VOC Monitoring and Ozone Generation Potential Analysis Based on a Single-Photon Ionization Time-of-Flight Mass Spectrometer
Abstract
1. Introduction
2. Instrumentation
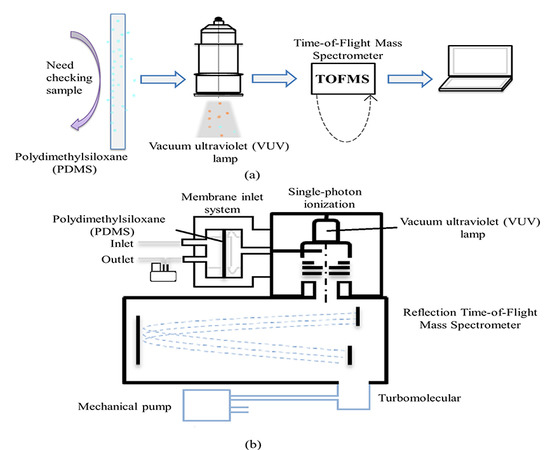
2.1. Vehicle Platform
2.2. The SPI-TOFMS Instrument
2.2.1. The Membrane Inlet System
2.2.2. Single-Photon Ionization
2.2.3. Time-of-Flight Mass Spectrometer
3. Experiment
4. Results and Discussion
4.1. Performance of the SPI-TOFMS
4.2. Environmental Factors
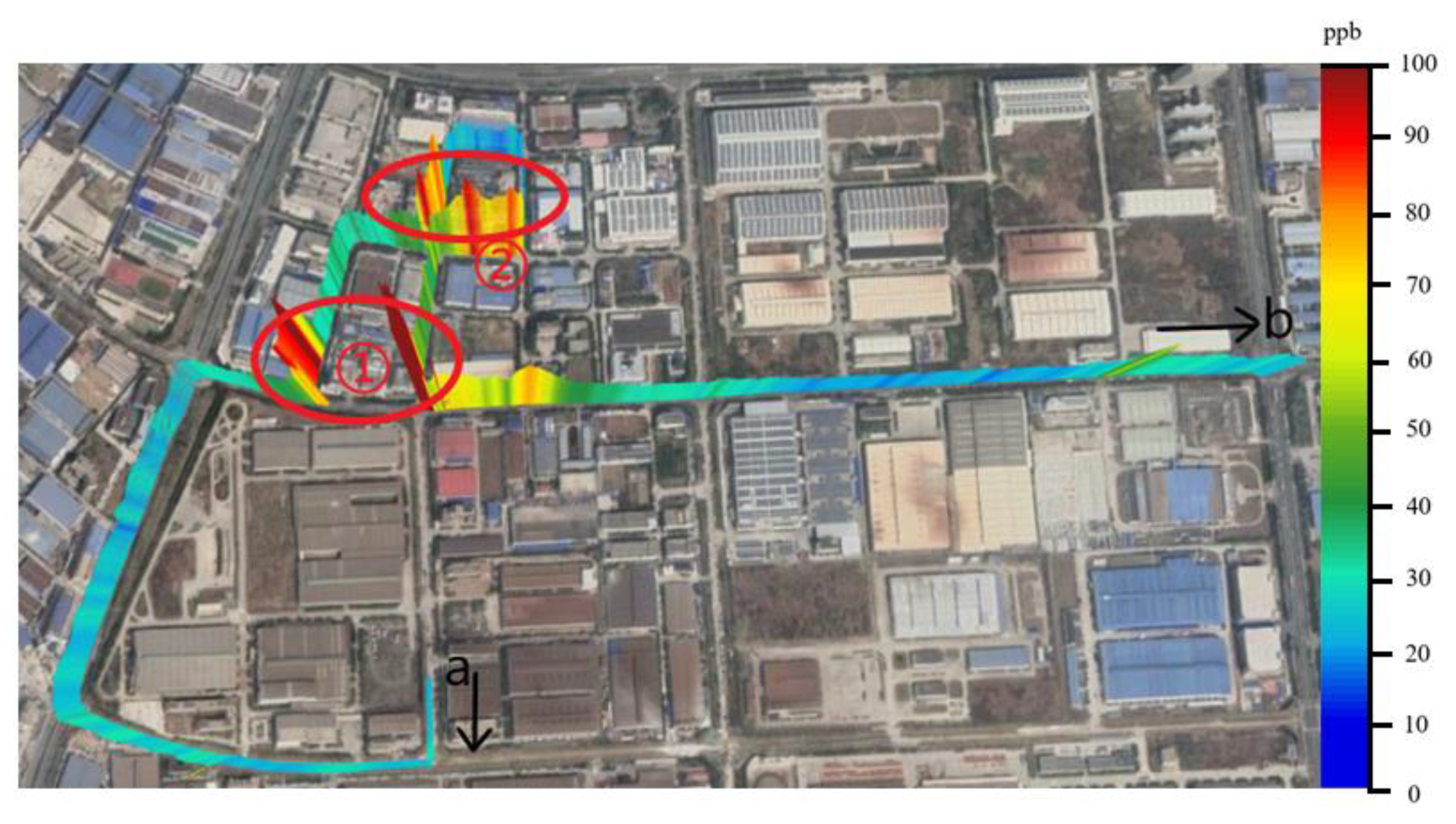
4.3. Analysis of the Navigation Monitoring Data
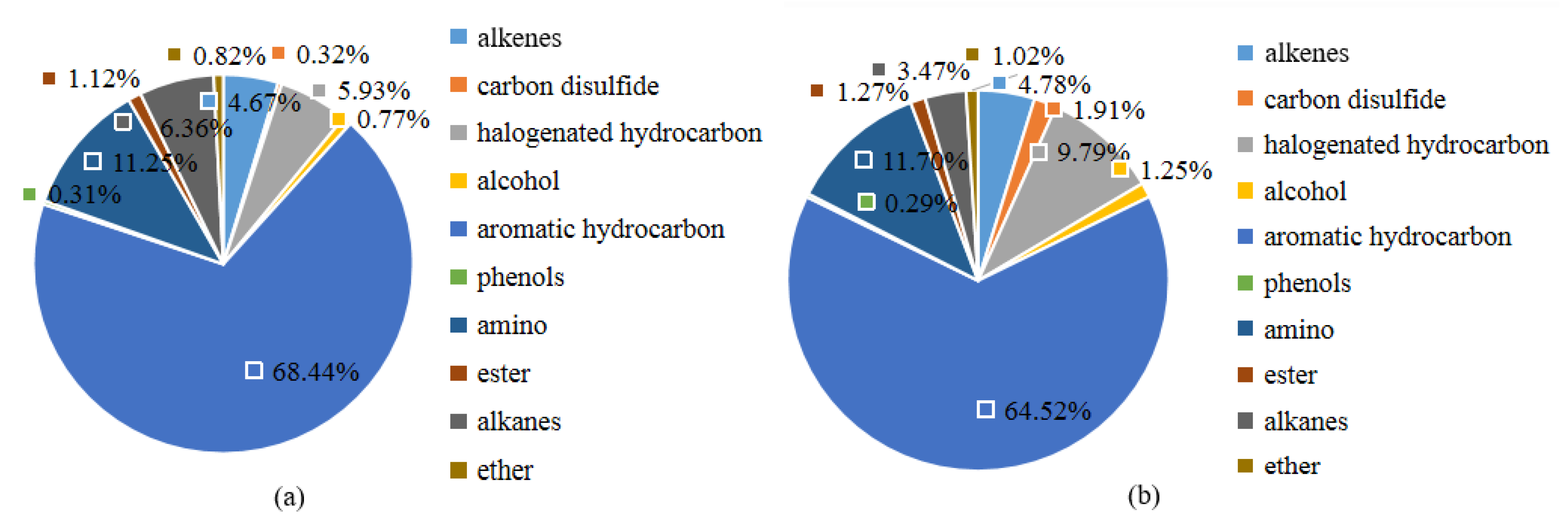
4.4. Analysis of the Contribution Rates of VOC Components to Ozone
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jenkin, M.E.; Clemitshaw, K.C. Ozone and other secondary photochemical pollutants: Chemical processes governing their formation in the planetary boundary layer. Atmos. Environ. 2000, 34, 2499–2527. [Google Scholar] [CrossRef]
- Jenkin, M.E.; Derwent, R.G.; Wallington, T.J. Photochemical ozone creation potentials for volatile organic compounds: Rationalization and estimation. Atmos. Environ. 2017, 163, 128–137. [Google Scholar] [CrossRef]
- Kjærgaard, S.K.; Mølhave, L.; Pedersen, O.F. Human reactions to a mixture of indoor air volatile organic compounds. Atmos. Environ. Part A 1991, 25, 1417–1426. [Google Scholar] [CrossRef]
- Shusterman, D. The effects of air pollutants and irritants on the upper airway. Proc. Am. Thorac. Soc. 2011, 8, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Ding, X.; Li, Q.; Sun, J.; Huang, C.; Yao, L.; Wang, X.; Ye, X.; Chen, Y.; He, H.; et al. Pollutants emitted from typical chinese vessels: Potential contributions to ozone and secondary organic aerosols. J. Clean. Prod. 2019, 238, 117862. [Google Scholar] [CrossRef]
- Zhang, G.; Hao, Z.; Jiang, M.; Wang, H. Study on foreign regulations and standards of stationary sources VOCs emission control. Huanjing Kexue 2011, 32, 3501–3508. [Google Scholar] [PubMed]
- Zhang, X.; Xue, Z.; Li, H.; Yan, L.; Yang, Y.; Wang, Y.; Duan, J.; Li, L.; Chai, F.; Cheng, M.; et al. Ambient volatile organic compounds pollution in China. J. Environ. Sci. 2017, 55, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-T. A comparative study on the statutory and technical regulations for controlling indoor volatile organic compounds in Taiwan and Japan. Atmosphere 2018, 9, 195. [Google Scholar] [CrossRef]
- Spinelle, L.; Gerboles, M.; Kok, G.; Persijn, S.; Sauerwald, T. Review of portable and low-cost sensors for the ambient air monitoring of benzene and other volatile organic compounds. Sensors 2017, 17, 1520. [Google Scholar] [CrossRef]
- Szulczyński, B.; Gębicki, J. Currently commercially available chemical sensors employed for detection of volatile organic compounds in outdoor and indoor air. Environments 2017, 4, 21. [Google Scholar] [CrossRef]
- Zhang, E.J.; Teng, C.C.; van Kessel, T.G.; Klein, L.; Muralidhar, R.; Wysocki, G.; Green, W.M.J. Field deployment of a portable optical spectrometer for methane fugitive emissions monitoring on oil and gas well pads. Sensors 2019, 19, 2707. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Van Stee, L.L.P.; Williams, J.; Beens, J.; Adahchour, M.; Vreuls, R.J.J.; Brinkman, U.A.T.; Lelieveld, J. Comprehensive two-dimensional gas chromatography (GC × GC) measurements of volatile organic compounds in the atmosphere. Atmos. Chem. Phys. 2003, 3, 665–682. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Yin, L.; Cheng, H.; Mao, T.; Zhang, K.; Lin, W.; Meng, Z.; Palasota, J. Coupling of comprehensive two-dimensional gas chromatography with quadrupole mass spectrometry: Application to the identification of atmospheric volatile organic compounds. J. Chromatogr. A 2014, 1361, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, B.; Zhang, H. Progress in research on on-line monitoring techniques for volatile organic compounds in ambient air. Chem. Ind. Eng. Prog. 2008, 27, 648–653. [Google Scholar]
- Gao, W.; Tan, G.; Hong, Y.; Li, M.; Nian, H.; Guo, C.; Huang, Z.; Fu, Z.; Dong, J.; Xu, X.; et al. Development of portable single photon ionization time-of-flight mass spectrometer combined with membrane inlet. Int. J. Mass Spectrom. 2013, 334, 8–12. [Google Scholar] [CrossRef]
- González-Méndez, R.; Reich, D.F.; Mullock, S.J.; Corlett, C.A.; Mayhew, C.A. Development and use of a thermal desorption unit and proton transfer reaction mass spectrometry for trace explosive detection: Determination of the instrumental limits of detection and an investigation of memory effects. Int. J. Mass Spectrom. 2015, 385, 13–18. [Google Scholar] [CrossRef]
- Kari, E.; Miettinen, P.; Yli-Pirilä, P.; Virtanen, A.; Faiola, C.L. PTR-TOF-MS product ion distributions and humidity-dependence of biogenic volatile organic compounds. Int. J. Mass Spectrom. 2018, 430, 87–97. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, J.; Hou, K.; Wang, W.; Qi, Y.; Wang, Y.; Xie, Y.; Hua, L.; Li, H. Online monitoring of trace chlorinated benzenes in flue gas of municipal solid waste incinerator by windowless VUV lamp single photon ionization TOFMS coupled with automatic enrichment system. Talanta 2016, 161, 693–699. [Google Scholar] [CrossRef]
- Li, Q.; Hua, L.; Xie, Y.; Jiang, J.; Li, H.; Hou, K.; Tian, D.; Li, H. Single photon ionization time-of-flight mass spectrometry with a windowless RF-discharge lamp for high temporal resolution monitoring of the initial stage of methanol-to-olefins reaction. Analyst 2019, 144, 1104–1109. [Google Scholar] [CrossRef]
- Wu, C.; Liu, W.; Jiang, J.; Wang, Y.; Hou, K.; Li, H. An in-source helical membrane inlet single photon ionization time-of-flight mass spectrometer for automatic monitoring of trace VOCs in water. Talanta 2019, 192, 46–51. [Google Scholar] [CrossRef]
- Sun, W.Q.; Shu, J.N.; Zhang, P.; Li, Z.; Li, N.N.; Liang, M.; Yang, B. Real-time monitoring of trace-level VOCs by an ultrasensitive lamp-based VUV photoionization mass spectrometer. Atmos. Meas. Tech. 2015, 8, 4637–4643. [Google Scholar] [CrossRef]
- Tonokura, K.; Kanno, N.; Yamamoto, Y.; Yamada, H. Development of a compact laser-based single photon ionization time-of-flight mass spectrometer. Int. J. Mass Spectrom. 2010, 290, 9–13. [Google Scholar] [CrossRef]
- Huo, L.; Gao, W.; Su, H.; Tan, G.; Mai, Z.; Huang, Z. Development of high sensitive online vacuum ultraviolet single-photon ionization time-of-flight mass spectrometer for VOCs. J. Chin. Mass Spectrom. Soc. 2018, 39, 171–179. [Google Scholar]
- Sun, W.Q.; Zhang, Y.; Fang, S.X. Application of vacuum ultraviolet single-photon ionization mass spectrometer in online analysis of volatile organic compounds. Chin. J. Anal. Chem. 2019, 47, 976–984. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, G.; Mai, Z.; Zhou, D.; Zhong, Y.; Li, X.; Gao, W.; Huang, Z. Monitoring VOCs of plastic company by single photon ionization time-of-flight mass spectrometry. J. Atmos. Environ. Opt. 2017, 12, 66–73. [Google Scholar]
- Liang, Q.; Bao, X.; Sun, Q.; Zhang, Q.; Zou, X.; Huang, C.; Shen, C.; Chu, Y. Imaging VOC distribution in cities and tracing VOC emission sources with a novel mobile proton transfer reaction mass spectrometer. Environ. Pollut. 2020, 265, 114628. [Google Scholar] [CrossRef]
- Tan, G.; Gao, W.; Hong, Y.; Huang, Z.; Li, M.; Dong, J.; Chen, P.; Fu, Z.; Zhou, Z. Development of high sensitivity membrane introduction mass spectrometer for on-line detecting VOC. Mod. Sci. Instrum. 2011, 10, 59–62. [Google Scholar]
- Han, W.; Xu, G.; Gao, Y.; Huang, C.; Zhao, X.; Qian, J.; Wang, Y.; Yang, P. Progress in field-portable and miniature mass spectrometry. J. Chin. Mass Spectrom. Soc. 2007, 28, 242–252. [Google Scholar]
- Shi, W.; Lu, X.; Zhang, J.; Zhao, J.; Yang, L.; Yu, Q.; Wang, X. Comparison of membrane inlet and capillary introduction miniature mass spectrometry for liquid analysis. Polymers 2019, 11, 567. [Google Scholar] [CrossRef]
- Tan, G.; Gao, W.; Huang, Z.; Hong, Y.; Fu, Z.; Dong, J.; Cheng, P.; Zhou, Z. Vacuum ultraviolet single-photon ionization time-of-flight mass spectrometer. Chin. J. Anal. Chem. 2011, 39, 1470–1475. [Google Scholar] [CrossRef]
- Kanno, N.; Tonokura, K. Vacuum ultraviolet photoionization mass spectra and cross-sections for volatile organic compounds at 10.5 eV. Appl. Spectrosc. 2007, 61, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Kronebusch, P.L.; Berkowitz, J. Photodissociative ionization in the 21–41 eV region: O2, N2, CO, NO, CO2, H2O, NH3 and CH4. Int. J. Mass Spectrom. Ion Phys. 1976, 22, 283–306. [Google Scholar] [CrossRef]
- Majety, V.P.; Scrinzi, A. Photo-ionization of noble gases: A demonstration of hybrid coupled channels approach. Photonics 2015, 2, 93–103. [Google Scholar] [CrossRef]
- Mühlberger, F.; Streibel, T.; Wieser, J.; Ulrich, A.; Zimmermann, R. Single photon ionization time-of-flight mass spectrometry with a pulsed electron beam pumped excimer VUV lamp for on-Line gas analysis: Setup and first results on cigarette smoke and human breath. Anal. Chem. 2005, 77, 7408–7414. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Huang, Z.; Nian, H.; Shen, X.; Wang, P.; Hu, S.; Li, M.; Cheng, P.; Dong, J.; Xu, X.; et al. A novel gas analysis system for metallurgical materials based on time-of-flight mass spectrometry. Int. J. Mass Spectrom. 2010, 294, 77–82. [Google Scholar] [CrossRef]
- Dodonov, A.F.; Kozlovski, V.I.; Soulimenkov, I.V.; Raznikov, V.V.; Loboda, A.V.; Zhen, Z.; Horwath, T.; Wollnik, H. High-resolution electrospray ionization orthogonal-injection time-of-flight mass spectrometer. Eur. J. Mass Spectrom. 2000, 6, 481–490. [Google Scholar] [CrossRef]
- Li, Z.; Wei, J.; Zhang, B. Laser mass specreometry: Principles and applications in environmental monitoring. Chin. J. Quantum Electron. 2001, 18, 1–8. [Google Scholar]
- Carter, W.P.L. Development of ozone reactivity scales for volatile organic compounds. Air Waste 1994, 44, 881–899. [Google Scholar] [CrossRef]
- Atlinson, R.; Arey, J. Atmospheric degradation of volatile organic compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, H.; Liang, S.; Hu, K.; Huang, Z.; Hu, Y.; Wang, Z.; Cheng, H.; Lyu, X. Ambient volatile organic compounds and their contributions to ozone formation in Wuhan. Environ. Sci. Technol. 2018, 41, 117–124. [Google Scholar]
- Lin, X.; Zhu, B.; An, J.; Yang, H. Potential contribution of secondary organic aerosols and ozone of VOCs in the northern suburb of Nanjing. China Environ. Sci. 2015, 35, 976–986. [Google Scholar]

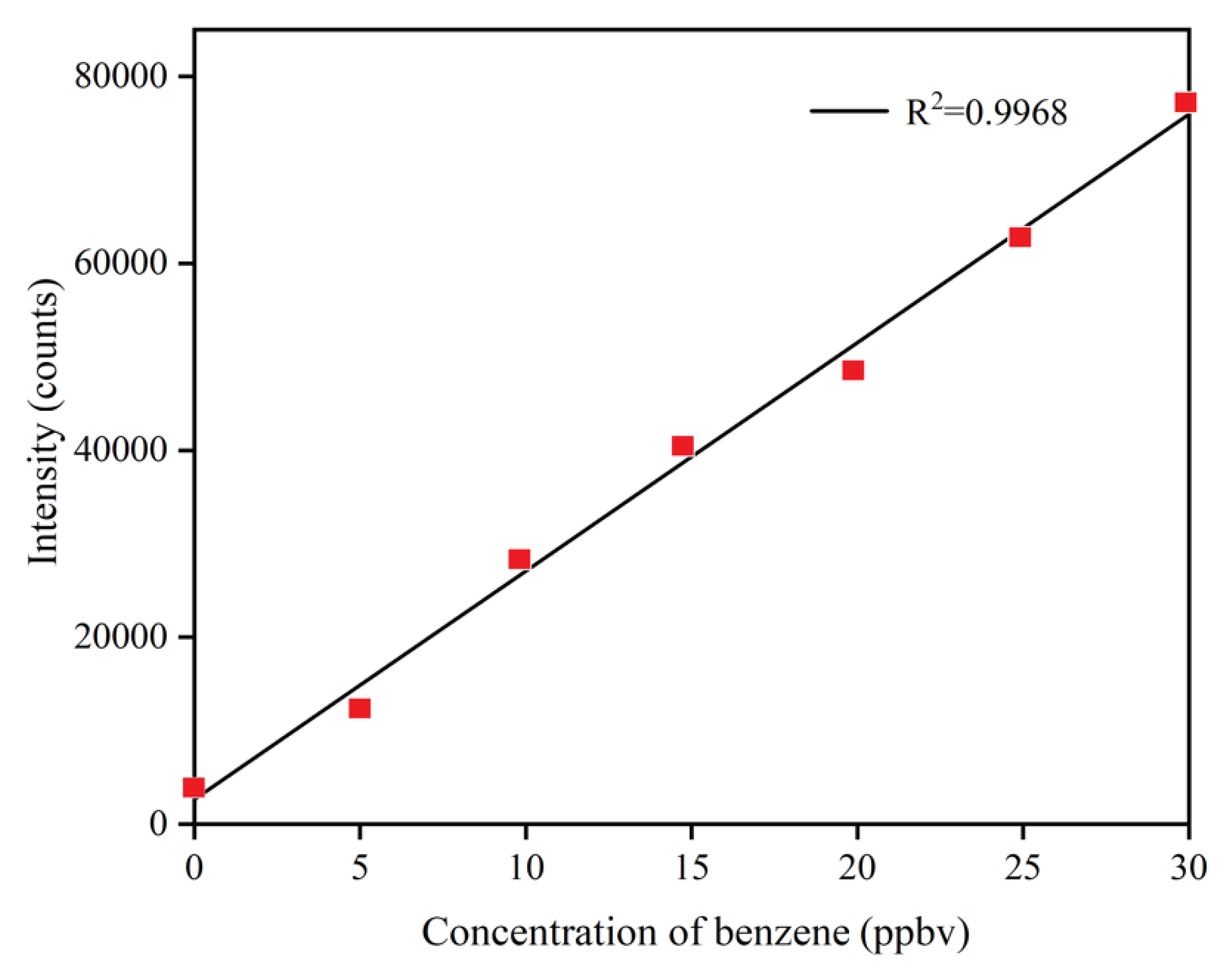

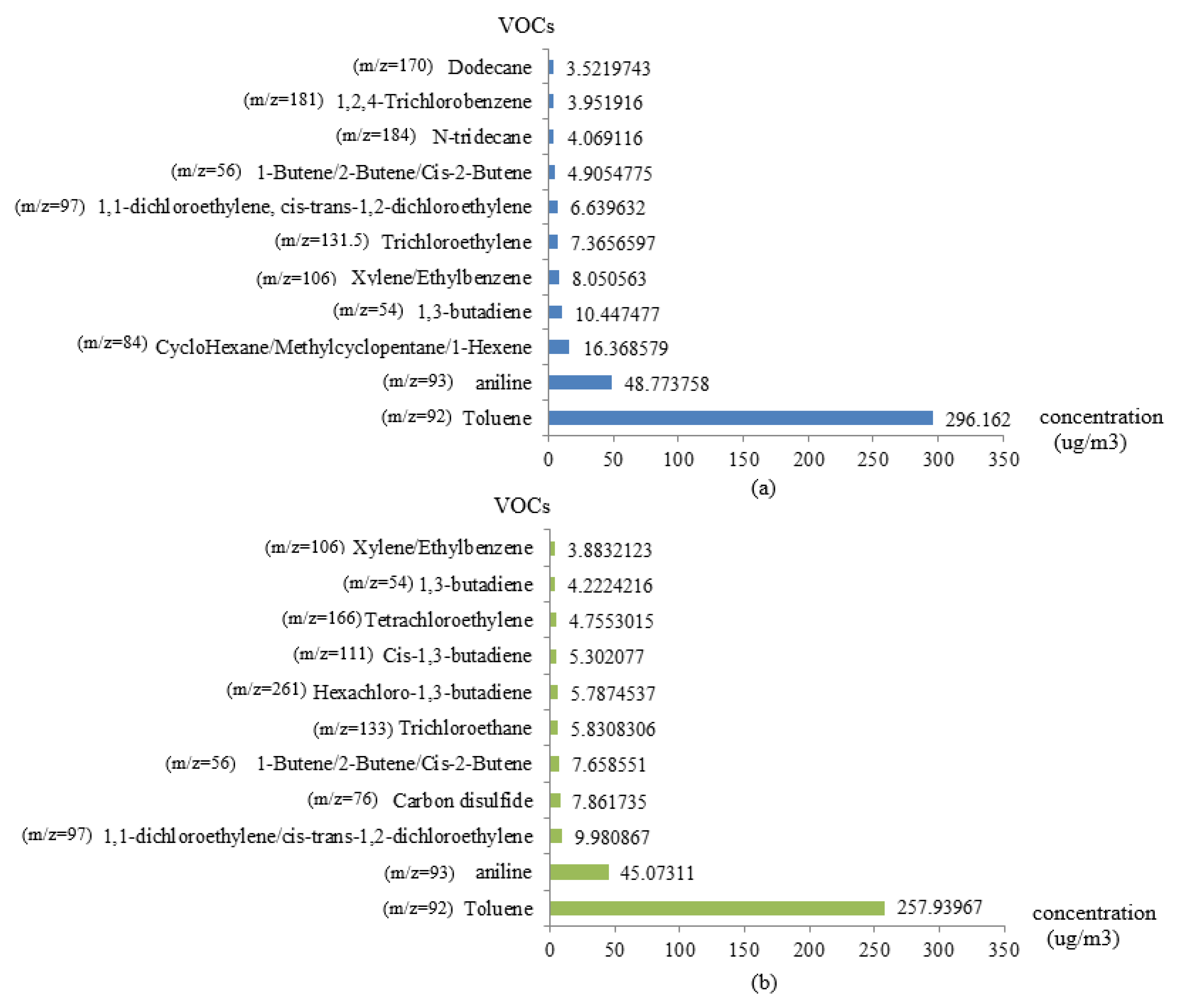
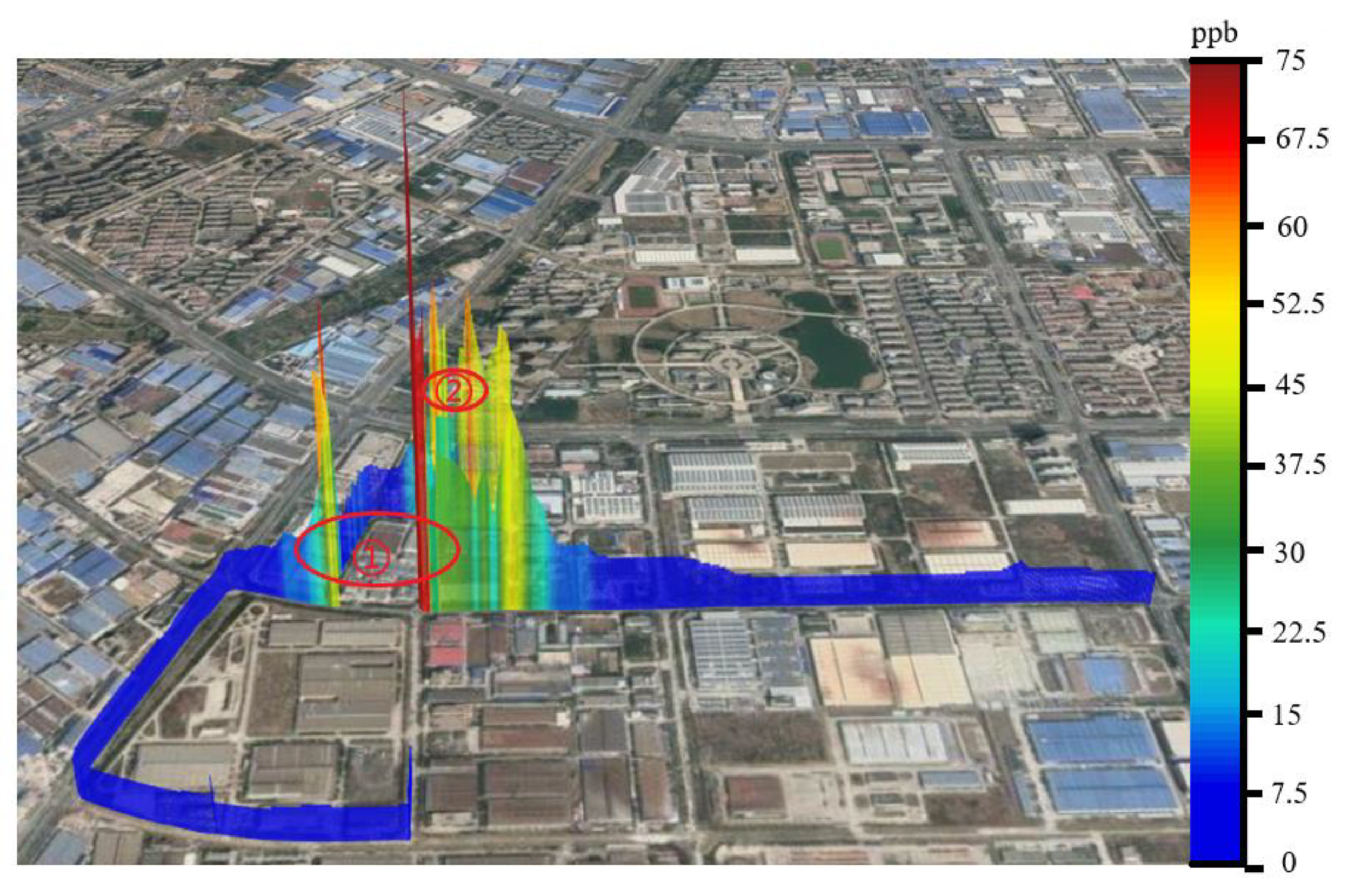
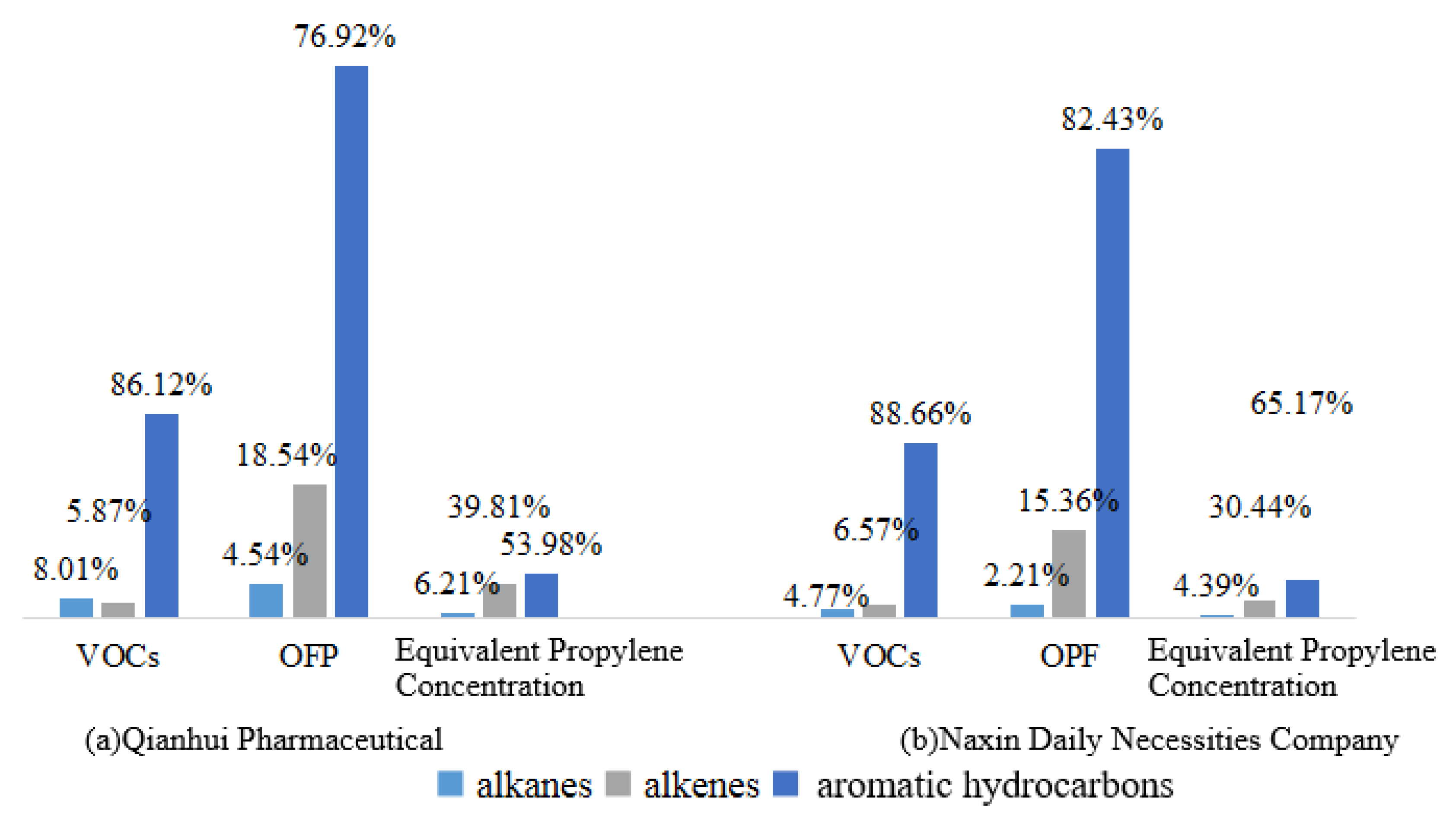
| Compounds | m/z | Actual Concentration (ppbv) | Mean of the Detected Concentration (ppbv) | Relative Standard Deviation (%) | Absolute Value of Relative Error (%) |
|---|---|---|---|---|---|
| Benzene | 78 | 30 | 29.202 | 3.11 | 2.66 |
| Toluene | 92 | 30 | 29.149 | 3.24 | 2.80 |
| Chloroethane | 64.5 | 30 | 28.787 | 2.45 | 4.04 |
| Isoprene | 68 | 40 | 40.197 | 2.19 | 0.49 |
| Pentanal | 86 | 30 | 31.042 | 1.22 | 3.47 |
| Qianhui Pharmaceutical | Naxin Daily Necessities Company | |||||||
|---|---|---|---|---|---|---|---|---|
| VOCs (μg/m3) | OPF min (×10−9) | OPF max (×10−9) | C(VOCs) * (×10−9) | VOCs (μg/m3) | OPF min (×10−9) | OPF max (×10−9) | C(VOCs) * (×10−9) | |
| Alkanes | 28.87 | 36.01 | 63.10 | 7.73 | 14.32 | 15.84 | 22.49 | 3.88 |
| Alkenes | 21.19 | 191.92 | 213.14 | 49.56 | 19.71 | 126.18 | 140.92 | 26.92 |
| Aromatic hydrocarbons | 310.52 | 822.59 | 858.01 | 67.21 | 266.03 | 707.95 | 725.03 | 57.62 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhang, Y.; Gao, J.; Wang, X.; Li, H.; Wang, Y.; Duan, M.; Cao, K.; Cai, Y.; Pan, J. VOC Monitoring and Ozone Generation Potential Analysis Based on a Single-Photon Ionization Time-of-Flight Mass Spectrometer. Photonics 2020, 7, 61. https://doi.org/10.3390/photonics7030061
Zhao Y, Zhang Y, Gao J, Wang X, Li H, Wang Y, Duan M, Cao K, Cai Y, Pan J. VOC Monitoring and Ozone Generation Potential Analysis Based on a Single-Photon Ionization Time-of-Flight Mass Spectrometer. Photonics. 2020; 7(3):61. https://doi.org/10.3390/photonics7030061
Chicago/Turabian StyleZhao, Yuefeng, Yurong Zhang, Jing Gao, Xu Wang, Hui Li, Yanqi Wang, Mengjun Duan, Kaifa Cao, Yangjian Cai, and Jie Pan. 2020. "VOC Monitoring and Ozone Generation Potential Analysis Based on a Single-Photon Ionization Time-of-Flight Mass Spectrometer" Photonics 7, no. 3: 61. https://doi.org/10.3390/photonics7030061
APA StyleZhao, Y., Zhang, Y., Gao, J., Wang, X., Li, H., Wang, Y., Duan, M., Cao, K., Cai, Y., & Pan, J. (2020). VOC Monitoring and Ozone Generation Potential Analysis Based on a Single-Photon Ionization Time-of-Flight Mass Spectrometer. Photonics, 7(3), 61. https://doi.org/10.3390/photonics7030061