Upconversion and Color Tunability in Er3+–Tm3+–Yb3+ Tri-Doped Fluorophosphate Glasses
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rivera-López, F.; Babu, P.; Basavapoornima, C.; Jayasankar, C.K.; Lavín, V. Efficient Nd3+→Yb3+ energy transfer processes in high phonon energy phosphate glasses for 1.0 μm Yb3+ laser. J. Appl. Phys. 2011, 109, 123514. [Google Scholar] [CrossRef]
- Rivera-López, F.; Lavín, V. Upconversion/back-transfer losses and emission dynamics in Nd3+-Yb3+ co-doped phosphate glasses for multiple pump channel laser. J. Non-Cryst. Solids 2018, 489, 84–90. [Google Scholar] [CrossRef]
- Pattnaik, S.; Mondal, M.; Mukhopadhyay, L.; Rai, V.K. Trivalent lanthanide doped orthovanadate phosphors for efficient upconversion. Mater. Today Proc. 2019, 46, 6363–6366. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, X.; Ren, Q.; Du, L.; Pei, M.; Hai, O. Triple luminescent center energy transfer enables color tuning in Na3Y. Polyhedron 2022, 225, 116047. [Google Scholar] [CrossRef]
- Babu, P.; Jang, K.H.; Kim, E.S.; Shi, L.; Seo, H.J.; Rivera-López, F.; Rodríguez-Mendoza, U.R.; Lavín, V.; Vijaya, R.; Jayasankar, C.K.; et al. Spectral investigations on Dy3+-doped transparent oxyfluoride glasses and nanocrystalline glass ceramics. J. Appl. Phys. 2009, 105, 013516. [Google Scholar] [CrossRef]
- Rivera-López, F.; Babu, P.; Jyothi, L.; Rodríguez-Mendoza, U.R.; Martín, I.R.; Jayasankar, C.K.; Lavín, V. Er3+–Yb3+ codoped phosphate glasses used for an efficient 1.5μm broadband gain medium. Opt. Mater. 2012, 34, 1235–1240. [Google Scholar] [CrossRef]
- Auzel, F. Upconversion and Anti-Stokes Processes with f and d Ions in Solids. Chem. Rev. 2004, 104, 139–173. [Google Scholar] [CrossRef] [PubMed]
- Rivera-López, F.; Torres, M.E.; de Cos, G.G. Upconversion and cooperative luminescence in YBO3:Yb3+–Er3+. Mater. Today Commun. 2021, 27, 102434. [Google Scholar] [CrossRef]
- Gaponenko, N.V.; Staskov, N.I.; Sudnik, L.V.; Vityaz, P.A.; Luchanok, A.R.; Karnilava, Y.D.; Lashkovskaya, E.I.; Stepikhova, M.V.; Yablonskiy, A.N.; Zhivulko, V.D.; et al. Upconversion Luminescence from Sol-Gel-Derived Erbium- and Ytterbium-Doped BaTiO3 Film Structures and the Target Form. Photonics 2023, 10, 359. [Google Scholar] [CrossRef]
- Pominova, D.; Proydakova, V.; Romanishkin, I.; Kuznetsov, S.; Linkov, K.; Tabachkova, N.; Ryabova, A. NaGdF4:Yb, Tm Upconversion Nanoparticles for Bioimaging in Shortwave-Infrared Range: Study of Energy Transfer Processes and Composition Optimization. Photonics 2024, 11, 38. [Google Scholar] [CrossRef]
- Sola, D.; Miguel, A.; Arias-Egido, E.; Peña, J.I. Spectroscopy and near-infrared to visible upconversion of Er3+ ions in aluminosilicate glasses manufactured with controlled optical transmission. Appl. Sci. 2021, 11, 1137. [Google Scholar] [CrossRef]
- Zi, Y.; Huang, A.; Zhao, H.; Bai, X.; Xu, Z.; Ullah, A.; Liu, Y.; Cun, Y.; Song, Z.; Qiu, J.; et al. Efficient Reversible Upconversion Luminescence Modulation based on Photochromism of Lanthanides-Doped BaMgSiO4 Glass Ceramics Toward Optical Storage Application. Laser Photonics Rev. 2024, 18, 2400882. [Google Scholar] [CrossRef]
- Patra, A.; Friend, C.S.; Kapoor, R.; Prasad, P.N. Upconversion in Er3+:ZrO2 Nanocrystals. J. Phys. Chem. B 2002, 106, 1909–1912. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, C.; Peng, C.; Li, C.; Wang, L.; Chai, R.; Lin, J. Controllable Red, Green, Green, Blue (RGB) and bright white upconversion luminescence of Lu2O3:Yb3+/Er 3+/Tm3+ nanocrystals through single laser excitation at 980 nm. Chem. A Eur. J. 2009, 15, 4649–4655. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Zhang, D.; Zhao, D.; Liu, N.; Shi, F.; Qin, W. Bright white upconversion emission from Yb3+, Er3+, and Tm3+-codoped Gd2O3 nanotubes. Phys. Chem. Chem. Phys. 2010, 12, 7620–7625. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.U.; Vijaya, N.; Oliva, J.; Jacinto, C.; de La Rosa, E.; Jayasankar, C.K. Multicolor upconversion emission and color tunability in Tm3+/Er3+/Yb3+ Tri-Doped NaNbO3 nanocrystals. Mater. Express 2012, 2, 294–302. [Google Scholar] [CrossRef]
- Yan, D.; Zhu, J.; Wu, H.; Yang, Z.; Qiu, J.; Song, Z.; Yu, X.; Yang, Y.; Zhou, D.; Yin, Z.; et al. Energy transfer and photoluminescence modification in Yb-Er-Tm triply doped Y2Ti2O7 upconversion inverse opal. J. Mater. Chem. 2012, 22, 18558–18563. [Google Scholar] [CrossRef]
- Carmo, F.F.D.; Nascimento, J.P.C.D.; Façanha, M.X.; Sales, T.O.; Santos, W.Q.; Gouveia-Neto, A.S.; Jacinto, C.; Sombra, A.S.B. White light upconversion emission and color tunability in Er3+/Tm3+/Yb3+ tri-doped YNbO4 phosphor. J. Lumin. 2018, 204, 676–684. [Google Scholar] [CrossRef]
- Du, S.; Wang, D.; Wang, Y.; Xin, S.; Qiang, Q.; Ma, X. Synthesis and up-conversion luminescence of Yb3+/Er3+/Tm3+ doped Ca9Y(PO4)7. New J. Chem. 2015, 39, 5605–5611. [Google Scholar] [CrossRef]
- Hassairi, M.A.; Hernández, A.G.; Dammak, M.; Zambon, D.; Chadeyron, G.; Mahiou, R. Tuning white upconversion emission in GdPO4:Er/Yb/Tm phosphors. J. Lumin. 2018, 203, 707–713. [Google Scholar] [CrossRef]
- Li, X.; Xiao, Z.; Luo, M.; Dong, X.; Du, T.; Wang, Y. Low melting glasses in ZnO-Fe2O3-P2O5 system with high chemical durability and thermal stability for sealing or waste immobilization. J. Non-Cryst. Solids 2017, 469, 62–69. [Google Scholar] [CrossRef]
- Zhu, S.; Xie, X.; Han, L.; Li, H.; Shi, C.; Yang, Y.; Sun, J. Co-doped NaYF4:Yb/Er/Tm upconversion luminescent coating to enhance the efficiency of photovoltaic cells. Phys. Chem. Chem. Phys. 2024, 26, 17882–17891. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Zhang, X.; Li, L.; Hao, H. Up-converting luminescence and temperature sensing of Er3+/Tm3+/Yb3+ co-doped NaYF4 phosphors operating in visible and the first biological window range. Nanomaterials 2021, 11, 2660. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Hu, L.; Fang, Y.; Zhang, J.; Sun, H.; Xu, S.; Zhang, L. Upconversion properties of Er3+, Yb3+ and Tm3+ codoped fluorophosphate glasses. Spectrochim. Acta–Part A Mol. Biomol. Spectrosc. 2007, 68, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wang, P.; Wan, R.; Guo, C.; Tian, S. Structural insight of fluorophosphate glasses through F/O ratio: Case study of Raman and NMR spectra. J. Non-Cryst. Solids 2024, 637, 123065. [Google Scholar] [CrossRef]
- Kaewjaeng, S.; Chanthima, N.; Thongdang, J.; Reungsri, S.; Kothan, S.; Kaewkhao, J. Synthesis and radiation properties of Li2O-BaO-Bi2O3-P2O5 glasses. Mater. Today Proc. 2018, 43, 2544–2553. [Google Scholar] [CrossRef]
- Liu, W.; Pan, Y.T.; Zhang, J.; Zhang, L.; Moya, J.S.; Cabal, B.; Wang, D.Y. Low-melting phosphate glasses as flame-retardant synergists to epoxy: Barrier effects vs flame retardancy. Polym. Degrad. Stab. 2021, 185, 109495. [Google Scholar] [CrossRef]
- Shi, X.Z.; Gu, Y.; Liu, T.Y.; Jiang, Z.H.; Li, R.; Zeng, F. Effect of different P2O5/SnF2 ratios on the structure and properties of phosphate glass. J. Non-Cryst. Solids 2022, 578, 121350. [Google Scholar] [CrossRef]
- Kityk, I.V.; Wasylak, J.; Kucharski, J.; Dorosz, D. PbO-Bi2O3-Ga2O3 -BaO-Dy3+ glasses for IR luminescence. J. Non-Cryst. Solids 2002, 297, 285–289. [Google Scholar] [CrossRef]
- Obaton, A.F.; Labbé, C.; Le Boulanger, P.; Elouadi, B.; Boulon, G. Excited state absorption in Yb3+ -Er3+ -codoped phosphate glasses (ZnO-Al2O3-La2O3-P2O5) around the 4I13/2→4I15/2 emission spectral range. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1999, 55, 263–271. [Google Scholar] [CrossRef]
- Lesniak, M.; Mach, G.; Starzyk, B.; Baranowska, A.; Bik, M.; Kochanowicz, M.; Zmojda, J.; Miluski, P.; Sitarz, M.; Dorosz, D. Investigation of the structure in oxyfluoride TeO2–P2O5 based glasses with the various BaF2 content. J. Mol. Struct. 2020, 1217, 128452. [Google Scholar] [CrossRef]
- Pollnau, M.; Gamelin, D.; Lüthi, S.; Güdel, H.; Hehlen, M. Power dependence of upconversion luminescence in lanthanide and transition-metal-ion systems. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 61, 3337–3346. [Google Scholar] [CrossRef]
- Jacinto, C.; Vermelho, M.V.D.; Gouveia, E.A.; De Araujo, M.T.; Udo, P.T.; Astrath, N.G.C.; Baesso, M.L. Pump-power-controlled luminescence switching in Yb3+ Tm3+ codoped water-free low silica calcium aluminosilicate glasses. Appl. Phys. Lett. 2007, 91, 2007–2009. [Google Scholar] [CrossRef]
- Pavitra, E.; Raju, G.S.R.; Oh, J.H.; Yu, J.S. Pump power induced tunable upconversion emissions from Er3+/Tm3+/Yb3+ ions tri-doped SrY2O4 nanocrystalline phosphors. New J. Chem. 2014, 38, 3413–3420. [Google Scholar] [CrossRef]
- Shi, L.; Li, C.; Shen, Q.; Qiu, Z. White upconversion emission in Er3+/Yb3+/Tm3+ codoped LiTaO3 polycrystals. J. Alloys Compd. 2014, 591, 105–109. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, B.; Qian, X.; Wang, J.; Zheng, L.; Hou, J.; Fang, Y.; Su, L. Upconversion color tunability and white light generation in Yb3+/Er3+/Tm3+ tri-doped CaF2 single crystals. Opt. Mater. 2019, 90, 40–45. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Y.; Peng, G.; Zhang, W.; Wang, Y.; Yang, K.; Zhang, X.; Song, Y. Enhanced white light emission in Er/Tm/Yb/Li codoped Y2O3 nanocrystals. Opt. Commun. 2009, 282, 1922–1924. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Luo, Y.; Zhang, X.; Hao, Z.; Wang, X. Color control and white light generation of upconversion luminescence by operating dopant concentrations and pump densities in Yb3+, Er3+ and Tm3+ tri-doped Lu2O3 nanocrystals. J. Mater. Chem. 2011, 21, 2895–2900. [Google Scholar] [CrossRef]
- Hassairi, M.A.; Dammak, M.; Zambon, D.; Chadeyron, G.; Mahiou, R. Red–green–blue upconversion luminescence and energy transfer in Yb3+/Er3+/Tm3+ doped YP5O14 ultraphosphates. J. Lumin. 2017, 181, 393–399. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Hong, G.; Yu, Y. Upconversion luminescence of Y2O3:Er3+, Yb3+ nanoparticles prepared by a homogeneous precipitation method. J. Rare Earths 2008, 26, 450–454. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Li, X.; Sun, J.; Zhang, J.; Zheng, H.; Zhong, H.; Hua, R.; Xia, H.; Chen, B. Concentration quenching of blue upconversion luminescence in Tm3+/Yb3+ co-doped Gd2(WO4)3 phosphors under 980 and 808 nm excitation. J. Alloys Compd. 2017, 709, 147–157. [Google Scholar] [CrossRef]
- Venkatramu, V.; León-Luis, S.F.; Rodríguez-Mendoza, U.R.; Monteseguro, V.; Manjón, F.J.; Lozano-Gorrín, A.D.; Valiente, R.; Navarro-Urrios, D.; Jayasankar, C.K.; Muñoz, A.; et al. Synthesis, structure and luminescence of Er3+-doped Y3Ga5O12 nano-garnets. J. Mater. Chem. 2012, 22, 13788–13799. [Google Scholar] [CrossRef]
- Liang, H.; Lei, W.; Liu, S.; Zhang, P.; Luo, Z.; Lu, A. Color tunable up-conversion luminescence characteristics of Yb3+-Er3+-Tm3+ tri-doped fluorosilicate glass potentially used in WLED field. Opt. Mater. 2021, 119, 111320. [Google Scholar] [CrossRef]
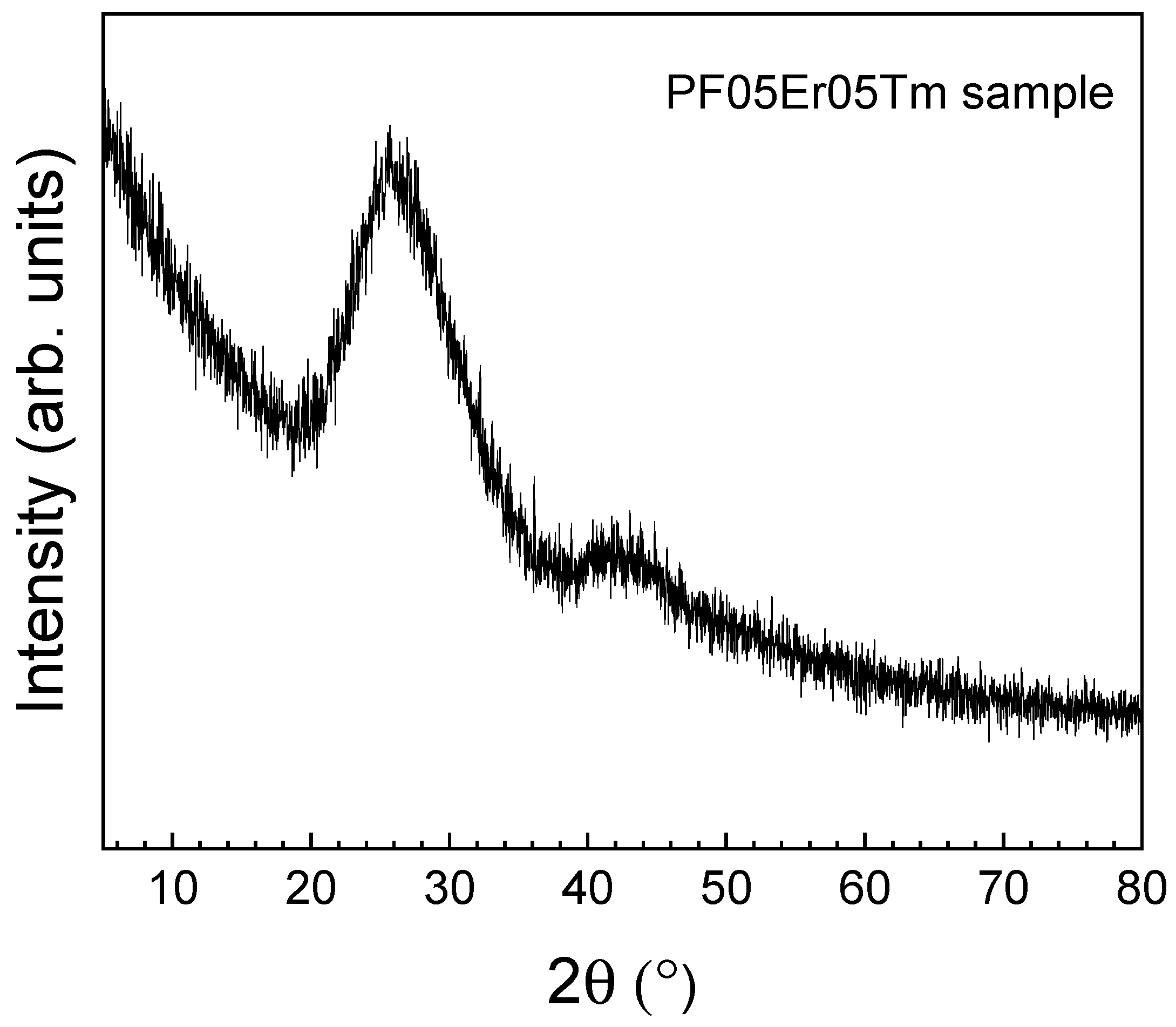
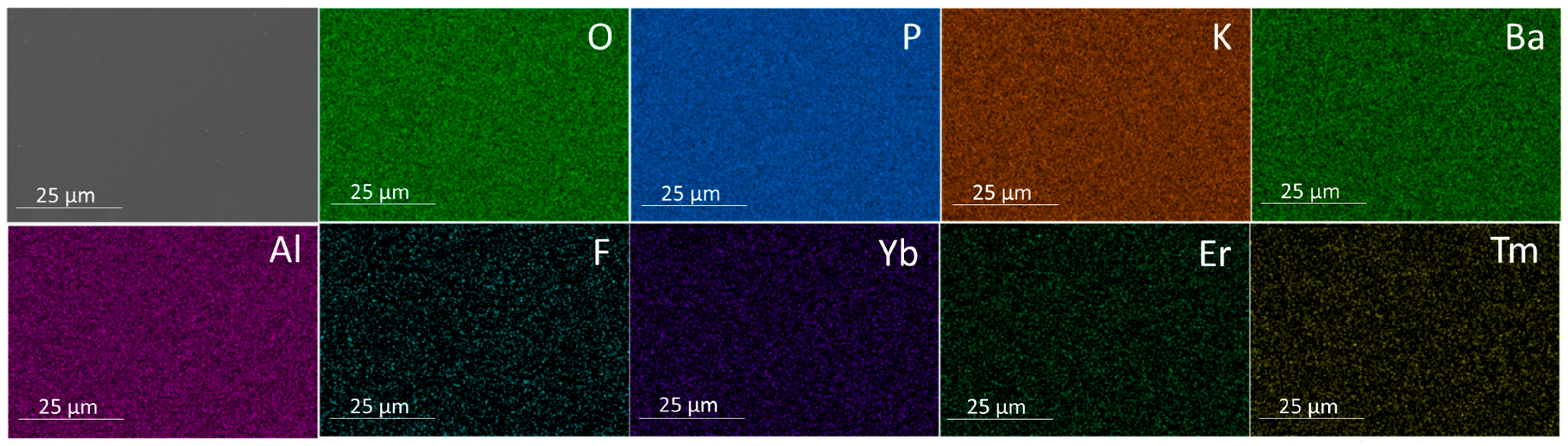
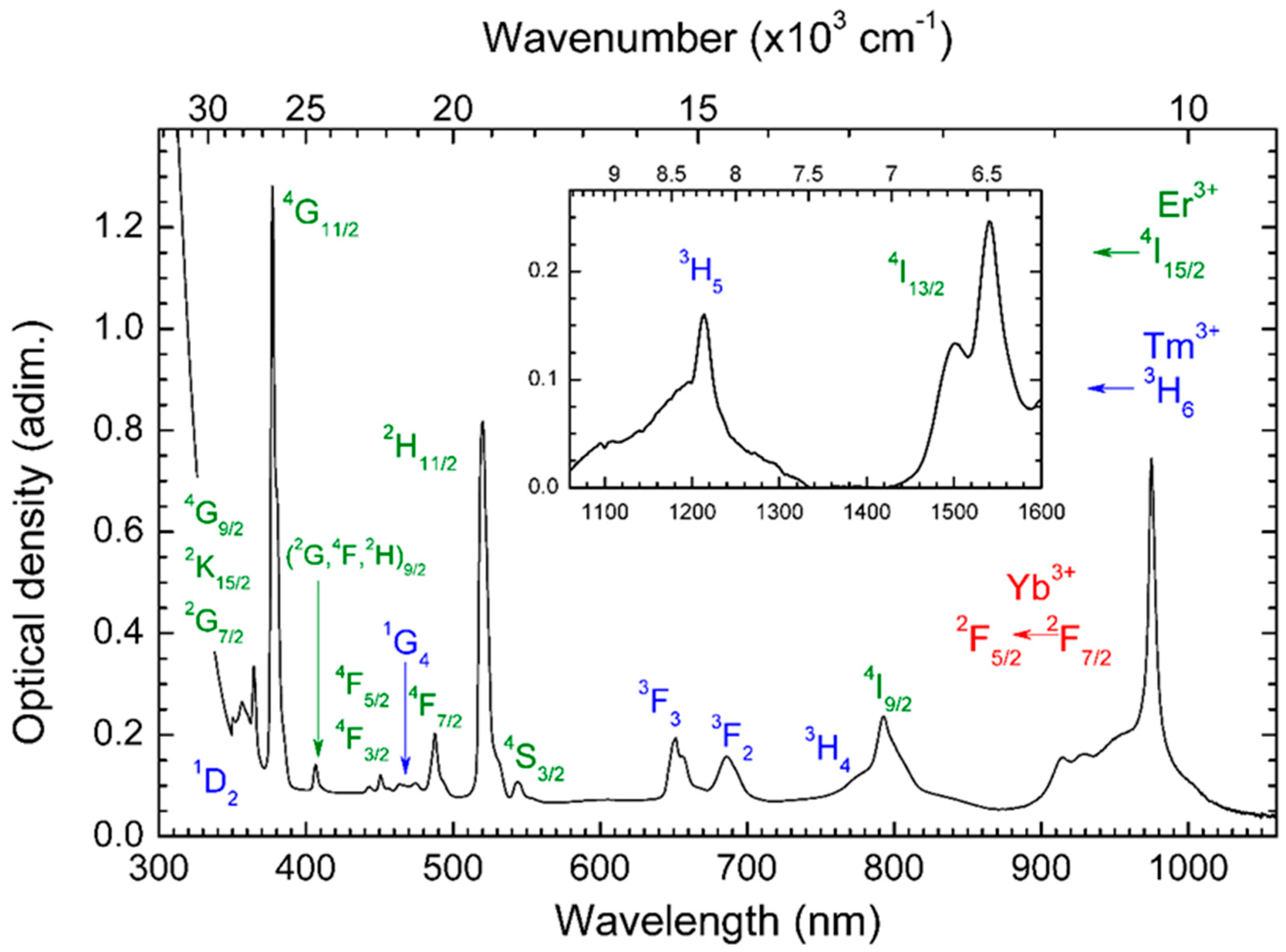
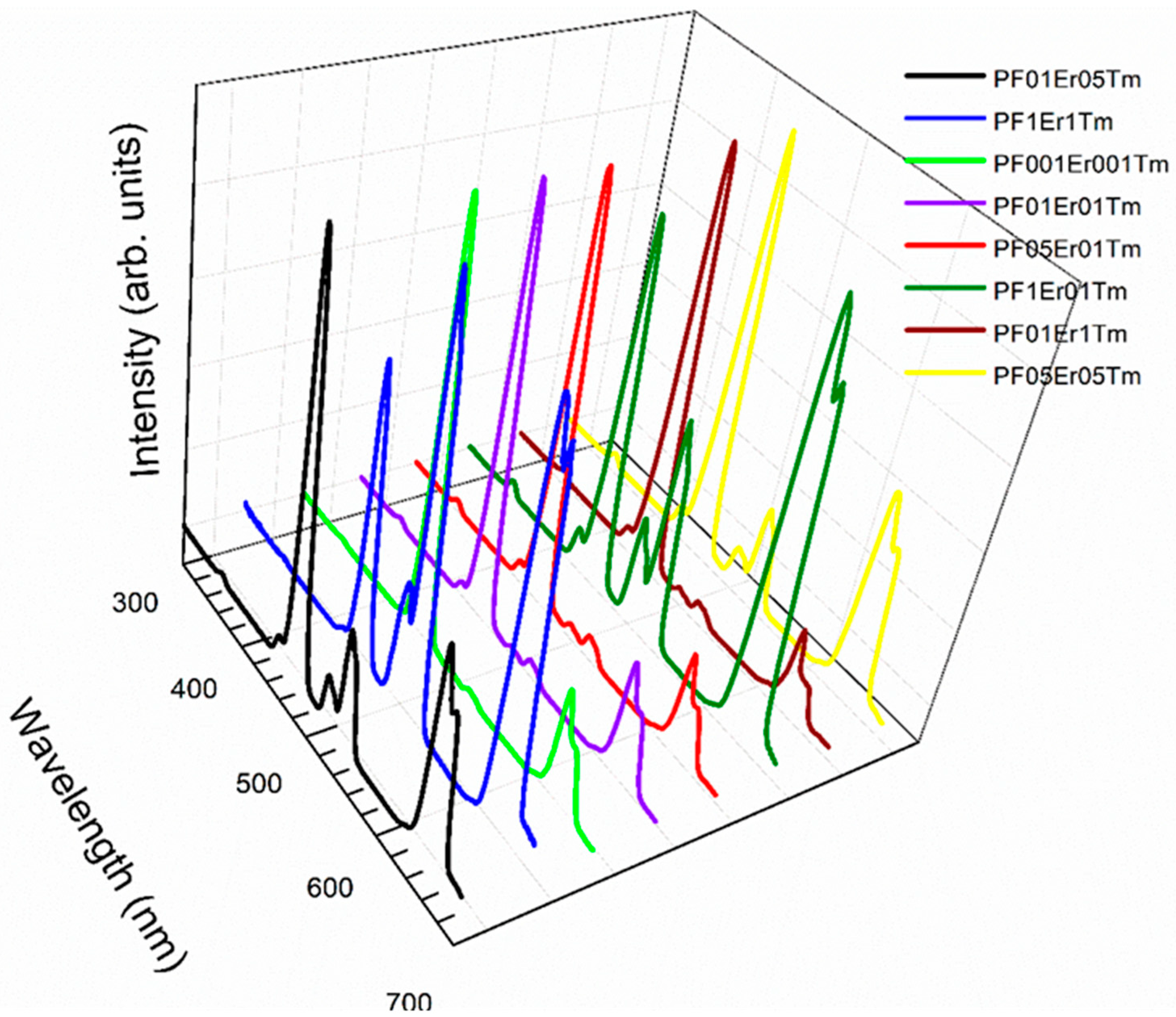
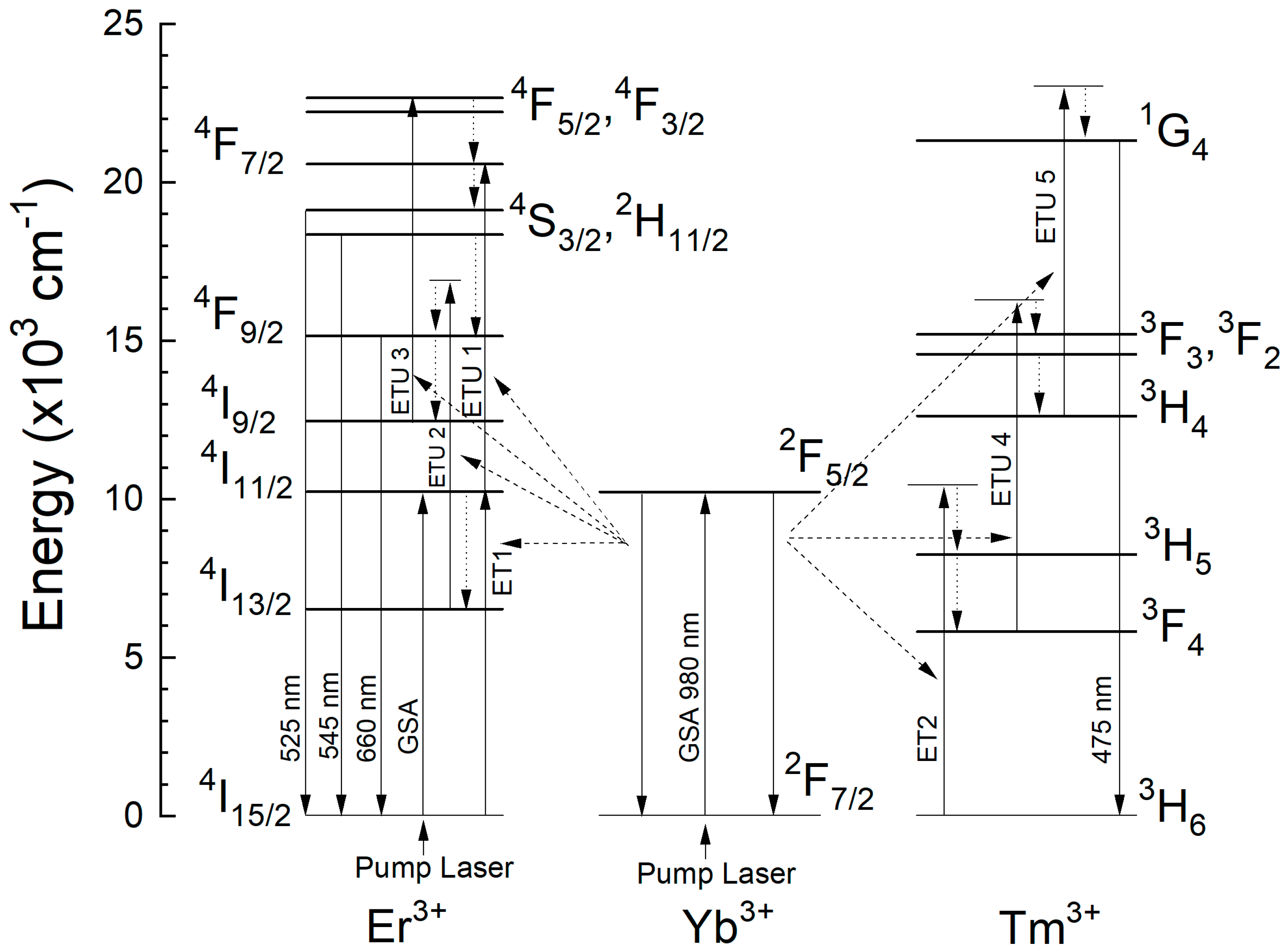
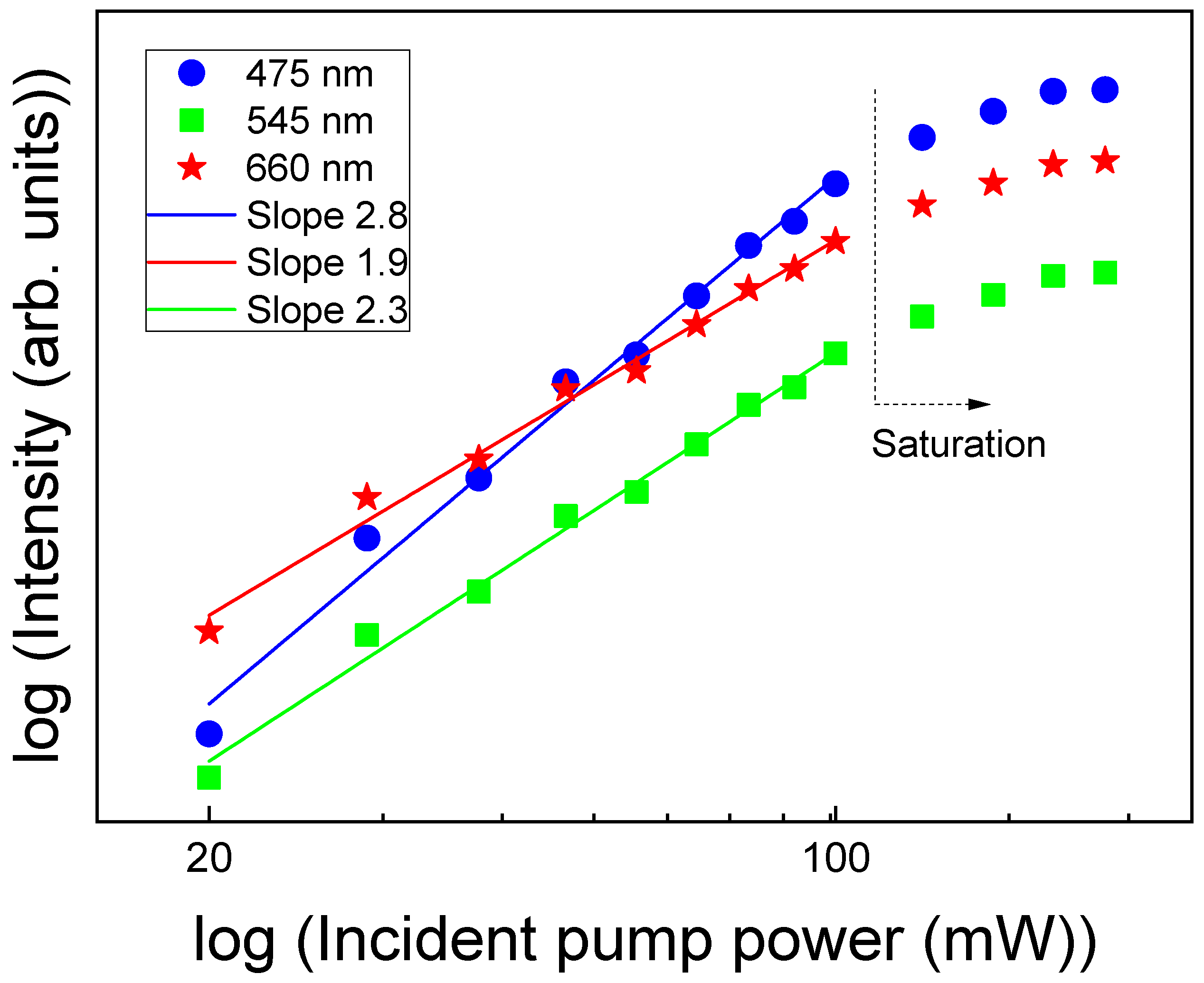
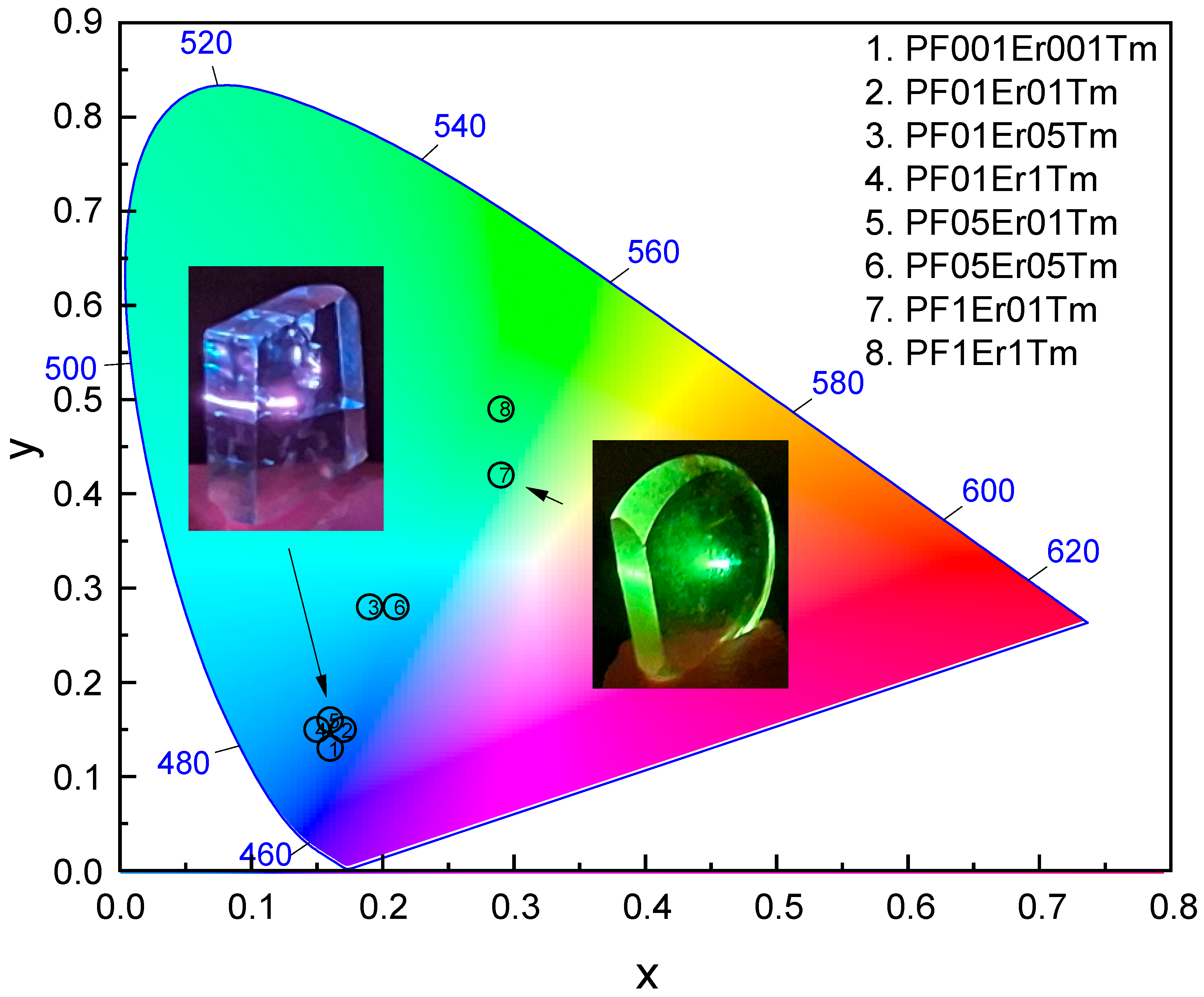
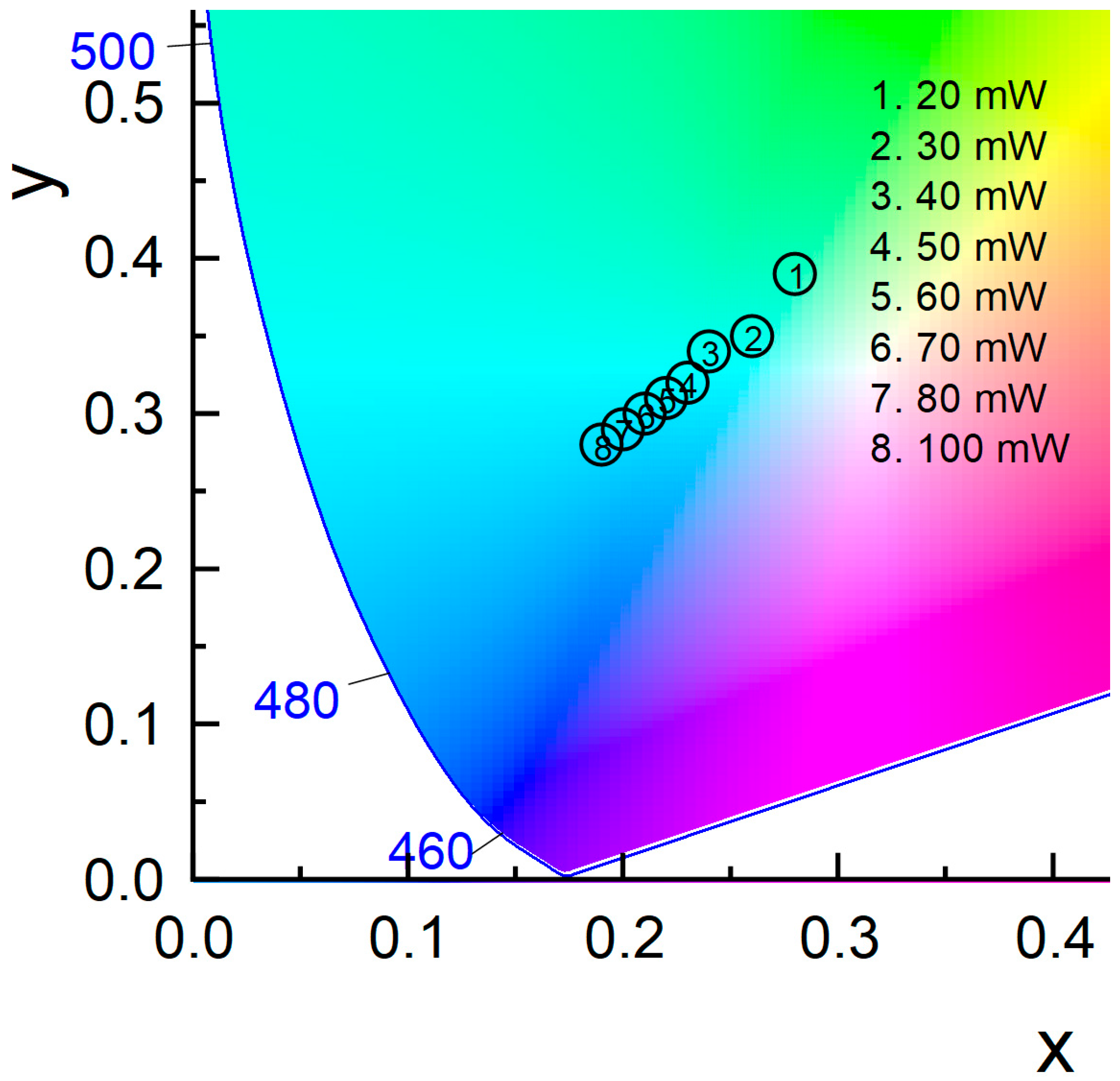
| Glass Composition (mol%) | Label |
|---|---|
| 54.99 P2O5 -17 K2O -10.99 BaO- 9 Al2O3 -6 BaF2 -2 Yb2O3 -0.01 Er2O3 -0.01 Tm2O3 | PF001Er001Tm |
| 54.90 P2O5 -17 K2O -10.90 BaO -9 Al2O3 -6 BaF2 -2 Yb2O3 -0.1 Er2O3 -0.1 Tm2O3 | PF01Er01Tm |
| 54.00 P2O5 -17 K2O -10.00 BaO -9 Al2O3 -6 BaF2 -2 Yb2O3 -1 Er2O3 -1 Tm2O3 | PF1Er1Tm |
| 54.50 P2O5 -17 K2O -10.50 BaO -9 Al2O3 -6 BaF2 -2 Yb2O3 -0.5 Er2O3 -0.5 Tm2O3 | PF05Er05Tm |
| 54.45 P2O5 -17 K2O -10.45 BaO -9 Al2O3 -6 BaF2 -2 Yb2O3 -0.1 Er2O3 -1 Tm2O3 | PF01Er1Tm |
| 54.70 P2O5 -17 K2O -10.70 BaO -9 Al2O3 -6 BaF2 -2 Yb2O3 -0.1 Er2O3 -0.5 Tm2O3 | PF01Er05Tm |
| 54.45 P2O5 -17 K2O -10.45 BaO -9 Al2O3 -6 BaF2 -2 Yb2O3 -1 Er2O3 -0.1 Tm2O3 | PF1Er01Tm |
| 54.70 P2O5 -17 K2O -10.70 BaO -9 Al2O3 -6 BaF2 -2 Yb2O3 -0.5 Er2O3 -0.1 Tm2O3 | PF05Er01Tm |
| Sample | Chromaticity Coordinates |
|---|---|
| PF001Er001Tm | x = 0.16, y = 0.13 |
| PF01Er01Tm | x = 0.17, y = 0.15 |
| PF1Er1Tm | x = 0.29, y = 0.49 |
| PF05Er05Tm | x = 0.21, y = 0.28 |
| PF01Er1Tm | x = 0.15, y = 0.15 |
| PF01Er05Tm | x = 0.19, y = 0.28 |
| PF1Er01Tm | x = 0.29, y = 0.42 |
| PF05Er01Tm | x = 0.16, y = 0.16 |
| Power (mW) | Chromaticity Coordinates |
|---|---|
| 20 | x = 0.28, y = 0.39 |
| 30 | x = 0.26, y = 0.35 |
| 40 | x = 0.24, y = 0.34 |
| 50 | x = 0.23, y = 0.32 |
| 60 | x = 0.22, y = 0.31 |
| 70 | x = 0.21, y = 0.30 |
| 80 | x = 0.20, y = 0.29 |
| 90 | x = 0.20, y = 0.29 |
| 100 | x = 0.19, y = 0.28 |
| 125 | x = 0.19, y = 0.27 |
| 150 | x = 0.19, y = 0.27 |
| 175 | x = 0.19, y = 0.27 |
| 200 | x = 0.19, y = 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-López, F.; Babu, P.; Venkatramu, V.; Lavín, V. Upconversion and Color Tunability in Er3+–Tm3+–Yb3+ Tri-Doped Fluorophosphate Glasses. Photonics 2025, 12, 745. https://doi.org/10.3390/photonics12080745
Rivera-López F, Babu P, Venkatramu V, Lavín V. Upconversion and Color Tunability in Er3+–Tm3+–Yb3+ Tri-Doped Fluorophosphate Glasses. Photonics. 2025; 12(8):745. https://doi.org/10.3390/photonics12080745
Chicago/Turabian StyleRivera-López, Fernando, Palamandala Babu, Vemula Venkatramu, and Víctor Lavín. 2025. "Upconversion and Color Tunability in Er3+–Tm3+–Yb3+ Tri-Doped Fluorophosphate Glasses" Photonics 12, no. 8: 745. https://doi.org/10.3390/photonics12080745
APA StyleRivera-López, F., Babu, P., Venkatramu, V., & Lavín, V. (2025). Upconversion and Color Tunability in Er3+–Tm3+–Yb3+ Tri-Doped Fluorophosphate Glasses. Photonics, 12(8), 745. https://doi.org/10.3390/photonics12080745






