Supramolecular Assemblies in Mn(II) and Zn(II) Metal–Organic Compounds Involving Phenanthroline and Benzoate: Experimental and Theoretical Studies
Abstract
1. Introduction
2. Results and Discussion
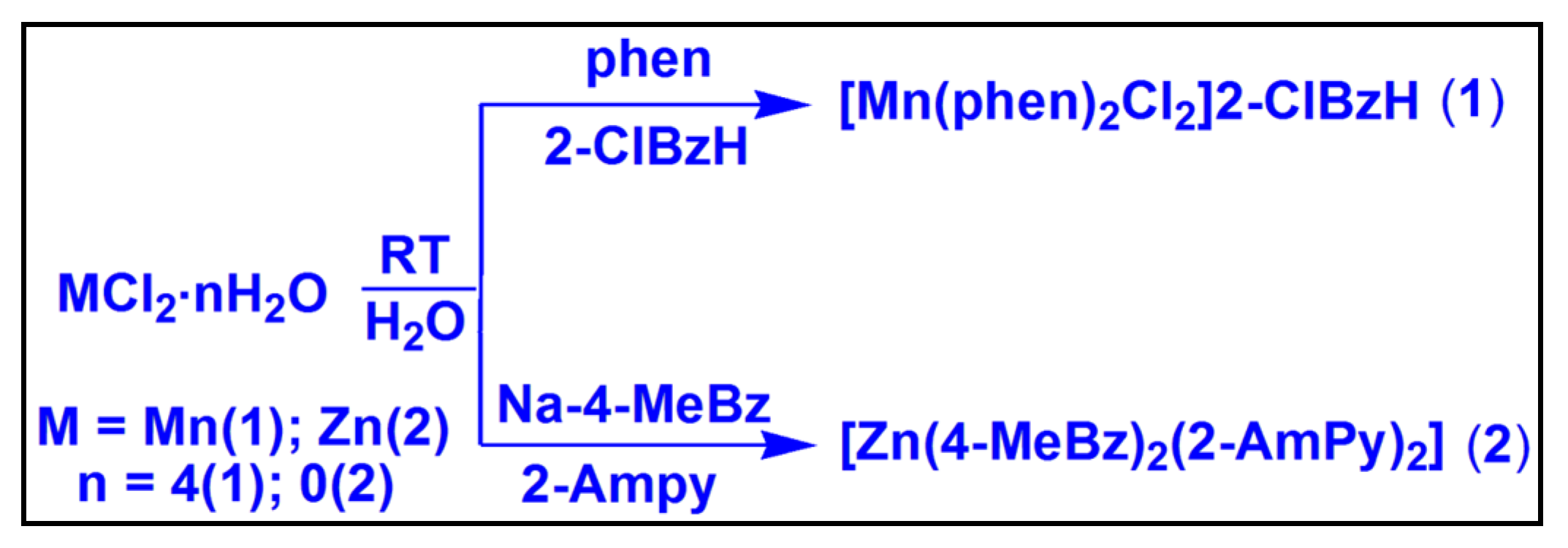
2.1. Syntheses and General Aspects
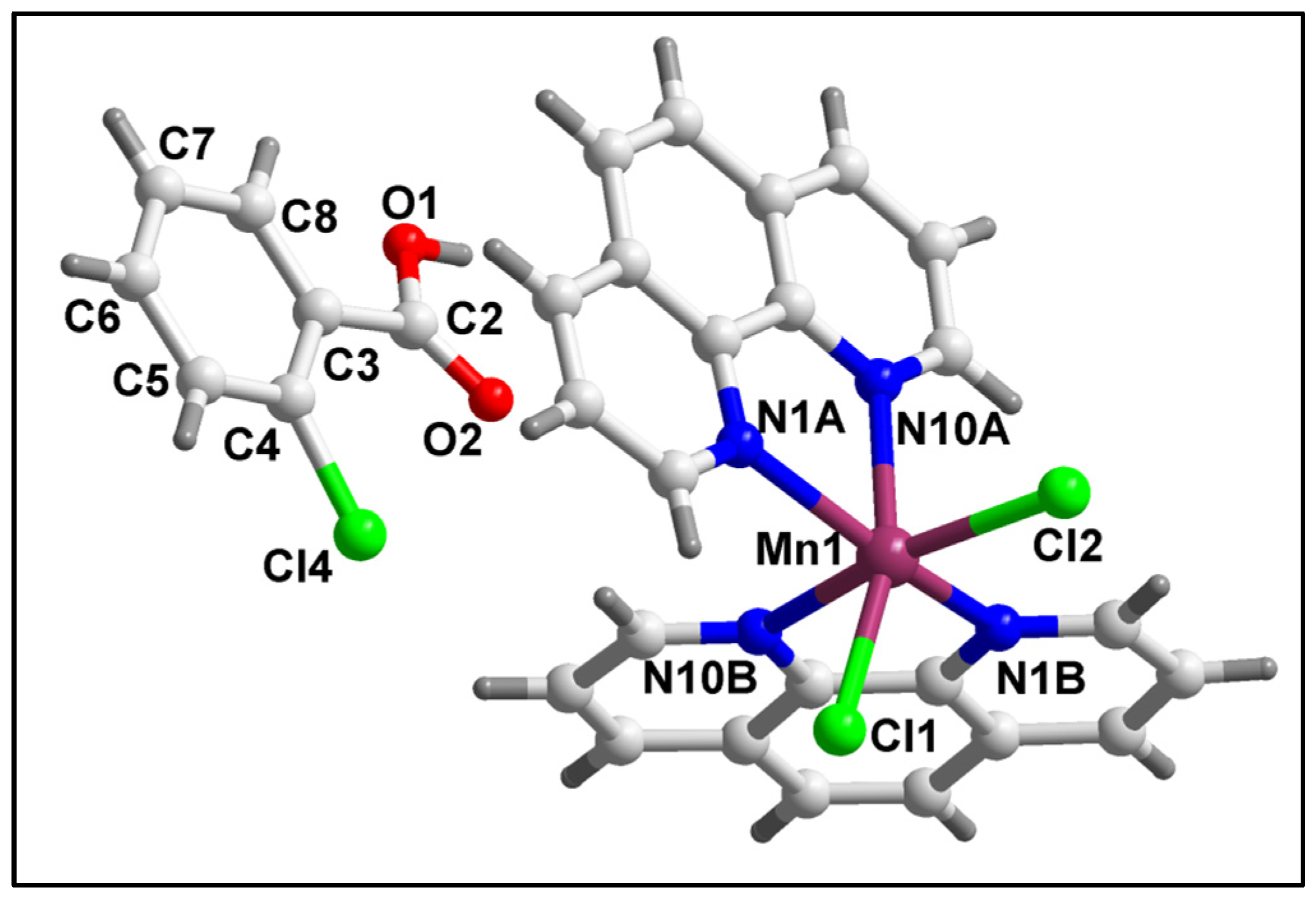
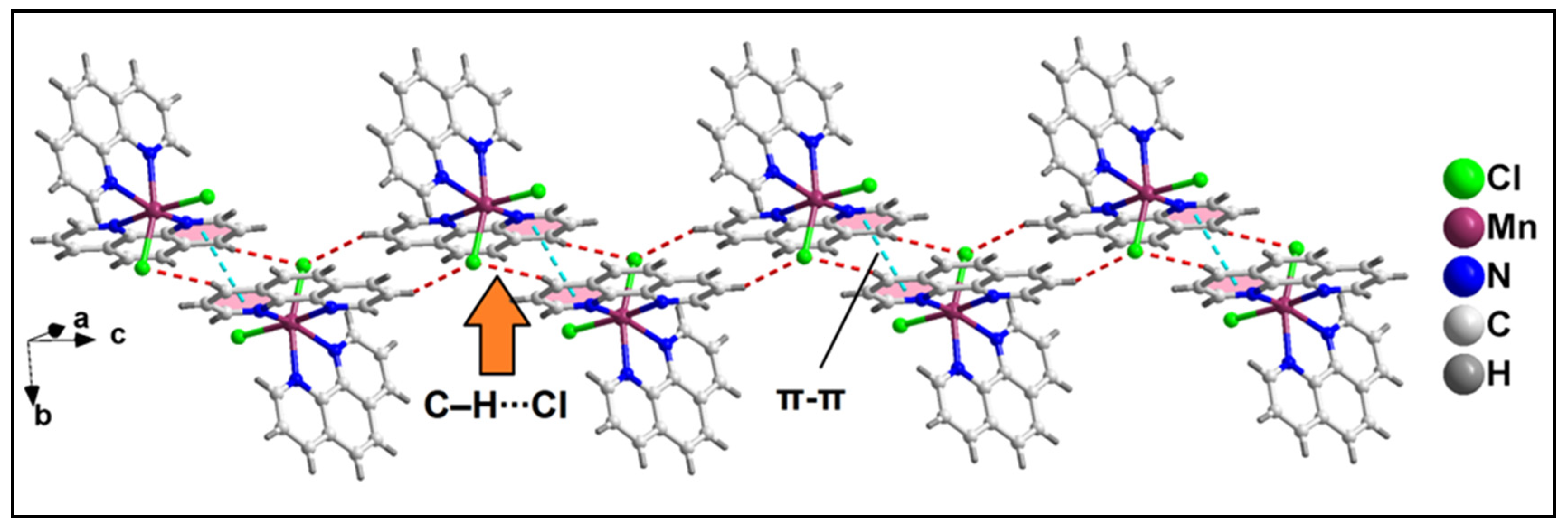
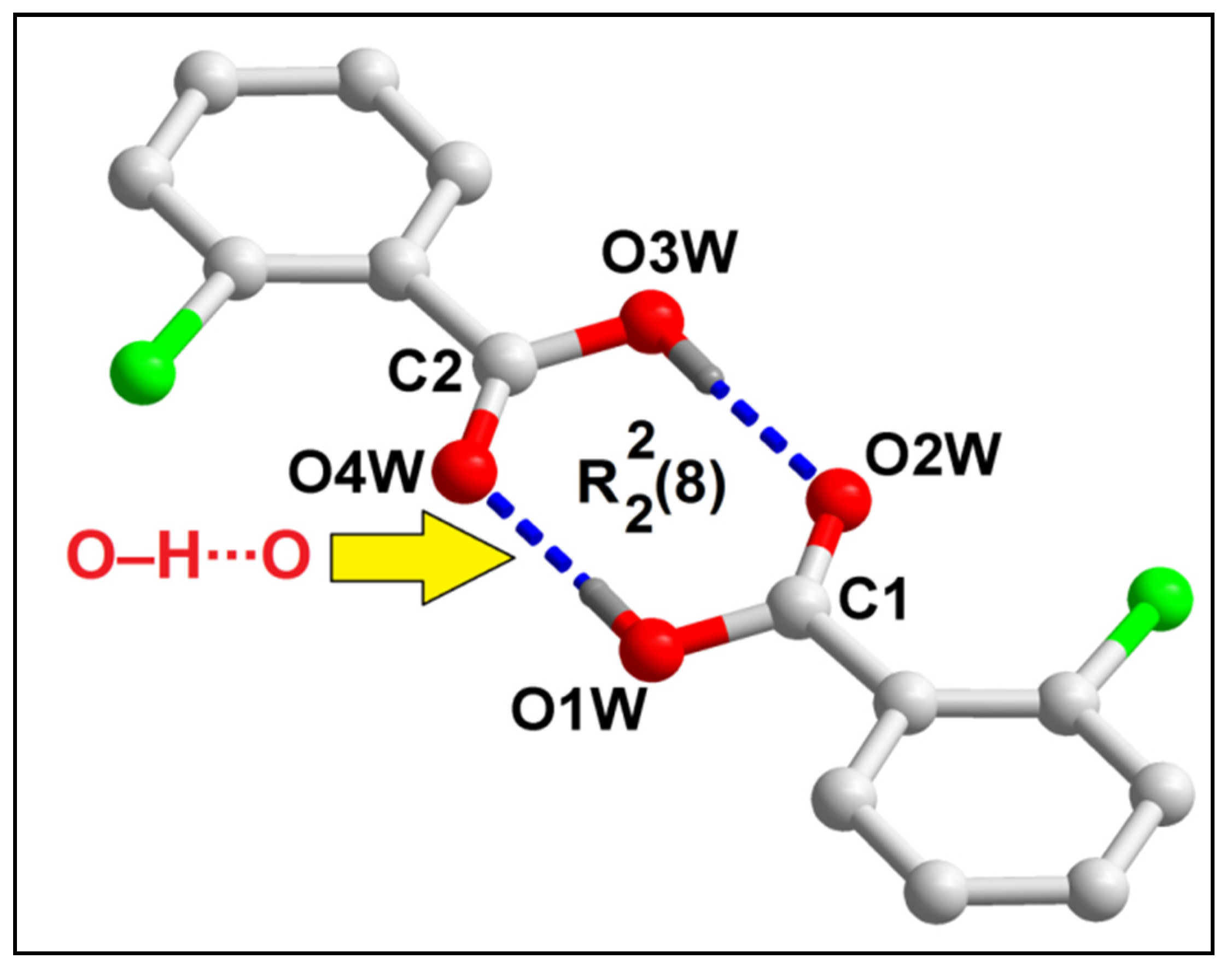
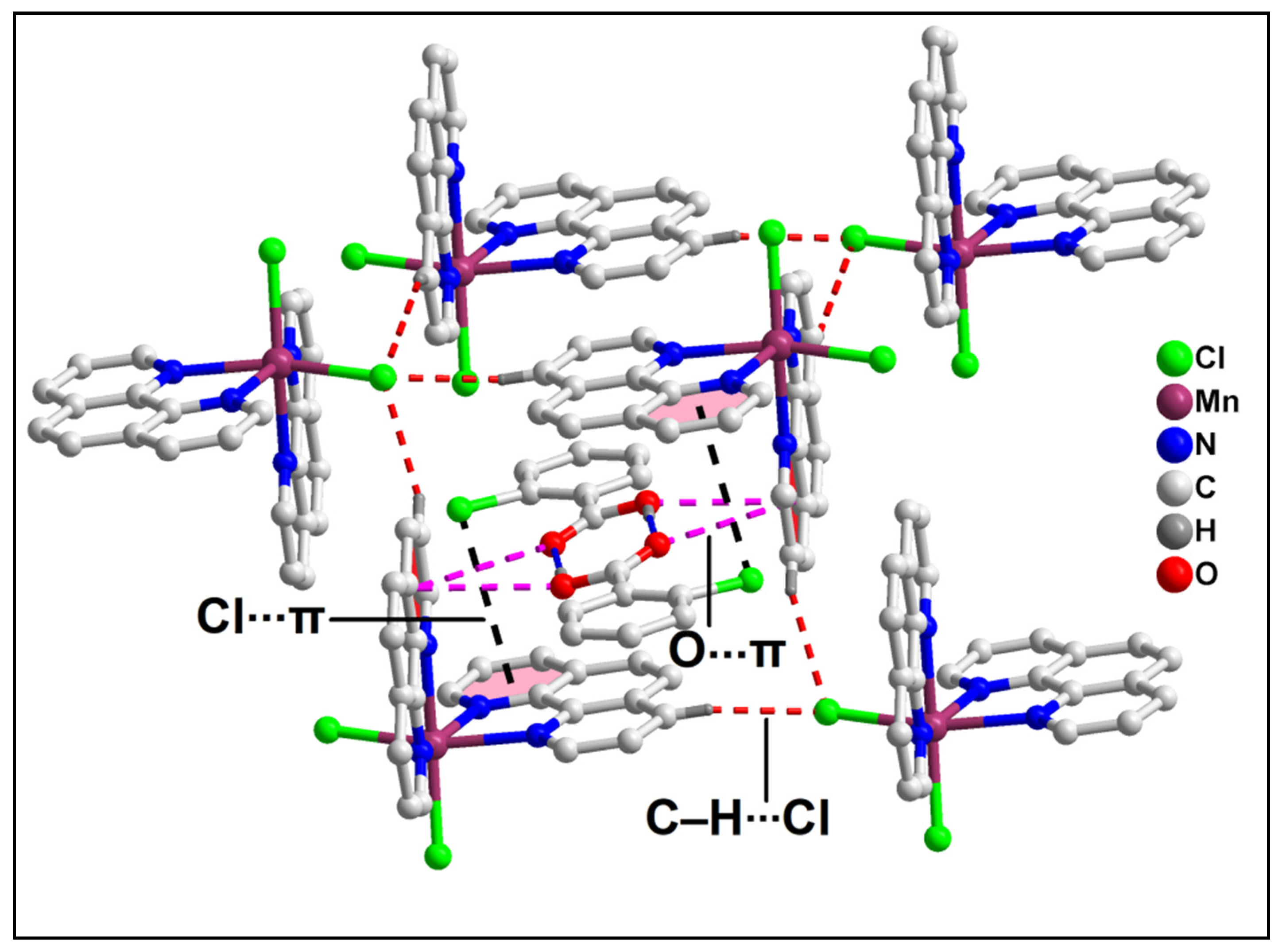
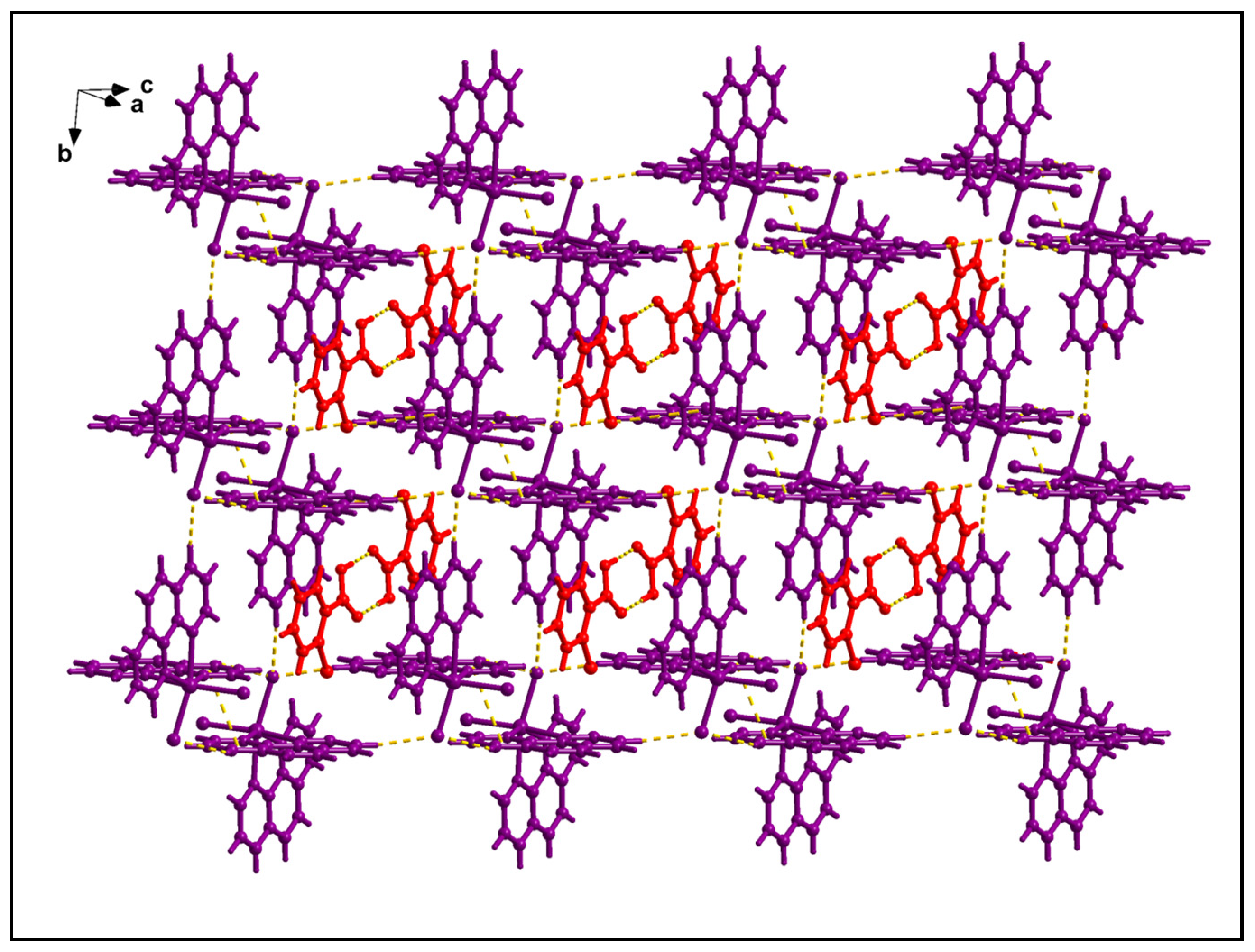
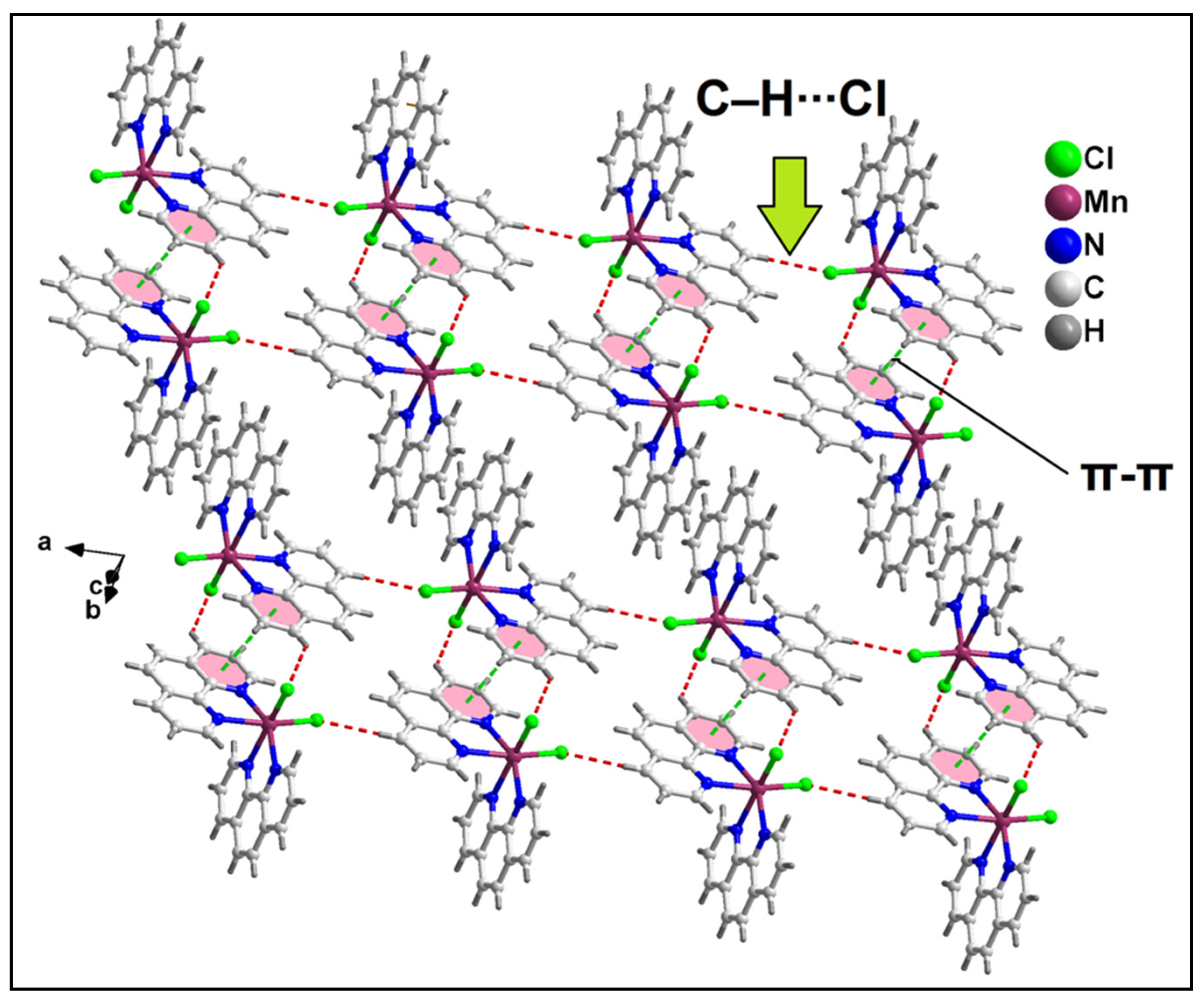
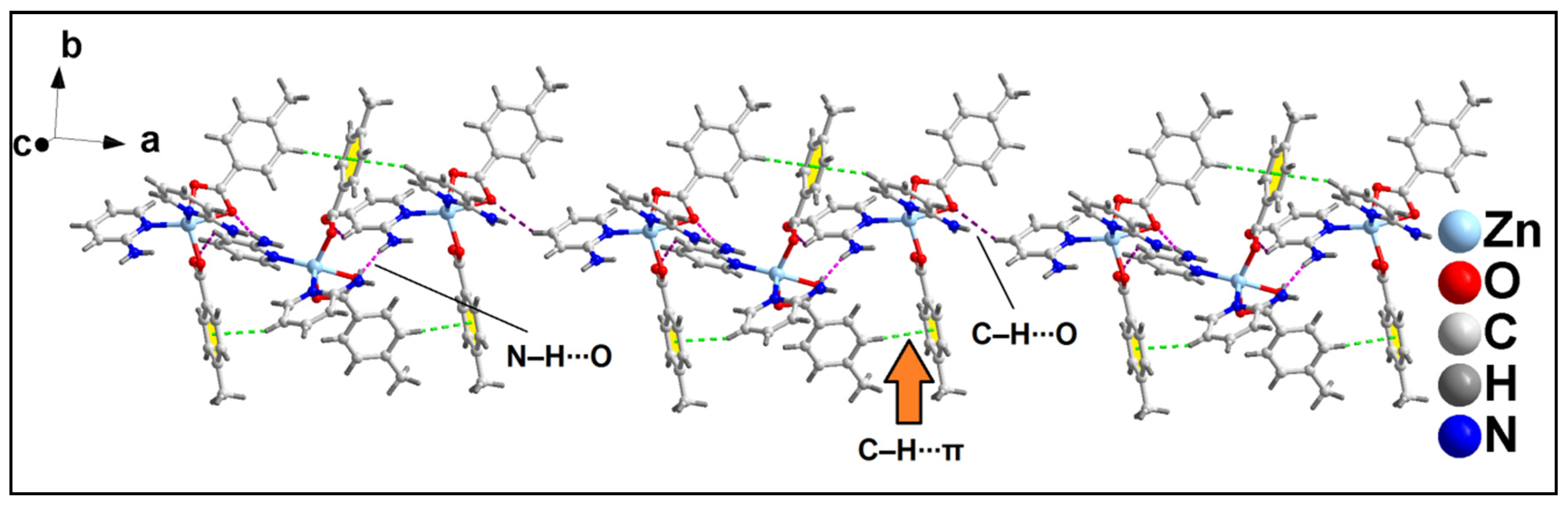
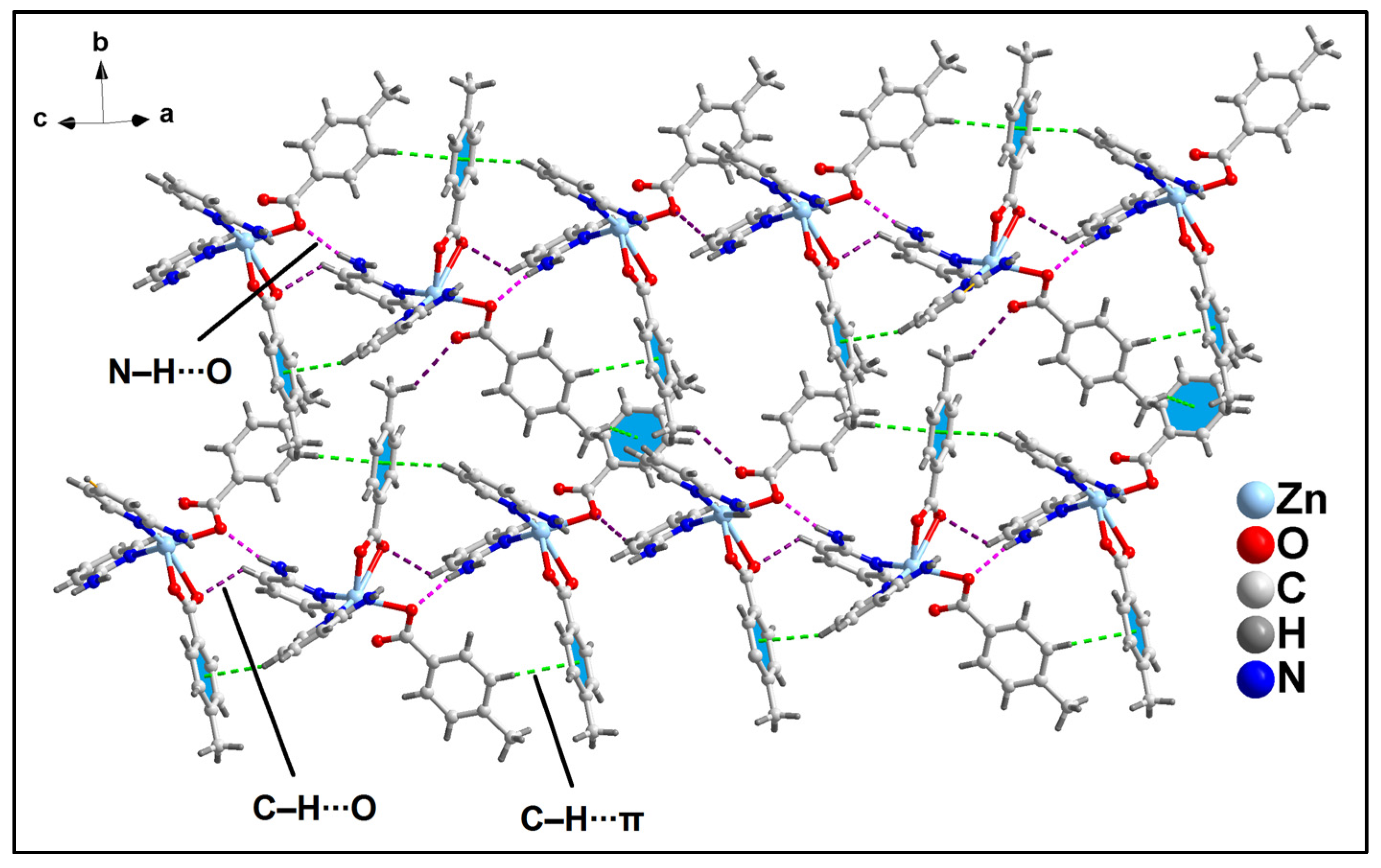
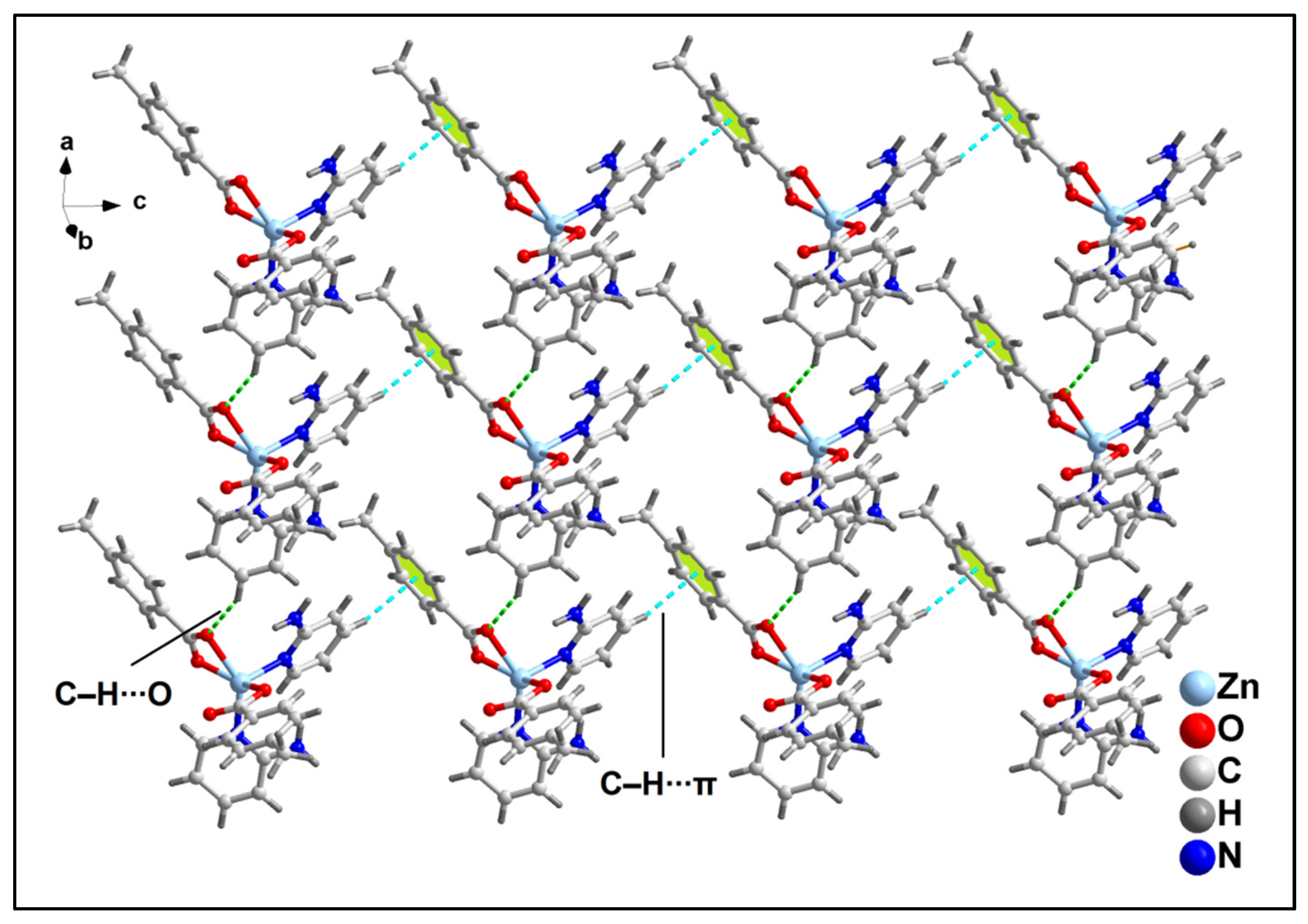
2.2. Crystal Structure Analysis
2.3. Spectral Studies
2.3.1. FT-IR Spectroscopy
2.3.2. Electronic Spectroscopy
2.4. Thermogravimetric Analysis
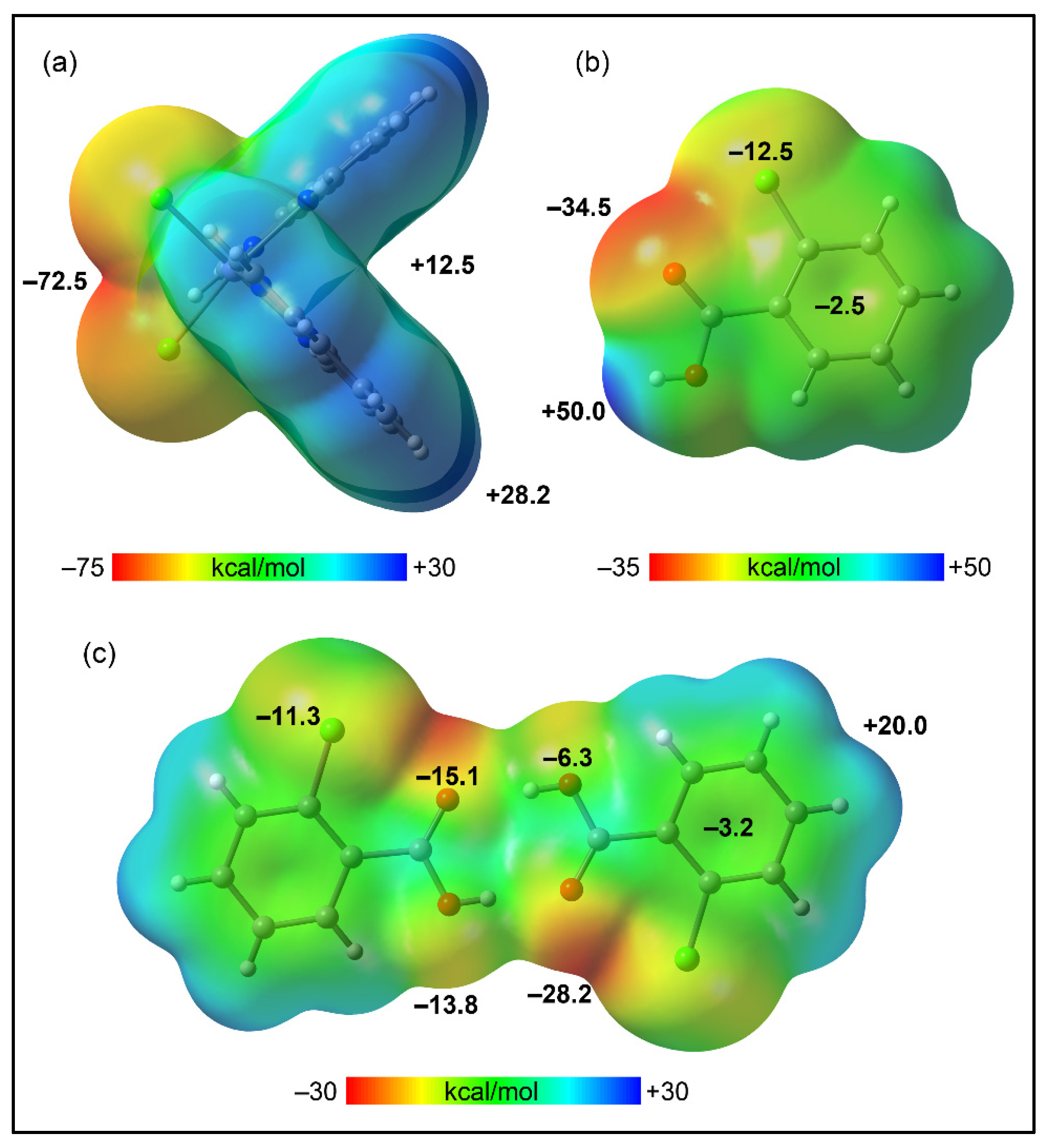
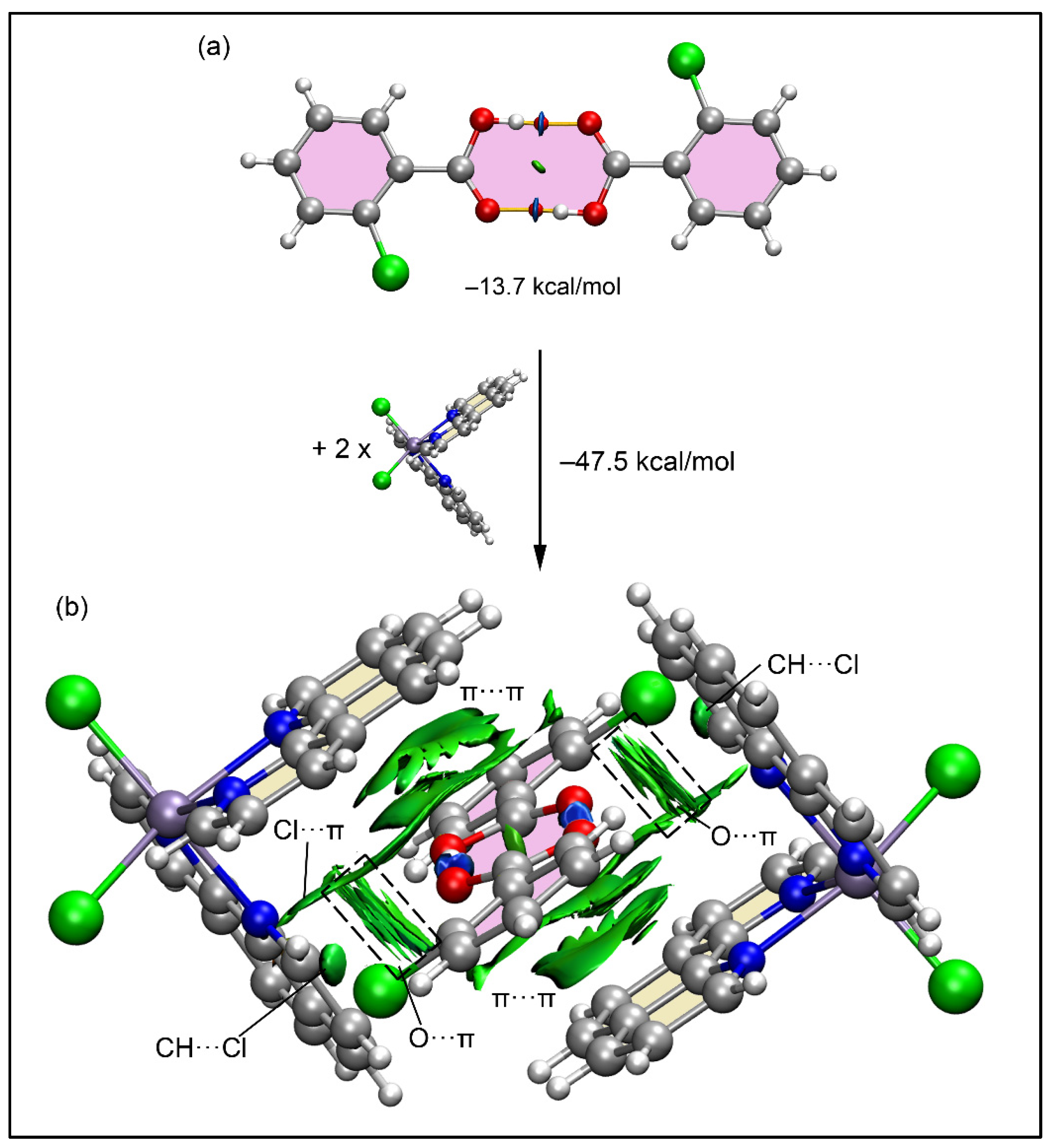
2.5. Theoretical Study
3. Materials and Methods
3.1. Syntheses
3.1.1. Synthesis of [Mn(phen)2Cl2]2-ClBzH (1)
3.1.2. Synthesis of [Zn(4-MeBz)2(2-AmPy)2] (2)
3.2. Crystallographic Data Collection and Refinement
3.3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Wang, H.S.; Wang, Y.H.; Ding, Y. Development of Biological Metal–Organic Frameworks Designed for Biomedical Applications: From Bio-Sensing/Bio-Imaging to Disease Treatment. Nanoscale Adv. 2020, 2, 3788–3797. [Google Scholar] [CrossRef] [PubMed]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal–Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Cheng, X.; Hao, H.; Han, J.; Lau, M.-T.; Li, Z.; Zhou, Z.; Dong, Q.; Wong, W.-Y. Metal-Containing Organic Compounds for Memory and Data Storage Applications. Chem. Soc. Rev. 2022, 51, 1926–1982. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Chen, Y.; Pan, W.; Li, N.; Liu, Z.; Tang, B. Antitumor Agents Based on Metal–Organic Frameworks. Angew. Chem. 2021, 133, 16901–16914. [Google Scholar] [CrossRef]
- Zheng, R.; Guo, J.; Cai, X.; Bin, L.; Lu, C.; Singh, A.; Liu, J. Manganese Complexes and Manganese-Based Metal-Organic Frameworks as Contrast Agents in MRI and Chemotherapeutics Agents: Applications and Prospects. Colloids Surf. B 2022, 213, 112432–112440. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kang, Q.; Liu, L.; Ma, J.; Dong, W. Trinuclear Co(II) and Mononuclear Ni(II) Salamo-Type Bisoxime Coordination Compounds. Crystals 2018, 8, 43–60. [Google Scholar] [CrossRef]
- Gu, J.; Wen, M.; Cai, Y.; Shi, Z.; Nesterov, D.S.; Kirillova, M.V.; Kirillov, A.M. Cobalt(II) Coordination Polymers Assembled from Unexplored Pyridine-Carboxylic Acids: Structural Diversity and Catalytic Oxidation of Alcohols. Inorg. Chem. 2019, 58, 5875–5885. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Tang, L.; Wang, D.; Deng, H.; Chen, K. Metal and Ligand Effects on the Stability and Electronic Properties of Crystalline Two-Dimensional Metal-Benzenehexathiolate Coordination Compounds. J. Phys. Condens. Matter 2018, 30, 465301–465306. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abdelhamid, A.A.; Abu-Dief, A.M.; Shehata, M.R.; Bakheeta, M.A. Facile Synthesis, X-Ray Structure of New Multi-Substituted Aryl Imidazole Ligand, Biological Screening and DNA Binding of its Cr(III), Fe(III) and Cu(II) Coordination Compounds as Potential Antibiotic and Anticancer Drugs. J. Mol. Struct. 2020, 1200, 127034–127045. [Google Scholar] [CrossRef]
- Jeoung, S.; Kim, S.; Kim, M.; Moon, H.R. Pore Engineering of Metal-Organic Frameworks with Coordinating Functionalities. Coord. Chem. Rev. 2020, 420, 213377–213392. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Champness, N.R.; Janiak, C. Recent Advances in Crystal Engineering. CrystEngComm 2010, 12, 22–43. [Google Scholar] [CrossRef]
- Schiebi, J.; Schulmeister, J.; Doppiu, A.; Worner, E.; Rudolph, M.; Karch, R.; Hashmi, A.S.K. An Industrial Perspective on Counter Anions in Gold Catalysis: On Alternative Counter Anions. Adv. Synth. Catal. 2018, 360, 3949–3954. [Google Scholar]
- Khavasi, H.R.; Sadegh, B.M.M. Temperature-Dependent Supramolecular Motif in Coordination Compounds. Inorg. Chem. 2010, 49, 5356–5358. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.Y.; Lv, X.Q.; Chai, W.L.; Jin, W.J.; Song, J.R.; Wong, W.K. Synthesis, Structure and Near-Infrared (NIR) Luminescence of Three Solvent-Induced Pseudo-Polymorphic Complexes from a Bimetallic Zn–Nd Schiff-Base Molecular Unit. Inorg. Chem. Commun. 2008, 11, 1316–1319. [Google Scholar] [CrossRef]
- Bhattacharyya, M.K.; Saha, U.; Dutta, D.; Das, A.; Verma, A.K.; Frontera, A. Solvent-Driven Structural Topology Involving Energetically Significant Intra- and Intermolecular Chelate Ring Contacts and Anticancer Activities of Cu(II) Phenanthroline Complexes Involving Benzoates: Experimental and Theoretical Studies. RSC Adv. 2019, 9, 16339–16356. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R. Chemistry Beyond the Molecule. Nature 2001, 412, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, R.H. Hypervalency, Secondary Bonding and HB: Siblings Under the Skin. Chem. Soc. Rev. 2017, 46, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Rather, I.A.; Wagay, S.A.; Ali, R. Emergence of Anion-π Interactions: The Land of Opportunity in Supramolecular Chemistry and Beyond. Coord. Chem. Rev. 2020, 415, 213327–213386. [Google Scholar] [CrossRef]
- Bauzá, A.; Frontera, A. σ/π-Hole Noble Gas Bonding Interactions: Insights from Theory and Experiment. Coord. Chem. Rev. 2020, 404, 213112–213222. [Google Scholar] [CrossRef]
- Pramanik, S.; Pathak, S.; Frontera, A.; Mukhopadhyay, S. Exploration of Supramolecular and Theoretical Aspects of Two New Cu(II) Complexes: On the Importance of Lone Pair…π(Chelate Ring) and π…π(Chelate Ring) Interactions. J. Mol. Struct. 2022, 1265, 133358–133369. [Google Scholar] [CrossRef]
- Speetzen, E.D.; Nwachukwu, C.I.; Bowling, N.P.; Bosch, E. Complementary, Cooperative Ditopic Halogen Bonding and Electron Donor-Acceptor π-π Complexation in the Formation of Co-Crystals. Molecules 2022, 27, 1527–1541. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, M.K.; Saha, U.; Dutta, D.; Frontera, A.; Verma, A.K.; Sharma, P.; Das, A. Unconventional DNA-Relevant π-Stacked Hydrogen Bonded Arrays Involving Supramolecular Guest Benzoate Dimers and Cooperative Anion–π/π–π/π–Anion Contacts in Coordination Compounds of Co(II) and Zn(II) Phenanthroline: Experimental and Theoretical Studies. New J. Chem. 2020, 44, 4504–4518. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Gogoleva, N.V.; Makarov, D.A.; Kiskin, M.A.; Yakushev, I.A.; Dolgushin, F.M.; Aleksandrov, G.G.; Varaksina, E.A.; Taidakov, I.V.; Aleksandrov, E.V.; et al. Synthesis of Coordination Polymers from the Heterometallic Carboxylate Complexes with Chelating N-Donor Ligands. Russ. J. Coord. Chem. 2020, 46, 1–14. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Y.; Xue, Z.; Shi, J.; Su, Y.; Sun, M.; Wang, S.; Wang, L.; Wang, Q.; Wei, Y. Syntheses, Crystal Structures and Properties of Four Metal Coordination Complexes Constructed from Aromatic Carboxylate and Benzimidazole-Based Ligands. Transit. Met. Chem. 2020, 45, 353–362. [Google Scholar] [CrossRef]
- Danilescu, O.; Bulhac, I.; Shova, S.; Novitchi, G.; Bourosh, P. Coordination Compounds of Copper(II) with Schiff Bases Based on Aromatic Carbonyl Compounds and Hydrazides of Carboxylic Acids: Synthesis, Structures, and Properties. Russ. J. Coord. Chem. 2020, 46, 838–849. [Google Scholar] [CrossRef]
- Gu, J.; Wan, S.; Kirillova, M.V.; Kirillov, A.M. H-Bonded and Metal(II)-Organic Architectures Assembled from an Unexplored Aromatic Tricarboxylic Acid: Structural Variety and Functional Properties. Dalton Trans. 2020, 49, 7197–7209. [Google Scholar] [CrossRef] [PubMed]
- Deegan, C.; McCann, M.; Devereux, M.; Coyle, B.; Egan, D.A. In Vitro Cancer Chemotherapeutic Activity of 1,10-Phenanthroline (phen), [Ag2(phen)3(mal)]·2H2O, [Cu(phen)2(mal)]·2H2O and [Mn(phen)2(mal)]·2H2O (malH2 = Malonic Acid) Using Human Cancer Cells. Cancer Lett. 2007, 247, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Prugovečki, B.; Vušak, D.; Ležaić, K.; Jurković, M. Ternary Coordination Compounds of Copper with Amino Acids and 1,10-Phenanthroline–Structural Insight and Biological Activity. Acta Cryst. 2021, A77, 975–983. [Google Scholar] [CrossRef]
- Vušak, D.; Ležaić, K.; Jurec, J.; Žilić, D.; Prugovečki, B. Solvent Effects on the Crystallization and Structure of Ternary Copper(II) Coordination Compounds with L-Threonine and 1,10-Phenanthroline. Heliyon 2022, 8, 09556–09570. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Malinina, E.A.; Zhizhin, K.Y.; Kuznetsov, N.T. Structural Diversity of Cationic Copper(II) Complexes with Neutral Nitrogen-Containing Organic Ligands in Compounds with Boron Cluster Anions and Their Derivatives. Russ. J. Inorg. Chem. 2020, 65, 514–534. [Google Scholar] [CrossRef]
- Accorsi, G.; Listorti, A.; Yoosaf, K.; Armaroli, N. 1,10-Phenanthrolines: Versatile Building Blocks for Luminescent Molecules, Materials and Metal Complexes. Chem. Soc. Rev. 2009, 38, 1690–1700. [Google Scholar] [CrossRef]
- Teixeira, F.J.; Flores, L.S.; Valverde, T.; Escobar, L.B.L.; Reis, M.S.; Corrêa, C.C. Synthesis and Magnetic Properties of Two Cobalt-Coordination Polymers Containing 1,10-Phenanthroline and Alkyl Dicarboxylates Ligands. J. Mol. Struct. 2022, 1261, 132820–132835. [Google Scholar] [CrossRef]
- Romo, A.I.B.; Reis, M.P.; Nascimento, O.R.; Bernhardt, P.V.; Rodríguez-López, J.; Diógenes, I.C.N. Interplay of Electronic and Geometric Structure on Cu Phenanthroline, Bipyridine and Derivative Complexes, Synthesis, Characterization, and Reactivity Towards Oxygen. Coord. Chem. Rev. 2023, 477, 214943–214960. [Google Scholar] [CrossRef]
- Bencini, A.; Lippolis, V. 1,10-Phenanthroline: A Versatile Building Block for the Construction of Ligands for Various Purposes. Coord. Chem. Rev. 2010, 254, 2096–2180. [Google Scholar] [CrossRef]
- Khan, E. Pyridine Derivatives as Biologically Active Precursors; Organics and Selected Coordination Complexes. ChemistrySelect 2021, 6, 3041–3064. [Google Scholar] [CrossRef]
- Raj, D.; Padhi, S.K. The Sporadic µ-Pyridine Bridge in Transition Metal Complexes: A Real Bond or an Interaction? Coord. Chem. Rev. 2022, 450, 214238–214245. [Google Scholar] [CrossRef]
- Rao, R.N.; Chanda, K. 2-Aminopyridine–An Unsung Hero in Drug Discovery. Chem. Commun. 2022, 58, 343–382. [Google Scholar] [CrossRef]
- Camidge, D.R.; Bang, Y.J.; Kwak, E.L.; Iafrate, A.J.; Varella-Garcia, M.; Fox, S.B.; Riely, G.J.; Solomon, B.; Ou, S.H.I.; Kim, D.W.; et al. Activity and Safety of Crizotinib in Patients with ALK-Positive Non-Small-Cell Lung Cancer: Updated Results from a Phase 1 Study. Lancet Oncol. 2012, 13, 1011–1019. [Google Scholar] [CrossRef]
- Marszaukowski, F.; Guimarães, I.D.L.; da Silva, J.P.; da Silveira Lacerda, L.H.; de Lazaro, S.R.; de Araujo, M.P.; Castellen, P.; Tominaga, T.T.; Boeré, R.T.; Wohnrath, K. Ruthenium (II)-Arene Complexes with Monodentate Aminopyridine Ligands: Insights into Redox Stability and Electronic Structures and Biological Activity. J. Organomet. Chem. 2019, 881, 66–78. [Google Scholar] [CrossRef]
- Fisher, M.H.; Lusi, A. Imidazo [1, 2-a] Pyridine Anthelmintic and Antifungal Agents. J. Med. Chem. 1972, 15, 982–985. [Google Scholar] [CrossRef]
- Rival, Y.; Grassy, G.; Taudou, A.; Ecalle, R. Antifungal Activity in Vitro of Some Imidazo [1, 2-a] Pyrimidine Derivatives. Eur. J. Med. Chem. 1991, 26, 13–18. [Google Scholar] [CrossRef]
- Gudmundsson, K.S.; Johns, B.A. Synthesis of Novel Imidazo [1, 2-a] Pyridines with Potent Activity against Herpesviruses. Org. Lett. 2003, 5, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, K.S.; Williams, J.D.; Drach, J.C.; Townsend, L.B. Synthesis and Antiviral Activity of Novel Erythrofuranosyl Imidazo [1, 2-a] Pyridine C-Nucleosides Constructed via Palladium Coupling of Iodoimidazo [1, 2-a] Pyridines and Dihydrofuran. J. Med. Chem. 2003, 46, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.A.; Brun, R.; Wenzler, T.; Tanious, F.A.; Wilson, W.D.; Boykin, D.W. Novel Dicationic Imidazo [1, 2-a] Pyridines and 5, 6, 7, 8-Tetrahydro-imidazo [1, 2-a] Pyridines as Antiprotozoal Agents. J. Med. Chem. 2004, 47, 3658–3664. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, J.J.; Perkins, D.G.; Frantz, J.D.; Solomon, D.M.; Elliott, A.J.; Chiu, P.J.S.; Long, J.F. Antiulcer Agents. 3. Structure-Activity-Toxicity Relationships of Substituted Imidazo [1, 2-a] Pyridines and a Related Imidazo [1, 2-a] Pyrazine. J. Med. Chem. 1987, 30, 2047–2051. [Google Scholar] [CrossRef] [PubMed]
- Phukan, N.; Baruah, J.B. Hydrolysis of 4-(4-Oxopentan-2-ylideneamino) Benzoic Acid and In-Situ Formation of Nickel (II), Zinc (II) and Cadmium (II) Complexes of 4-Aminobenzoic Acid. Inorg. Chim. Acta 2013, 396, 430–435. [Google Scholar] [CrossRef]
- Dutta, D.; Islam, S.M.N.; Saha, U.; Chetry, S.; Guha, A.K.; Bhattacharyya, M.K. Structural Topology of Weak Non-Covalent Interactions in a Layered Supramolecular Coordination Solid of Zinc Involving 3-Aminopyridine and Benzoate: Experimental and Theoretical Studies. J. Chem. Crystallogr. 2018, 48, 156–163. [Google Scholar] [CrossRef]
- Zhang, F.; Lin, Q.-Y.; Zheng, X.-L.; Zhang, L.-L.; Yang, Q.; Gu, J.-W. Crystal Structures, Interactions with Biomacromolecules and Anticancer Activities of Mn(II), Ni(II), Cu(II) Complexes of Demethylcantharate and 2-Aminopyridine. J. Fluoresc. 2012, 22, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Aitipamula, S.; Nangia, A. Guest-Induced Supramolecular Isomerism in Inclusion Complexes of T-Shaped Host 4,4-Bis(40-Hydroxyphenyl)cyclohexanone. Chem. Eur. J. 2005, 11, 6727–6742. [Google Scholar] [CrossRef]
- Liu, K.; Kang, Y.; Wang, Z.; Zhang, X. 25th Anniversary Article: Reversible and Adaptive Functional Supramolecular Materials: “Noncovalent Interaction” Matters. Adv. Mater. 2013, 25, 5530–5548. [Google Scholar] [CrossRef]
- Morimoto, M.; Bierschenk, S.M.; Xia, K.T.; Bergman, R.G.; Raymond, K.N.; Toste, F.D. Advances in Supramolecular Host-Mediated Reactivity. Nat. Catal. 2020, 3, 969–984. [Google Scholar] [CrossRef]
- Rizzuto, F.J.; von Krbek, L.K.S.; Nitschke, J.R. Strategies for Binding Multiple Guests in Metal–Organic Cages. Nat. Rev. Chem. 2019, 3, 204–222. [Google Scholar] [CrossRef]
- Yi, J.W.; Barry, N.P.E.; Furrer, M.A.; Zava, O.; Dyson, P.J.; Therrien, B.; Kim, B.H. Delivery of Floxuridine Derivatives to Cancer Cells by Water-Soluble Organometallic Cages. Bioconjug. Chem. 2012, 23, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-W.; Sun, Y.-L.; Song, N. Switchable Host–Guest Systems on Surfaces. Acc. Chem. Res. 2014, 47, 1950–1960. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, H.; Shi, J.; Bai, Q.; Xu, Y.; Zhang, Z.; Hao, X.-Q.; Li, B.; Wang, P.; Wu, L.; et al. From Mechanically Interlocked Structures to Host–Guest Chemistry Based on Twisted Dimeric Architectures by Adjusting Space Constraints. CCS Chem. 2021, 4, 2127–2139. [Google Scholar] [CrossRef]
- Sarma, R.; Karmakar, A.; Baruah, J.B. Synthesis and Characterization of Pyridine N-Oxide Complexes of Manganese, Copper and Zinc. Inorg. Chim. Acta 2008, 361, 2081–2086. [Google Scholar] [CrossRef]
- Singh, N.K.; Singh, S.B. Complexes of 1-Isonicotinoyl-4-Benzoyl-3-Thiosemicarbazide with Manganese(II), Iron(III), Chromium(III), Cobalt(II), Nickel(II), Copper(II) and Zinc(II). Transit. Met. Chem. 2001, 26, 487–495. [Google Scholar] [CrossRef]
- Etaiw, S.E.H.; El-Bendary, M.M.; Abdelazim, H. Synthesis, Characterization, and Biological Activity of Cd(II) and Mn(II) Coordination Polymers Based on Pyridine-2,6-Dicarboxylic Acid. Russ. J. Coord. Chem. 2017, 43, 320–330. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Zhang, T.X.; Wang, P.; Gao, S. Four Coordination Compounds Constructed from 1,10-Phenanthroline and Semi-Flexible and Flexible Carboxylic Acids: Hydrothermal Synthesis, Optical Properties and Photocatalytic Performance. Polyhedron 2015, 90, 58–68. [Google Scholar] [CrossRef]
- Janiak, C. A Critical Account on π-π Stacking in Metal Complexes with Aromatic Nitrogen-Containing Ligands. Dalton Trans. 2000, 21, 3885–3895. [Google Scholar] [CrossRef]
- Etter, M.C. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Li, S.-L.; Wu, J.-Y.; Tian, Y.-P.; Fun, H.-K.; Chantrapromma, S. Bis(Thio-semicarbazide)Zinc(II) Bis-(Maleate) Dihydrate. Acta Cryst. E 2005, 61, 2701–2703. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, X.M.; Bai, F.Y.; Sun, L.X. Novel Vanadium Complexes with Rigid Carboxylate Ligands: Synthesis, Structure and Catalytic Bromine Dynamics of Phenol Red. J. Mol. Struct. 2017, 1149, 379–386. [Google Scholar] [CrossRef]
- Sharma, R.P.; Saini, A.; Kumar, J.; Kumar, S.; Venugopalan, P.; Ferretti, V. Coordination Complexes of Copper(II) with Herbicide Trichlorophenoxyacetate: Syntheses, Characterization, Single Crystal X-ray Structure and Packing Analyses of Monomeric [Cu(γ-pic)3(2,4,5-Trichlorophenoxyacetate)].H2O, [Trans-Cu(en)2(2,4,5-Trichlorophenoxyacetate)2].2H2O and dimeric [Cu2(H2tea)2(2,4,5-Trichlorophenoxyacetate)2].2(H2O). Inorg. Chim. Acta 2017, 457, 59–68. [Google Scholar]
- Diab, M.A.; Mohamed, G.G.; Mahmoud, W.H.; El-Sonbati, A.Z.; Morgan, S.M.; Abbas, S.Y. Metal- and Covalent Organic Frameworks as Catalyst for Organic Transformation: Comparative Overview and Future Perspectives. Appl. Organomet. Chem. 2019, 33, 4945–4962. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 5th ed.; John Wiley & Sons: New York, NY, USA, 1997. [Google Scholar]
- Tao, J.; Tong, M.L.; Chen, X.M. Hydrothermal Synthesis and Crystal Structures of Three-Dimensional Co-ordination Frameworks Constructed with Mixed Terephthalate (tp) and 4, 4′-Bipyridine (4, 4′-Bipy) Ligands: [M(tp)(4, 4′-Bipy)] (M = Co II, Cd II or Zn II). J. Chem. Soc. Dalton Trans. 2000, 20, 3669–3674. [Google Scholar] [CrossRef]
- Titi, A.; Shiga, T.; Oshio, H.; Touzani, R.; Hammouti, B.; Mouslim, M.; Warad, I. Synthesis of Novel Cl2Co4L6 Cluster Using 1-Hydroxymethyl-3, 5-Dimethylpyrazole (LH) Ligand: Crystal Structure, Spectral, Thermal, Hirschfeld Surface Analysis and Catalytic Oxidation Evaluation. J. Mol. Struct. 2020, 1199, 126995–127010. [Google Scholar] [CrossRef]
- Bhattacharyya, M.K.; Dutta, D.; Nashre-ul-Islam, S.M.; Frontera, A.; Sharma, P.; Verma, A.K.; Das, A. Energetically Significant Antiparallel π-Stacking Contacts in Co(II), Ni(II), and Cu(II) Coordination Compounds of Pyridine-2,6-dicarboxylates: Antiproliferative Evaluation and Theoretical Studies. Inorg. Chim. Acta 2020, 501, 119233–119240. [Google Scholar] [CrossRef]
- Sarma, P.; Gomila, R.M.; Frontera, A.; Barcelo-Oliver, M.; Verma, A.K.; Saikia, S.; Bhattacharyya, M.K. Terephthalato and Succinato Bridged Mn(II) and Zn(II) Coordination Polymers Involving Structure-Guiding H-Bonded Tetrameric Assemblies: Antiproliferative Evaluation and Theoretical Studies. Polyhedron 2022, 224, 115982–115995. [Google Scholar] [CrossRef]
- Akyüz, S. The FT-IR Spectra of Transition Metal 3-Aminopyridine Tetracyanonickelate Complexes. J. Mol. Struct. 1998, 449, 23–27. [Google Scholar] [CrossRef]
- Shirvan, S.A.; Khazali, F.; Dezfuli, S.H.; Borsalani, A. Distorted Square-Based Pyramidal and Trigonal Bipyramidal Geometries in a Mercury(II) Coordination Compound Containing 2-(Aminomethyl) Pyridine Ligand. Mol. Cryst. Liq. Cryst. 2017, 656, 105–112. [Google Scholar] [CrossRef]
- Chan, S.; Wong, W.T. Ruthenium 1992. Coord. Chem. Rev. 1995, 138, 219–296. [Google Scholar] [CrossRef]
- Prashanthi, Y.; Kiranmai, K.; Subhashini, N.J.P. Synthesis, Potentiometric and Antimicrobial Studies on Metal Complexes of Isoxazole Schiff Bases. Spectrochim. Acta Part A 2008, 70, 30–35. [Google Scholar] [CrossRef]
- Batool, S.S.; Ahmad, S.; Khan, I.U.; Ejaz; Harrison, W.T.A. Structural Characterization of a New Copper(II) Complex of 1,10-Phenanthroline and Benzoate [Cu(Phen)(C6H5CO2−)2]. J. Struct. Chem. 2015, 56, 387–391. [Google Scholar] [CrossRef]
- Nashre-ul-Islam, S.M.; Dutta, D.; Guha, A.K.; Bhattacharyya, M.K. An Unusual Werner Type Clathrate of Mn(II) Benzoate Involving Energetically Significant Weak CH⋯C Contacts: A Combined Experimental and Theoretical Study. J. Mol. Struct. 2019, 1175, 130–138. [Google Scholar] [CrossRef]
- de Araújo, E.L.; Barbosa, H.F.G.; Dockal, E.R.; Cavalheiro, É.T.G. Synthesis, Characterization and Biological Activity of Cu(II), Ni(II), and Zn(II) Complexes of Biopolymeric Schiff Bases of Salicylaldehydes and Chitosan. Int. J. Biol. Macromol. 2017, 95, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Aligo, J.A.; Smith, L.; Eglin, J.L.; Pence, L.E. Solution and Solid-State Variation of Cupric Phenanthroline Complexes. Inorg. Chem. 2005, 44, 4001–4007. [Google Scholar] [CrossRef] [PubMed]
- Ekennia, A.C.; Onwudiwe, D.C.; Osowole, A.A.; Olasunkanmi, L.O.; Ebenso, E.E. Synthesis, Biological, and Quantum Chemical Studies of Zn(II) and Ni(II) Mixed-Ligand Complexes Derived from N,N-Disubstituted Dithiocarbamate and Benzoic Acid. J. Chem. 2016, 2016, 5129010–5129025. [Google Scholar] [CrossRef]
- Kalarani, R.; Sankarganesh, M.; Kumar, G.V.; Kalanithi, M. Synthesis, Spectral, DFT Calculation, Sensor, Antimicrobial and DNA Binding Studies of Co(II), Cu(II), and Zn(II) Metal Complexes with 2-Amino Benzimidazole Schiff Base. J. Mol. Struct. 2020, 1206, 127725–127740. [Google Scholar] [CrossRef]
- Ghosh, M.; Majee, A.; Nethaji, M.; Chattopadhyay, T. Syntheses and Characterization of trans-[NiL2(NCS)2][L = 2-(Aminomethyl) Pyridine], trans-[NiL2′(NSC)2][L′ = 2-(2-Aminoethyl) Pyridine] and trans-[NiL2″(NSC)2][L″ = 2-(2-Methylaminoethyl) Pyridine] Complexes: X-ray Single Crystal Structure of trans-[NiL2′(NSC)2][L′ = 2-(2-Aminoethyl) Pyridine]. Inorg. Chim. Acta 2009, 362, 2052–2055. [Google Scholar]
- Yenikaya, C.; Poyraz, M.; Sarı, M.; Demirci, F.; İlkimen, H.; Büyükgüngör, O. Synthesis, Characterization and Biological Evaluation of a Novel Cu(II) Complex with the Mixed Ligands 2,6-Pyridinedicarboxylic Acid and 2-Aminopyridine. Polyhedron 2009, 28, 3526–3532. [Google Scholar] [CrossRef]
- Sizova, O.V.; Ershov, A.Y.; Ivanova, N.V.; Shashko, A.D.; Kuteikina-Teplyakova, A.V. Ru(II) Chloro-bis(bipyridyl) Complexes with Substituted Pyridine Ligands: Interpretation of Their Electronic Absorption Spectra. Russ. J. Coord. Chem. 2003, 29, 494–500. [Google Scholar] [CrossRef]
- de Mesquita, M.E.; Junior, S.A.; Oliveira, F.C.; Freire, R.O.; Júnior, N.B.C.; De Sá, G.F. Synthesis, Spectroscopic Studies and Structure Prediction of the New Tb(3-NH2PIC)3·3H2O Complex. Inorg. Chem. Commun. 2002, 5, 292–295. [Google Scholar] [CrossRef]
- Batool, S.S.; Harrison, W.T.A.; Syed, Q.; Haider, M.S. Syntheses and Crystal Structures of Mixed-Ligand Copper(II)–Imidazole–Carboxylate Complexes. J. Coord. Chem. 2018, 71, 1380–1391. [Google Scholar] [CrossRef]
- Nath, H.; Sharma, P.; Frontera, A.; Barcelo-Oliver, M.; Verma, A.K.; Das, J.; Bhattacharyya, M.K. Phenanthroline-Based Ni(II) Coordination Compounds Involving Unconventional Discrete Fumarate-Water-Nitrate Clusters and Energetically Significant Cooperative Ternary π-Stacked Assemblies: Antiproliferative Evaluation and Theoretical Studies. J. Mol. Struct. 2022, 1248, 131424–131440. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, S.; Gao, S. Two Mn(II) Chloride Complexes Containing Guest Molecules. J. Therm. Anal. Calorim. 2007, 89, 567–571. [Google Scholar] [CrossRef]
- Al-Fakeh, M.S.; Allazzam, G.A.; Yarkandi, N.H. Ni(II), Cu(II), Mn(II), and Fe(II) Metal Complexes Containing 1,3-Bis(Diphenylphosphino)Propane and Pyridine Derivative: Synthesis, Characterization, and Antimicrobial Activity. Int. J. Biomater. 2021, 2021, 4981367–4981381. [Google Scholar] [CrossRef]
- Porterfield, J.P.; Bross, D.H.; Ruscic, B.; Thorpe, J.H.; Nguyen, T.L.; Baraban, J.H.; Stanton, J.F.; Daily, J.W.; Ellison, G.B. Thermal Decomposition of Potential Ester Biofuels. Part I: Methyl Acetate and Methyl Butanoate. J. Phys. Chem. A 2017, 121, 4658–4677. [Google Scholar] [CrossRef]
- Mirabella, F.M. Modern Techniques in Applied Molecular Spectroscopy, 14th ed.; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- SADABS, V2.05; Bruker AXS: Madison, WI, USA, 1999.
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–117. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond 3.1f.; Crystal Impact GbR: Bonn, Germany, 2008. [Google Scholar]
- Ahlrichs, R.; Bär, M.; Häser, M.; Horn, H.; Kölmel, C. Electronic Structure Calculations on Workstation Computers: The Program System TURBOMOLE. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A. 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Caldeweyher, E.; Mewes, J.-M.; Ehlert, S.; Grimme, S. Extension and evaluation of the D4 London-dispersion model for periodic systems. Phys. Chem. Chem. Phys. 2020, 22, 8499–8512. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyze. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, J.W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graphics. 1996, 14, 33–38. [Google Scholar] [CrossRef]

| D–H⋯A | d(D⋯A) | d(H⋯A) | <(DHA) |
|---|---|---|---|
| C7B–H7B∙∙∙Cl2 | 3.701(4) | 2.92(1) | 139.3(3) |
| O1–H1∙∙∙O2 | 2.635(5) | 1.79(3) | 177.2(3) |
| C8B–H8B∙∙∙Cl1 | 3.566(6) | 2.75(1) | 144.3(3) |
| C4B–H4B∙∙∙Cl1 | 3.502(5) | 2.81(1) | 130.4(3) |
| D–H⋯A | d(D⋯A) | d(H⋯A) | <(DHA) |
|---|---|---|---|
| N2–H2B∙∙∙O2 | 2.906(4) | 2.03(2) | 168.435(2) |
| C19–H19∙∙∙O2 | 3.623(5) | 2.80(3) | 145.031(2) |
| C20–H20∙∙∙O4 | 3.197(4) | 2.58(2) | 122.597(2) |
| C16–H16B∙∙∙O1 | 3.522(6) | 2.68(3) | 143.144(3) |
| Parameters | 1 | 2 |
|---|---|---|
| Formula | C31H21Cl3MnN4O2 | C26H26N4O4Zn |
| Formula weight | 642.81 | 523.88 |
| Temp, [K] | 100 | 100 |
| Crystal system | Triclinic | Monoclinic |
| Space group | Cc | |
| a, [Å] | 10.6563(18) | 9.9347(14) |
| b, [Å] | 10.9066(19) | 23.521(3) |
| c, [Å] | 12.790(2) | 10.5889(15) |
| α, [°] | 89.159(9) | 90 |
| β, [°] | 66.391(7) | 93.195(4) |
| γ, [°] | 86.483(8) | 90 |
| V, [Å3] | 1359.4(4) | 2470.5(6) |
| Z | 2 | 4 |
| Absorption coefficient (mm−1) | 6.977 | 1.709 |
| F(0 0 0) | 654.0 | 1088.0 |
| ρcalcg/cm3 | 1.570 | 1.409 |
| index ranges | −12 ≤ h ≤ 12, −12 ≤ k ≤ 13, | −11 ≤ h ≤ 11, −28 ≤ k ≤ 28, |
| −15 ≤ l ≤ 15 | −12 ≤ l ≤ 12 | |
| Crystal size, [mm3] | 0.38 × 0.28 × 0.25 | 0.38 × 0.31 × 0.15 |
| 2θ range, [°] | 8.122 to 137.828 | 9.676 to 136.904 |
| Independent Reflections | 4832 | 4216 |
| Reflections collected | 53,812 | 26,679 |
| Refinement method | Fullmatrix | Fullmatrix |
| Leastsquares for F2 | Leastsquares for F2 | |
| Data/restraints/parameters | 4832/0/371 | 4216/2/319 |
| Goodness-of-fit on F2 | 1.060 | 0.831 |
| Final R indices [I > 2σ(I)] | R1 = 0.0773, wR2 = 0.2158 | R1 = 0.0281, wR2 = 0.0725 |
| R indices (all data) | R1 = 0.0812, wR2 = 0.2233 | R1 = 0.0281, wR2 = 0.0725 |
| Largest hole and peak [e·Å−3] | 1.00/−1.06 | 0.73/−0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boro, M.; Banik, S.; Gomila, R.M.; Frontera, A.; Barcelo-Oliver, M.; Bhattacharyya, M.K. Supramolecular Assemblies in Mn(II) and Zn(II) Metal–Organic Compounds Involving Phenanthroline and Benzoate: Experimental and Theoretical Studies. Inorganics 2024, 12, 139. https://doi.org/10.3390/inorganics12050139
Boro M, Banik S, Gomila RM, Frontera A, Barcelo-Oliver M, Bhattacharyya MK. Supramolecular Assemblies in Mn(II) and Zn(II) Metal–Organic Compounds Involving Phenanthroline and Benzoate: Experimental and Theoretical Studies. Inorganics. 2024; 12(5):139. https://doi.org/10.3390/inorganics12050139
Chicago/Turabian StyleBoro, Mridul, Subham Banik, Rosa M. Gomila, Antonio Frontera, Miquel Barcelo-Oliver, and Manjit K. Bhattacharyya. 2024. "Supramolecular Assemblies in Mn(II) and Zn(II) Metal–Organic Compounds Involving Phenanthroline and Benzoate: Experimental and Theoretical Studies" Inorganics 12, no. 5: 139. https://doi.org/10.3390/inorganics12050139
APA StyleBoro, M., Banik, S., Gomila, R. M., Frontera, A., Barcelo-Oliver, M., & Bhattacharyya, M. K. (2024). Supramolecular Assemblies in Mn(II) and Zn(II) Metal–Organic Compounds Involving Phenanthroline and Benzoate: Experimental and Theoretical Studies. Inorganics, 12(5), 139. https://doi.org/10.3390/inorganics12050139










