Abstract
A family of silver(I) and copper(I) complexes containing carboxylate ligands were prepared from the corresponding carboxylic acids and Ag2O. The compounds were characterized by various spectroscopic methods and X-ray diffraction. In the solid state, the silver(I) salts are coordination polymers based on dinuclear silver species with bridging carboxylate ligands. The reaction of these silver salts with Ph3P gives four-coordinate, tetrahedral bis(phosphine) complexes. Analogous copper(I) bis(phosphine) compounds were prepared by the reduction of copper(II) carboxylates with Ph3P. Decomposition temperatures and thermal decomposition products were studied by TGA/DSC measurements. The metal compounds decomposed cleanly to their respective metals (silver or copper) at temperatures ranging from 206 to 338 °C.
1. Introduction
The use of silver(I) oxide both as a base and source of silver ions in organometallic chemistry was pioneered by Ivan Lin, demonstrating that N-heterocyclic carbene complexes of silver can be easily prepared from imidazolium salts and Ag2O (Figure 1) [1]. This method is in fact so general that it has become known as the silver oxide route.
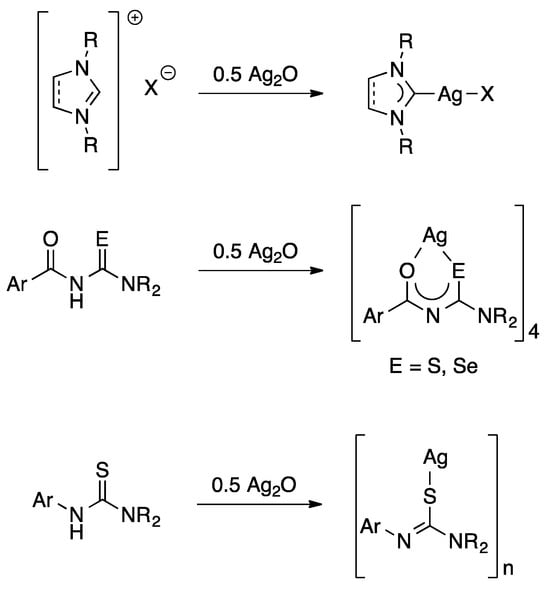
Figure 1.
General examples of reactions involving Ag2O both as a base and source of silver ions.
More recently, our group showed that Ag2O also reacts with acylthioureas and acylselenoureas of the type ArC(O)NHC(E)NR2 (E = S, Se) to give tetranuclear silver(I) clusters (Figure 1) [2]. We have subsequently extended this approach to other thiourea derivatives and perfluorinated carboxylic acids [3,4]. In all these reactions, water is the only side product and no additional base is required, which simplifies the work-up and increases both atom economy and yield. We have now extended this work to include other carboxylic acid derivatives and report herein their structures, reactivity and thermal behaviour.
2. Results and Discussion
Initially, we examined the reaction of glutarimide with Ag2O in water as an alternative route to obtain silver glutarimidate. Indeed, when refluxing an aqueous solution of glutarimide with Ag2O, a colourless, insoluble product was isolated. The IR spectrum of this material was, however, very different from that previously reported for silver glutarimidate [5]. The IR spectrum of our isolated product features two bands at 3398 and 3203 cm−1, which are typical for NH2 groups. Furthermore, there are intense bands at 1661 and 1515 cm−1, consistent with the presence of both an amide functionality and a metal-bound carboxylate. These data suggest that under these reaction conditions, glutarimide undergoes hydrolytic ring-opening forming glutaric acid monoamide, which, in its deprotonated form, coordinates to the silver ion (Scheme 1). Thus, the product may be formulated as the polymer [Ag{O2C(CH2)3C(O)NH2}]n (1). Whilst we were unable to characterize the insoluble material by single-crystal X-ray diffraction or NMR spectroscopy, the molecular structure of its bis(triphenylphosphine) derivative (see below) unambiguously confirms the presence of a glutaric acid monoamide O-bound to silver. Although the formation of glutaric acid monoamide by heating glutarimide in the presence of NaOH in water has been known for some time [6], complex 1 appears to be the first reported metal complex containing a glutaric acid monoamide ligand derived from the in situ hydrolytic ring-opening of glutarimide. It is also important to note that the reaction of glutarimide with AgNO3 and NaOH at room temperature affords the coordination polymer [Ag(glutarimidato)]n, in which the glutarimide ring remains intact [7].
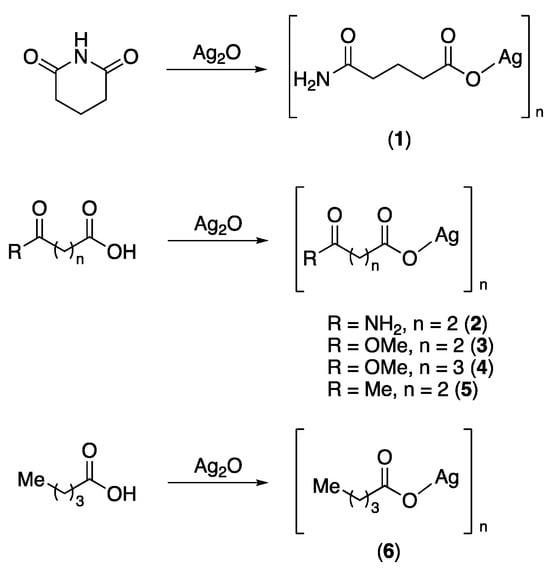
Scheme 1.
Synthesis of polymeric silver(I) salts.
In order to learn more about such silver carboxylates, we examined the reactivity of other structurally related carboxylic acids including succinic acid monoamide, succinic acid monomethyl ester, glutaric acid monomethyl ester, levulinic acid and valeric acid with silver(I) oxide. Thus, heating aqueous solutions of these carboxylic acid derivatives with Ag2O results in formation of solutions, out of which the colourless silver salts 2–6 are deposited upon standing in the dark for several days (Scheme 1).
These silver carboxylates are all colourless solids, which appear to be light-stable for several weeks. As is typical for silver salts, they tend to be poorly soluble or insoluble in common solvents. Some of the complexes reported here are, however, slightly soluble in hot water, out of which they crystalize or precipitate on cooling. An exception seems to be the succinic acid monomethyl ester salt (3), which is quite soluble in water even at room temperature, allowing us to record proton- and carbon-NMR spectra. In addition, IR spectroscopy was used to characterize compounds 1–6. Most diagnostic are the signals in the carbonyl region of the spectrum, where we could identify the C=O band of the amide, ester or ketone as well as the asymmetric and the symmetric OCO bands of the carboxylate group. In principle, the difference between the asymmetric and symmetric OCO bands (Δ) of a carboxylate group can be used to identify its coordination mode [8,9]. However, the method is of course only reliable when both bands can be unambiguously identified, which is often not trivial. In complexes 1–6, the values for Δ range from 120 to 190 cm−1, consistent with a bridging, bidentate coordination mode. Similar values have been reported by others for structurally related silver carboxylates [10]. In addition, we were able to determine the molecular structures of [Ag{O2C(CH2)2C(O)NH2}]n (2) and [Ag{O2C(CH2)3C(O)OMe}]n (4) by single-crystal X-ray diffraction experiments, which indeed confirmed the bridging bidentate O,O-coordination mode (Figure 2).
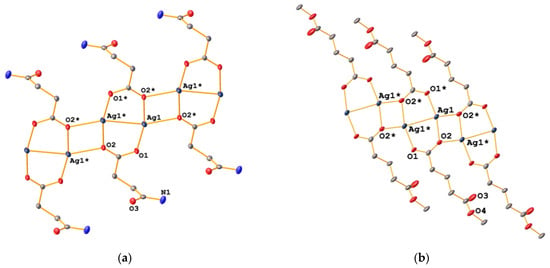
Figure 2.
Molecular structures of 2 (a) and 4 (b). Atoms generated by symmetry are indicated by an asterisk. Ellipsoids are drawn at the 50% level and hydrogen atoms have been omitted for clarity.
In both cases, the salts are coordination polymers consisting of dinuclear silver units containing two bridging carboxylate ligands with Ag···Ag distances of about 2.08 Å, resulting in eight-membered rings. In addition, one of the carboxylate oxygen atoms binds to a silver atom of a neighbouring ring, resulting in the formation of a polymeric sheet-like structure. This motif has been previously observed in other silver alkyl-carboxylates including silver acetate [11], silver tiglate [12] and silver stearate [13]. The 2-D structure of 2 is further assembled through short Ag···Ag contacts of ca. 3.09 Å between silver atoms in neighbouring polymer sheets. Such argentophilic interactions with Ag···Ag distances shorter than the van der Waals contact of 3.44 Å are often observed in solid-state structures of silver(I) compounds [14]. Furthermore, there is intermolecular hydrogen bonding between an NH-proton and the amide carbonyl oxygen atom in the adjacent sheet. In contrast, the 2-D assembly of 4 is achieved solely through Ag-O bonds between the different sheets. As a result, the sheets of 2 are flat, whilst those of 4 have a zig-zag-shape (Figure 3).
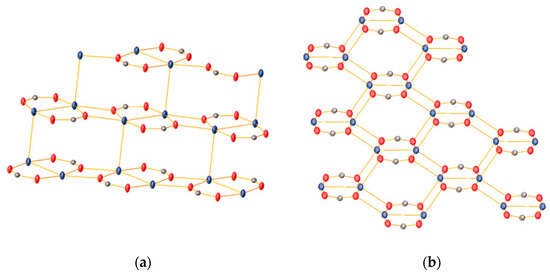
Figure 3.
The 2-D sheet structures of 2 (a) and 4 (b). Only the silver atoms and the bridging carboxylate groups are shown for clarity. Atom colours: Ag (teal), O (red) and C (grey).
TGA scans of the six silver carboxylates show a single decomposition process commencing at temperatures ranging from 206 to 254 °C (Figure 4a).
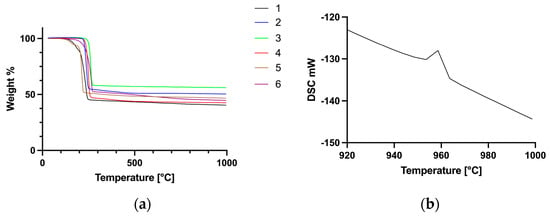
Figure 4.
TGA traces of complexes 1–6 (a) and an expansion of the DSC scan of 5, showing the endothermic peak corresponding to the melting of metallic silver (b).
The remaining mass in each case corresponds to elemental silver, which is confirmed by an endothermic peak at 961 °C in the DSC scan, consistent with the melting point of metallic silver (Figure 4b). In the case of compound 5, the enthalpy of melting calculated from the DSC data was determined to be 11.1 kJ mol−1, which agrees well with 11.3 kJ mol−1 reported for silver metal. Out of these six compounds, silver levulinate (5) has the lowest onset temperature of decomposition (206 °C). Overall, the range of temperatures of decomposition is quite narrow, indicating that amongst these six compounds, the nature of the carboxylate ligand has little influence on the decomposition temperature.
Since these silver carboxylates are poorly soluble or insoluble in most solvents, they were converted into their respective bis(triphenylphosphine) complexes. The silver carboxylates readily dissolve in CH2Cl2 solutions containing two equivalents of Ph3P to afford the bis(phosphine) complexes [(Ph3P)2Ag{O2C(CH2)nC(O)R}] (n = 2 or 3; R = NH2, OMe, Me) 1a–5a and [(Ph3P)2Ag{O2C(CH2)3Me}] (6a) (Scheme 2).
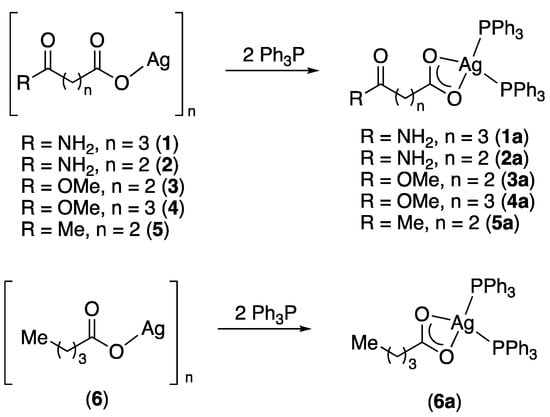
Scheme 2.
Synthesis of silver bis(triphenylphosphine) complexes.
The phosphine complexes are soluble in a variety of organic solvents including CH2Cl2, CHCl3, toluene, alcohols and acetone, allowing for their characterization in solution by NMR spectroscopy. In the proton NMR spectra of compounds 1a–6a, signals from the respective carboxylates and Ph3P can be observed in a 1:2 ratio. Most diagnostic are the 31P-NMR spectra, in which singlet resonances are observed, the chemical shifts of which are consistent with Ph3P bound to silver. The observed shifts of around 8 ppm are similar to what has been reported for related bis(triphenylphosphine) complexes of silver carboxylates [12,15,16]. At room temperature, no coupling between 31P and the 107/109Ag nuclei is observed; however, upon cooling a sample of 3a to −50 °C, two doublets due to P-Ag coupling begin to evolve. Such dynamic behaviour is often seen in bis(phosphine) complexes of silver carboxylates and has been studied by variable-temperature NMR spectroscopy in the myristate complex [(Ph3P)2Ag{O2C(CH2)12Me}], where the Ag-P coupling constants were determined to be 472 and 414 Hz [16].
In compounds 1a–6a, the carboxylate ligand is O,O-chelating towards the metal, which is reflected in their IR spectra. The values for Δ in the bis(phosphine) complexes range from 149 to 164 cm−1, consistent with such a chelating coordination mode [9]. This was indeed confirmed by X-ray diffraction studies of several complexes. The molecular structures of 1a, 2a, 5a and 6a are depicted in Figure 5 and Figure 6. Given their similarity, the structures will be discussed together in the following section.
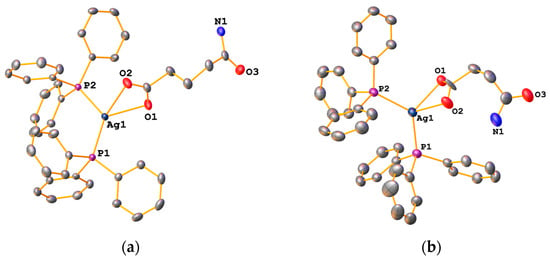
Figure 5.
Molecular structures of 1a (a) and 2a (b). Ellipsoids are drawn at the 50% level. Hydrogen atoms have been omitted for clarity.
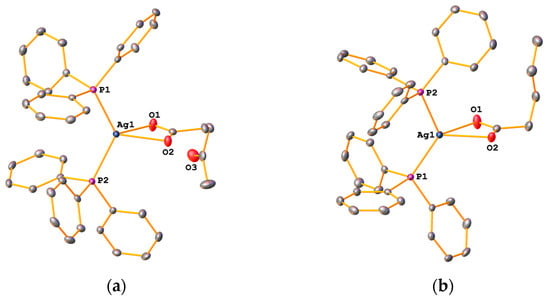
Figure 6.
Molecular structures of 5a (a) and 6a (b). Ellipsoids are drawn at the 50% level. Hydrogen atoms have been omitted for clarity.
The complexes consist of a silver atom coordinated to two Ph3P molecules as well as to two oxygen atoms from the deprotonated carboxylic acid in a distorted trigonal pyramidal manner. Houser introduced the τ4-parameter to describe structures of four-coordinate transition-metal compounds [17]. From the crystallographic data, we computed τ4-values of 0.78 and 0.80 (Table 1), which are reasonably close to the τ4-value of 0.85 expected for an ideal trigonal pyramidal structure. The two Ag-O bond lengths are slightly different, with values of around 2.5 Å and 2.3 Å for the longer and shorter bond lengths, respectively (Table 1). The P-Ag-P and O-Ag-P angles at the metal centre are substantially different, the former being around 126°, the latter ca. 53°, reflecting the distorted trigonal pyramidal environment.

Table 1.
Selected structural parameters of the silver bis(triphenylphosphine) complexes.
In other silver(I) bis(triphenylphosphine) carboxylate complexes including [(Ph3P)2Ag{O2C(CH2)nMe}] (n = 13, 16) and [(Ph3P)2Ag(O2CCH2tBu)], similar values are found [16,18,19]. It should be noted that in the structure of compound 2a (Figure 5b), the succinic acid monoamide group is disordered over two positions, resulting in two slightly different long Ag-O distances (2.53 and 2.63 Å). One would be tempted to suggest that in one position the succinate is O,O-chelating, whilst in the other it is O-monodentate. However, this is not the case: in structures where the carboxylate ligand is monodentate, the long Ag-O distances are greater than 2.9 Å.
The amide-derivative 1a packs such that dimers are formed by two intermolecular H-bonds (N-H···O = ca. 2.97 Å) between the amide NH2 group and the carbonyl oxygen atom (Figure 7). Furthermore, the second NH2-proton forms a hydrogen bond with the oxygen atom of a neighbouring carboxylate group (N-H···O = ca. 2.91 Å). A similar packing motif, dimer formation through N-H···O bonds, is also observed in the structure of 2a.
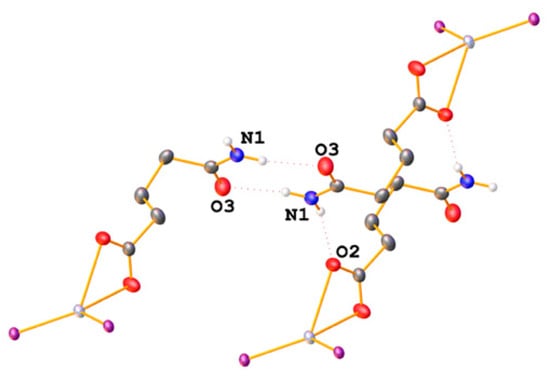
Figure 7.
Intermolecular hydrogen bonds in the packing of 1a. Only hydrogen atoms involved in H-bonding are depicted and only the phosphorus atoms of the Ph3P ligands are shown for clarity.
TGA scans of the bis(triphenylphosphine) complexes 1a–6a (Figure 8a) show a single decomposition step leading to metallic silver commencing between 287 and 313 °C. As above, the formation of metallic silver was confirmed from the melting temperature and enthalpy of melting from DCS data (shown for 3a in Figure 8b). It is apparent that the nature of the carboxylate ligand in this series of compounds has no significant effect on the temperature of decomposition onset.
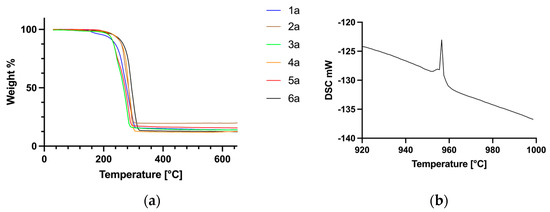
Figure 8.
TGA curves of the bis(phosphine) complexes 1a–6a (a) and an expansion of the DSC trace of 3a (b).
In addition to the silver(I) compounds discussed above, we also examined the copper(I) counterparts of levulinic and valeric acid for comparison. The bis(triphenylphosphine) copper(I) complexes were prepared from the respective copper(II) carboxylates by reduction with Ph3P in wet MeOH (Scheme 3) [20,21]. Compound 8a has recently also been prepared from [Cu(PPh3)2(NO3)] and levulinic acid in hexane; the reported spectroscopic data, however, are somewhat different to what we observe [22]. In contrast to the silver compounds, the copper counterparts could not be prepared from the reaction between the acid derivatives and copper(I) oxide.
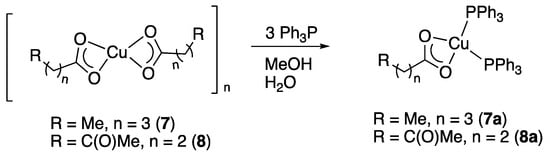
Scheme 3.
Synthesis of copper(I) bis(triphenylphosphine) complexes.
In the solid state, these copper(I) compounds are stable towards oxidation but solutions turn green-blue after several days. The diamagnetic species could be characterized by NMR spectroscopy as well as IR spectroscopy and X-ray diffraction. In the 31P-NMR spectra of 7a and 8a, singlet resonances are observed with chemical shifts of around −2.9 ppm. Similar chemical shifts have been reported for bis(triphenylphosphine) complexes containing ethylene glycol-functionalized carboxylates [20]. From the proton NMR spectra, a 2:1 ratio of phosphine to carboxylate ligands is evident. Furthermore, IR data suggest an O,O-chelating coordination mode of the carboxylate ligands, which was confirmed by X-ray diffraction. The molecular structures of 7a and 8a (Figure 9) are quite similar and will be discussed together.
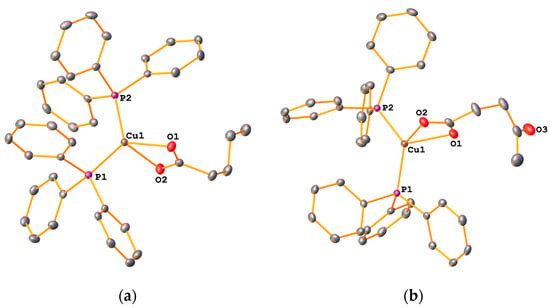
Figure 9.
Molecular structure of 7a (a) and 8a (b). Ellipsoids are drawn at the 50% levels. Hydrogen atoms have been omitted for clarity.
The complexes consist of a four-coordinate copper centre containing two Ph3P ligands as well as the O,O-chelating carboxylate. The geometry at the metal can be described as distorted trigonal pyramidal. The τ4-values for these complexes are 0.80 and 0.82, comparable to those of the silver compounds discussed above. Whilst the Cu-P bond lengths are similar in each molecule, there are two different Cu-O bonds, the longer being around 2.2 Å and the shorter one around 2.13 Å. There are not many reported structures of bis(triphenylphosphine) copper(I) complexes with alkyl-chain carboxylates available for comparison. In the vinyl acetate complex [(Ph3P)2Cu(O2CCH2CHCH2)] as well as in the polyethylene glycol-functionalized carboxylate [(Ph3P)2Cu{O2CCH2O(CH2)2OMe}], the Cu-O and Cu-P bond distances are quite similar to those observed here [20,23]. The TGA curves of 7a and 8a (Figure 10) show a single decomposition step commencing at 338 °C (7a) and 299 °C (8a) leading to the formation of elemental copper, based on the observed residual mass. DSC data indicate a very weak endothermic signal roughly corresponding to the melting point of copper.
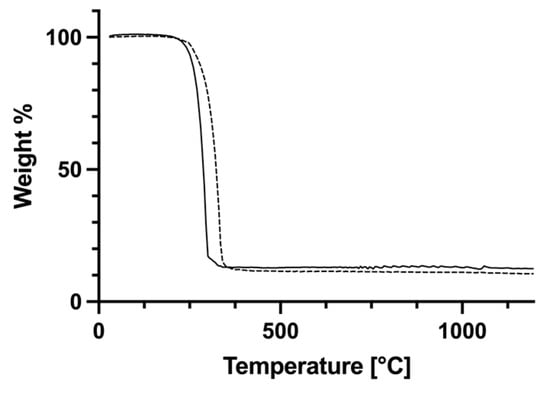
Figure 10.
TGA curves of complexes 7a (dashed line) and 8a (solid line).
Metal-containing compounds with such thermal properties (i.e., clean decomposition to the metal at not too high temperatures) are potentially suitable ink precursors for the inkjet printing of metallic patterns [24,25]. Related phosphine complexes containing silver(I) or copper(I) have indeed been used for this purpose [26,27,28]. Crucial for any useful inkjet ink is of course its formulation. An optimal concentration of the metal compound is needed and the solvent system itself must have a specific viscosity, volatility and surface tension for optimal droplet formation during printing. While we have not undertaken any optimization to date, some very preliminary experiments have shown that the silver(I) levulinate complex (5a) dissolved in nBuOH can in principle be used to inkjet print a silver pattern onto a glass substrate. Further optimization of ink formulations using the compounds reported herein and investigating suitable conditions for inkjet printing is currently ongoing.
3. Materials and Methods
3.1. General Methods
Unless specified otherwise, reactions were carried out under ambient conditions using HPLC-grade solvents in vessels shielded from light. All chemicals and solvents were commercial products and were used as received. The copper(II) carboxylates were prepared from Cu(OAc)2⋅H2O and the respective carboxylic acids in toluene as previously described [20]. NMR spectra were measured on a Bruker (Rheinstetten, Germany) Avance 400 instrument. Spectra were referenced externally to Me4Si (1H, 13C) or 85% H3PO4 (31P). IR spectra were recorded on a ThermoFisher Scientific (Waltham, MA, USA) Nicolet iS5 spectrometer equipped with an iD7 diamond ATR unit. TGA and DSC measurements were simultaneously carried out using a Netzsch (Selb, Germany) STA 449 F5 Jupiter instrument. Experiments were conducted in 40 μL alumina crucibles, which were closed with alumina lids. Samples were heated from 25 °C to 1000 °C or 1200 °C with a heating rate of 5 K min−1 in a nitrogen atmosphere, applying a constant nitrogen flow of 25 mL min−1 during the experiment.
3.2. Synthesis of Silver Carboxylates 1–6
[Ag{O2C(CH2)3C(O)NH2}]n (1). To a solution of glutarimide (0.573 g, 5.07 mmol) in H2O (30 mL), Ag2O (0.667 g, 2.93 mmol) was added and the mixture was heated to reflux for ca. 2 h. The hot solution was filtered through Celite paper and the filtrate was subsequently left to stand in the dark. The resulting colourless crystals were isolated by filtration, washed with water and dried in air. The product was found to be insoluble in all common solvents. A total of 1.044 g (87%) of product was isolated. IR (ATR): 3398, 3204 ν(NH2), 1661 ν (CO amide), 1516 νa(OCO), 1325 νs(OCO) cm−1.
[Ag{O2C(CH2)2C(O)NH2}]n (2). To a solution of succinic acid monoamide (0.205 g, 1.75 mmol) in H2O (20 mL), Ag2O (0.203 g, 0.876 mmol) was added. The mixture was heated to reflux for ca. 10 min by which time most of the silver(I) oxide had dissolved. The hot solution was filtered and the filtrate was left to stand protected from light. After two days, colourless crystals were deposited, which were isolated by filtration and dried in air. A total of 0.233 g (59%) of product was isolated. IR (ATR): 3377, 3179 ν(NH2), 1662 ν(CO amide), 1511 νa(OCO), 1392 νs(OCO) cm−1. X-ray quality crystals were selected from the bulk sample.
[Ag{O2C(CH2)2C(O)OMe}]n (3). To a solution of succinic acid monomethyl ester (0.511 g, 3.87 mmol) in H2O (20 mL), Ag2O (0.448 g, 1.93 mmol) was added. The mixture was heated to reflux for ca. 30 min by which time the black suspension had turned into a cream-coloured suspension. The solid was isolated by filtration and was dried in air. A total of 0.310 g (34%) of product was isolated. IR (ATR): 1724 ν(CO ester), 1507 νa(OCO), 1358 νs(OCO) cm−1. 1H-NMR (400 MHz, D2O): δ = 2.47–2.53 (m, 2 H, CH2), 2.56–2.62 (m, 2 H, CH2), 3.67 (s, 3 H, OMe). 13C-NMR (101 MHz, D2O): δ = 31.81 (CH2), 32.92 (CH2), 54.05 (OMe), 178.21 (CO ester), 181.87 (CO carboxylate).
[Ag{O2C(CH2)3C(O)OMe}]n (4). To a solution of glutaric acid monomethyl ester (0.298 g, 2.04 mmol) in H2O (30 mL), Ag2O (0.235 g, 1.01 mmol) was added. The mixture was heated to reflux for ca. 30 min, by which time most of the silver(I) oxide had dissolved. The hot solution was filtered and the filtrate was left to stand protected from light. After two days, colourless crystals were deposited, which were isolated by filtration and dried in air. A total of 0.228 g (44%) of product was isolated. IR (ATR): 1724 ν(CO ester), 1499 νa(OCO), 1325 νs(OCO) cm−1. X-ray-quality crystals were selected from the bulk sample.
[Ag{O2C(CH2)2C(O)Me}]n (5). To a solution of levulinic acid (0.406 g, 1.75 mmol) in H2O (30 mL), Ag2O (0.406 g, 1.75 mmol) was added. The mixture was heated to reflux for ca. 30 min, by which time most of the silver(I) oxide had dissolved. The hot solution was filtered and the filtrate was left to stand protected from light. After two days, colourless crystals were deposited, which were isolated by filtration and dried in air. A total of 0.836 g (75%) of product was isolated. IR (ATR): 1697 ν(CO ketone), 1507 νa(OCO), 1356 νs(OCO) cm−1.
[Ag{O2C(CH2)3Me}]n (6). To a solution of valeric acid (0.511 g, 5.00 mmol) in H2O (25 mL), Ag2O (0.579 g, 2.50 mmol) was added. The mixture was heated to reflux for ca. 30 min, by which time most of the silver(I) oxide had dissolved. The hot solution was filtered and the filtrate was left to stand protected from light. After two days, colourless crystals were deposited, which were isolated by filtration and dried in air. A total of 0.272 g (26%) of product was isolated. IR (ATR): 1508 νa(OCO), 1357 νs(OCO) cm−1.
3.3. Synthesis of Bis(Triphenylphosphine) Silver(I) Complexes 1a–6a
[(Ph3P)2Ag{O2C(CH2)3C(O)NH2}] (1a). To a suspension of [Ag{O2C(CH2)3C(O)NH2}]n (0.110 g, 0.462 mmol) in CH2Cl2 (20 mL), Ph3P (0.262 g, 1.00 mmol) was added. After ca. 2 h at room temperature, all solids had dissolved. The colourless solution was passed through Celite and the filtrate was evaporated to a small volume. The addition of hexanes caused the precipitation of colourless crystals, which were isolated by filtration and dried in air. A total of 0.316 g (89%) of colourless crystals were obtained. IR (ATR): 3305, 3155 ν(NH2), 1676 ν(CO amide), 1547 νa(OCO), 1394 νs(OCO) cm−1. 1H-NMR (400 MHz, CD2Cl2): δ = 7.34–7.49 (m, 30 H, Ph3P), 7.06 (br. s, 1 H NH2), 5.08 (br. s, 1 H NH2), 2.32 (t, J = 7.1 Hz, 2 H, CH2), 2.12 (t, J = 7.1 Hz, 2 H, CH2), 1.88 (pent., J = 7.1 Hz, 2 H, CH2). 13C-NMR (101 MHz, CD2Cl2): δ = 23.38 (CH2), 36.01 (CH2), 36.57 (CH2), 126.16 (d, J = 9.5 Hz, m-Ph3P), 130.52 (p-Ph3P), 132.98 (d, J = 25.3 Hz, ipso-Ph3P), 134.21 (d, J = 16.7 Hz, o-Ph3P), 176.20 (CO amide), 179.86 (CO carboxylate). 31P-NMR (162 MHz, CD2Cl2): δ = 7.86 (s). X-ray-quality crystals were obtained by recrystallization from iPrOH.
[(Ph3P)2Ag{O2C(CH2)2C(O)NH2}] (2a). This was prepared as described above using [Ag{O2C(CH2)2C(O)NH2}]n (0.066 g, 0.295 mmol) and Ph3P (0.155 g, 0.591 mmol). Colourless plates were isolated at an 88% yield (0.194 g). IR (ATR): 3300 ν(NH2), 1689 ν(CO amide), 1553 νa(OCO), 1393 νs(OCO) cm−1. 1H-NMR (400 MHz, CD2Cl2): δ = 2.38–2.43 (m, 2 H, CH2), 2.47–2.54 (m, 2 H, CH2), 4.91 (br. s, 1 H, NH2), 7.33–7.51 (m, 30 H, Ph3P), 7.74 (br. s, 1 H, NH2). 13C-NMR (101 MHz, CD2Cl2): δ = 33.57 (CH2), 33.87 (CH2), 129.21 58 (d, J = 9.8 Hz, m-Ph3P), 130.65 (d, J = 1.6 Hz, p-Ph3P), 132.67 (d, J = 27.2 Hz, ipso-Ph3P), 134.21 (d, J = 16.8 Hz, o-Ph3P), 176.70 (CO amide), 179.35 (CO carboxylate). 31P-NMR (162 MHz, CD2Cl2): δ = 9.04 (s). X-ray-quality crystals were selected from the bulk sample.
[(Ph3P)2Ag{O2C(CH2)2C(O)OMe}] (3a). This was prepared as described above using [Ag{O2C(CH2)2C(O)OMe}]n (0.050 g, 0.21 mmol) and Ph3P (0.108 g, 0.41 mmol). Colourless crystals were isolated at a 71% yield (0.114 g). IR (ATR): 1741 ν(CO ester), 1563 νa(OCO), 1406 νs(OCO) cm−1. 1H-NMR (400 MHz, CD2Cl2): δ = 7.34–7.48 (m, 30 H, Ph3P), 3.63 (s, 3 H, OMe), 2.47–2.61 (m, 4 H, CH2). 13C-NMR (101 MHz, CD2Cl2): δ = 31.56 (CH2), 32.23 (CH2), 51.43 (OMe), 129.16 (d, J = 9.8 Hz, m-Ph3P), 130.54 (p-Ph3P), 132.80 (d, J = 26.8 Hz, ipso-Ph3P), 134.23 (d, J = 15.9 Hz, o-Ph3P), 174.56 (CO ester), 178.36 (CO carboxylate). 31P-NMR (162 MHz, CD2Cl2): δ = 8.67 (s).
[(Ph3P)2Ag{O2C(CH2)3C(O)OMe}] (4a). This was prepared as described above using [Ag{O2C(CH2)3C(O)OMe}]n (0.046 g, 0.182 mmol) and Ph3P (0.095 g, 0.362 mmol). A total of 0.098 g (86%) of product was isolated. IR (ATR): 1730 ν(C=O ester), 1549 νa(OCO), 1398 νs(OCO) cm−1. 1H-NMR (400 MHz, CD2Cl2): δ = 7.33–7.49 (m, 30 H, Ph3P), 3.68 (s, 3 H, OMe), 2.36 (t, J = 7.2 Hz, 2 H, γ-CH2), 2.25 (t, J = 7.2 Hz, 2 H, α-CH2), 1.90 (pent., J = 7.3 Hz, 2 H, β-CH2). 13C{1H}-NMR (101 MHz, CD2Cl2): δ = 22.68 (β-CH2), 34.29 (γ-CH2), 36.66 (α-CH2), 51.39 (OMe), 129.15 (d, J = 9.6 Hz, m-Ph3P), 131.51 (d, J = 1.7 Hz, p-Ph3P), 132.96 (d, J = 26.1 Hz, ipso-Ph3P), 134.24 (d, J = 16.8 Hz, o-Ph3P), 174.50 (CO ester), 179.42 (CO carboxylate). 31P-NMR (162 MHz, CD2Cl2): δ = 8.45 (s). X-ray-quality crystals were selected from the bulk sample.
[(Ph3P)2Ag{O2C(CH2)2C(O)Me}] (5a). This was prepared as described above using [Ag{O2C(CH2)2C(O)Me}]n (0.104 g, 0.47 mmol) and Ph3P (0.250 g, 0.95 mmol). A total of 0.317 g (95%) of product was isolated. IR (ATR): 1718 ν(CO ketone), 1557 νa(OCO), 1393 νs(OCO) cm−1. 1H-NMR (400 MHz, CD2Cl2): d = 7.30–7.44 (m, 30 H, Ph3P), 2.64 (t, J = 6.9 Hz, 2 H, CH2), 2.43 (t, J = 6.9 Hz, 2 H, CH2), 2.09 (s, 3 H, Me). 13C{1H}-NMR (101 MHz, CD2Cl2): d = 29.89 (Me), 31.73 (CH2), 41.05 (CH2), 129.15 (d, J = 9.8 Hz, m-Ph3P), 130.49 (d, J = 1.6 Hz, p-Ph3P), 132.98 (d, J = 25.7 Hz, ipso-Ph3P), 134.24 (d, J = 16.9 Hz, o-Ph3P), 178.66 (CO carboxylate), 209.11 (CO ketone). 31P-NMR (162 MHz, CD2Cl2): d = 8.03 (s). X-ray-quality crystals were selected from the bulk sample.
[(Ph3P)2Ag{O2C(CH2)3Me}] (6a). This was prepared as described above using [Ag{O2C(CH2)3Me}]n (0.099 g, 0.48 mmol) and Ph3P (0.252 g, 0.96 mmol). A total of 0.309 g (88%) of product was isolated. IR (ATR): 1585 νa(OCO), 1478 νs(OCO) cm−1. 1H-NMR (400 MHz, CD2Cl2): δ = 7.41–7.49 (m, 18 H, m/p-Ph3P), 7.32–7.39 (m, 12 H, o-Ph3P), 2.23 (t, J = 7.5 Hz, 2 H, α-CH2), 1.61 (pent., J = 7.3 Hz, 2 H, β-CH2), 1.36 (sext., J = 7.3 Hz, 2 H, γ-CH2), 0.93 (t, J = 7.3 Hz, 3 H, Me). 13C{1H}-NMR (101 MHz, CD2Cl2): δ = 14.23 (Me), 23.26 (CH2), 29.67 (CH2), 37.52 (CH2), 129.15 (d, J = 9.6 Hz, m-Ph3P), 130.41 (d, J = 1.6 Hz, p-Ph3P), 133.26 (d, J = 24.2 Hz, ipso-Ph3P), 134.25 (d, J = 16.8 Hz, o-Ph3P), 180.63 (CO). 31P-NMR (162 MHz, CD2Cl2): δ = 7.77 (s). X-ray-quality crystals were selected from the bulk sample.
3.4. Synthesis of Bis(Triphenylphosphine) Copper(I) Complexes 7a–8a
[(Ph3P)2Cu{O2C(CH2)3Me}] (7a). To a solution of [Cu{O2C(CH2)3Me}2] (0.099 g, 0.37 mmol) in MeOH (30 mL) containing 2 drops of water, Ph3P (0.344 g, 1.31 mmol) was added. The blue-green solution was heated to reflux for ca. 4 h, during which time it became completely colourless. Upon cooling the solution to −20 °C for several hours, a colourless solid precipitated. Recrystallization from CH2Cl2/hexane afforded colourless crystals, which were isolated by filtration, washed with Et2O and dried in air. A total of 0.232 g (90%) of product was isolated. IR (ATR): 1585 νa(OCO), 1478 νs(OCO) cm−1. 1H-NMR (400 MHz, CD2Cl2): δ = 7.23–7.47 (m, 30 H, Ph3P), 2.12–2.68 (br. m, 2 H, CH2), 1.61 (br. m, 2 H, CH2), 1.34 (sext., J = 7.2 Hz, 2 H, γ-CH2), 0.92 (t, J = 7.2 Hz, 3 H, Me). 13C{1H}-NMR (101 MHz, CD2Cl2): δ = 14.25 (Me), 23.37 (CH2), 29.29 (CH2), 128.90 (d, J = 8.2 Hz, m-Ph3P), 130.13 (p-Ph3P), 133.34 (d, J = 29.9 Hz, ipso-Ph3P), 134.10 (d, J = 14.7 Hz, o-Ph3P). The signal for C=O was not observed. 31P-NMR (162 MHz, CD2Cl2): δ = −2.93 (s). X-ray-quality crystals were selected from the bulk sample.
[(Ph3P)2Cu{O2C(CH2)2C(O)Me}] (8a). This was prepared as described above using [Cu{O2C(CH2)2C(O)Me}2] (0.199 g, 0.68 mmol) and Ph3P (0.625 g, 2.38 mmol). A total of 0.335 g (70%) of product was isolated. IR (ATR): 1705 ν(C=O ketone), 1585 νa(OCO), 1478 νs(OCO) cm−1. 1H-NMR (400 MHz, CD2Cl2): δ = 7.25–7.46 (m, 30 H, Ph3P), 2.72 (br. m, 4 H, CH2), 2.12 (s, 3 H, Me). 13C{1H}-NMR (101 MHz, CD2Cl2): δ = 23.47 (Me), 30.05 (CH2), 128.93 (d, J = 8.8 Hz, m-Ph3P), 130.18 (p-Ph3P), 133.20 (d, J = 30.1 Hz, ipso-Ph3P), 134.08 (d, J = 14.9 Hz, o-Ph3P), 209.02 (CO ketone). The signal for CO (carboxylate) was not observed. 31P-NMR (162 MHz, CD2Cl2): δ = −2.85 (s). X-ray-quality crystals were selected from the bulk sample.
3.5. X-ray Crystallography
Diffraction data were collected at either 150 K (1a, 2, 4) or 90 K (2a, 5a, 6a, 7a, 8a) using a Rigaku Oxford Diffraction Gemini E Ultra diffractometer with Mo Ka-radiation (λ = 0.71073 nm). Data integration, scaling and empirical absorption correction were carried out using the CrysAlis Pro version 42_32.73a program package [29]. The structures were solved using SHELXT [30] and refined with SHELXL [31] operated through the Olex2 interface [32]. Olex2 was also used to generate the ortep-style figures. In the structure of 2a, the succinic acid monoamide unit was found to be disordered over two positions. This was best modelled as a 50:50 disorder. One of the groups is O,O-chelating to the silver, whilst the other acts a monodentate carboxylate ligand. One of the phenyl-rigs of a Ph3P ligand was also disordered. The respective atoms were modelled with a 50:50 occupancy. There was also evidence of a highly disordered residual solvent, for which we were unable to develop a suitable model. The data were therefore subjected to the solvent mask procedure implemented in Olex2. Crystallographic and refinement details are collected in Table S1.
4. Conclusions
In summary, we present a series of silver(I) and copper(I) complexes with various O,O-chelating or bridging carboxylate ligands together with their spectroscopic and structural characterization. In addition, TGA data recorded for the bis(triphenylphosphine) compounds indicate clean decomposition to the respective metals (Ag or Cu) at temperatures ranging from 287 to 338 °C. The silver(I) levulinate-complex 5a had the lowest decomposition temperature and is thus a potential candidate for an inkjet printing ink.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/inorganics12050140/s1, Table S1. Crystallographic and refinement details.
Author Contributions
Conceptualization, F.M.; formal analysis, K.H.n.R., F.B. and B.B.B.; investigation, K.H.n.R. and F.B.; writing—original draft preparation, F.M.; writing—review and editing, F.M and B.B.B.; visualization, K.H.n.R. and F.B.; supervision, F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We thank the University of Wuppertal for supporting this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, H.M.J.; Lin, I.J.B. Facile synthesis of silver(I)–carbene complexes. Useful carbene transfer agents. Organometallics 1998, 17, 972–975. [Google Scholar] [CrossRef]
- Dörner, M.; Rautiainen, J.M.; Rust, J.; Lehmann, C.W.; Mohr, F. Acylchalcogenourea complexes of silver(I). Eur. J. Inorg. Chem. 2017, 2017, 789–797. [Google Scholar] [CrossRef]
- Pilz, S.K.; Mohr, F. On the reactivity of thiourea derivatives with silver(I) oxide. Eur. J. Inorg. Chem. 2020, 2020, 2285–2294. [Google Scholar] [CrossRef]
- Piani, R.; Beele, B.B.; Rust, J.; Lehmann, C.W.; Mohr, F. Coinage metal complexes containing perfluorinated carboxylates. Chemistry 2023, 5, 813–833. [Google Scholar] [CrossRef]
- Morzyk-Ociepa, B.; Michalska, D. FT-Raman and infrared spectra of silver(I) complexes with glutarimidate and 3,3-dimethylglutarimidate anions. Spectrochim. Acta Part A 1999, 55, 2671–2676. [Google Scholar] [CrossRef]
- Handley, G.J.; Nelson, E.R.; Sommers, T.C. Compounds derived from b-substituted glutaric acids: Glutarimides, glutaramic acids, 1,5-pentane diols. Aust. J. Chem. 1960, 13, 127–144. [Google Scholar] [CrossRef]
- Perron, J.; Beauchamp, A.L. Interactions de l’ion Ag+ avec la glutarmidie. Can. J. Chem. 1984, 62, 1287–1291. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Edwards, D.A.; Harker, R.M.; Mahon, M.F.; Molloy, K.C. Aerosol-assisted chemical vapour deposition (AACVD) of silver films from triorganophosphine adducts of silver carboxylates, including the structure of [Ag(O2CC3F7)(PPh3)2]. Inorg. Chim. Acta 2002, 328, 134–146. [Google Scholar] [CrossRef]
- Olson, L.P.; Whitcomb, D.R.; Rajeswaran, M.; Blanton, T.N.; Stwertka, B.J. The simple yet elusive crystal structure of silver acetate and the role of the Ag–Ag bond in the formation of silver nanoparticles during the thermally induced reduction of silver carboxylates. Chem. Mater. 2006, 18, 1667–1674. [Google Scholar] [CrossRef]
- Edwards, D.A.; Mahon, M.F.; Molloy, K.C.; Ogrodnik, V. Aerosol-assisted chemical vapour deposition of silver thin films from adducts of functionalised silver carboxylates. J. Mater. Chem. 2003, 13, 563–570. [Google Scholar] [CrossRef]
- Tolochko, B.P.; Chernov, S.V.; Nikitenko, S.G.; Whitcomb, D.R. EXAFS determination of the structure of silver stearate, [Ag(O2C(CH2)16CH3]2, and the effect of temperature on the silver coordination sphere. Nucl. Instrum. Methods Phys. Res. Sect. A 1998, 405, 428–434. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. Argentophilic interactions. Angew. Chem. Int. Ed. 2015, 54, 746–784. [Google Scholar] [CrossRef]
- Han, J.; Shen, Y.; Li, C.; Li, Y.; Pan, Y. Synthesis and characterization of triphenylphosphine stabilized silver α,β-unsaturated carboxylate: Crystal structure of [Ag(O2CCH=C(CH3)2)(PPh3)2]. Inorg. Chim. Acta 2005, 358, 4417–4422. [Google Scholar] [CrossRef]
- Richards, V.N.; Rath, N.P.; Buhro, W.E. Pathway from a molecular precursor to silver Nanoparticles: The prominent role of aggregative growth. Chem. Mater. 2010, 22, 3556–3567. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef]
- Whitcomb, D.R.; Rogers, R.D. The molecular structure of [bis-triphenylphosphine-silver(I) stearate], [((C6H5)3P)2Ag(O2C(CH2)16CH3)], solubilization of long alkyl chain silver carboxylates. J. Chem. Crystallogr. 1996, 26, 99–105. [Google Scholar] [CrossRef]
- Grabowsky, S.; White, A.H.; Healy, P.C.; Lapere, K.M.; Ng, S.W.; Skelton, B.W.; Wild, D.A.; Bowmaker, G.A.; Hanna, J.V. Solid-state NMR, X-ray diffraction, and theoretical studies of neutral mononuclear molecular bis(triphenylphosphine)silver(I) mono-carboxylate and -nitrate systems. Aust. J. Chem. 2020, 73, 556–569. [Google Scholar] [CrossRef]
- Adner, D.; Möckel, S.; Korb, M.; Buschbeck, R.; Rüffer, T.; Schulze, S.; Mertens, L.; Hietschold, M.; Mehring, M.; Lang, H. Copper(II) and triphenylphosphine copper(I) ethylene glycol carboxylates: Synthesis, characterisation and copper nanoparticle generation. Dalton Trans. 2013, 42, 15599–15609. [Google Scholar] [CrossRef]
- Adner, D.; Korb, M.; Schulze, S.; Hietschold, M.; Lang, H. A straightforward approach to oxide-free copper nanoparticles by thermal decomposition of a copper(I) precursor. Chem. Commun. 2013, 49, 6855–6857. [Google Scholar] [CrossRef]
- Jurado-Vázquez, T.; Rosaldo, E.; Arévalo, A.; García, J.J. Levulinic acid hydrogenation with homogeneous Cu(I) catalyst. ChemCatChem 2022, 14, e202200628. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Holtcamp, M.W.; Longridge, E.M.; Klausmeyer, K.K.; Reibenspies, J.H. Structural characterization of bidentate carboxylate derivatives of copper(I) bistriphenylphosphine. Inorg. Chim. Acta 1994, 227, 223–232. [Google Scholar] [CrossRef]
- Yang, W.; List-Kratochvil, E.J.W.; Wang, C. Metal particle-free inks for printed flexible electronics. J. Mater. Chem. C 2019, 7, 15098–15117. [Google Scholar] [CrossRef]
- Cummins, G.; Desmulliez, M.P.Y. Inkjet printing of conductive materials: A review. Curcuit World 2012, 38, 193–213. [Google Scholar] [CrossRef]
- Jahn, S.F.; Blaudeck, T.; Baumann, R.R.; Jakob, A.; Ecorchard, P.; Rüffer, T.; Lang, H.; Schmidt, P. Inkjet printing of conductive silver patterns by using the first aqueous particle-free MOD ink without additional stabilizing ligands. Chem. Mater. 2010, 22, 3067–3071. [Google Scholar] [CrossRef]
- Jahn, S.F.; Jakob, A.; Blaudeck, T.; Schmidt, P.; Lang, H.; Baumann, R.R. Inkjet printing of conductive patterns with an aqueous solution of [AgO2C(CH2OCH2)3H] without any additional stabilizing ligands. This Solid Films 2010, 518, 3218–3222. [Google Scholar] [CrossRef]
- Kintzel, S.; Eckhardt, K.; Getzschmann, J.; Bon, V.; Grothe, J.; Kaskel, S. Synthesis and structure of the silver complexes [Ag2(C4H6O4N)NO3] H2O and Ag6(C6H6O6N)2 for the formulation of silver inks in nanoimprint lithography. Eur. J. Inorg. Chem. 2020, 2020, 3167–3173. [Google Scholar] [CrossRef]
- CrysAlisPro, version 42_32.73a; Rigaku Oxford Diffraction Ltd.: Oxford, UK, 2023.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).