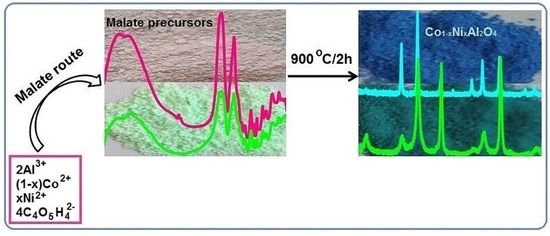
Structural, Morphological, and Optical Properties of Single and Mixed Ni-Co Aluminates Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Malate Precursors
2.2. Characterization of Co1−xNixAl2O4 Samples
2.2.1. X-ray Diffraction
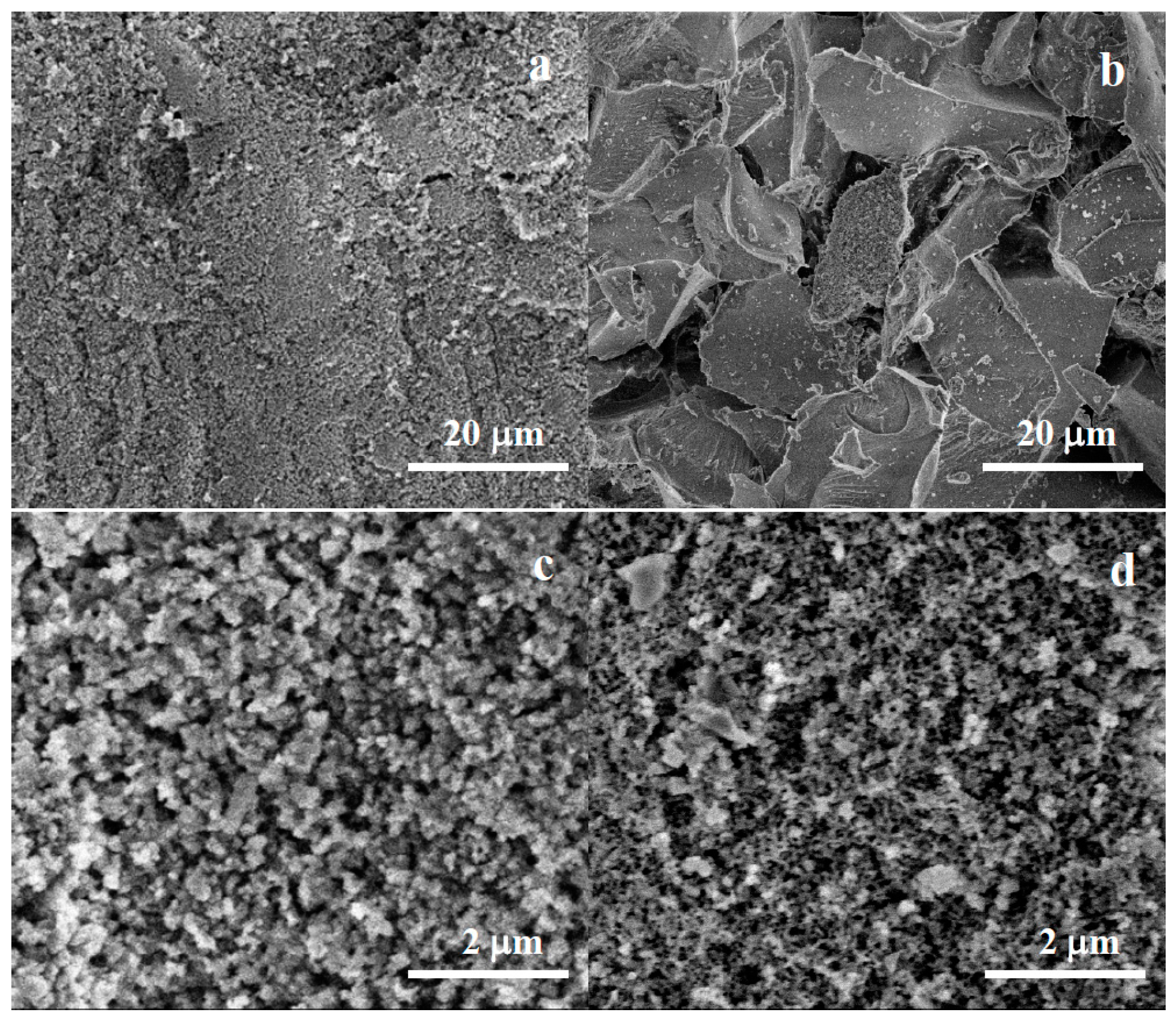
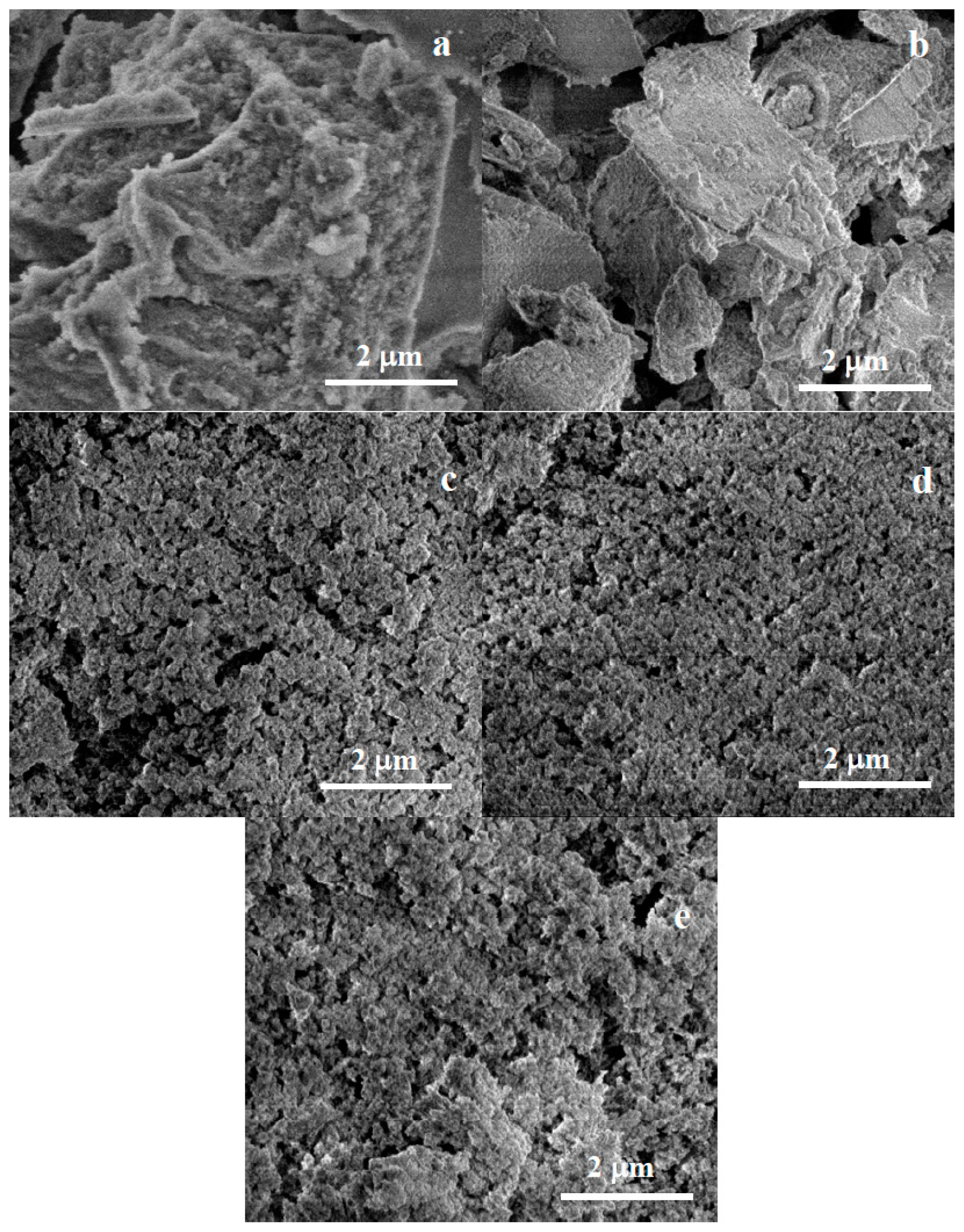
2.2.2. Scanning Electron Microscopy
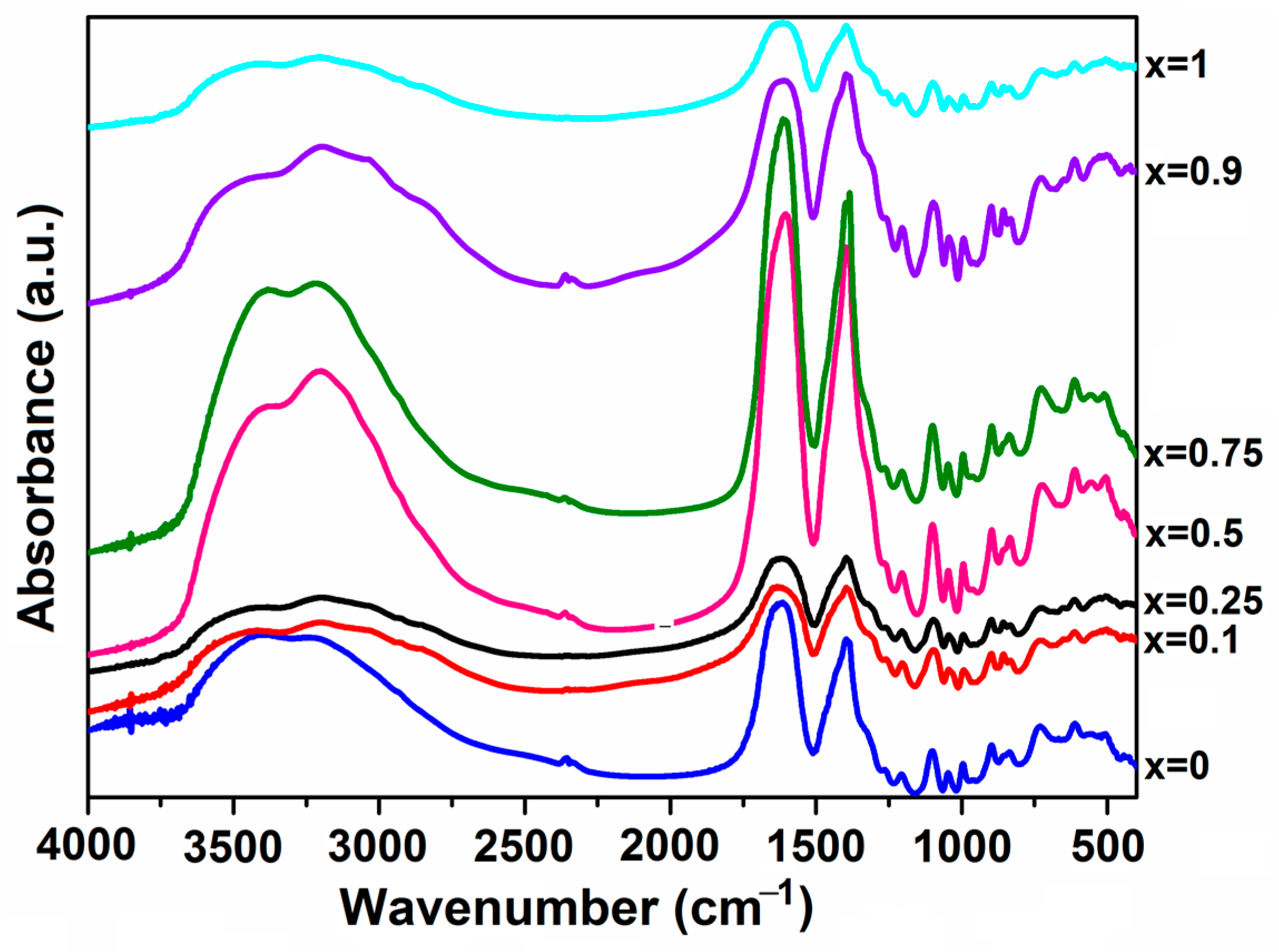
2.2.3. Infrared Spectroscopy
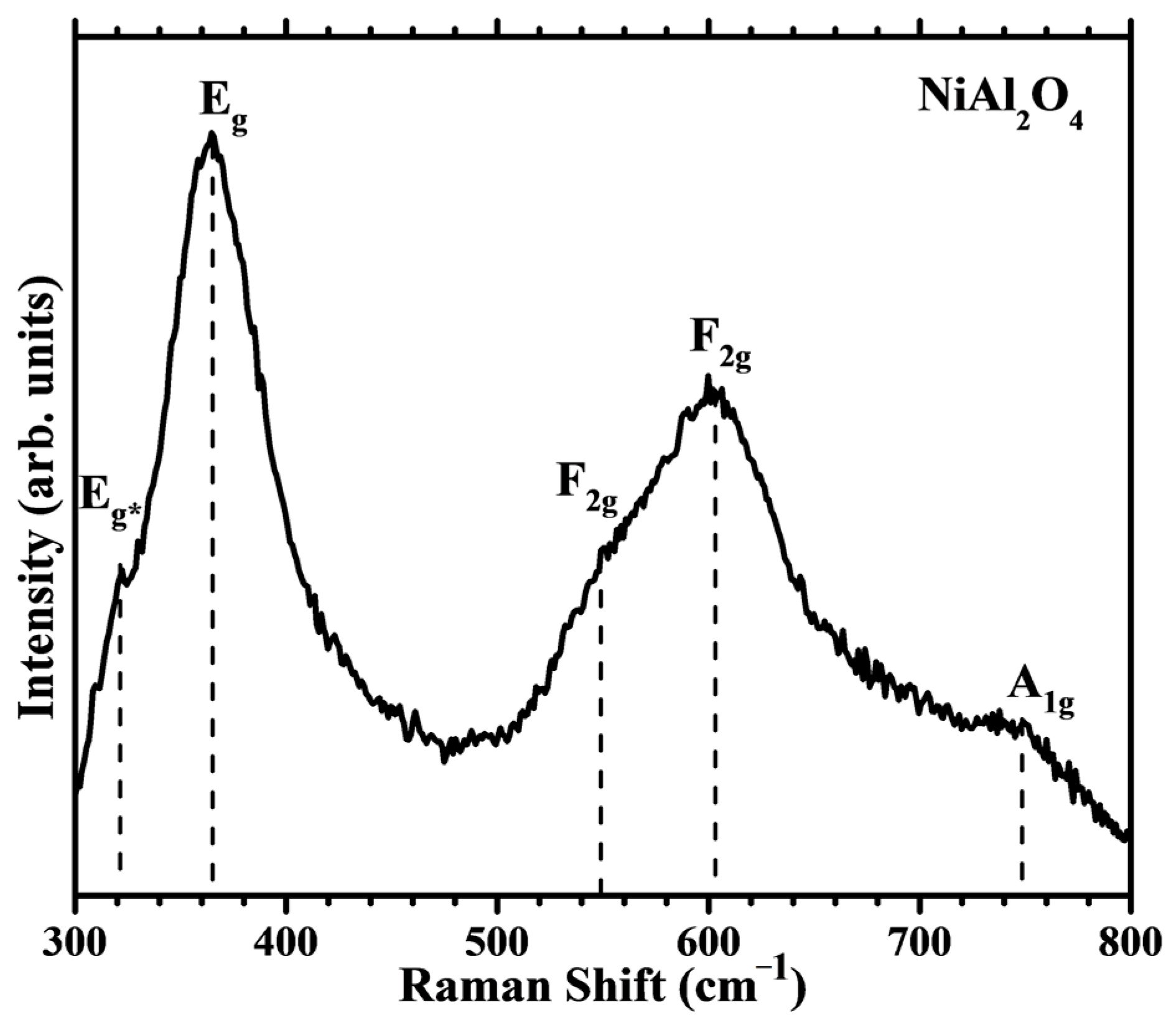
2.2.4. Raman Spectroscopy
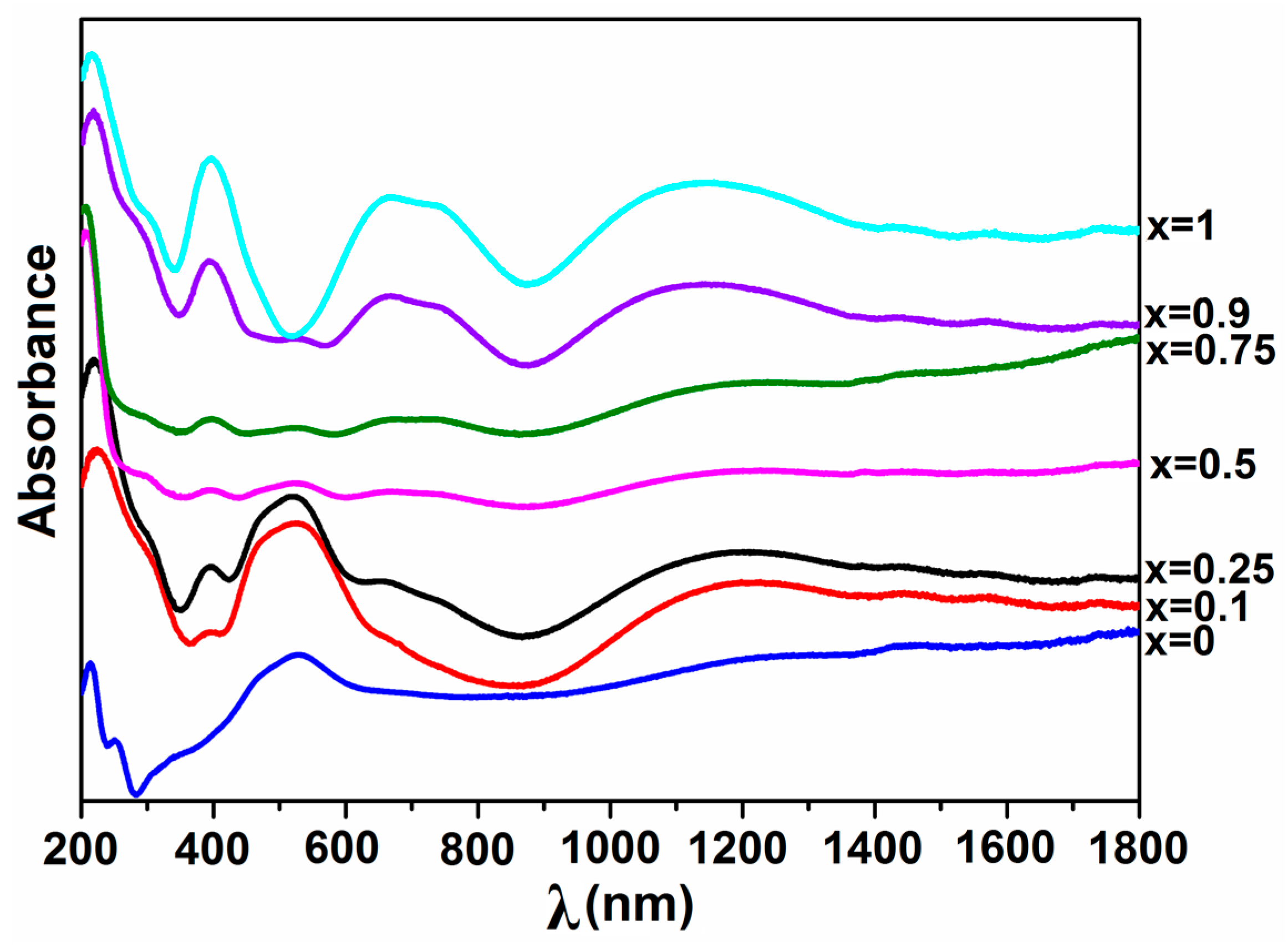
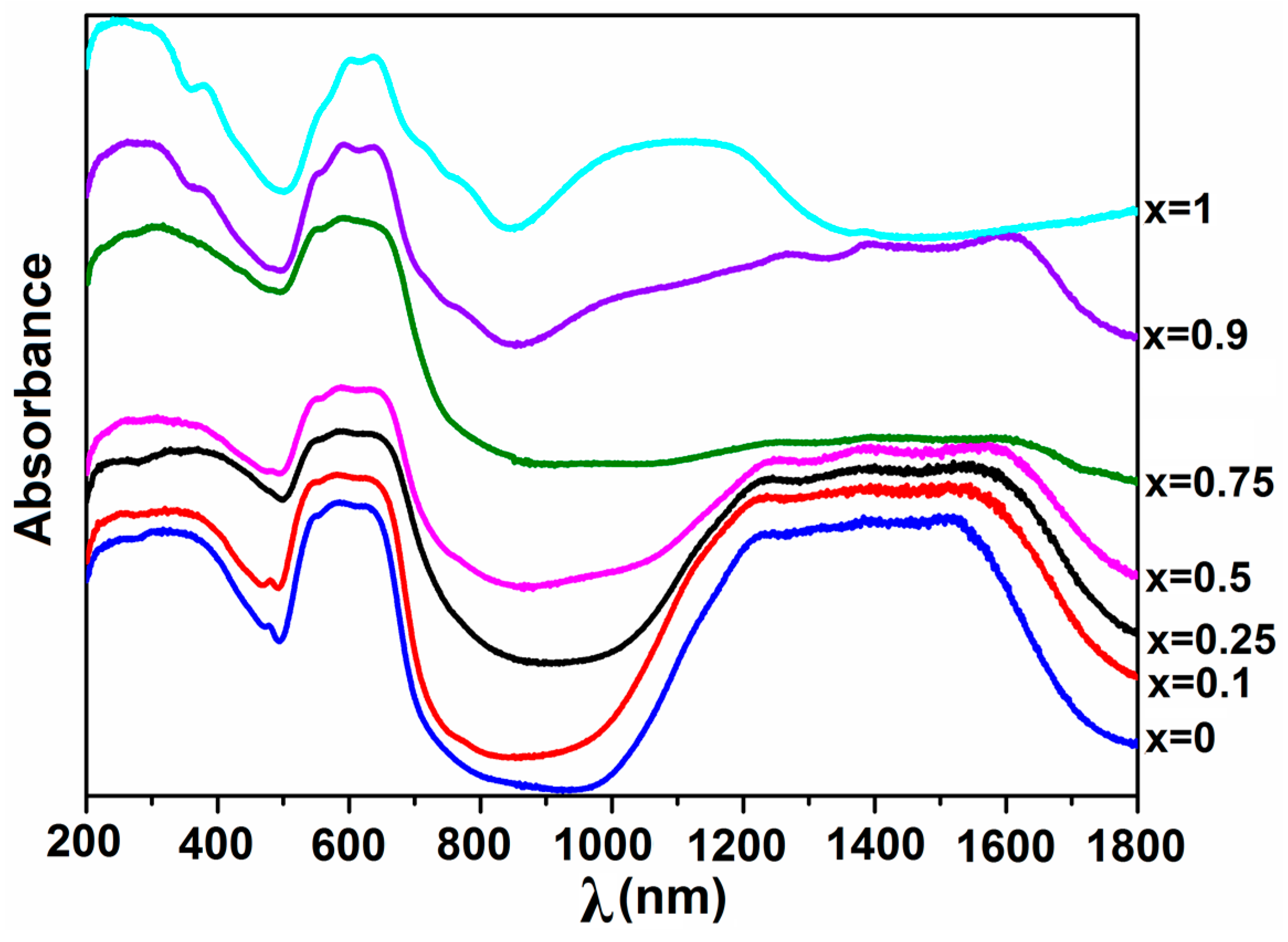
2.2.5. UV–Vis–NIR Spectroscopy
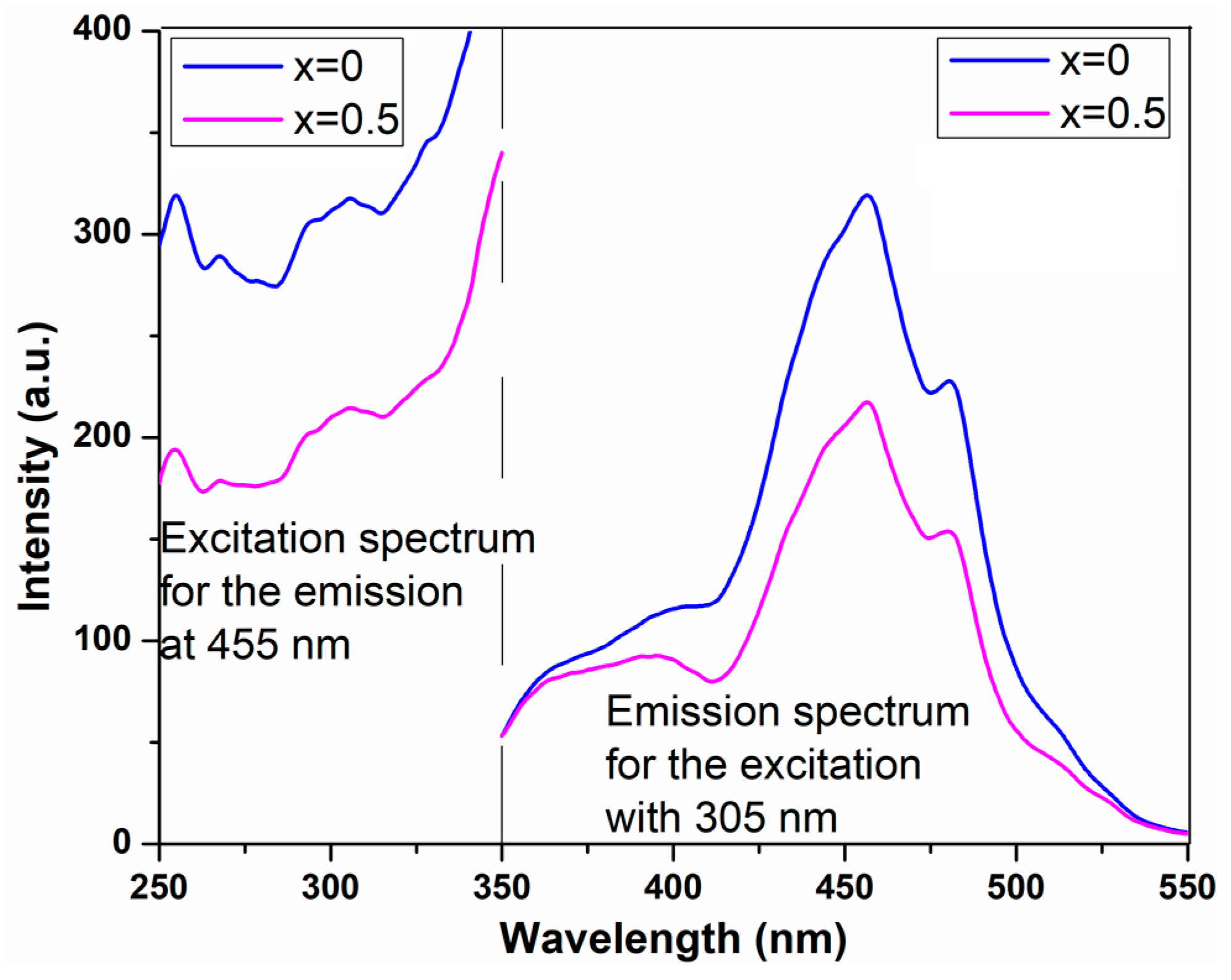
2.2.6. Photoluminescence Spectra
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Co1−xNixAl2O4 (x = 0, 0.1, 0.25, 0.5, 0.75, 0.9, 1)
3.3. Characterization Techniques
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tangcharoen, T.; Klysubun, W.; T-Thienprasert, J.; Kongmark, C. Cation exchange in Ni–Cu–Zn aluminate spinels revealed by EXAFS. J. Solid State Chem. 2020, 292, 121695. [Google Scholar] [CrossRef]
- Ali, A.A.; El Fadaly, E.; Ahmed, I.S. Near-infrared reflecting blue inorganic nano-pigment based on cobalt aluminate spinel via combustion synthesis method. Dye. Pigment. 2018, 158, 451–462. [Google Scholar] [CrossRef]
- Álvarez-Docio, C.M.; Portela, R.; Reinosa, J.J.; Rubio-Marcos, F.; Pascual, L.; Fernández, J.F. Performance and stability of wet-milled CoAl2O4, Ni/CoAl2O4, and Pt,Ni/CoAl2O4 for soot combustion. Catalysts 2020, 10, 406. [Google Scholar] [CrossRef]
- Suguna, S.; Shankar, S.; Jaganathan, S.K.; Manikandan, A. Novel synthesis and characterization studies of spinel NixCo1−xAl2O4 (x = 0.0 to 1.0) nano-catalysts for the catalytic oxidation of benzyl alcohol. J. Nanosci. Nanotechnol. 2018, 18, 1019–1026. [Google Scholar] [CrossRef]
- Boudiaf, S.; Nasrallah, N.; Mellal, M.; Belabed, C.; Belhamdi, B.; Meziani, D.; Mehdi, B.; Trari, M. Synthesis and characterization of semiconductor CoAl2O4 for optical and dielectric studies: Application to photodegradation of organic pollutants under visible light. Optik 2020, 219, 165038. [Google Scholar] [CrossRef]
- Gaudonn, M.; Robertson, L.C.; Lataste, E.; Duttine, M.; Ménétrier, M.; Demourgues, A. Cobalt and nickel aluminate spinels: Blue and cyan pigments. Ceram. Int. 2014, 40, 5201–5207. [Google Scholar] [CrossRef]
- Yancheshmeh, M.S.; Sahraei, O.A.; Aissaoui, M.; Iliuta, M.C. A novel synthesis of NiAl2O4 spinel from a Ni-Al mixed-metal alkoxide as a highly efficient catalyst for hydrogen production by glycerol steam reforming. Appl. Catal. B 2020, 265, 118535. [Google Scholar] [CrossRef]
- Rahimi-Nasrabadi, M.; Ahmadi, F.; Eghbali-Arani, M. Different morphologies fabrication of NiAl2O4 nanostructures with the aid of new template and its photocatalyst application. J. Mater. Sci. Mater. Electron. 2017, 28, 2415–2420. [Google Scholar] [CrossRef]
- Rahman, M.A.; Faruque, M.R.I.; Islam, M.T. Preparation of flexible substrate for patch antenna based on nickel aluminate (NiAl2O4) synthesized by sol–gel method. J. Electron. Mater. 2019, 48, 2932–2939. [Google Scholar] [CrossRef]
- Irshad, A.; Shahid, M.; El-Bahy, S.M.; El Azab, I.H.; Mersal, G.A.M.; Ibrahim, M.M.; Agboola, P.O.; Shakir, I. Nickel doped CoAl2O4@CNT nanocomposite: Synthesis, characterization, and evaluation of sunlight driven catalytic studies. Phys. B Condens. Matter 2022, 636, 413873. [Google Scholar] [CrossRef]
- Roniboss, A.; Subramani, A.; Ramamoorthy, R.; Yuvaraj, S.; Sundararajan, M.; Dash, C.S. Investigation of structural, optical and magnetic behavior of MAl2O4 (M=Zn and Co) nanoparticles via microwave combustion technique. Mater. Sci. Semicond. Process. 2021, 123, 105507. [Google Scholar] [CrossRef]
- Deraz, N.M. Formation and characterization of cobalt aluminate nano-particles. Int. J. Electrochem. Sci. 2013, 8, 4036–4046. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, J.; Li, W.; Guo, H.; You, B. Fabrication of cobalt aluminate nanopigments by coprecipitation method in threonine waterborne solution. Dye. Pigment. 2018, 151, 130–139. [Google Scholar] [CrossRef]
- Akika, F.Z.; Benamira, M.; Lahmar, H.; Tibera, A.; Chabi, R.; Avramova, I.; Suzer, Ş.; Trari, M. Structural and optical properties of Cu-substitution of NiAl2O4 and their photocatalytic activity towards Congo red under solar light irradiation. J. Photochem. Photobiol. A 2018, 364, 542–550. [Google Scholar] [CrossRef]
- Srisawad, N.; Chaitree, W.; Mekasuwandumrong, O.; Praserthdam, P.; Panpranot, J. Formation of CoAl2O4 nanoparticles via low-temperature solid-state reaction of fine gibbsite and cobalt precursor. J. Nanomater. 2012, 2012, 108369. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A.; Sanjuan, M.L.; Merino, R.I. Raman spectroscopic study of cation disorder in poly- and single crystals of the nickel aluminate spinel. J. Phys. Condens. Matter. 2007, 19, 186217. [Google Scholar] [CrossRef]
- Kim, J.-H.; Son, B.-R.; Yoon, D.-H.; Hwang, K.-T.; Noh, H.-G.; Cho, W.-S.; Kim, U.-S. Characterization of blue CoAl2O4 nano-pigment synthesized by ultrasonic hydrothermal method. Ceram. Int. 2012, 38, 5707–5712. [Google Scholar] [CrossRef]
- Aghaali, M.H.; Firoozi, S. Enhancing the catalytic performance of Co substituted NiAl2O4 spinel by ultrasonic spray pyrolysis method for steam and dry reforming of methane. Int. J. Hydrogen Energy 2021, 46, 357–373. [Google Scholar] [CrossRef]
- Mindru, I.; Marinescu, G.; Gingasu, D.; Patron, L.; Ghica, C.; Giurginca, M. Blue CoAl2O4 spinel via complexation method. Mater. Chem. Phys. 2010, 122, 491–497. [Google Scholar] [CrossRef]
- Gingasu, D.; Patron, L.; Mindru, I.; Stanica, N.; Balint, I. Copper ferrite prepared by soft chemical method. Rev. Roum. Chim. 2004, 49, 669–674. [Google Scholar]
- Suciu, C.; Patron, L.; Mindru, I.; Carp, O. Nickel aluminate spinel by thermal decomposition of polynuclear malate complexes. Rev. Roum. Chim. 2006, 51, 385–389. [Google Scholar]
- Mitran, G.; Nguyen, T.L.P.; Seo, D.-K. The influence of complexing agents on the cobalt-based catalysts properties and activities. J. Ind. Eng. Chem. 2022, 114, 446–455. [Google Scholar] [CrossRef]
- Zhou, N.; Li, Y.; Zhang, Y.; Shu, Y.; Nian, S.; Cao, W.; Wu, Z. Synthesis and characterization of Co1−xCaxAl2O4 composite blue nanopigments by the polyacrylamide gel method. Dye. Pigment. 2018, 148, 25–30. [Google Scholar] [CrossRef]
- Jafari, M.; Hassanzadeh-Tabrizi, S.A.; Ghashang, M.; Pournajaf, R. Characterization of Ba2+-added alumina/cobalt nanoceramic pigment prepared by polyacrylamide gel method. Ceram. Int. 2014, 40, 11877–11881. [Google Scholar] [CrossRef]
- De Souza, L.K.C.; Zamian, J.R.; Da Rocha Filho, G.N.; Soledade, L.E.B.; Dos Santos, I.M.G.; Souza, A.G.; Scheller, T.; Angelica, R.S.; Da Costa, C.E.F. Blue pigments based on CoxZn1−xAl2O4 spinels synthesized by the polymeric precursor method. Dye. Pigment. 2009, 81, 187–192. [Google Scholar] [CrossRef]
- Kanithan, S.; Vignesh, N.A.; Baskar, S.; Nagaraja, S.; Abbas, M.; Aabid, A.; Baig, M. Structural, morphology and optical properties of strontium-doped cobalt aluminate nanoparticles synthesized by the combustion method. Materials 2022, 15, 8180. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Wei, Z.; Wu, X.; Huang, S.; Ding, M.; Ma, J. Electronic structure and optical properties of spinel structure Zn1−xNixAl2O4 nanopowders synthesized by sol–gel method. Chem. Phys. Lett. 2021, 772, 138582. [Google Scholar] [CrossRef]
- Patil, P.S.; Dhivare, R.S.; Mirgane, S.R.; Pawar, B.G.; Mane, T.R. Cobalt-Doped Nickel Aluminate Nanomaterials Synthesis, Characterization, and Catalytic Properties. Macromol. Symp. 2020, 393, 2000163. [Google Scholar] [CrossRef]
- Max, J.-J.; Chapados, C. Infrared Spectroscopy of Aqueous Carboxylic Acids: Malic Acid. J. Phys. Chem. A 2002, 106, 6452–6461. [Google Scholar] [CrossRef]
- Lever, A.B.P. Inorganic Electronic Spectroscopy; Elsevier Publishing Company: Amsterdam, The Netherlands, 1968. [Google Scholar]
- Rotan, M.; Tolchard, J.; Rytter, E.; Einarsrud, M.-A.; Grande, T. On the solid solution of the spinel phase in the system NiO–Al2O3. J. Solid State Chem. 2009, 182, 3412–3415. [Google Scholar] [CrossRef]
- Chokkaram, S.; Srinivasan, R.; Milburn, D.R.; Davis, B.H. Conversion of 2-octanol over nickel-alumina, cobalt-alumina, and alumina catalysts. J. Mol. Catal A 1997, 121, 157–169. [Google Scholar] [CrossRef]
- Shamsi, A.; Hashemian, S. Structural, magnetic and adsorptive properties of nano spinel of cobalt aluminate doped with nickel for Cr(VI) removal. J. Mol. Struct. 2022, 1255, 132412. [Google Scholar] [CrossRef]
- Gholami, T.; Salavati-Niasari, M.; Varshoy, S. Electrochemical hydrogen storage capacity and optical properties of NiAl2O4/NiO nanocomposite synthesized by green method. Int. J. Hydrogen Energy 2017, 42, 5235–5245. [Google Scholar] [CrossRef]
- Kock, L.D.; De Waal, D. Raman studies of the underglaze blue pigment on ceramic artefacts of the Ming dynasty and of unknown origins. J. Raman Spectrosc. 2007, 38, 1480–1487. [Google Scholar] [CrossRef]
- Bouchard, M.; Gambardella, A. Raman microscopy study of synthetic cobalt blue spinels used in the field of art. J. Raman Spectrosc. 2010, 41, 1477–1485. [Google Scholar] [CrossRef]
- D’Ippolito, V.; Andreozzi, G.B.; Bersani, D.; Lottici, P.P. Raman fingerprint of chromate, aluminate and ferrite spinels. J. Raman Spectrosc. 2015, 46, 1255–1264. [Google Scholar] [CrossRef]
- Visinescu, D.; Papa, F.; Ianculescu, A.C.; Balint, I.; Carp, O. Nickel-doped zinc aluminate oxides: Starch-assisted synthesis, structural, optical properties, and their catalytic activity in oxidative coupling of methane. J. Nanoparticle Res. 2013, 15, 1456. [Google Scholar] [CrossRef]
- Goga, F.; Bortnic, R.A.; Avram, A.; Zagrai, M.; Tudoran, L.B.; Mereu, R.A. The effect of Ni2+ ions substitution on structural, morphological, and optical properties in CoCr2O4 matrix as pigments in ceramic glazes. Materials 2022, 15, 8713. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yang, H.; Wang, S.; Li, D.; Wang, F.; Fang, L.; Lei, L.; Xiao, Y.; Yang, G. A new route for the preparation of CoAl2O4 nanoblue pigments with high uniformity and its optical properties. J. Sol-Gel Sci. Technol. 2018, 86, 206–216. [Google Scholar] [CrossRef]
- Motelica, L.; Oprea, O.-C.; Vasile, B.-S.; Ficai, A.; Ficai, D.; Andronescu, E.; Holban, A.M. Antibacterial activity of solvothermal obtained ZnO nanoparticles with different morphology and photocatalytic activity against a dye mixture: Methylene Blue, Rhodamine B and Methyl Orange. Int. J. Mol. Sci. 2023, 24, 5677. [Google Scholar] [CrossRef]
- Motelica, L.; Vasile, B.-S.; Ficai, A.; Surdu, A.-V.; Ficai, D.; Oprea, O.-C.; Andronescu, E.; Jinga, D.C.; Holban, A.M. Influence of the alcohols on the ZnO synthesis and its properties: The photocatalytic and antimicrobial activities. Pharmaceutics 2022, 14, 2842. [Google Scholar] [CrossRef]
- Agilandeswari, K.; Kumar, A.R. Synthesis, characterisation, optical and luminescence properties of CoAl2O4. AIP Conf. Proc. 2015, 1665, 120022. [Google Scholar] [CrossRef]
- Ahn, K.-S.; Yan, Y.; Kang, M.-S.; Kim, J.-Y.; Shet, S.; Wang, H.; Turner, J.; Al-Jassim, M. CoAl2O4–Fe2O3 p-n nanocomposite electrodes for photoelectrochemical cells. Appl. Phys. Lett. 2009, 95, 022116. [Google Scholar] [CrossRef]
- Islam, M.A.; Akther Hossain, A.K.M.; Ahsan, M.Z.; Bally, M.A.A.; Samir Ullah, M.; Hoque, S.M.; Khan, F.A. Structural characteristics, cation distribution, and elastic properties of Cr3+ substituted stoichiometric and non-stoichiometric cobalt ferrites. RSC Adv. 2022, 12, 8502–8519. [Google Scholar] [CrossRef]
- Bindu, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef]
- Ferreira, T.A.S.; Waerenborgh, J.C.; Mendonça, M.H.R.M.; Nunes, M.R.; Costa, F.M. Structural and morphological characterization of FeCo2O4 and CoFe2O4 spinels prepared by a coprecipitation method. Solid State Sci. 2003, 5, 383–392. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Knyazev, A.V.; Demidov, D.N.; Knyazeva, S.S. High- and Low-Temperature X-Ray Diffraction Studies of Aluminate Spinels in the CoAl2O4–NiAl2O4 System. Inorg. Mater. 2017, 53, 190–199. [Google Scholar] [CrossRef]
- O’Neill, H.S.C.; Navrotsky, A. Cation distributions and thermodynamic properties of binary spinel solid solutions. Am. Min. 1984, 69, 733–753. [Google Scholar]
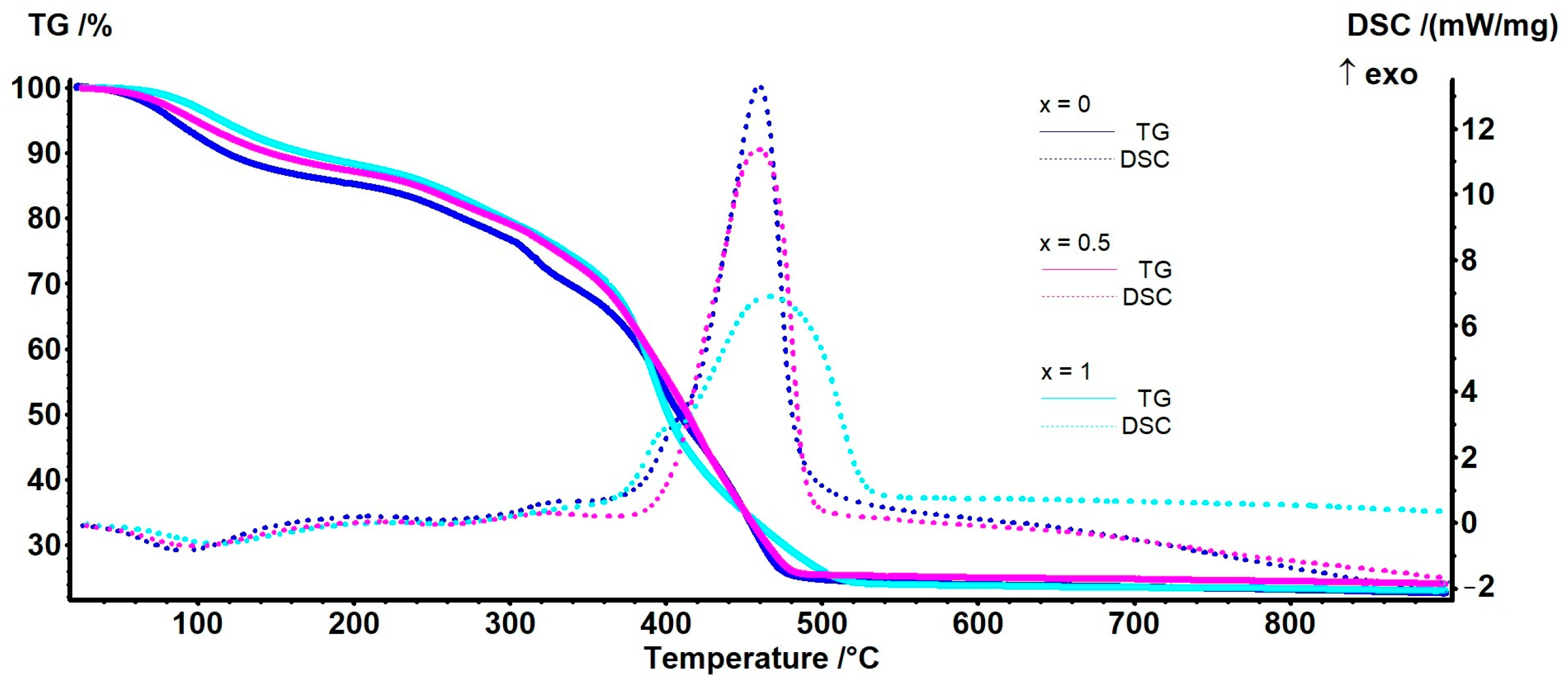







| Samples | Mass Loss RT–200 °C | Endo Effect | Mass Loss 200–360 °C | Mass Loss 360–550 °C | Exo Effect | Residual Mass |
|---|---|---|---|---|---|---|
| x = 0 | 14.79% | 91.1 °C | 18.70% | 42.37% | 459.9 °C | 22.56% |
| x = 0.1 | 11.97% | 98.7 °C | 20.09% | 44.69% | 474.9 °C | 21.96% |
| x = 0.25 | 12.89% | 106.2 °C | 22.61% | 42.35% | 469.9 °C | 21.18% |
| x = 0.5 | 12.82% | 97.9 °C | 17.55% | 44.47% | 459.4 °C | 24.01% |
| x = 0.75 | 9.68% | 101.1 °C | 26.52% | 43.20% | 455.0 °C | 19.18% |
| x = 0.9 | 11.41% | 107.0 °C | 35.17% | 34.81% | 453.3 °C | 18.04% |
| x = 1 | 11.72% | 112.0 °C | 17.77% | 46.58% | 465.9 °C | 22.96% |
| Co1−xNixAl2O4 Samples | Molar Mass (g·mol−1) | Lattice Constant (Å) | Unit Cell Volume (Å3) | Crystallite Size (nm) | X-ray Density, ρx (g·cm−3) | Dislocation Density, δ (Å−2) |
|---|---|---|---|---|---|---|
| x = 0 | 176.9 | 8.1111 | 533.6229 | 18 | 4.40385 | 2.12 × 10−3 |
| x = 0.1 | 176.87 | 8.1091 | 533.2342 | 16 | 4.40631 | 2.32 × 10−3 |
| x = 0.25 | 176.825 | 8.1047 | 532.3666 | 16 | 4.41237 | 2.30 × 10−3 |
| x = 0.5 | 176.75 | 8.0894 | 529.3593 | 12 | 4.43555 | 3.75 × 10−3 |
| x = 0.75 | 176.675 | 8.0522 | 522.0879 | 7 | 4.49542 | 1.53 × 10−2 |
| x = 0.9 | 176.63 | 8.0554 | 522.7106 | 6 | 4.48892 | 1.48 × 10−2 |
| x = 1 | 176.6 | 8.0600 | 523.6066 | 5 | 4.48048 | 1.89 × 10−2 |
| Co1−xNixAl2O4 Samples | Oxygen Occupancy, u | Degree of Inversion, λ | Bond Length | Structural Radii | Average Cation Radii | |||
|---|---|---|---|---|---|---|---|---|
| dt | do | rt | ro | <rt> | <rt> | |||
| Å | Å | Å | Å | Å | Å | |||
| x = 0 | 0.264517 | 0.198 | 1.9600 | 1.9173 | 0.5800 | 0.5373 | 0.54238 | 0.55579 |
| x = 0.1 | 0.263328 | 0.208 | 1.9429 | 1.9253 | 0.5629 | 0.5453 | 0.53810 | 0.55626 |
| x = 0.25 | 0.256609 | 0.283 | 1.8475 | 1.9741 | 0.4675 | 0.5941 | 0.52085 | 0.56276 |
| x = 0.5 | 0.260236 | 0.502 | 1.8948 | 1.9431 | 0.5148 | 0.5631 | 0.47715 | 0.58080 |
| x = 0.75 | 0.252727 | 0.864 | 1.7814 | 1.9913 | 0.4014 | 0.6113 | 0.41278 | 0.6079 |
| x = 0.9 | 0.251866 | 0.872 | 1.7701 | 1.9989 | 0.3901 | 0.6189 | 0.41086 | 0.60497 |
| x = 1 | 0.251526 | 0.918 | 1.7663 | 2.0028 | 0.3863 | 0.6228 | 0.40312 | 0.60614 |
| Sample | Synthesis Method, Thermal Treatment | Lattice Constant (Å) | Crystallite Size (nm) | Reference |
|---|---|---|---|---|
| CoAl2O4 | Microwave combustion | 8.125 | 21.47 | [4] |
| CoAl2O4 | Polyacrylamide gel, 1000 °C/2 h | - | 51 | [23] |
| Co0.8Ni0.2Al2O4 | Microwave combustion | 8.202 | 20.59 | [4] |
| Co0.75Ni0.25Al2O4 | Sol–gel combustion, 900 °C | 8.12 | 31.31 | [28] |
| Co0.5Ni0.5Al2O4 | Sol–gel, 900 °C | - | 38 | [33] |
| Co0.25Ni0.75Al2O4 | Sol–gel combustion, 900 °C | 8.01 | 22.26 | [28] |
| NiAl2O4 | Co-precipitation, 800 °C/5 h | 8.0692 | 20.70 | [14] |
| NiAl2O4 | Sol–gel, 450 °C | 8.048 | 13 | [9] |
| NiAl2O4 | Microwave combustion | 8.122 | 18.93 | [4] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gingasu, D.; Oprea, O.; Marinescu, G.; Calderon Moreno, J.M.; Culita, D.C.; Preda, S.; Surdu, V.-A. Structural, Morphological, and Optical Properties of Single and Mixed Ni-Co Aluminates Nanoparticles. Inorganics 2023, 11, 371. https://doi.org/10.3390/inorganics11090371
Gingasu D, Oprea O, Marinescu G, Calderon Moreno JM, Culita DC, Preda S, Surdu V-A. Structural, Morphological, and Optical Properties of Single and Mixed Ni-Co Aluminates Nanoparticles. Inorganics. 2023; 11(9):371. https://doi.org/10.3390/inorganics11090371
Chicago/Turabian StyleGingasu, Dana, Ovidiu Oprea, Gabriela Marinescu, Jose Maria Calderon Moreno, Daniela C. Culita, Silviu Preda, and Vasile-Adrian Surdu. 2023. "Structural, Morphological, and Optical Properties of Single and Mixed Ni-Co Aluminates Nanoparticles" Inorganics 11, no. 9: 371. https://doi.org/10.3390/inorganics11090371
APA StyleGingasu, D., Oprea, O., Marinescu, G., Calderon Moreno, J. M., Culita, D. C., Preda, S., & Surdu, V.-A. (2023). Structural, Morphological, and Optical Properties of Single and Mixed Ni-Co Aluminates Nanoparticles. Inorganics, 11(9), 371. https://doi.org/10.3390/inorganics11090371