Abstract
The esterification of terephthalic acid (PTA) with methanol to dimethyl terephthalate (DMT) was investigated using commercially available zeolite catalysts as the eco-friendly solid acids. Six typical zeolites (ZSM-5-25, ZSM-5-50, ZSM-5-100, ZSM-35, MOR, and β) were systematically evaluated. Among them, β zeolite showed excellent catalytic performance, achieving nearly 100% PTA conversion and 76.1% DMT selectivity under the conditions of 200 °C, of 0.5 MPa N2 pressure, m(PTA):V(methanol) of 1:40 (g/mL), m(PTA):m(catalyst) of 10:1 over 4 h. The characterization results show that the catalytic efficiency was correlated with acid site strength, specific surface area, and mesoporous structure of the zeolite. After optimization, β zeolite achieved 100% PTA conversion and 94.1% DMT selectivity under the conditions of 200 °C, of 1 MPa N2 pressure, m(PTA)/V(methanol) of 1:30 (g/mL), m(PTA)/m(catalyst) of 8:1 over 8 h. Moreover, β zeolite exhibited superior stability, maintaining over 92% of its initial activity after five cycles, highlighting its potential for sustainable DMT production.
1. Introduction
Dimethyl terephthalate (DMT) is an essential chemical intermediate and organic raw material widely utilized in various industries. Its versatile applications include the production of plastics, polyester fibers, and coatings. Especially in coatings, DMT acts as a plasticizer, significantly enhancing flexibility, weather resistance, and substrate adhesion [1]. Additionally, DMT serves as a crucial precursor for polyester polymers such as polyethylene terephthalate (PET) and polybutylene terephthalate (PBT), as well as in the synthesis of specialty chemicals.
Industrially, DMT is primarily produced via the esterification of terephthalic acid (PTA) with methanol, a key step in synthesizing PET polymers and dimethyl cyclohexane dicarboxylate (DMCD). The esterification reaction is a fundamental organic process and holds significant industrial importance, particularly in the polymer industry. Conventionally, the esterification process utilizes homogeneous acid catalysts, such as sulfuric acid or metal salts like AlCl3 and ZnCl3, due to their high catalytic efficiency [2]. Nevertheless, homogeneous catalysts present several limitations, including high corrosivity, extensive acid consumption, severe environmental impact, numerous side reactions, and complex post-treatment purification procedures [3]. Consequently, the development of alternative, environmentally friendly catalytic systems that can overcome these drawbacks has become imperative [4]. Solid acid catalysts have emerged as promising alternatives due to their ease of separation from reaction mixtures, recyclability, reduced corrosiveness, and minimal environmental impact [5,6]. The use of solid catalysts not only improves the esterification reaction efficiency and selectivity but also significantly reduces both production costs and environmental harm [7,8,9].
Among the promising solid acid catalysts, zeolites exhibit exceptional performance due to their robust thermal stability, unique molecular sieving capabilities, tunable pore structures, and strong surface acidity [10,11,12,13]. These properties enable zeolites to function effectively in high-temperature and high-pressure environments, ensuring catalyst stability and prolonging catalyst life. The regular pore structure and high surface area facilitate the selective adsorption of reactants and products, improving the reaction efficiency and product selectivity [14,15,16]. The presence of robust acidic sites and strong Coulombic fields within zeolite frameworks further enhances their catalytic activity. Additionally, the amount and strength of zeolite active sites can be adjusted to meet the catalytic requirements of different esterification reactions, thereby enabling precise control over catalytic activity and selectivity [17,18]. Compared to homogeneous catalysts, zeolites also exhibit the advantages of separation, regeneration, and reuse, significantly reducing the environmental impacts associated with traditional catalytic methods [19,20,21,22].
Recent studies have demonstrated that zeolite properties, such as crystal structure and surface acidity, can be modulated effectively through modification methods, enhancing their catalytic performance [23]. Fan et al. [24] reported that adjusting crystallization times effectively modified the acidity and structure of β/ZSM-5 composite zeolites, improving catalytic activity in rearrangement reactions. Tong et al. [25] demonstrated that hydrothermal-citric acid washing could modify zeolites, effectively reducing framework aluminum, thereby adjusting acidity and improving both selectivity and stability. Similarly, Kang et al. [26] emphasized the significance of controlling Brønsted and Lewis acidic site concentrations within ZSM-5 zeolites by varying their Si/Al ratios, correlating these parameters directly with catalytic activity in depolymerization reactions.
In this work, we systematically evaluated the catalytic performance of six commercial zeolites (ZSM-5-25, ZSM-5-50, ZSM-5-100, ZSM-35, MOR, and β) for the esterification of PTA with methanol to produce DMT. The comprehensive characterization of the catalysts’ structural and acidic properties was performed using X-ray diffraction (XRD), scanning electron microscopy (SEM), ammonia temperature-programmed desorption (NH3-TPD), and N2 physisorption methods. The results indicate that the pore structure of the zeolites plays a crucial role in the diffusion of reactants and the desorption of products, while the strength and density of the acid sites directly influence the activity and selectivity of the esterification reaction. The findings provide some valuable insights into optimizing zeolite-based catalytic systems, presenting β zeolite as an exceptionally promising candidate for the sustainable and efficient industrial production of DMT.
2. Results and Discussion
2.1. Physiochemical Characterization of the Zeolite Catalysts
Figure 1 presents the XRD patterns of the zeolite catalysts, which were recorded in the range of 2θ = 5~80°. It is evident that the ZSM-5-25, ZSM-5-50 and ZSM-5-100 zeolite catalysts exhibit the characteristic diffraction peaks corresponding to the ZSM-5 series within the 2θ ranges of 8–10° and 22–25°, which aligns with the typical MFI crystal structure [27]. The ZSM-35 zeolite catalyst displays distinctive diffraction peaks at 2θ = 9.2°, 25.2°, and 25.7° [28], which is in good agreement with the characteristic of the typical ZSM-35 zeolite. The MOR zeolite catalyst reveals characteristic diffraction peaks associated with the MOR structure at 2θ of 9.8°, 19.6°, 22.3°, 25.7°, and 26.3° [29]. The β zeolite catalyst is characterized by a series of BEA topology diffraction peaks observed at 2θ = 7.7°, 16.5°, 21.4°, 25.3°, 26.8°, 29.5°, and 30.5° [30]. Notably, no impurity peaks are present in the XRD patterns of the six zeolites, indicating that the commercially obtained samples are devoid of extraneous impurities and their crystalline structures are consistent with the available literature [31].
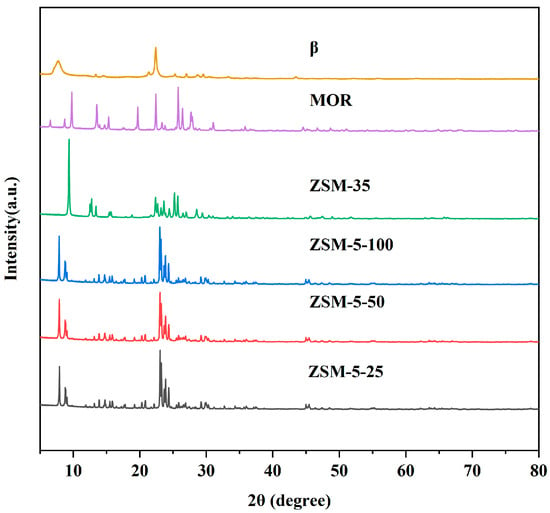
Figure 1.
XRD patterns of the zeolite catalysts.
Figure 2 illustrates a uniform sample distribution and regular morphology. The ZSM-35 zeolite catalyst crystals exhibit a relatively regular arrangement in flake-like aggregates, with well-defined layered and needle-like structures, and numerous fine fragments are observed on the surfaces of these flakes. The dimensions of the ZSM-35 zeolite crystals are approximately 2–4 μm in length and 2 μm in width for the flake-like crystals [32]. The MOR zeolite catalyst crystals display a morphology of small, elongated hexagonal prisms measuring around 10 μm, with evidence of aggregation [33]. The β zeolite exhibits a regular distribution of uniform spherical microcrystals, possessing substantial porosity and surface roughness, and the grain size is approximately in the range of 0.4–0.5 μm [12].
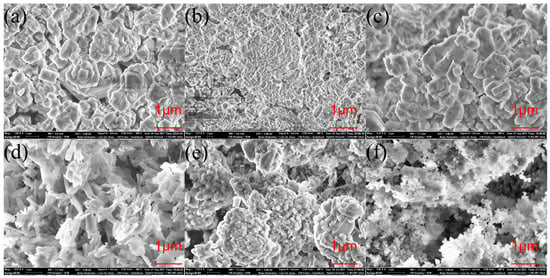
Figure 2.
SEM images of the employed zeolite catalysts: (a) ZSM-5-25, (b) ZSM-5-50, (c) ZSM-5-100, (d) ZSM-35, (e) MOR, and (f) β.
NH3-TPD was conducted to elucidate the acid strength of six zeolites, as shown in Figure 3. The NH3-TPD profiles reveal that ZSM-5 zeolites with Si/Al ratios of 25, 50, and 100 exhibit pronounced desorption peaks at approximately 230 °C and 420 °C. These peaks correspond to the desorption of ammonia from weak and strong acid sites, respectively, indicating the presence of weak and strong acid centers within the zeolite framework [13]. The intensities and areas under the peaks for both weak and strong acid sites differ between ZSM-5 zeolites with varying Si/Al ratios, suggesting that the Si/Al ratio significantly influences the acid strength and quantity of acid sites present in the zeolites. Notably, zeolites with a lower Si/Al ratio demonstrate enhanced acid strength and a higher density of acid sites [10,11,14,15]. The acid amount of the catalysts is listed in Table 1. Among the six catalysts, ZSM-5 exhibits the highest acidity, which is consistent with the NH3-TPD results.
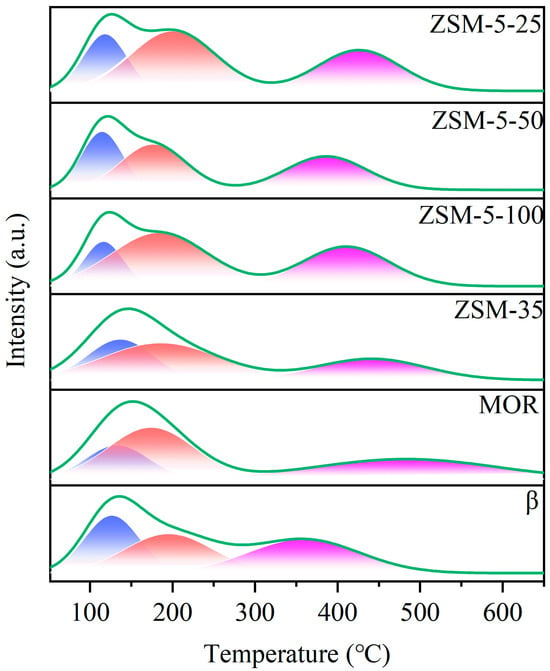
Figure 3.
NH3-TPD profiles of the employed zeolite catalysts.

Table 1.
NH3-TPD characterization results of acid amount of zeolite catalysts.
The remaining ZSM-35, MOR, and β zeolites each exhibit two desorption peaks in both the low-temperature and high-temperature regions, which are attributed to the weak acid centers and strong acid centers, respectively. It is likely that the relatively weak hydrogen bond breakage between the adsorbed NH3 and the surface acidic groups results in an additional desorption peak around 120 °C for all catalysts [16,34,35]. A comparison of the six zeolites indicates that the order of acid strength is as follows: ZSM-5-25 > ZSM-5-50 > ZSM-5-100 > ZSM-35 > β > MOR. This correlation is positively associated with the catalytic performance of the zeolites in PTA esterification, indicating that the acid strength and the number of acidic sites on the zeolites are the significant factors affecting the catalytic activity [17].
Based on the N2 physical adsorption data from Table 2 and the pore size distribution diagram of the zeolites in Figure 4, it can be noted that the zeolites possess a microporous nanostructure. The N2 adsorption–desorption isotherms for the six types of zeolites are shown in Figure 4. According to the classification rules of IUPAC, the adsorption and desorption curves of the six types of zeolites shown in Figure 4 are typical Type I isotherms, with distinct H1-type hysteresis loops, indicating that the materials have a microporous structure. This is also consistent with the N2 physisorption data and the pore size distributions.

Table 2.
Physical properties of zeolite catalysts.
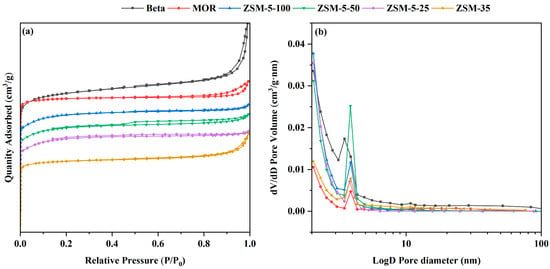
Figure 4.
(a) N2 adsorption–desorption isotherms and (b) pore size distributions of the employed zeolites.
Table 2 details the physical parameters of all samples, including the specific surface area (SBET), micropore specific surface area (Smicro), mesopore specific surface area (Smeso), pore diameter (Dp), total pore volume (Vp), micropore volume (Vmicro), and mesopore volume (Vmeso) for six types of zeolites. The structural characteristic of the ZSM-5 series zeolites are their crystalline silicoaluminate structure composed of 10-membered ring channels [36]. According to Table 2, the ZSM-5-25 zeolite has a pore diameter of 2.03 nm and a specific surface area of 347 m2/g. The ZSM-5-50 zeolite has a pore diameter of 2.15 nm and a specific surface area of 361 m2/g. The ZSM-5-100 catalyst has a pore diameter of 2.05 nm and a specific surface area of 334 m2/g. This indicates that the silica-to-alumina molar ratio in the ZSM-5 series zeolites affects their structure [30,37,38]. As the Si/Al ratio increases, the pore diameter of the zeolites first increases and then decreases, while the specific surface area first increases and then decreases [39,40,41]. The morphology changes of different ZSM-5 samples are mainly related to their synthesis conditions and Si/Al ratios. As the Si/Al ratio varies, the crystal growth rate and the exposure of crystal facets differ, leading to noticeable differences in crystal morphology. For example, a lower Si/Al ratio usually involves more aluminum atoms participating in the crystal structure, promoting more regular crystal growth, while a higher Si/Al ratio may cause uneven crystal growth and more diverse morphologies. In addition, factors such as synthesis temperature, time, and template concentration also influence the changes in crystal morphology. The combined effect of these factors results in different morphology dependencies observed in the ZSM-5 samples. The ZSM-35 zeolite features a two-dimensional pore channel system composed of perpendicularly intersecting 8-membered and 10-membered rings [42]. It has a pore diameter of 2.33 nm and a specific surface area of 303 m2/g. The MOR zeolite has a layered structure made up of multiple 8-membered and 12-membered rings, with a pore diameter of 2.17 nm and a specific surface area of 423 m2/g. The β zeolite possesses a unique three-dimensional structure with 12-membered ring intersecting channels, composed of two closely related polymorphs that share the same central symmetric layer arrangement structural units [43]. It has a pore diameter of 2.36 nm and a specific surface area of 556 m2/g. A larger specific surface area and pore diameter are more conducive to the surface adsorption of various reaction components, thereby enhancing esterification reactions and improving catalytic activity [44]. The ranking order of the specific surface area (SBET) and total pore volume (Vp) for the six zeolites is: β > MOR > ZSM-5-50 > ZSM-5-25 > ZSM-5-100 > ZSM-35.
2.2. Catalytic Activity for Terephthalic Acid Esterification
From Figure 5, it can be observed that among the six zeolites catalysts, the β zeolite catalyst exhibits the best performance, with a conversion rate of PTA approaching 100% and a selectivity of 76.1%. In contrast, the ZSM-35 zeolite catalyst performs the worst, with a PTA conversion rate of only 45.1% and a selectivity of just 24.7%. This may be related to the differences in their zeolites pore structures. The β zeolite possesses three intersecting 12-membered ring channels, while ZSM-35 zeolite belongs to the FER-type topological framework structure, featuring a two-dimensional pore system with perpendicular intersections of 8-membered and 10-membered rings. This structure is more conducive to the diffusion of product molecules. Due to the size limitation of the 12-membered ring pores in β zeolite, it exhibits strong shape selectivity, is less prone to carbon deposition within the pores, which helps maintain catalytic activity, and has an ideal acid distribution, thus showing better performance in catalyzing esterification reactions. Furthermore, analysis of the gas chromatography results suggests that the poorer catalytic performance and lower selectivity of ZSM-35 zeolite may be due to the production of more by-products during the reaction process The main by-product is monomethyl terephthalate (MMT), which is an intermediate product of the reaction with a lower degree of esterification. [21,36,45]. Therefore, β zeolite is selected as the catalyst for the esterification reaction of PTA.
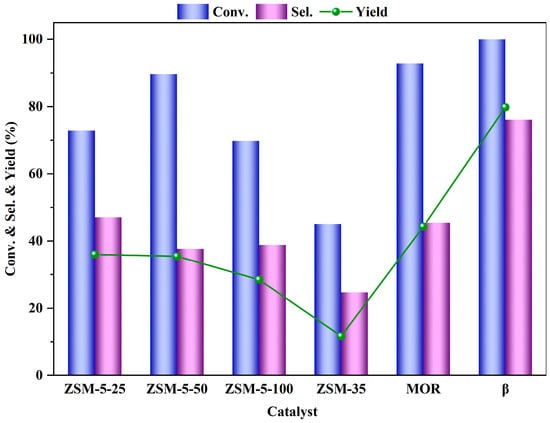
Figure 5.
Dependence of the PTA esterification performance over the employed zeolites (reaction conditions: m (PTA) = 1 g, m (Cat.) = 0.1 g, V (methanol) = 40 mL, t = 4 h, 200 °C, 0.5 MPa N2).
The effect of the reaction temperature (160 to 240 °C) on the esterification reaction of PTA is shown in Figure 6. As the reaction temperature increases, the conversion rate of PTA also increases, and when the temperature reaches 180 °C, the conversion of PTA is essentially complete, with a conversion rate approaching 100%. The yield and selectivity of DMT first increase and then decrease, reaching a peak at 200 °C, with a selectivity of 76.0%, and then decrease as the reaction temperature increases further. The esterification of PTA to prepare DMT is an endothermic reaction. From the perspective of chemical equilibrium, an increase in temperature favors an increase in the diffusion rate of reactant molecules, which enhances the diffusion and mass transfer of reactants on the catalyst surface, accelerating the reaction rate and thus increasing the conversion rate of PTA. Consequently, the yield and selectivity of DMT also improve.
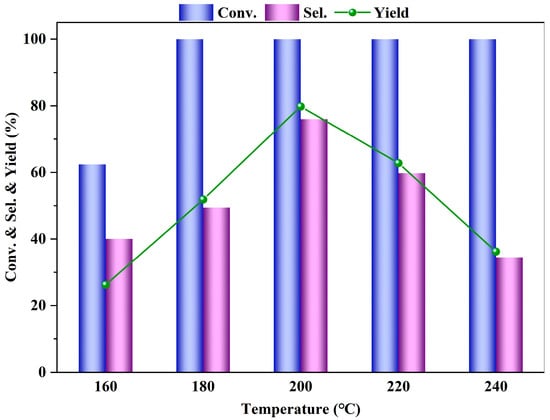
Figure 6.
Influence of reaction temperature on the PTA esterification performance (reaction conditions: m (PTA) = 1 g, m(β) = 0.1 g, V (methanol) = 40 mL, t = 4 h, 0.5 MPa N2).
However, when the reaction temperature exceeds 200 °C, the yield and selectivity of DMT begin to decline. This is because at high temperatures, reactants desorb from the catalyst surface, preventing the catalyst from functioning properly and slowing down the reaction rate. Additionally, excessively high temperatures can lead to the reverse reaction of esterification, resulting in a decrease in DMT yield. Therefore, reaction temperatures that are too low or too high are not conducive to the esterification reaction of PTA and can affect the selectivity of the target product. In summary, the most suitable reaction temperature for the esterification reaction of PTA is 200 °C.
The influence of the reaction pressure on the esterification reaction of PTA, particularly on the conversion of PTA and the selectivity of DMT, is significant. As shown in Figure 7, the effect of the reaction pressure on the esterification reaction of PTA is such that an increase in pressure leads to a rapid increase in the yield and selectivity of DMT. When the nitrogen pressure increases from 0.5 MPa to 1 MPa, the yield and selectivity of DMT reach their peak values. However, with further increases in pressure, both the yield and selectivity of DMT begin to decline. Esterification is a reversible reaction, and within a certain range, elevating the reaction pressure can shift the equilibrium towards the desired direction and prevent the catalyst from deactivating. However, when the pressure becomes excessively high, it may promote the rapid transformation of side products, leading to a decrease in DMT yield and selectivity. Therefore, the most suitable reaction pressure for the esterification of PTA is determined to be 1 MPa.
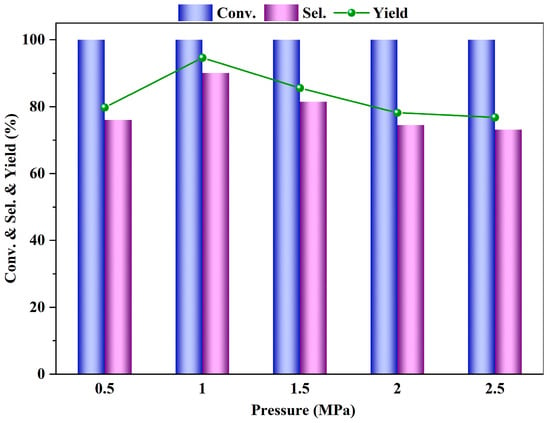
Figure 7.
Influence of reaction pressure on the PTA esterification performance (reaction conditions: m (PTA) = 1 g, m (β) = 0.1 g, V (methanol) = 40 mL, t = 4 h, 200 °C).
Figure 8 illustrates the impact of the reaction time on the esterification reaction of PTA. Under appropriate fixed conditions, the conversion rate of DMT is 100% at various reaction times. However, as the reaction time increases, the yield and selectivity of DMT reach their peak at 8 h, after which they decline with further prolongation of the reaction time. This implies that an excessively long reaction time may lead to further side reactions and the formation of other by-products from DMT. In summary, the reaction time affects the yield of the target product. If the reaction time is too short, the raw materials will not be completely converted; if the reaction time is too long, it will result in an increase in by-products and a decrease in the selectivity of the target product. Therefore, selecting the appropriate reaction time is crucial, and choosing a reaction time of 8 h for the esterification reaction of PTA is most suitable.
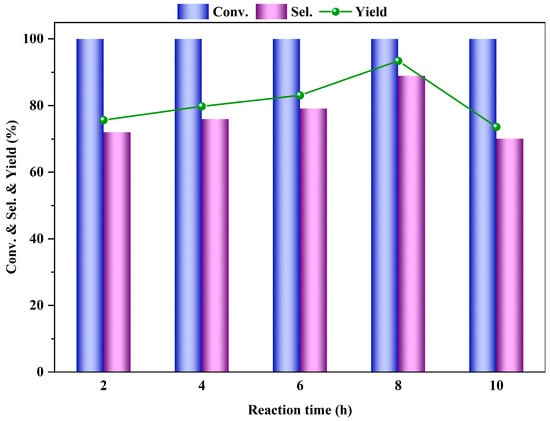
Figure 8.
Influence of reaction time on PTA esterification performance (reaction conditions: m (PTA) = 1 g, m (β) = 0.1 g, V (methanol) = 30 mL, 200 °C, 0.5 MPa N2).
The experiment explored the impact of different ratios of PTA to methanol on the esterification reaction of PTA. The experiment compared the PTA to methanol ratios (g/mL) of 1:20, 1:30, 1:40, 1:50, and 1:60 to observe their effects on the reaction outcomes. From Figure 9, it can be seen that when the PTA to methanol ratio is 1:30 (g/mL), the conversion rate of PTA, and the yield and selectivity of DMT are the best. The esterification reaction between PTA and methanol is a reversible reaction. Increasing the proportion of methanol can promote the equilibrium point of the reaction to move in the forward direction. However, when the proportion of methanol is too high, the yield and selectivity of DMT decrease. This is because an excess of methanol dilutes the concentration of the catalyst in the reaction system, reducing the effective contact between the reactants and the active sites.
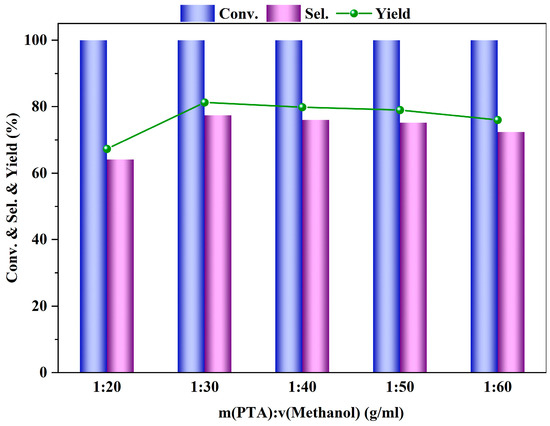
Figure 9.
Influence of m (PTA): V (methanol) on PTA esterification performance (reaction conditions: m (β) = 0.1 g, V (methanol) = 40 mL, t = 4 h, 200 °C, 0.5 MPa N2).
Figure 10 illustrates the impact of the amount of catalyst on the esterification reaction of PTA. The experiment compared the effects on the reaction outcomes when the mass ratio of PTA to catalyst was 1:1, 2:1, 4:1, 8:1, and 10:1. From Figure 10, it can be observed that when the mass ratio of PTA to catalyst is 8:1, the conversion rate of PTA, as well as the yield and selectivity of DMT, are at their best. To further investigate, the stability of the catalyst was also explored.
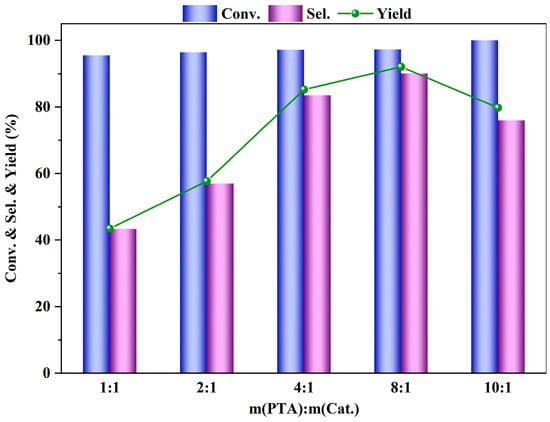
Figure 10.
Influence of m (PTA): m (catalyst) on PTA esterification performance (reaction conditions: m (PTA) = 1 g, V (methanol) = 40 mL, t = 4 h, 200 °C, 0.5 MPa N2).
2.3. Stability of the β Zeolite Catalyst
The stability of the catalyst is indeed a complex multi-dimensional concept, which encompasses not only the ability of the catalyst to maintain its original activity, selectivity, resistance to poisoning, and thermal stability during the chemical reaction process, but also its capacity to retain these characteristics over extended periods of use. This stability is crucial for the long-term effective operation of the catalyst and for maintaining the expected catalytic effects. Particularly in the esterification reaction of purified PTA with methanol, the microporous structure of zeolite catalysts is particularly susceptible to blockage, leading to the loss of active components and a significant decrease in catalytic efficiency. Therefore, in-depth research into the stability of catalysts, along with multiple cycles of experiments targeting the PTA and methanol esterification reaction, is essential for evaluating and optimizing the performance of catalysts.
Finally, the reaction stability of the β-zeolite catalyst was assessed to investigate its reusability for the esterification reaction of PTA and methanol under fixed reaction conditions. After the reaction, the solid catalyst was separated from the liquid products using a centrifuge, and the recovered catalyst was washed 2–3 times with deionized water and then dried in a vacuum oven at 70 °C for 12 h, before being used for the DMT hydrogenation reaction under the same reaction conditions as the fresh catalyst. The results, as shown in Figure 11, indicate that the selectivity for DMT remained essentially unchanged over five cycles, while the conversion rate of PTA and the yield of DMCD slightly decreased with increasing cycle numbers, possibly due to the catalyst not being 100% recoverable in each cycle. The experiments demonstrate that zeolite β, with its unique three-dimensional dodecasil 3R pore structure, exhibits sufficient stability as a catalyst for the esterification reaction of PTA and can be used as a catalyst for this reaction.
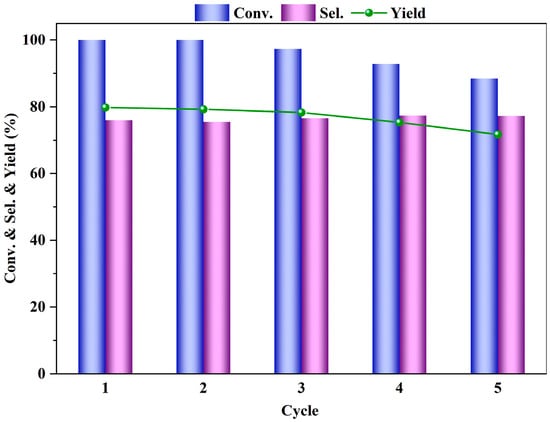
Figure 11.
Cycling stability of the catalyst in the esterification of PTA (reaction conditions: m (PTA) = 1 g, m (β) = 0.1 g, V (methanol) = 40 mL, t = 4 h, 200 °C, 0.5 MPa N2).
3. Experimental
3.1. Materials
Terephthalic acid (PTA, 99.9%) and methanol (CH3OH, 99.9%), were obtained from the Shanghai Maclean’s Biochemical Technology Co., Ltd. (Shanghai, China). p-xylene (99.9%) was obtained from the Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China).
3.2. Catalyst Preparation
The ZSM-5 zeolites, characterized by Si/Al ratios of 25, 50, and 100, and the β zeolite characterized by Si/Al ratios of 25 were acquired from the Nankai University Catalyst Co., Ltd. (Tianjin, China). The ZSM-35 zeolite and MOR zeolite, characterized by Si/Al ratios of 25 were sourced from the Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). All the chemicals were used as received without further purification. Before the test, the zeolites were calcined at 550 °C for 6 h to obtain the protonic form (H-zeolites). The ZSM-5 zeolites with different Si/Al ratios mentioned in the paper were named as ZSM-5-X (X was the Si/Al ratio).
3.3. Catalyst Characterization
The crystal structure of the catalyst was measured using a Rigaku Smart Lab SE X-ray powder diffractometer (Tokyo, Japan). Before the test, the catalyst was pressed flat and secured in the sample holder. The scanning range was set from 5° to 90° with a scan rate of 10°/min. A Cu Kα X-ray source (λ = 1.5406 Å) was utilized with a tube voltage of 40 kV and a tube current of 30 mA.
The morphologies of the catalysts were investigated with a ZEISS Gemini SEM 500 scanning electron microscope (SEM) (Oberkochen, Germany). Before testing, the sample is first attached to the sample stage using a conductive adhesive. The sample stage is then placed in a gold sputter coater for conductive treatment (i.e., gold coating). After gold coating, the sample stage is removed and placed onto the sample holder in the scanning electron microscope (SEM) chamber. The chamber door is closed, and vacuum is applied. High-energy electron beams are then used to scan the sample surface, characterizing and analyzing the microstructure, morphology, and size of the sample.
NH3-temperature programmed desorption (NH3-TPD) experiments were performed on a Micromeritics AutoChem II chemisorption analyzer (Norcross, GA, USA). Approximately 200 mg of the catalyst sample was loaded into a U-shaped quartz tube. The sample was first pretreated in a He flow at 200 °C for 2 h. After cooling to room temperature, 5% NH3/He was introduced for adsorption until saturation. The system was then purged with He for 30 min to remove the physiosorbed NH3. Subsequently, the desorption was carried out from room temperature to 700 °C at a heating rate of 10 °C/min to record the NH3-TPD profiles. The quantified acid strength of the zeolite catalysts was determined based on the NH3-TPD experiments, where the weak acid sites were identified within the temperature range of 190–250 °C, while the strong acid sites corresponded to the range of 390–500 °C.
The specific surface area and porosity of the catalyst were determined by N2 physisorption measurement using a Micromeritics ASAP 2460 instrument (Norcross, GA, USA). The catalysts were degassed at 200 °C for 4 h before acquiring the adsorption isotherm. The specific surface area of the catalysts was determined using the Brunauer–Emmett–Teller (BET) equation from adsorption data at P/P0 values between 0.05 and 0.30. The micropore surface area and micropore volume were determined by the t-plot method. The mesopore volume of the catalyst was calculated by subtracting the micropore volume from the total pore volume. The average pore diameter and pore size distribution of the synthesized catalysts were assessed by employing the BET (desorption branch) and Barrett–Joyner–Halenda (BJH) methods, respectively.
3.4. Product Analysis
The esterification reaction of PTA was conducted in a sealed 100 mL high-pressure reactor (YanZheng Co., Ltd., Shanghai, China). Firstly, the reactant and catalyst were loaded into the reactor liner. After sealing the reactor, the inlet and outlet valves were closed. To ensure airtightness, the reactor was purged three times with N2 at 0.5 MPa to remove air. The airtightness was then verified by pressurizing the reactor with N2 and holding it for 15 min, where a stable pressure indicated no leakage. Once the airtightness was confirmed, the reactor was pressurized with N2 to the desired initial pressure. Subsequently, the temperature was set to the required value, the stirring speed was adjusted to 750 rpm, and the experiment was started. The reaction time was noted when the desired temperature was achieved.
Following the PTA esterification reaction, samples of the reaction mixture were taken and mixed according to a y-fold dilution ratio. To one portion, a precise amount of the internal standard, p-xylene, was added, and the mixture was stirred for 1 min. The solution was then filtered through a 0.2 μm membrane filter. The filtrate was subsequently analyzed using an Agilent GC-8890 gas chromatograph, with the peak areas of PTA, DMT, and the internal standard recorded as APTA, ADMT, and APX, respectively. The DMT yield, DMT selectivity, and PTA conversion rate were calculated via Equations (1)–(3) by applying the relative correction factor derived from the external standard calibration curves. By substituting the relative correction factor (slope KDMT = 1.862) obtained from the internal standard calibration curve, the DMT yield, DMT selectivity, and PTA conversion rate can be calculated.
where MPTA was the quantity of raw material PTA (g); APX, ADMT, and AProduct were the peak areas of the external standard, para-xylene (PX), DMT, and products, respectively; NPX and NPTA were the molar quantity of the external standard para-xylene (PX) and PTA (mol), respectively; y was the dilution ratio; KDMT was the relative correction factor for DMT.
4. Conclusions
In this study, six commercially available zeolite catalysts (ZSM-5-25, ZSM-5-50, ZSM-5-100, ZSM-35, MOR, and β) were systematically evaluated for the esterification of terephthalic acid (PTA) to produce dimethyl terephthalate (DMT). Among them, the β zeolite exhibited the best catalytic performance, which can be attributed to its optimal combination of strong acid sites, high specific surface area, and the presence of secondary mesoporous structures, as confirmed by NH3-TPD and N2 physisorption analyses. After a comprehensive optimization of the reaction conditions, complete PTA conversion and a high DMT selectivity of 94.11% were achieved under the following conditions: 200 °C, 1 MPa N2 pressure, a PTA-to-methanol mass-to-volume ratio of 1:30 (g/mL), a PTA-to-catalyst mass ratio of 8:1, and a reaction time of 8 h. Furthermore, the β zeolite catalyst demonstrated excellent recyclability, retaining over 92% of its initial activity after five consecutive reaction cycles. Overall, this work highlights β zeolite as a highly efficient, stable, and environmentally benign solid acid catalyst, offering significant potential for the sustainable and scalable industrial production of DMT, and representing a viable alternative to traditional liquid acid catalysts. The findings provide some valuable insights into optimizing zeolite-based catalytic systems, presenting β zeolite as an exceptionally promising candidate for the sustainable and efficient industrial production of DMT.
Author Contributions
Conceptualization, M.Z. and Z.L.; Methodology, H.H.; Formal analysis, N.J. and H.H.; Investigation, N.J., H.H. and T.Y.; Resources, Z.L.; Writing—original draft, N.J.; Writing—review & editing, T.Y. and M.Z.; Supervision, Z.L.; Project administration, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peterson, R.L.; Neppel, E.P.; Peereboom, L.; Trinh, P.A.; Ofoli, R.Y.; Dorgan, J.R. Upcycling Waste PET: I. Ammonolysis Kinetics of Model Dimethyl Terephthalate and the Catalytic Effects of Ethylene Glycol. Sustain. Chem. Eng. 2025, 13, 4120–4131. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Zhang, Y.; Su, Y.; Hu, H.; Huang, A.; Huang, Z.; Chen, D.; Yang, M.; Wu, J. Esterification of bagasse cellulose with metal salts as efficient catalyst in mechanical activation-assisted solid phase reaction system. Cellulose 2017, 24, 5371–5387. [Google Scholar] [CrossRef]
- Iliopoulou, E.F.; Triantafyllidis, K.S.; Lappas, A.A. Overview of catalytic upgrading of biomass pyrolysis vapors toward the production of fuels and high-value chemicals. Wiley Interdiscip. Rev. Energy Environ. 2018, 8, 1. [Google Scholar] [CrossRef]
- Pereira, P.; Savage, P.E.; Pester, C.W. Acid catalyst screening for hydrolysis of post-consumer PET waste and exploration of acidolysis. Green Chem. 2024, 26, 1964–1974. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, Y.; Wang, M.; Gou, G.; Li, L. Metal−organic frameworks as recyclable catalysts for efficient esterification to synthesize traditional plasticizers. Appl. Catal. A Gen. 2021, 622, 118212. [Google Scholar] [CrossRef]
- Li, J.; Liang, X. Magnetic solid acid catalyst for biodiesel synthesis from waste oil. Energy Convers. Manag. 2017, 141, 126–132. [Google Scholar] [CrossRef]
- Xue, D.; Jiang, Y.; Zheng, F. Magnetic-responsive solid acid catalysts for esterification. RSC Adv. 2023, 13, 27579–27588. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Yin, P.; Ju, H.X.; Chen, Z.Q.; Li, C.; Li, S.C.; Liang, H.W.; Zhu, J.F.; Yu, S.H. Natural Nanofibrous Cellulose-Derived Solid Acid Catalysts. Research 2019, 2019, 6262719. [Google Scholar] [CrossRef]
- Wu, L.; Hu, X.; Wang, S.; Mahmudul Hasan, M.D.; Jiang, S.; Li, T.; Li, C.-Z. Acid-treatment of bio-oil in methanol: The distinct catalytic behaviours of a mineral acid catalyst and a solid acid catalyst. Fuel 2018, 212, 412–421. [Google Scholar] [CrossRef]
- Liu, J.; Wang, N.; Liu, S.; Liu, G. Catalytic Hydrodeoxygenation of Mixed Plastic Wastes into Sustainable Naphthenes. JACS Au 2024, 4, 4361–4373. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, W.; Cui, W.; Dong, X.; Liu, G.; Xu, Y.; Liu, Z. Optimization and modification of ZSM-5 zeolite for efficient catalytic cracking of 1,2-dichloroethane. Mol. Catal. 2023, 545, 113189. [Google Scholar] [CrossRef]
- Jiang, C.; Cai, Y.; Xu, T.; Xiao, B.; Hu, Z.; Wang, X. Vapor-phase hydrodeoxygenation of guaiacol for phenol production using bifunctional Ni/Cu-Beta zeolite catalysts. J. Energy Inst. 2023, 109, 101273. [Google Scholar] [CrossRef]
- Jiang, W.; Cao, J.-P.; Yao, N.-Y.; Xie, J.-X.; Zhao, L.; Yi, F.-J.; Zhang, C.; Zhu, C.; Zhao, X.-Y.; Zhao, Y.-P.; et al. Hydrodeoxygenation of Lignin-Derived Diphenyl Ether to Cyclohexane over a Bifunctional Ru Supported on Synthesis HZSM-5 Catalyst under Mild Conditions. Ind. Eng. Chem. Res. 2022, 61, 2937–2946. [Google Scholar] [CrossRef]
- Yu, L.; Xu, C.; Zhou, Q.; Fu, X.; Liang, Y.; Wang, W. Facile synthesis of hierarchical porous ZSM-5 zeolite with tunable mesostructure and its application in catalytic cracking of LDPE. J. Alloys Compd. 2023, 965, 171454. [Google Scholar] [CrossRef]
- Yang, X.; Ma, X.; Wang, X.; Qin, B.; Zhang, L.; Du, Y.; Liu, Y.; Wang, Q.; Wang, Y.; Zheng, J. Caterpillar-shaped hierarchical ZSM-5 resulted from the self-assembly of regularly primary nano-sized zeolite crystals. J. Porous Mater. 2023, 30, 1543–1553. [Google Scholar] [CrossRef]
- Tang, H.; Hu, Y.; Li, G.; Wang, A.; Xu, G.; Yu, C.; Wang, X.; Zhang, T.; Li, N. Synthesis of jet fuel range high-density polycycloalkanes with polycarbonate waste. Green Chem. 2019, 21, 3789–3795. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Liu, X.; Liao, X.; Huang, J.; Jiang, Y. Co-upcycling of Plastic Waste and Biowaste via Tandem Transesterification Reactions. JACS Au 2024, 4, 3135–3145. [Google Scholar] [CrossRef]
- Yu, S.; Wang, H.-M.; Xiong, S.-J.; Zhou, S.-J.; Wang, H.-H.; Yuan, T.-Q. Sustainable Wood-Based Poly(butylene adipate-co-terephthalate) Biodegradable Composite Films Reinforced by a Rapid Homogeneous Esterification Strategy. ACS Sustain. Chem. Eng. 2022, 10, 14568–14578. [Google Scholar] [CrossRef]
- Liou, T.-H.; Liu, R.-T.; Wen, S.-D. Fabricating mesoporous titanium nanocomposites with high adsorption and photolysis performance by using rice husk ash as a renewable support source. Environ. Technol. Innov. 2024, 34, 103605. [Google Scholar] [CrossRef]
- Lan, K.; Zhao, D. Functional Ordered Mesoporous Materials: Present and Future. Nano Lett. 2022, 22, 3177–3179. [Google Scholar] [CrossRef]
- Trejda, M.; Jądrzak, A.; Nurwita, A.; Kryszak, D. An efficient synthesis of acidic mesoporous materials. Catal. Today 2020, 354, 61–66. [Google Scholar] [CrossRef]
- Lende, A.B.; Bhattacharjee, S.; Lu, W.-Y.; Tan, C.-S. Hydrogenation of dioctyl phthalate over a Rh-supported Al modified mesocellular foam catalyst. New J. Chem. 2019, 43, 5623–5631. [Google Scholar] [CrossRef]
- Cai, S.-J.; Cao, J.-P.; Yao, N.-Y.; Zhao, J.-P.; Pang, X.-B.; Xu, M.; Lu, Y.; Zhao, X.-Y.; Bold, T. Modification of highly acid ZSM-5 composed of nanocrystal stacks for reforming of lignite pyrolysis volatiles to light aromatics. J. Anal. Appl. Pyrolysis 2025, 187, 107009. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, B.; Su, Z.; Ma, W. Preparation, surface acidity and catalytic performance of Beta/ZSM-5 composite molecular sieve. Chem. Phys. 2022, 558, 111512. [Google Scholar] [CrossRef]
- Tong, Y.; Ke, M. Study on the Acidic Modification of Mesoporous HZSM-5 Zeolite and Its Catalytic Cracking Performance. Catalysts 2024, 14, 713. [Google Scholar] [CrossRef]
- Kang, M.J.; Yu, H.J.; Jegal, J.; Kim, H.S.; Cha, H.G. Depolymerization of PET into terephthalic acid in neutral media catalyzed by the ZSM-5 acidic catalyst. Chem. Eng. J. 2020, 398, 125655. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Li, L.; Li, M.; Liu, Z.-Q.; Wei, X.-Y.; Ma, H.; Cong, X.-S. Catalytic hydrodeoxygenation of lignin enhanced by selectively etching ZSM-5. J. Energy Inst. 2024, 117, 101838. [Google Scholar] [CrossRef]
- Wei, C.; Li, J.; Liu, Z. Selective Synthesis of Butadiene from γ-Valerolactone over ZSM-35 Zeolite. ACS Sustain. Chem. Eng. 2023, 11, 14103–14111. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Liu, W.; Yu, Q.; Liu, Z.; Xu, S.; An, J.; Li, X.; Zhu, X. An efficient synthesis strategy for MOR zeolite. Microporous Mesoporous Mater. 2022, 346, 112282. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, S.; Zhang, H.; Lü, E.; Ren, J. Investigation of synthesis and hydroisomerization performance of SAPO-11/Beta composite molecular sieve. Chin. J. Catal. 2014, 35, 1676–1686. [Google Scholar] [CrossRef]
- Jia, Y.; Tao, J.; Bai, T.; Liu, H.; Niu, M.; Huang, W.; Li, R.R.; Wei, Q.; Zhou, Y. Study on the synthesis of nanosheets petal-shaped ZSM-5 Zeolites. Mater. Today Commun. 2024, 41, 110478. [Google Scholar] [CrossRef]
- Dai, S.; Tan, Y.; Yang, Y.; Zhu, L.; Liu, B.; Du, Y.; Cao, X. Organotemplate-free synthesis of Al-rich ZSM-35 and ZSM-22 zeolites with the addition of ZSM-57 zeolite seeds. CrystEngComm 2022, 24, 6987–6995. [Google Scholar] [CrossRef]
- Gao, W.; Amoo, C.C.; Zhang, G.; Javed, M.; Mazonde, B.; Lu, C.; Yang, R.; Xing, C.; Tsubaki, N. Insight into solvent-free synthesis of MOR zeolite and its laboratory scale production. Microporous Mesoporous Mater. 2019, 280, 187–194. [Google Scholar] [CrossRef]
- Manal, A.K.; Shanbhag, G.V.; Srivastava, R. Design of a bifunctional catalyst by alloying Ni with Ru-supported H-beta for selective hydrodeoxygenation of bisphenol A and polycarbonate plastic waste. Appl. Catal. B Environ. 2023, 338, 123021. [Google Scholar] [CrossRef]
- Wang, X.; Lv, Y.; Zhu, S.; Wang, X.; Deng, C. Phosphoric Acid Modification of Hβ Zeolite for Guaiacol Hydrodeoxygenation. Catalysts 2021, 11, 962. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, S.; Wei, Y.; Li, J.; Chen, J.; Wang, J.; Zhang, W.; Gao, S.; Li, X.; Wang, C.; et al. Methanol conversion on ZSM-22, ZSM-35 and ZSM-5 zeolites: Effects of 10-membered ring zeolite structures on methylcyclopentenyl cations and dual cycle mechanism. RSC Adv. 2016, 6, 95855–95864. [Google Scholar] [CrossRef]
- Li, C.; Ren, Y.; Gou, J.; Liu, B.; Xi, H. Facile synthesis of mesostructured ZSM-5 zeolite with enhanced mass transport and catalytic performances. Appl. Surf. Sci. 2017, 392, 785–794. [Google Scholar] [CrossRef]
- Li, H.; Chu, H.; Ma, X.; Wang, G.; Liu, F.; Guo, M.; Lu, W.; Zhou, S.; Yu, M. Efficient heterogeneous acid synthesis and stability enhancement of UiO-66 impregnated with ammonium sulfate for biodiesel production. Chem. Eng. J. 2021, 408, 127277. [Google Scholar] [CrossRef]
- Sun, H.; Chen, Z.; Zhou, J.; Chen, L.; Zuo, W. Recovery of high-quality terephthalic acid from waste polyester textiles via a neutral hydrolysis method. J. Environ. Chem. Eng. 2024, 12, 112558. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, Y.; Wu, Y.; Zheng, M.; Zhang, C.; Yang, M.; Cao, G. Production of mesoporous materials with high hydrothermal stability by doping metal heteroatoms. Microporous Mesoporous Mater. 2016, 224, 420–425. [Google Scholar] [CrossRef]
- Tian, K.; Tan, D.; Fu, X.; Zhang, Y.; Yao, D.; Zhong, M.; Chen, R.; Dong, Y.; Liu, Y. Adsorption performance of 1,4-dioxane by MCM-22 and Beta zeolites and their bio-zeolite composite system in the presence of co-contaminants. Sep. Purif. Technol. 2025, 354, 128752. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, W.; Chen, Z.; Ye, Y.; Luo, Y.; Street, J.; Zhou, H.; Xu, C. Formation and Regeneration of Shape-Selective ZSM-35 Catalysts for n-Butene Skeletal Isomerization to Isobutene. ACS Omega 2018, 3, 8202–8211. [Google Scholar] [CrossRef]
- Azam, M.U.; Fernandes, A.; Ferreira, M.J.; Afzal, W.; Graça, I. Pore-Structure Engineering of Hierarchical β Zeolites for the Enhanced Hydrocracking of Waste Plastics to Liquid Fuels. ACS Catal. 2024, 14, 16148–16165. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, R.; Yang, L.; Ge, J.; Hu, F.; Zhang, T.; Lu, F.; Wang, H.; Qi, J. In Situ Growth of Mn-Co3O4 on Mesoporous ZSM-5 Zeolite for Boosting Lean Methane Catalytic Oxidation. Catalysts 2024, 14, 397. [Google Scholar] [CrossRef]
- Ye, B.; Zhou, R.; Zhong, Z.; Wang, S.; Wang, H.; Hou, Z. Upcycling of waste polyethylene terephthalate to dimethyl terephthalate over solid acids under mild conditions. Green Chem. 2023, 25, 7243–7252. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).











