. Soc. 2025, 147, 3129–3139. [Google Scholar] [CrossRef]
- Aguilà, D.; Prado, Y.; Koumousi, E.S.; Mathonière, C.; Clèrac, R. Switchable Fe/Co Prussian blue networks and molecular analogues. Chem. Soc. Rev. 2016, 45, 203–224. [Google Scholar] [CrossRef] [PubMed]
- Dolai, M.; Saha, U.; Kumar, G.S.; Ali, M. Amidoxime-Based Mononuclear Mn(II) complexes: Synthesis, Characterization, and Studies on DNA Binding and Nuclease Activity. ChemistrySelect 2018, 3, 6935–6941. [Google Scholar] [CrossRef]
- Ran, J.; Tong, Y.-P. Synthesis, structures, photoluminescence, and theoretical investigations on two new Zn(II)/Mn(II) complexes with pyridine-2-amidoxime and carboxylate ligands. Struct. Chem. 2011, 22, 1113–1118. [Google Scholar] [CrossRef]
- Mylonas-Margaritis, I.; Winterlich, M.; Efthymiou, C.G.; Lazarides, T.; McArdle, P.; Papatriantafyllopoulou, C. New insights into oximic ligands: Synthesis and characterization of 1D chains by the use of pyridine-2-amidoxime and polycarboxylates. Polyhedron 2018, 151, 360–368. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Chen, Y.-M.; Gao, Q.; Liu, W.; Li, Y.-W. Zn(II), Ni(II), and Mn(II) Complexes Supported by N-Hydroxy-pyridine-2-carboxamidine: Syntheses, Structures, and Luminescent and Magnetic Properties. Chin. J. Struct. Chem. 2014, 33, 1171–1183. [Google Scholar]
- Pearse, G.A.; Raithby, P.R.; Hay, C.M.; Lewis, J. Synthesis and X-ray Crystal Structure of two Complexes of Nickel(II) Nitrate with Pyridine-2-amidoxime (C6H7N3O): [Ni(C6H7N3O)2(NO3)2] and [Ni(C6H7N3O)3](NO3)2·H2O. Polyhedron 1989, 8, 305–310. [Google Scholar] [CrossRef]
- Werner, M.; Berner, J.; Jones, P.G. Bis(acetate)bis(pyridine-2-amidoxime-N,N′)nickel(II)-Ethanol (1/2). Acta Crystallogr. Sect. C 1996, 52, 72–74. [Google Scholar] [CrossRef]
- Näsäkkälä, M.; Saarinen, H.; Korvenranta, J.; Orama, M. Aquabis(pyridine-2-carboxamide oxime)copper(II) Chloride. Acta Crystallogr. Sect. C 1989, 45, 1514–1517. [Google Scholar] [CrossRef]
- Pearse, G.A.; Raithby, P.R.; Lewis, J. Synthesis and X-ray Crystal Structure of Pyridine-2-amidoxime, C6H7N3O, and Aqua-bis(pyridine-2-amidoxime)copper(II) chloride, [Cu(C6H7N3O)2(H2O)]Cl2. Polyhedron 1989, 8, 301–304. [Google Scholar] [CrossRef]
- Konidaris, K.F.; Bekiari, V.; Katsoulakou, E.; Raptopoulou, C.P.; Psycharis, V.; Perlepes, S.P.; Stamatatos, T.C.; Manessi-Zoupa, E. Initial employment of pyridine-2-amidoxime in zinc(II) chemistry: Synthetic, structural and spectroscopic studies of mononuclear and dinuclear complexes. Inorg. Chim. Acta 2011, 376, 470–478. [Google Scholar] [CrossRef]
- Liu, J. Crystal structure of bis(acetate-κO)bis(pyridine-2-carboxamide oxime-κ2N,N′)cadmium ethanol disolvate. Acta Crystallogr. Sect. E 2014, 70, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Danelli, P.; Lada, Z.G.; Raptopoulou, C.P.; Psycharis, V.; Stamatatos, T.C.; Perlepes, S.P. Doubly Thiocyanato (S,N)-Bridged Dinuclear Complexes of Mercury(II) from the Use of 2-pyridyl Oximes as Capping Ligands. Curr. Inorg. Chem. 2015, 5, 26–37. [Google Scholar] [CrossRef]
- Costa, R.; Barone, N.; Gorczycka, C.; Powers, E.F.; Cupelo, W.; Lopez, J.; Herrick, R.S.; Ziegler, C.J. Dioxime and pyridine-2-aldoxime complexes of Re(CO)3+. J. Organomet. Chem. 2009, 694, 2163–2170. [Google Scholar] [CrossRef]
- Palepu, N.R.; Adhikari, S.; Premkumar, R.; Verma, A.K.; Shepherd, S.L.; Phillips, R.M.; Kaminsky, W.; Kollipara, M.R. Half-sandwich ruthenium, rhodium and iridium complexes featuring oxime ligands: Structural studies and preliminary investigation of in vitro and in vivo anti-tumour activities. Appl. Organomet. Chem. 2017, 31, 3640. [Google Scholar] [CrossRef]
- Cui, A.-L.; Han, P.; Yang, H.-J.; Wang, R.-J.; Kou, H.-Z. Poly[μ2-(N-hydroxypyridine-2-carboxamide)-μ2-nitrato-silver(I)]. Acta Crystallogr. Sect. C 2007, 63, m560–m562. [Google Scholar] [CrossRef]
- Nikolaou, H.; Terzis, A.; Raptopoulou, C.P.; Psycharis, V.; Bekiari, V.; Perlepes, S.P. Unique Dinuclear, Tetrakis(nitrato-O,O’)-Bridged Lanthanide(III) Complexes from the Use of Pyridine-2-Amidoxime: Synthesis, Structural Studies and Spectroscopic Characterization. J. Surf. Interfac. Mater. 2014, 2, 311–318. [Google Scholar] [CrossRef]
- Deng, X.-H.; Ran, J.-W. [Bis(μ2-pyridine-2-carboxamide oximato)-bis[pyridine-2-carboxamide oxime)zinc]dinitrate. Acta Crystallogr. Sect. E 2011, 67, m1323–m1324. [Google Scholar] [CrossRef]
- Tomsa, A.-R.; Li, Y.; Blanchard, S.; Herson, P.; Boubekeur, K.; Gouzerh, P.; Proust, A. Oxo-Centered Trinuclear Chromium(III) Complexes with both Carboxylate and Amidoximate Ligands. J. Clust. Sci. 2014, 25, 825–838. [Google Scholar] [CrossRef]
- An, G.-Y.; Wang, H.-B.; Cui, A.-L.; Kou, H.-Z. Ferromagnetic versus antiferromagnetic exchange in oximato-bridged nickel(II) complexes. New J. Chem. 2014, 38, 5037–5042. [Google Scholar] [CrossRef]
- Jiang, X.; An, G.-Y.; Liu, C.-M.; Kou, H.-Z. Mixed-Valent {Mn14} Single-Molecule Magnet Based on Pyridine-2-amidoxime. Eur. J. Inorg. Chem. 2015, 5314–5317. [Google Scholar] [CrossRef]
- Ji, C.-M.; Yang, H.-J.; Zhao, C.-C.; Tangoulis, V.; Cui, A.-L.; Kou, H.-Z. Self-Assembly of Multidecker NiII Clusters from Preformed Ni4 Decks. Cryst. Growth Des. 2009, 9, 4606–4609. [Google Scholar] [CrossRef]
- Deng, X.-H.; Ran, J.-W. Bis[μ3-Ν′-oxidopyridine-2-carboximidamidato(2-)]bis[μ2-Ν′-oxidopyridine-2-carboximidamidato (1-)]tetrapyridinetetranickel(II) dinitrate. Acta Crystallogr. Sect. E 2012, 68, m40. [Google Scholar] [CrossRef]
- Kou, H.-Z.; An, G.-Y.; Ji, C.-M.; Wang, B.-W.; Cui, A.-L. Ferromagnetic coupling in oximato-bridged multi-decker NiII clusters. Dalton Trans. 2010, 39, 9604–9610. [Google Scholar] [CrossRef]
- Dimakopoulou, F.; Efthymiou, C.; O’Malley, C.; Kourtellaris, A.; Moushi, E.; Tasiopoulos, A.; Perlepes, S.P.; McArdle, P.; Costa-Villén, E.; Mayans, J.; et al. Novel Co5 and Ni4 Metal Complexes and Ferromagnets by the Combination of 2-Pyridyl Oximes with Polycarboxylic Ligands. Molecules 2022, 47, 4701. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; Wiley: New York, NY, USA, 1986; pp. 121–124, 130–138. [Google Scholar]
- Dollish, F.R.; Fateley, W.G.; Bentley, F.F. Characteristic Raman Frequencies of Organic Compounds; Wiley: New York, NY, USA, 1974; pp. 135–137. [Google Scholar]
- Geary, W.J. The Use of Conductivity Measurements in Organic Solvents for the Characterization of Coordination Compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Rao, C.N.R. Ultra-Violet and Visible Spectroscopy, 2nd ed.; Butteworths: London, UK, 1967; pp. 20–33, 58–73. [Google Scholar]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1984; pp. 329–332, 452, 453, 457–458, 473–478. [Google Scholar]
- Greenwood, N.N.; Gibbs, T.C. Mössbauer Spectroscopy; Chapman and Hall: London, UK, 1971; pp. 1–660. [Google Scholar]
- Bernasek, E. Pyridineamidoximes. J. Org. Chem. 1957, 22, 1263. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, ver. 2.03; Brucker Analytical X-Ray Systems: Madison, WI, USA, 2000.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).